Key Points
In vivo and in vitro thrombus formation is altered in MRP4-deficient mice.
MRP4 modulates the cAMP–protein kinase A platelet signaling pathway.
Abstract
Molecules that reduce the level of cyclic adenosine 5′-monophosphate (cAMP) in the platelet cytosol, such as adenosine 5′-diphosphate (ADP) secreted from dense granules, trigger platelet activation. Therefore, any change in the distribution and/or availability of cyclic nucleotides or ADP may interfere with platelet reactivity. In this study, we evaluated the role of multidrug resistance protein 4 (MRP4, or ABCC4), a nucleotide transporter, in platelet functions in vivo and in vitro by investigating MRP4-deficient mice. MRP4 deletion resulted in a slight increase in platelet count but had no impact on platelet ultrastructure. In MRP4-deficient mice, the arterial occlusion was delayed and the tail bleeding time was prolonged. In a model of platelet depletion and transfusion mimicking a platelet-specific knockout, mice injected with MRP4−/− platelets also showed a significant increase in blood loss compared with mice injected with wild-type platelets. Defective thrombus formation and platelet activation were confirmed in vitro by studying platelet adhesion to collagen in flow conditions, integrin αIIbβ3 activation, washed platelet secretion, and aggregation induced by low concentrations of proteinase-activated receptor 4–activating peptide, U46619, or ADP. We found no role of MRP4 in ADP dense-granule storage, but MRP4 redistributed cAMP from the cytosol to dense granules, as confirmed by increased vasodilator-stimulated phosphoprotein phosphorylation in MRP4-deficient platelets. These data suggest that MRP4 promotes platelet aggregation by modulating the cAMP–protein kinase A signaling pathway, suggesting that MRP4 might serve as a target for novel antiplatelet agents.
Introduction
Circulating platelets are maintained in an inactive state, thus avoiding inappropriate activation and clot formation. This resting state is dependent on cyclic nucleotide homeostasis. Endothelial prostacyclin and adenosine generated through adenosine triphosphate (ATP) metabolism bind to their respective receptors and thereby activate adenylate cyclase (AC) and consequently cyclic adenosine 5′-monophosphate (cAMP) synthesis.1 Nitric oxide (NO), a free radical messenger mainly produced by endothelial cells, activates soluble guanylate cyclases to generate cyclic guanosine monophosphate (cGMP) (for a review, see Smolenski2). An increase in cGMP levels during platelet stimulation3,4 has also been shown to occur via an NO synthase–independent pathway.5 cAMP and cGMP elevation results in the activation of cyclic nucleotide–dependent protein kinases (cAMP-dependent protein kinase [PKA] and cGMP-dependent protein kinase [PKG], respectively), which phosphorylate a broad panel of substrate proteins.6 The rise in platelet cyclic nucleotide levels results in a downregulation of activating signaling pathways that in turn inhibits platelet cytoskeleton rearrangement, fibrinogen receptor activation, secretion processes, and procoagulant activity.2 Common substrates of these kinases include signaling regulators such as vasodilator-stimulated phosphoprotein (VASP), one of the major PKA and PKG substrates that is an important regulator of actin dynamics2. VASP has 3 phosphorylation sites (Ser157, Ser239, and Thr278) that are used by both PKA and PKG, Ser157 being mainly phosphorylated by PKA.7 To limit the inhibitory effect of cyclic nucleotides, phosphodiesterases (PDEs) rapidly hydrolyze them.8 Thus, the cytosolic cAMP level results from a balance between its synthesis from ATP and its degradation into AMP by PDEs and, potentially, its transport from cytosol to dense granules.
Molecules such as adenosine 5′-diphosphate (ADP) that reduce the level of cytosolic cyclic nucleotides act as platelet agonists. ADP is secreted by activated platelets from dense granules, and its binding to the G-coupled P2Y12 receptor inhibits AC via the Gαi subunit.9 Modulation of cellular cyclic nucleotide levels has become an important focus of drug development, including anti-P2Y12 agents, PDE inhibitors, NO donors, and prostacyclin analogs.10 Of interest, the pharmacodynamics of molecules that act by increasing cAMP level can be specifically and routinely monitored by measuring VASP phosphorylation level.11
Although the major role of cAMP in platelet regulation has been known for many years, the underlying molecular mechanisms are only beginning to emerge. One candidate in the regulation of cyclic nucleotide levels in platelet cytosol is multidrug resistance protein 4 (MRP4, or ABCC4), a membrane transporter capable of pumping structurally diverse endogenous compounds, including cyclic nucleotides and nucleotide analogs12 and xenobiotics out of various cells (for reviews, see Russel et al13 and Cheepala et al14). In particular, MRP4 has been shown to play a role in cAMP homeostasis in vascular smooth cells and cardiac myocytes.15,16 MRP4 inhibition was found to protect mice from pulmonary hypertension by increasing intracellular cAMP levels and preventing activation of cAMP-mediated signaling pathways.17 MRP4 inhibition was thus associated with reduced small pulmonary artery remodeling.17 In platelets, MRP4 is located on the membrane of dense (δ) granules and, to a lesser extent, the plasma membrane, and could therefore be involved in the transport of molecules, notably adenine nucleotides, from cytosol into dense granules.18 Depletion of MRP4 on dense granules has been found in some Hermansky-Pudlak syndrome patients, correlating with defective adenine nucleotide storage.18 However, in contrast to “classical” δ storage pool disease, these patients had normal dense-granule serotonin levels.19 Borgognone and Pulcinelli20 inferred that MRP4 is involved in the control of platelet cyclic nucleotide levels, and that MRP4 inhibition enhances PKA and PKG activity and thereby inhibits platelet activation. Therefore, the identification of MRP4 as a candidate transporter for adenine nucleotides in platelet dense granules represents a first step toward the elucidation of a role for MRP4 in platelets. In this study, we further examined the role of MRP4 in platelet functions by using an MRP4-deficient mouse model. Our results show that MRP4 is involved in the vesicular storage of cAMP and consequently plays a role in the modulation of platelet functions.
Methods
Animals
MRP4-deficient mice (MRP4−/−) were originally generated in the laboratory of John Schuetz21 and were repeatedly backcrossed to Friend virus B-type (FVB) mice to greater than 99% FVB. All our experiments compared MRP4−/− mice with age- and gender-matched FVB wild-type (WT) mice. Anesthesia was induced by intraperitoneal injection of ketamine (80 mg/kg) plus xylazine (10 mg/kg) or by isofluorane for tail venous thrombosis experiments. All animal studies were approved by the Ethics Committee on Animal Resources of Paris Descartes University (registration number CEEA34.CBL.131.12.).
Methods are available in supplemental Methods, found on the Blood Web site.
Results
MRP4-deficient platelet characterization
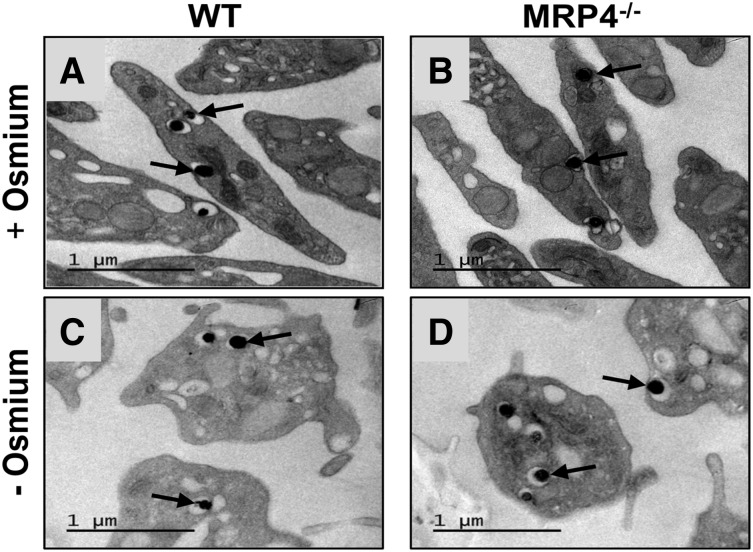
Western blot analysis of platelet proteins confirmed the expression of MRP4 in WT platelets and its absence in platelets from MRP4−/− mice (supplemental Figure 1). In line with previous reports,22,23 we did not find expression of MRP5, another member of the ABC family that can efflux cyclic nucleotide analogs24 (data not shown). Cell counts on anticoagulated blood showed a slight but significant increase in the platelet count and platelet volume in MRP4−/− mice compared with WT mice, whereas hematocrit was similar (supplemental Table 1). However, bone marrow smears showed no morphologic differences in megakaryocytes from WT and MRP4−/− mice, and no difference in cell ploidy was found by flow cytometry (supplemental Figure 2). Transmission electron microscopy showed no differences in platelet or granule ultrastructure between WT and MRP4−/− mice (Figure 1A-B), even when osmium was omitted to specifically contrast nucleotide analogs with uranyl acetate (Figure 1C-D). No differences were found in glycoprotein (GP)Ibα, GPIX, GPV, GPVI, CD49b, or CD41 platelet surface expression (supplemental Table 2).
Figure 1.
Platelet transmission electron microscopy. Representative transmission electron micrographs of resting WT (A,C) and MRP4−/− (B,D) platelets labeled with uranyl acetate in the presence (A,B) or absence (C,D) of osmium. The arrows indicate dense granules.
MRP4−/− mice have delayed arterial thrombosis and a longer bleeding time
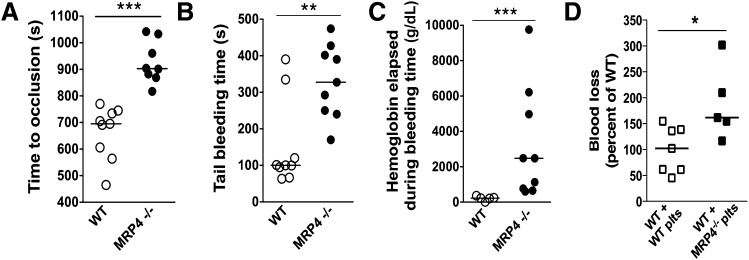
The impact of MRP4 deletion on in vivo hemostasis was evaluated using thrombosis and bleeding experiments. In the model of carotid artery thrombosis in response to a 15% ferric chloride patch, MRP4−/− mice showed a significantly longer time to occlusion (903 seconds [95% confidence interval (CI), 817-1042] vs 695 seconds [95% CI, 564-745] for MRP4−/− and WT mice, respectively; P < .0001; Figure 2A).
Figure 2.
Role of MRP4 in bleeding and thrombosis. (A) Carotid artery thrombosis was induced by placing a 15% ferric chloride patch on the artery for 4 minutes, and the time to occlusion was recorded (n ≥ 8 animals in each group) (***P < .0001). (B) Tail bleeding time in WT and MRP4−/− mice (n = 9) (**P < .001). (C) Hemoglobin concentration in the chamber effluent (containing 10 ml NaCl) was measured by the Drabkin method (n ≥ 5 animals in each group) (***P < .001). (D) Platelet-depleted WT mice were injected with WT or MRP4−/− washed platelets (plts). Platelet count was checked to be higher than 3 × 108 platelets per milliliter in each animal, and the lateral tail vein was cut as described in supplemental Methods. Blood loss was measured and normalized to WT (n ≥ 5 animals in each group) (*P < .05).
Concerning bleeding, MRP4−/− mice did not bleed spontaneously but showed a significantly longer tail bleeding time than WT mice (328 seconds [95% CI, 240-428] vs 100 seconds [95% CI, 67-335], respectively; P < .001; Figure 2B), which was associated with more blood loss, as estimated by the hemoglobin content (2.48g/dL [95% CI, 0.65-6.21] vs 0.23g/dL [95% CI, 0.12-0.36] for MRP4−/− and WT mice, respectively; P < .001; Figure 2C). Rebleeding was observed in none of the WT mice but in 30% of MRP4−/− mice.
However, because the MRP4−/− model used is not specific for platelets, a contribution of MRP4 deletion in the vascular bed might interfere with in vivo results. Therefore, we investigated the specific role of MRP4 in platelets by performing tail venous thrombosis in WT mice depleted in platelets and transfused with WT or MRP4−/− platelets. In this model mimicking a platelet-specific knockout, mice injected with MRP4−/− platelets also showed a significant increase in blood loss compared with mice injected with WT platelets (162% [95% CI, 101-277] vs 102% [95% CI, 59-141] normalized to WT, for mice transfused with MRP4−/− and WT platelets, respectively; P < .05; Figure 2D).
These results indicate that MRP4, and especially platelet MRP4, is involved in positive regulation of hemostasis and arterial thrombosis in vivo.
Decreased thrombus formation during perfusion of MRP4−/− blood on collagen in flow conditions
For ex vivo experiments, we considered the flow adhesion model on collagen as the first step to analyze the impact of MRP4 deletion on platelet functions because it is a well-recognized model that mimics platelet adhesion and activation under arterial flow.
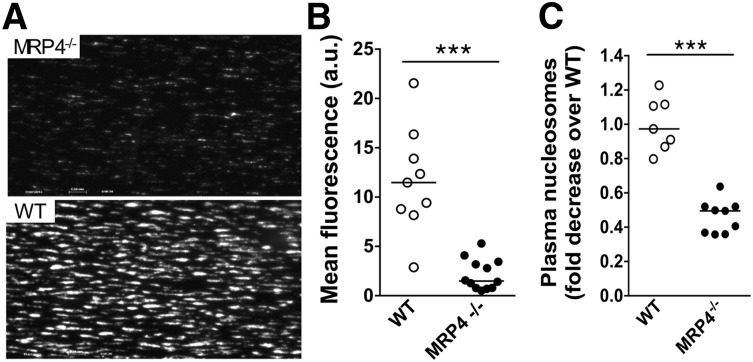
In vitro thrombus formation on collagen was studied in a whole-blood perfusion system at an arterial shear rate of 1800 seconds−1. During early steps of adhesion (30 seconds), no significant difference was noted in fluorescence intensity between MRP4−/− (4.3 arbitrary units [au] [95% CI, 0.64-7.7]) and WT (0.9 au [95% CI, 0.14-2.8]; P > .05). However, at 3 minutes, thrombus formation by MRP4−/− platelets was strongly reduced in terms of number and size (Figure 3A, top panel) compared to WT platelets (Figure 3A, bottom panel). Mean fluorescence intensity, reflecting the 3-dimensional size/volume of aggregates, was significantly (∼fivefold) lower with MRP4−/− than with WT blood (1.5 au [95% CI, 0.8-3.4] vs 11.5 au [95% CI, 8.2-16.4] for MRP4−/− and WT mice, respectively; P < .0001; Figure 3B).
Figure 3.
Role of MRP4 in whole-blood platelet adhesion ex vivo. Whole blood from MRP4−/− and WT mice was collected on PPACK (80 μM)/lepirudin (100 U/mL). Dioc6-labeled treated blood was perfused over a fibrillar collagen matrix (50 μg/mL) at a shear rate of 1800 seconds−1 for 3 minutes. (A) The formation of Dioc6-stained thrombi was recorded with a CoolSnap camera on a Leica DM IRB microscope. Images are representative of a minimum of 7 experiments in each group. (B) The mean fluorescence intensity was quantified with ImageJ software. Each point, corresponding to an independent experiment (n ≥ 7), represents the mean fluorescence intensity of at least 8 fields. (C) Nucleosome content was quantified in plasma prepared from the flow chamber effluent, using an enzyme-linked immunosorbent assay cell-death detection kit. One arbitrary unit (a.u.) of nucleosomes corresponds to the average amount of nucleosomes contained in plasma from a minimum of 8 WT control animals (***P < .0001).
We then quantified nucleosome production in plasma obtained from the flow chamber effluent to assess the capacity of platelet activation on collagen to induce neutrophil extracellular trap (NET) formation. Nucleosomes are a complex of DNA and histones released from neutrophils during clot formation, resulting in the formation of NETs that contribute to thrombus formation and stability. NET formation was used as another tool to reflect platelet activation leading to P-selectin exposure, platelet binding to leukocytes, and subsequent cell activation. As shown in Figure 3C, a significantly lower nucleosome level was obtained with MRP4−/− blood (0.5-fold decrease [95% CI, 0.36-0.52]) compared to WT blood (0.97-fold decrease [95% CI, 0.8-1.2]; P < .0001). These results suggest that MRP4 deficiency results in impaired platelet activation.
Impaired MRP4-deficient platelet functions in vitro
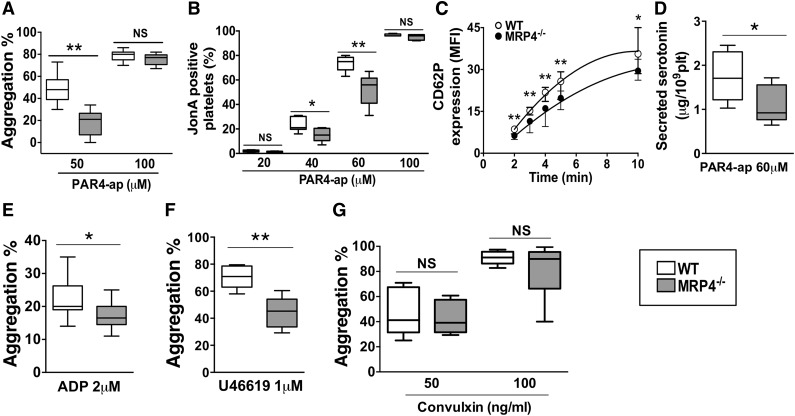
The influence of MRP4 on washed platelet reactivity in response to different agonists was studied. No differences were noted in platelet aggregation induced by a high PAR4–activating peptide (ap) concentration of 100 μM (77% [95% CI, 67-82] vs 80% [95% CI, 70-86] in MRP4−/− and WT mice, respectively). In contrast, at a lower PAR4-ap concentration of 50 μM, which induces activation highly dependent on secreted ADP, MRP4−/− platelet aggregation was halved with respect to WT platelets (21% [95% CI, 0-28] vs 48% [95% CI, 36-62]; P < .01; Figure 4A and supplemental Figure 3). Of interest, low ADP dose (0.5 µM) rescued aggregation of MRP4−/− platelets (supplemental Figure 4).
Figure 4.
Role of MRP4 in platelet activity in vitro. Washed-platelet aggregation was monitored by light transmission through a platelet suspension at a concentration of 3.5 × 108 platelets per milliliter. (A) PAR4-ap (50 and 100 μM) induced platelet aggregation; results are expressed as the percentage of maximal aggregation (n ≥ 9) (**P < .01). (B) αIIbβ3 activation was evaluated in WT or MRP4−/− washed platelets activated for 10 minutes with increasing concentrations of PAR4-ap in the presence of phycoerythrin-labeled JonA antibody. The experiment was performed without stirring to prevent platelet aggregation. The level of activated integrin is indicated by the percentage of JonA-positive platelets measured by flow cytometry (n ≥ 4) (*P < .05; **P < .01). (C) α-Granule secretion was measured by P-selectin (CD62P) plasma membrane expression in response to PAR4-ap (100 μM) and was analyzed using flow cytometry after platelet incubation with fluorescein isothiocyanate–labeled rat anti-mouse CD62P. P-selectin expression is expressed as the mean fluorescence intensity (MFI) (n ≥ 5) (*P < .05; **P < .01). (D) Dense granule secretion was measured by serotonin release in platelet supernatants after PAR4-ap (60 μM) induced activation (n ≥ 6) (*P < .05). (E-G) Agonist-induced platelet aggregation: platelets were incubated at 37°C under stirring and then activated with ADP in the presence of fibrinogen (5 μg/mL) (n = 13) (*P < .05) (E); with U46619 (n = 6) (**P < .01) (F); and with 50 and 100 ng/mL convulxin (n ≥ 6) (G). Each box represents the interquartile range with median maximal aggregation (horizontal line in the box); the whiskers represent the fifth to 95th percentiles. NS, not significant.
To specifically analyze the involvement of MRP4 in αIIbβ3 activation mediated by inside-out signaling, we investigated JonA binding in response to PAR4-ap. Compared to WT platelets, the percentage of MRP4−/− JonA-positive platelets was significantly lower when activated with 40 to 60 µM PAR4-ap (56% [95% CI, 36-69] vs 75% [95% CI, 67-80] for WT platelets, in the presence of 60 µM PAR4-ap; P < .01; Figure 4B). No differences were noted in response to high PAR4-ap concentration (100 μM) with respect to JonA binding.
To confirm the defect of platelet activation, we examined the P-selectin (CD62P) exposure by flow cytometry as an indicator of α-granule secretion. Upon PAR4-ap–induced activation, MRP4-deficient platelets showed lower CD62P expression than WT platelets at 2 minutes (geometric mean 6.3 [95% CI, 4.9-7.2] vs 8.6 [95% CI, 7-9.7]; P < .01; Figure 4C) and at 5 minutes (geometric mean 19.8 [95% CI, 15.6-22.3] vs 25.8 [95% CI, 19.3-29.2], respectively; P < .01). Although weaker, this respective decrease was still observed at 10 minutes (geometric mean 29.5 [95% CI, 26.2-33.7] vs 35.6 [95% CI, 28.5-45]; P < .05). To explore dense-granule secretion, we quantified serotonin, which is passively stored in granules independently of MRP4. Secreted serotonin was decreased for MRP4−/− platelets after low PAR4 activation (PAR4-ap 60 μM) (0.9 μg per 109 MRP4−/− platelets [95% CI, 0.6-1.7] vs 1.7 μg per 109 WT platelets [95% CI, 1.0-2.5]; P < .05; Figure 4D). After PAR4 strong activation (PAR4-ap 200 μM), serotonin secretion did not differ (1.9 μg per 109 MRP4−/− platelets [95% CI, 1.7-2.4] vs 1.9 μg per 109 WT platelets [95% CI, 1.7-2.6]; P > .05), nor did total serotonin content (data not shown).
To investigate the involvement of MRP4 in platelet aggregation in response to other G-coupled receptor agonists, we used ADP and U46619. At a low ADP concentration (2 µM), MRP4−/− platelet aggregation was significantly lower than WT aggregation (16.5% [95% CI, 14-21] vs 20% [95% CI, 19-27], respectively; P < .05; Figure 4E), whereas no difference was noted at a higher ADP concentration (10 μM; 42% [95% CI, 36-46] vs 42% [95% CI, 32-53], respectively; data not shown). Washed platelet aggregation tests showed that loss of function of MRP4-deficient platelets was less pronounced when aggregation was induced by ADP (17.7% ± 5.2% inhibition relative to WT) rather than PAR4-ap (54.9% ± 6.7% inhibition relative to WT). At 1 μM U46619 concentration, aggregation was also significantly decreased for MRP4−/− compared to WT platelets (45% [95% CI, 33-56] vs 71% [95% CI, 62-79], respectively; P < .01; Figure 4F).
Finally, no differences between WT and MRP4−/− were denoted when aggregation was induced by soluble convulxin (a specific GPVI agonist) (Figure 4G) or collagen (supplemental Figure 5).
Normal storage of ADP and ATP in MRP4-deficient platelets
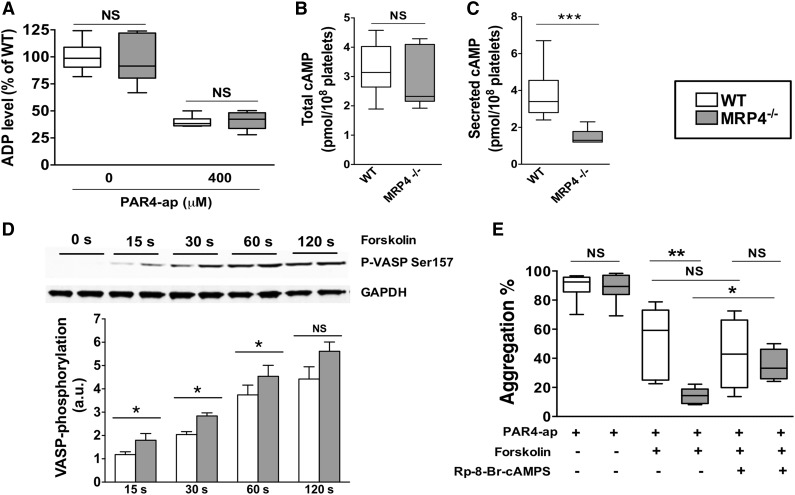
Considering our results showing a global defect in MRP4−/− platelet activation, we hypothesized a lack of secreted-ADP amplification in MRP4-deficient platelets that could be due to defective ADP storage in MRP4−/− dense granules. To explore this hypothesis, we investigated ADP dense-granule storage. As shown in Figure 5A, the ADP level did not differ between WT and MRP4−/− in resting platelets (left panel, 0 μM PAR4-ap). After strong activation, the residual cytosolic ADP level in platelets also did not differ between WT and MRP4−/− (right panel, 400 μM PAR4-ap), showing that the estimated secreted fraction was similar. These results strongly suggest that MRP4 does not play a major role in ADP dense-granule storage in mouse platelets. The amount of ATP secreted upon strong platelet activation, allowing full aggregation in both animal groups (PAR4-ap 400 μM), was also similar between WT and MRP4-deficient platelets (data not shown).
Figure 5.
Role of MRP4 in ADP and cAMP dense-granule storage. (A) ADP was quantified either in lysates of unstimulated or degranulated platelet pellets (obtained by a centrifugation of 2 minutes at 12 000g). Degranulated platelets were obtained after 10 minutes of activation with 400 μM PAR4-ap. Platelets were centrifuged, the supernatant was discarded, and lysis buffer was added to the pellets before quantifying ADP (n = 6). cAMP was quantified in total platelets at rest (n ≥ 6) (B) and in the supernatant of activated platelets (n ≥ 6) (***P < .001) (C). (D) WT and MRP4−/− platelets were incubated with forskolin for the indicated times, and lysates were harvested for western blot analysis and densitometry. Blots were probed with anti P-VASP Ser 157 and loading was controlled with anti-GAPDH. Quantitative analysis is obtained by densitometry using Image J software. Values are mean ± standard error of the mean (n ≥ 4) (*P < .05). Upper panel shows a representative western blot of the phosphorylation kinetics of P-VASP Ser 157 in WT and MRP4−/−. (E) Effect of cAMP-elevating agent and PKA inhibitor on PAR4-ap–induced platelet aggregation. Platelets were preincubated with the PKA inhibitor Rp-8-Br-cAMPS (500 μM) or with vehicle for 10 minutes. Forskolin (5 μM) or vehicle was then added for 15 seconds at 37°C under stirring before activation with PAR4-ap (50 μM) (n ≥ 4) (*P < .05; **P < .001). Boxes represent the interquartile range with median maximal aggregation (horizontal line); whiskers represent the fifth to 95th percentiles. GAPDH, glyceraldehyde-3-phosphate dehydrogenase.
Altered cAMP distribution and increased in PKA pathway activity in MRP4-deficient platelets
We sought to evaluate whether MRP4 deficiency was associated with an increase in the cyclic nucleotide level due to impaired redistribution from the cytosol to dense granules and, consequently, a lack of ADP secretion owing to lower platelet reactivity. The total cAMP level did not differ between MRP4−/− and WT platelets (2.3 pmol per 108 platelets [95% CI, 2.1-3.8] vs 3.1 pmol per 108 platelets [95% CI, 2.6-3.9], respectively; P > .05; Figure 5B). However, a significant decrease in the amount of secreted cAMP was observed with MRP4-deficient platelets upon full activation by a saturating PAR4-ap concentration (400 µM) (1.3 pmol per 108 platelets [95% CI, 1.0-1.9] vs 3.4 pmol per 108 platelets [95% CI, 2.7-4.8] for WT platelets; P < .001; Figure 5C). Moreover, in contrast to WT (Figure 5B-C, open boxes), secreted cAMP was significantly lower than total cAMP for MRP4−/− (P < .01, gray boxes). The role of MRP4 in the regulation of cytosolic cGMP was also investigated. Total and secreted cGMP was quantified in PAR4-ap activated platelets preincubated with sodium nitroprusside. No differences were observed in total cGMP (9.3 pmol per 108 platelets [95% CI, 6.5-11.2] vs 9.0 pmol per 108 platelets [95% CI, 5.6-10.9] for WT and MRP4−/− platelets, respectively), and, in contrast to cAMP, no differences were observed in secreted cGMP (6.7 pmol per 108 platelets [95% CI, 5.6-10.0] vs 6.6 pmol per 108 platelets [95% CI, 5.0-8.5], for WT and MRP4−/− platelets, respectively).
These results suggest a role of MRP4 in vesicular cAMP storage and, thus, an altered cAMP distribution in MRP4−/− platelets. To determine whether these differences in the cAMP level affected downstream signaling, we specifically analyzed VASP phosphorylation on Ser157, a preferential cAMP-dependent protein kinase phosphorylation site. VASP phosphorylation kinetics were measured in the presence of the AC activator forskolin (5 μM), used to sensitize the system. As shown in Figure 5D, the kinetic response of PKA was significantly increased in MRP4−/− platelets, as shown by a higher VASP phosphorylation level on Ser157 at 15, 30, and 60 seconds. We did not denote a significant difference after 120 seconds. To confirm that MRP4−/− platelets are more sensitive to inhibition mediated by AC pathway activation, platelets were pretreated with forskolin before PAR4-ap–induced aggregation. Aggregation in the presence of forskolin was significantly decreased for MRP4−/− platelets compared to WT (14% [95% CI, 7-21] vs 59% [95% CI, 28-78], respectively; P < .001; Figure 5E). Preincubation with the PKA inhibitor Rp-8-Br-cAMPS abrogated the difference between WT and MRP4−/− platelet aggregation (43% [95% CI, 4-82] vs 33% [95% CI, 18-52], respectively; P > .05; Figure 5E).
Of interest, no compensatory change in PDE activity was observed (supplemental Figure 6).
Discussion
MRP4 has been shown to play an important role in conveying molecules involved in cellular signaling and, more recently, in platelet activation.18,20 In this study, we examined the mechanism by which MRP4 influences platelet reactivity in a model of MRP4 invalidation in vivo and in vitro. First, hemostasis and thrombosis were both disrupted in MRP4−/− mice, with a prolonged bleeding time and delayed carotid occlusion. The specific MRP4 platelet involvement in in vivo hemostasis was further confirmed using platelet-depleted WT mice transfused with MRP4-deficient platelets. These results are in line with those of previous studies showing that the absence or inhibition of MRP4 leads to a decrease in human platelet function.19,20 It was initially proposed that MRP4 was responsible for ATP and ADP storage in dense granules. Indeed, Jedlitschky et al,18,19 studying Hermansky-Pudlak syndrome patients, found in few patients an absence of MRP4 (or an abnormal location on the plasma membrane) associated with defective ADP storage in dense granules and diminished ATP secretion. However, these investigators did not study cyclic nucleotide levels. Given the polyspecificity of MRP4 and its affinity for various substrates (for a review, see Russel et al13), recent studies have also focused on the role of MRP4 in cyclic nucleotide regulation in platelets. Borgognone and Pulcinelli20 showed that cyclic nucleotides are physiologically transported into dense granules and that this transport can be inhibited by the MRP4 inhibitor MK571, leading to platelet inhibition. However, using MK571 at the same concentrations of 50 to 100 μM, we observed functional inhibition of WT and MRP4−/− mouse platelets, confirming a nonspecific inhibitory effect of MK571 (data not shown). This nonspecific effect has been recently attributed to inhibition of Akt and JNK phosphorylation.25 Therefore, the MRP4−/− mouse model is a useful tool to avoid such drawbacks.
As shown by Sassi et al,15 the absence of MRP4 impacts vascular muscle cell proliferation and thus likely affects vascular reactivity to injury. We therefore studied platelet MRP4−/− functions in vitro. After determining that MRP4 deletion did not have a major impact on platelet and dense-granule structure or on adhesion receptor expression, we showed a loss-of-function phenotype with defective platelet activation on a collagen matrix under flow, whereas adhesion was not impaired. MRP4-deficient platelet aggregation and, more precisely, αIIbβ3 activation due to inside-out signaling, was reduced in the presence of low concentrations of agonists, especially G protein–coupled receptor agonists. In contrast, platelet aggregation consecutive to GPVI activation was not impaired. This result suggests that this signaling pathway is less sensitive to MRP4 regulation than G protein–coupled receptor pathways. However, because of the strong defect in thrombus formation on collagen under flow, we can also assume that the lack of difference between groups may be due to large between-experiment variability in the dose response to collagen and convulxin.
The defect in MRP4−/− platelet activation was confirmed by P-selectin exposure and serotonin secretion, which showed decreased α- and dense-granule secretion by MRP4−/− platelets, respectively.
Suspecting defective ADP storage and/or modified cyclic nucleotide regulation in MRP4−/− platelets, we then examined ADP-induced aggregation. ADP is not a strong agonist and induces platelet aggregation without secretion (especially ADP stored in dense granules). In this condition, platelet activation is specifically dependent on P2Y12 and P2Y1 activation and on AC inhibition by the P2Y12-associated subunit Gαi. Despite an absence of amplification by stored ADP, a significant decrease in MRP4−/− platelet aggregation was observed, suggesting defective cyclic nucleotide regulation. By measuring the level of ADP in resting and activating platelets, we further confirmed the minor role of MRP4 in ADP storage in dense granules, at least in the MRP4−/− mouse model. This finding was also supported by the transmission electron microscopy performed with uranyl acetate in the absence of osmium to specifically label nucleotide analogs, because ADP is the most abundant nucleotide in platelet dense granules and no difference was found between MRP4−/− and WT in this labeling condition. Because ADP is stored during dense-granule formation in megakaryocytes, another transporter is probably involved. The vesicular nucleotide transporter (VNUT, SLC17A9) has recently been proposed as a candidate for ADP and ATP accumulation in dense granules. Indeed, Hiasa et al26 showed the presence of functional VNUT in the dense-granule membrane and found that inhibition of VNUT expression by small interfering RNA in MEG-01 cells led to a decrease in ADP and ATP release. Biochemical studies have shown that VNUT requires an inside positive hydrogen ion gradient to transport negatively charged ATP.27 The transport experiments performed by Jedlitschky et al18 probably did not create the conditions required to obtain such a hydrogen ion gradient, which is likely why they did not find functional VNUT in their platelet membrane vesicles.
The present study suggests that the defective MRP4−/− platelet function is due to accumulation of cyclic nucleotides in the platelet cytosol. Indeed, dense-granule “secretable” cAMP was strongly decreased in these platelets, whereas total cAMP levels were unaffected. A deregulation of the cAMP pathway was further supported by the observation that inhibition of MRP4-deficient platelets by forskolin was significantly more pronounced compared to WT platelets, this difference being abrogated by a PKA inhibitor. Moreover, the increased phosphorylation of a PKA substrate (VASP ser157) in MRP4−/− platelets is also consistent with a cytosolic cAMP increase in these platelets. In agreement with results obtained by Sassi et al15 in smooth muscle cells, MRP4 deletion shifted the forskolin response without affecting the maximal effect of the drug, supporting the absence of difference in VASP phosphorylation between genotypes after 120 seconds of AC stimulation. In contrast to cAMP, MRP4 appears not to interfere with cGMP homeostasis in our model. These results are in apparent contradiction to those reported by Jedlitschky et al18 and by Borgognone and Pulcinelli.20 One explanation would be that they used a subcellular fraction of enriched dense granules and measured the transport of exogenous cGMP in the presence or absence of inhibitors. Otherwise, we cannot exclude a lack of sensitivity of the cGMP assay to evidence a role of MRP4 in our model using platelets instead of an enriched granule fraction.
It has been suggested, however, that cAMP and cGMP are secreted by 2 different pathways, cGMP being transported by MRP5.28 Of interest, MRP5 has been found on the plasma membrane of megakaryocytes.29 Therefore, we can assume that a remnant of MRP5 sufficient to behave as a membrane transporter of cGMP may still be present in platelets, although not detectable by western blot analysis.
These results suggest an important role of MRP4 in platelet cAMP homeostasis, controlled not only by its classical synthesis and degradation but also by its compartmentation in microdomains14,30 or in subcellular compartments. This finding is in agreement with a previously described role of MRP4 as a physiological cAMP transporter in smooth cells and cardiac myocytes.15,16
MRP4 could also play a role in megakaryocytopoiesis. Indeed, Oevermann et al29 showed that MRP4 expression is increased in the megakaryocyte lineage during hematopoietic stem cell differentiation. Moreover, Begonja et al31 reported evidence of a dominant role of cyclic nucleotides in megakaryocyte differentiation. However, because platelet counts were only slightly increased in MRP4−/− mice, MRP4 would not appear to play a major role in megakaryocyte maturation or platelet formation, at least in this mouse model. Finally, a recent study by Bröderdorf et al32 showed that MRP4 expression can be regulated by cAMP in vascular smooth muscle cells and hematopoietic cells, suggesting a feedback control mechanism.
In addition, it has been shown that MRP4 transports aspirin and contributes to aspirin resistance, particularly in coronary patients, with MRP4 upregulation on the platelet surface resulting in aspirin extrusion.33 It has been shown that MRP4 expression and aspirin are linked and that aspirin treatment enhances MRP4 expression by megacaryocytes.34
In conclusion, our results provide evidence for a novel role of MRP4 in platelet cyclic nucleotide regulation. They also show that MRP4 is not the main transporter responsible for ADP and ATP accumulation in dense granules. MRP4 could represent a target for new antiplatelet agents that would provide weak, controlled, nondeleterious inhibition of platelet activation, particularly in combination with aspirin.
Acknowledgments
The authors thank R. Lai-Kuen and B. Saubamea (Cellular and Molecular Imaging Platform, Centre de Recherche Pharmaceutique de Paris–Unité Mixte de Service 3612, Centre National de la Recherche scientifique–Unité de Services 25, INSERM–Institut de recherche pour le Développement–Université Paris Descartes) and V. Vieillefond for support with the experiments; Y. Sassi, M. Canault, and C. Dubois for helpful discussions; and S. Poirault-Chassac and F. Atassi for excellent technical assistance. The authors also thank M. Andrieu and K. Labroquère from the Cochin Cytometry and Immunobiology Facility for access to the BD LSR II cytometer.
This work received grants from INSERM, Agence Nationale de la Recherche (09-JCJC-0112), and the Promex Stiftung für die Forschung. B. B. Decouture was the recipient of a Research Fellowship from the New French Atherosclerosis Society and the Promex Stiftung für die Forschung.
Footnotes
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Authorship
Contribution: B. Decouture designed the study; performed the research; collected, analyzed, and interpreted the data; and wrote the initial manuscript; E.D., T.B.-R., and O.K. performed the experiments; B. Dizier, A.B., and B.C. performed the animal experiments; A.-M.L. and J.-S.H. provided the animals and critically reviewed the manuscript; C.V.D. designed the tail venous thrombosis model and discussed the results; and C.B.-L. and P.G. designed the research, performed some experiments, interpreted the data, and wrote the manuscript.
Conflict-of-interest disclosure: J.-S.H. and A.-M.L. have a patent on the use of MRP4 inhibitors for the treatment of cardiovascular disorders (EP07290433.7, INSERM). The remaining authors declare no competing financial interests.
Correspondence: Christilla Bachelot-Loza, INSERM UMRS 1140, Faculté de Pharmacie, 4 Avenue de l’Observatoire, 75006 Paris, France; e-mail: christilla.bachelot-loza@parisdescartes.fr.
References
- 1.Radomski MW, Palmer RM, Moncada S. Comparative pharmacology of endothelium-derived relaxing factor, nitric oxide and prostacyclin in platelets. Br J Pharmacol. 1987;92(1):181–187. doi: 10.1111/j.1476-5381.1987.tb11310.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Smolenski A. Novel roles of cAMP/cGMP-dependent signaling in platelets. J Thromb Haemost. 2012;10(2):167–176. doi: 10.1111/j.1538-7836.2011.04576.x. [DOI] [PubMed] [Google Scholar]
- 3.Haslam RJ, McClenaghan MD. Effects of collagen and of aspirin on the concentration of guanosine 3′:5′-cyclic monophosphate in human blood platelets: measurement by a prelabelling technique. Biochem J. 1974;138(2):317–320. doi: 10.1042/bj1380317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Riba R, Oberprieler NG, Roberts W, Naseem KM. Von Willebrand factor activates endothelial nitric oxide synthase in blood platelets by a glycoprotein Ib-dependent mechanism. J Thromb Haemost. 2006;4(12):2636–2644. doi: 10.1111/j.1538-7836.2006.02195.x. [DOI] [PubMed] [Google Scholar]
- 5.Gambaryan S, Kobsar A, Hartmann S, et al. NO-synthase-/NO-independent regulation of human and murine platelet soluble guanylyl cyclase activity. J Thromb Haemost. 2008;6(8):1376–1384. doi: 10.1111/j.1538-7836.2008.03014.x. [DOI] [PubMed] [Google Scholar]
- 6.Beck F, Geiger J, Gambaryan S, et al. Time-resolved characterization of cAMP/PKA-dependent signaling reveals that platelet inhibition is a concerted process involving multiple signaling pathways. Blood. 2014;123(5):e1–e10. doi: 10.1182/blood-2013-07-512384. [DOI] [PubMed] [Google Scholar]
- 7.Smolenski A, Bachmann C, Reinhard K, et al. Analysis and regulation of vasodilator-stimulated phosphoprotein serine 239 phosphorylation in vitro and in intact cells using a phosphospecific monoclonal antibody. J Biol Chem. 1998;273(32):20029–20035. doi: 10.1074/jbc.273.32.20029. [DOI] [PubMed] [Google Scholar]
- 8.Haslam RJ, Dickinson NT, Jang EK. Cyclic nucleotides and phosphodiesterases in platelets. Thromb Haemost. 1999;82(2):412–423. [PubMed] [Google Scholar]
- 9.Hechler B, Gachet C. P2 receptors and platelet function. Purinergic Signal. 2011;7(3):293–303. doi: 10.1007/s11302-011-9247-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gurbel PA, Tantry US. Combination antithrombotic therapies. Circulation. 2010;121(4):569–583. doi: 10.1161/CIRCULATIONAHA.109.853085. [DOI] [PubMed] [Google Scholar]
- 11.Tantry US, Bonello L, Aradi D, et al. Working Group on On-Treatment Platelet Reactivity. Consensus and update on the definition of on-treatment platelet reactivity to adenosine diphosphate associated with ischemia and bleeding. J Am Coll Cardiol. 2013;62(24):2261–2273. doi: 10.1016/j.jacc.2013.07.101. [DOI] [PubMed] [Google Scholar]
- 12.Chen ZS, Lee K, Kruh GD. Transport of cyclic nucleotides and estradiol 17-beta-D-glucuronide by multidrug resistance protein 4. Resistance to 6-mercaptopurine and 6-thioguanine. J Biol Chem. 2001;276(36):33747–33754. doi: 10.1074/jbc.M104833200. [DOI] [PubMed] [Google Scholar]
- 13.Russel FG, Koenderink JB, Masereeuw R. Multidrug resistance protein 4 (MRP4/ABCC4): a versatile efflux transporter for drugs and signalling molecules. Trends Pharmacol Sci. 2008;29(4):200–207. doi: 10.1016/j.tips.2008.01.006. [DOI] [PubMed] [Google Scholar]
- 14.Cheepala S, Hulot JS, Morgan JA, et al. Cyclic nucleotide compartmentalization: contributions of phosphodiesterases and ATP-binding cassette transporters. Annu Rev Pharmacol Toxicol. 2013;53:231–253. doi: 10.1146/annurev-pharmtox-010611-134609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sassi Y, Lipskaia L, Vandecasteele G, et al. Multidrug resistance-associated protein 4 regulates cAMP-dependent signaling pathways and controls human and rat SMC proliferation. J Clin Invest. 2008;118(8):2747–2757. doi: 10.1172/JCI35067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sassi Y, Abi-Gerges A, Fauconnier J, et al. Regulation of cAMP homeostasis by the efflux protein MRP4 in cardiac myocytes. FASEB J. 2012;26(3):1009–1017. doi: 10.1096/fj.11-194027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hara Y, Sassi Y, Guibert C, et al. Inhibition of MRP4 prevents and reverses pulmonary hypertension in mice. J Clin Invest. 2011;121(7):2888–2897. doi: 10.1172/JCI45023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jedlitschky G, Tirschmann K, Lubenow LE, et al. The nucleotide transporter MRP4 (ABCC4) is highly expressed in human platelets and present in dense granules, indicating a role in mediator storage. Blood. 2004;104(12):3603–3610. doi: 10.1182/blood-2003-12-4330. [DOI] [PubMed] [Google Scholar]
- 19.Jedlitschky G, Cattaneo M, Lubenow LE, et al. Role of MRP4 (ABCC4) in platelet adenine nucleotide-storage: evidence from patients with delta-storage pool deficiencies. Am J Pathol. 2010;176(3):1097–1103. doi: 10.2353/ajpath.2010.090425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Borgognone A, Pulcinelli FM. Reduction of cAMP and cGMP inhibitory effects in human platelets by MRP4-mediated transport. Thromb Haemost. 2012;108(5):955–962. doi: 10.1160/TH12-04-0232. [DOI] [PubMed] [Google Scholar]
- 21.Leggas M, Adachi M, Scheffer GL, et al. Mrp4 confers resistance to topotecan and protects the brain from chemotherapy. Mol Cell Biol. 2004;24(17):7612–7621. doi: 10.1128/MCB.24.17.7612-7621.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ritter CA, Jedlitschky G, Meyer zu Schwabedissen H, Grube M, Köck K, Kroemer HK. Cellular export of drugs and signaling molecules by the ATP-binding cassette transporters MRP4 (ABCC4) and MRP5 (ABCC5). Drug Metab Rev. 2005;37(1):253–278. doi: 10.1081/dmr-200047984. [DOI] [PubMed] [Google Scholar]
- 23.Köck K, Grube M, Jedlitschky G, et al. Expression of adenosine triphosphate-binding cassette (ABC) drug transporters in peripheral blood cells: relevance for physiology and pharmacotherapy. Clin Pharmacokinet. 2007;46(6):449–470. doi: 10.2165/00003088-200746060-00001. [DOI] [PubMed] [Google Scholar]
- 24.Jedlitschky G, Burchell B, Keppler D. The multidrug resistance protein 5 functions as an ATP-dependent export pump for cyclic nucleotides. J Biol Chem. 2000;275(39):30069–30074. doi: 10.1074/jbc.M005463200. [DOI] [PubMed] [Google Scholar]
- 25.Lien LM, Chen ZC, Chung CL, et al. Multidrug resistance protein 4 (MRP4/ABCC4) regulates thrombus formation in vitro and in vivo. Eur J Pharmacol. 2014;737:159–167. doi: 10.1016/j.ejphar.2014.05.001. [DOI] [PubMed] [Google Scholar]
- 26.Hiasa M, Togawa N, Miyaji T, Omote H, Yamamoto A, Moriyama Y. Essential role of vesicular nucleotide transporter in vesicular storage and release of nucleotides in platelets. Physiol Rep. 2014;2(6):e12034. doi: 10.14814/phy2.12034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sawada K, Echigo N, Juge N, et al. Identification of a vesicular nucleotide transporter. Proc Natl Acad Sci USA. 2008;105(15):5683–5686. doi: 10.1073/pnas.0800141105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Andric SA, Kostic TS, Stojilkovic SS. Contribution of multidrug resistance protein MRP5 in control of cyclic guanosine 5′-monophosphate intracellular signaling in anterior pituitary cells. Endocrinology. 2006;147(7):3435–3445. doi: 10.1210/en.2006-0091. [DOI] [PubMed] [Google Scholar]
- 29.Oevermann L, Scheitz J, Starke K, et al. Hematopoietic stem cell differentiation affects expression and function of MRP4 (ABCC4), a transport protein for signaling molecules and drugs. Int J Cancer. 2009;124(10):2303–2311. doi: 10.1002/ijc.24207. [DOI] [PubMed] [Google Scholar]
- 30.Agarwal SR, Yang PC, Rice M, et al. Role of membrane microdomains in compartmentation of cAMP signaling. PLoS One. 2014;9(4):e95835. doi: 10.1371/journal.pone.0095835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Begonja AJ, Gambaryan S, Schulze H, et al. Differential roles of cAMP and cGMP in megakaryocyte maturation and platelet biogenesis. Exp Hematol. 2013;41(1):91–101.e4. doi: 10.1016/j.exphem.2012.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bröderdorf S, Zang S, Schaletzki Y, Grube M, Kroemer HK, Jedlitschky G. cAMP regulates expression of the cyclic nucleotide transporter MRP4 (ABCC4) through the EPAC pathway. Pharmacogenet Genomics. 2014;24(10):522–526. doi: 10.1097/FPC.0000000000000084. [DOI] [PubMed] [Google Scholar]
- 33.Mattiello T, Guerriero R, Lotti LV, et al. Aspirin extrusion from human platelets through multidrug resistance protein-4-mediated transport: evidence of a reduced drug action in patients after coronary artery bypass grafting. J Am Coll Cardiol. 2011;58(7):752–761. doi: 10.1016/j.jacc.2011.03.049. [DOI] [PubMed] [Google Scholar]
- 34.Massimi I, Guerriero R, Lotti LV, et al. Aspirin influences megakaryocytic gene expression leading to up-regulation of multidrug resistance protein-4 in human platelets. Br J Clin Pharmacol. 2014;78(6):1343–1353. doi: 10.1111/bcp.12432. [DOI] [PMC free article] [PubMed] [Google Scholar]