Abstract
Pineapple production in Costa Rica increased nearly 300-fold during the last 30 yr, and >40,000 hectares of land are currently dedicated to this crop. At the end of the pineapple cropping cycle, plants are chopped and residues incorporated into the soil in preparation for replanting. Associated with increased pineapple production has been a large increase in stable fly, Stomoxys calcitrans (L.), populations. Stable flies are attracted to, and oviposit in, the decomposing, chopped pineapple residues. In conjunction with chemical control of developing larvae, adult trapping is an important control strategy. In this study, four blue-black fabric traps, Nzi, Vavoua, Model H, and Ngu, were compared with a white sticky trap currently used for stable fly control in Costa Rica. Overall, the white sticky trap caught the highest number of stable flies, followed by the Nzi, Vavoua, Model H, and Ngu. Collections on the white sticky trap increased 16 d after residues were chopped; coinciding with the expected emergence of flies developing in the pineapple residues. During this same time period, collections in the blue-black fabric traps decreased. Sex ratio decreased from >7:1 (females:males) 3–7 d after chopping to 1:1 at 24–28 d. White sticky, Nzi and Vavoua traps collected similar numbers of colonizing flies 3–7 d after residues were chopped. However, white sticky traps collected more flies once emergence from the pineapple residues began. Although white sticky traps collected more flies than fabric traps, they remain labor intensive and environmentally unsound because of their disposable and nonbiodegradable nature.
Keywords: mass trapping, Vavoua, Nzi, Stomoxys calcitrans
Stable flies (Stomoxys calcitrans (L.), Diptera: Muscidae) are important pests of livestock worldwide with heavy economic impact (Kunz et al. 1991, Foil and Hogsette 1994, Taylor and Berkebile 2006). Annual losses in the United States have been estimated to exceed $2 billion (Taylor et al. 2012). This pest reproduces in decomposing organic matter including crop residues and animal manure. Adults require multiple blood meals to complete sexual maturation and ovarian development (Anderson 1978, Axtell 1986).
Outbreaks of stable flies affecting cattle in Costa Rica were first observed in 1987 (Herrero et al. 1989, 1991) in pineapple (Ananas comosus (L.) Merrill) producing regions of the country. Populations of this pest have increased in the last 15 yr, mainly on dairies and farms near pineapple fields in the northern and Caribbean coastal regions of the country. Infestations in excess of 700 flies per animal are not uncommon (J.-A.S., unpublished data). The accepted economic threshold for stable flies is 5 per front leg (Campbell and Berry 1989), which is equivalent to 14 flies per animal (Berry et al. 1983). High infestations cause stress, which manifests defensive behavior and significant production losses (Taylor et al. 2012). Tropical conditions in Costa Rica with high temperatures and humidity shorten the life cycle of stable flies to 15–27 d (Lysyk 1998, Taylor and Berkebile 2011, Vargas and Solórzano 2015). The number of stable fly outbreaks has increased significantly in recent years with 230 reported in 2012 (Servicio Nacional de Salud Animal [SENASA] 2012).
Since the introduction of the pineapple variety MD-2 (“Golden” or “Sweet yellow”), acreage dedicated to pineapple production in Costa Rica has increased 4-fold, from ≈8,000 ha in 2001 to >45,000 in 2008 (Secretaria Ejecutiva de Planificacion Agropecuaria [SEPSA] 2009). MD-2 plants are larger and more succulent than other commercial varieties of pineapple. In Costa Rica, pineapples are grown with a 1.5–2-yr crop cycle. The first crop of fruit is harvested approximately 1 yr after planting. A second is harvested 6 mo later. After the second harvest, plants are chopped and incorporated into the soil in preparation for replanting. The chopped plant residues, ≈230 tons/ha, combined with the warm, humid climate, and high precipitation make an excellent substrate for the development of stable fly larvae.
Pineapple producers in Costa Rica are mandated to control stable flies developing in their fields and adult trapping is widely used. White plastic bags with a brushed on adhesive are a primary tool. Traps are mounted on wooden stakes and placed every 10–20 m surrounding fields with fresh chopped pineapple residues and in surrounding pastures (Solórzano et al. 2013). While these traps collect large numbers of flies, their efficacy in reducing the intensity of the stable fly outbreaks remains questionable. The traps are not considered to be environmental friendly and efficacy decreases quickly with time. During the rainy season, traps must be serviced twice a week. Finally, they are of little use for monitoring fly populations because removal of insects from the traps for sexing and identification is very difficult.
Since 1970s, various types of traps have been developed for the study and control of stable flies. Classical examples are sticky traps using Alsynite such as the Williams (1973) and Broce (1988) traps. Meifert et al. (1978) and Rugg (1982) evaluated these traps for stable fly control. More recently, blue fabric traps (Vavoua and F3 trap) initially designed for tse-tse fly (Glossina spp.) have been evaluated for Stomoxys spp. (Gilles et al. 2007). In parts of Africa, they are being used for the control of diverse species of Stomoxys (Holloway and Phelps 1991, Mihok et al. 1995). Gilles et al. (2005) collected up to 1,250 stomoxyines per day in Vavoua traps.
In this study, we compare five different types of traps using a Latin square design to assess their efficacy for capturing stable flies and usefulness as monitoring tools to increase our understanding of their biology in Costa Rica.
Materials and Methods
Study Site
The study was conducted on a PINDECO, Del Monte Co., plantation with MD-2 pineapples near Pital, San Carlos, Costa Rica (10.454 N, 84.265 W). The site has an annual rainfall of 2,450 mm, red (Ultisol) soils, and is classified as tropical wet forest (Holdridge 1967). The study was conducted in February and March 2013. A 300 by 6 m strip of 2-yr-old pineapple was chopped with a Seppi Midiforst M dt 150 at 5,000 revolutions per minute pulled by a John Deere 150 HP tractor at 0.5 km/h on 2 February 2013, 2 d before the setup of traps.
Climate Record
An automated weather station (Davis Vantage Pro, Vernon Hills, IL) was placed near the study site. Daily data with readings every 30 min for precipitation, relative humidity, temperature, sunshine (solar radiation), and wind speed and direction were recorded. Degree-days (DD10) were calculated from daily maximum-minimum temperatures using sine-wave integration (Allen 1976) with a threshold of 10°C (Lysyk 1993).
Traps
Efficacy of four models of blue-black polyester and polyethylene fabric traps, Vavoua (Laveissière and Grébaut 1990), Nzi (Mihok 2002), H (Kappmeier 2000), and Ngu (Brightwell et al. 1987, 1991) obtained from Vestergaard Frandsen S.A. (Lausanne, Switzerland), was compared with that of standard white sticky traps for trapping stable flies (Fig. 1). Fabric traps were fitted with transparent plastic 2-liter bottles for collection chambers. Flies collected in the two collection chambers of the model H trap were combined for analysis. White sticky traps were made from white plastic bags 0.80 by 0. 95 m high, coated with Zapicol adhesive (Zapi, San Jose, Costa Rica) diluted with gasoline, placed with the bottom 10 cm above the ground and supported by two wooden stakes (Fig. 1E). Stable flies collected in the fabric traps were sexed, but flies collected on the white sticky traps could not be removed for sexing because of the strength of the adhesive.
Fig. 1.
Traps. (A) Vavoua, (B) Nzi, (C) Model H, (D) Ngu, and (E) white sticky.
Experimental Design and Statistical Analysis
A Latin Square design with daily rotation of traps among sites was used. Traps were set in a row 300 m long, ≈50 m between trap sites. Flies were collected and traps relocated daily at 8 a.m. A complete rotation of traps through sites was completed each week for four consecutive weekly blocks. Daily trap catches were evaluated with General Mixed Linear Models (Proc GLIMMIX, SAS 2012) using a log transformation and negative binomial distribution. Differences between LSMeans were determined with Tukey’s adjustment for multiple comparisons (α = 0.05 for all comparisons). Sex ratio was analyzed with logistic regression. For those collections with 0 for either sex, one of each sex was added to the observed values. Block was considered a random variable.
Results
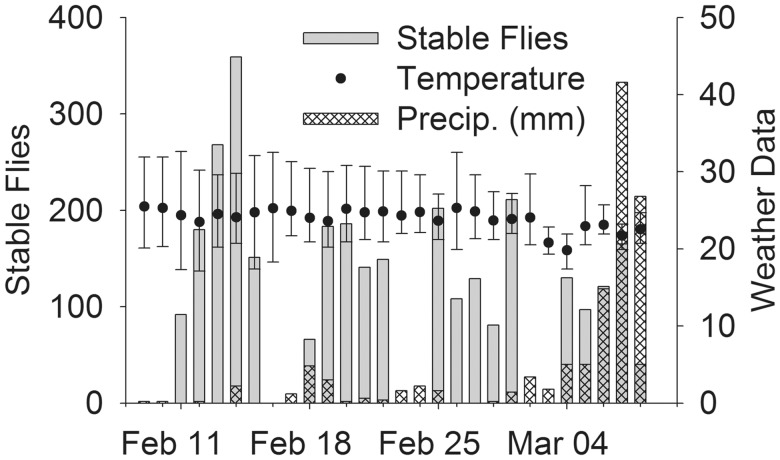
In total, 6,777 stable flies were collected in five traps over the 20 trapping days of this study (Fig. 2). Temperature ranged from 17.1 to 32.6°C with an average of 23.7°C and relative humidity was 75–98%. During the first 3 wk, precipitation was low, in the 4th week daily rainfalls increased, ranging from 15 to 41 mm. The cumulative 232 DD10 required for stable fly egg to adult development (Lysyk 1993) was attained 15 d after the pineapple was chopped.
Fig. 2.
Summary of stable fly collections and weather conditions during study period. Stable flies are total daily collections of the five traps combined. Temperatures (°C) are daily average and range. Precipitation is daily total in mm.
Trap Collections
Trap collections did not vary among trapping sites (F = 0.14, df = 4,68, P = 0.97) nor was there an interaction between trap type and site (F = 0.88, df = 16,68, P = 0.60). White sticky traps collected 2-fold more stable flies per day than did any of the fabric traps (Table 1; F = 4.53, df = 4,68, P < 0.01). The interaction between trap type and time since chopping the substrate was significant as well (F = 4.71, df = 4,68, P < 0.01) indicating that trap efficacy differed relative to the age of the substrate. Collections on the white sticky traps increased with the age of the substrate (Fig. 3; F = 11.63, df = 1,15, P < 0.01), whereas those of fabric traps decreased (F = 11.73, df = 1,69, P < 0.01) similarly among trap models (F = 0.28, df = 3,69, P = 0.84).
Table 1.
Mean daily S. calcitrans trap catches and sex ratios
| Trap | Catch/day |
% ♀ |
||
|---|---|---|---|---|
| 95% CI | 95% CI | |||
| White sticky | 140a | 113 –173 | ||
| Nzi | 57b | 46 –71 | 73a | 70 –75 |
| Vavoua | 44bc | 35 –55 | 72a | 69 –75 |
| Model H | 31c | 25 –38 | 71a | 67 –74 |
| Ngu | 17d | 14 –22 | 81b | 77 –86 |
Means followed by the same letter within a column do not differ (α = 0.05).
Fig. 3.
Mean daily trap collections for four time periods after chopping of pineapple residues.
Sex Ratio
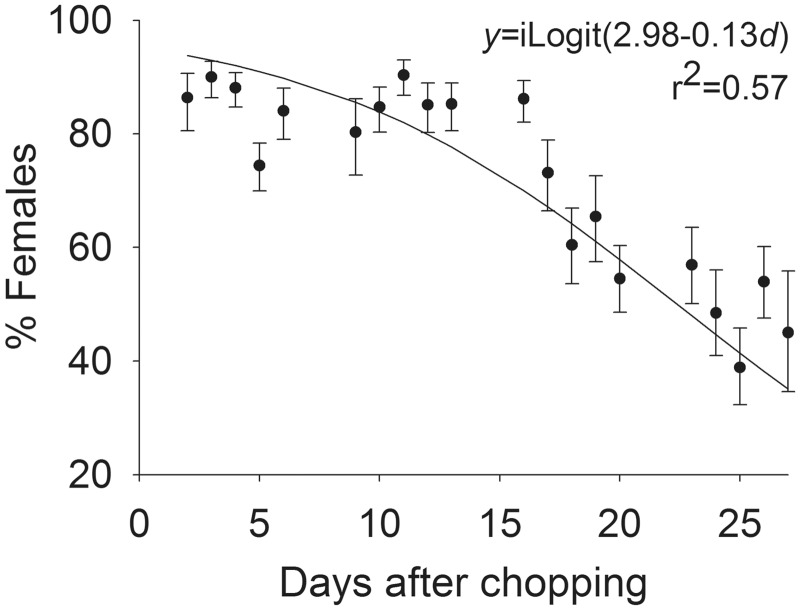
The percentage of female flies collected in the fabric traps did not differ among the four models of fabric trap (Table 1; F = 1.82, df = 3,67, P = 0.15) but declined (Fig. 4; F = 19.16, df = 1,67, P < 0.01) similarly among the trap models (F = 1.70, df = 3,67, P = 0.17) with time after chopping.
Fig. 4.
Sex ratio for flies collected in four fabric traps (pooled) relative to time after chopping of pineapple residues.
Discussion
Climatic conditions observed during this study were conducive to survival and reproduction of stable flies (Lysyk 1998, Gilles et al. 2005). Stable flies were not observed in the pineapple fields prior to chopping and, based upon degree-day accumulations, eggs deposited in the chopped substrate would not be expected to complete development and begin emerging as adults for 16 d (Lysyk 1993). Therefore, we assume that the flies collected prior to 16 d were immigrants from outside the field. These flies were primarily females (Fig. 4) and were presumably attracted to the fields for oviposition. Odors from freshly chopped pineapple plants are highly attractive for female stable flies (unpublished data).
White sticky traps caught more stable flies than any of the fabric traps. During the study, we frequently observed stable flies attracted to, and landing on, the fabric traps without entering them. Similar observations were reported for fabric traps used for tsetse fly (Glossina spp.) control in West Africa (Rayaisse et al. 2012). Insecticide-treated fabric traps or targets may be more efficacious than the untreated traps used for this study. Also, during the study, sticky traps were replaced daily, not every 2 wk as is the usual practice. Therefore, only first-day collections were evaluated. It is likely that the efficacy of sticky traps drops with time due to degradation of the adhesive and saturation of the surface.
The number of flies collected on the white sticky traps increased and sex ratio (females per male) of the flies collected in the fabric traps decreased at ≈16 d after chopping, when, according to degree-day accumulations, flies developing in the residues were expected to start emerging. This observation validates the degree-day models of Lysyk (1993, 1998). Unexpectedly, collections in the fabric traps decreased during this time frame. Previous studies found that sticky traps, both Alsynite and white coroplast, tend to collect young, nulliparous flies (Hogsette et al. 1989, Beresford and Sutcliffe 2006) and that blue-black fabric traps tend to catch an older component of the stable fly population (Taylor and Berkebile 2006). Results of this study are in accord with those findings. The fabric and white sticky traps collected similar numbers of stable flies when the population was expected to be dominated by older, presumably gravid, females seeking oviposition sites. However, once flies completed development and young, presumably nulliparous, adults began to emerge from the pineapple substrate, collections on the white sticky traps increased.
Fabric traps, especially the Nzi and Vavoua, collected significant numbers of female stable flies attempting to colonize the freshly cut pineapple residues. Use of fabric traps for 2 wk after chopping the pineapple residues in the fields reduces the number of ovipositing females and hence the number of flies developing in the fields. The fabric traps have advantages for use in bio-ecological studies as well because flies can be easily removed for species identification and sexing. Although the white sticky traps collected more flies than the fabric traps, they remain labor intensive and environmentally unsound because of their disposable and nonbiodegradable nature. Further studies on these traps are warranted to evaluate effects of long lasting insecticide treatment of traps, possible age and sex biases trap positioning, timing, and number of traps required per hectare.
Acknowledgments
We thank Jairo Treviño and Deiber Porras (PINDECO-Del Monte, Finca Pital) for their assistance in daily set of traps and all collaboration for the field records and climate data. We thank Mr. Jesus Reyes of the Food and Agriculture Organization/International Atomic Energy Agency (FAO/IAEA) Insect Pest Control Section and the Technical Cooperation Department of the IAEA for their technical and financial support through project COS5030. Vestergaard Frandsen S.A. kindly provided fabric traps for this study. This work was partially supported by Innovación y Transferencia de Tecnología Agropecuaria (INTA) Costa Rica and it is a component of national stable fly management program at Ministerio de Agricultura y Ganadería (MAG/INTA).
References Cited
- Allen J. C. 1976. A modified sine wave method for calculating degree-days. Environ. Entomol. 5: 388–396. [Google Scholar]
- Anderson J. R. 1978. Mating behavior of Stomoxys calcitrans: effects of a blood meal on the mating drive of males and its necessity as a prerequisite for proper insemination of females. J. Econ. Entomol. 71: 379–386. [DOI] [PubMed] [Google Scholar]
- Axtell R. C. 1986. Fly control in confined livestock and poultry production. Ciba Geigy Corporation, Agricultural Division, Greensboro, NC. [Google Scholar]
- Beresford D. V., Sutcliffe J. F. 2006. Studies on the effectiveness of coroplast sticky traps for sampling stable flies (Diptera: Muscidae), including a comparison to Alsynite. J. Econ. Entomol. 99: 1025–1035. [DOI] [PubMed] [Google Scholar]
- Berry I. L., Stage D. A., Campbell J. B. 1983. Populations and economic impacts of stable flies on cattle. Trans. Am. Soc. Agric. Eng. 26: 873–877. [Google Scholar]
- Brightwell R., Dransfield R. D., Kyorku C., Golder T. K., Tarimo S. A., Mungai D. 1987. A new trap for Glossina pallidipes. Trop. Pest. Manage. 33: 151–159. [Google Scholar]
- Brightwell R., Dransfield R., Kyorku C. 1991. Development of a low-cost tsetse trap and odour baits for Glossina pallidipes and G. longipennis in Kenya. Med. Vet. Entomol. 5: 153–164. [DOI] [PubMed] [Google Scholar]
- Broce A. B. 1988. An improved Alsynite trap for stable flies, Stomoxys calcitrans (Diptera: Muscidae). J. Med. Entomol. 25: 406–409 [DOI] [PubMed] [Google Scholar]
- Campbell J. B., Berry I. L. 1989. Economic threshold for stable flies on confined livestock, pp. 18–22. In Petersen J. J., Greene G. L. [eds.], Current status of stable fly (Diptera: Muscidae) research. Misc. Publ. Entomol. Soc. Am. Vol. 74. [Google Scholar]
- Foil L. D., Hogsette J. A. 1994. Biology and control of tabanids, stable flies and horn flies. Rev. Sci. Tech. Int. Epiz. 13: 1125–1158. [DOI] [PubMed] [Google Scholar]
- Gilles. J., David J.-F., Duvallet G. 2005. Temperature effects on development and survival of two stable flies, Stomoxys calcitrans and Stomoxys niger niger (Diptera: Muscidade), in La Reunion island. J. Med. Entomol. 42: 959–965. [DOI] [PubMed] [Google Scholar]
- Gilles J., David J.-F., Duvallet G., De la Rocque S., Tillard E. 2007. Efficiency of traps for Stomoxys calcitrans and Stomoxys niger niger on Reunion Island. Med. Vet. Entomol. 21: 65–69. [DOI] [PubMed] [Google Scholar]
- Herrero M. V., Montes. L., Sanabria C., Sánchez A., Hernández R. 1989. Estudio inicial sobre la mosca de los establos Stomoxys calcitrans (Diptera: Muscidae), en la región del pacífico sur de Costa Rica. Cienc. Vet. 11:11–14. [Google Scholar]
- Herrero M. V., Montes-Pico L., Hernández R. 1991. Abundancia relativa de Stomoxys calcitrans (L.) (Diptera: Muscidae) en seis localidades del Pacífico Sur de Costa Rica. Rev. Biol. Trop. 39: 309–310. [Google Scholar]
- Hogsette J. A., Ruff J. P., Jones C. J. 1989. Dispersal behavior of stable flies (Diptera: Muscidae), pp. 23–32. In Petersen J. J., Greene G. L. [eds.], Current status of stable fly (Diptera: Muscidae) research. Misc. Publ. Entomol. Soc. Am. Vol. 74. [Google Scholar]
- Holloway M.T.P., Phelps R. J. 1991. The responses of Stomoxys spp. (Diptera: Muscidae) to traps and artificial host odours in the field. Bull. Entomol. Res. 81: 51–55. [Google Scholar]
- Holdridge L. 1967. Life zone ecology. Tropical Science Center, San José, Costa Rica. [Google Scholar]
- Kappmeier K. 2000. A newly developed odour-baited “H-trap” for the live collection of Glossina brevipalpis and Glossina austeni (Diptera: Glossinidae) in South Africa. Onderstepoort J. Vet. Res. 67: 15–26. [PubMed] [Google Scholar]
- Kunz S. E., Murrell K. D., Lambert G., James L. F., Terrill C. E. 1991. Estimated losses of livestock to pests, pp. 69–98. In Pimentel D. (ed.), CRC handbook of pest management in agriculture, vol. 1. CRC Press, Boca Raton, FL. [Google Scholar]
- Laveissière C., Grébaut P. 1990. Recherches sur les pièges à glossines (Diptera, Glossinidae). Mise au point d’un modèle économique: le piège Vavoua . Trop. Med. Parasitol. 41: 185–192. [PubMed] [Google Scholar]
- Lysyk T. J. 1993. Seasonal abundance of stable flies and house flies (Diptera: Muscidae) in dairies in Alberta, Canada. J. Med. Entomol. 30: 888–895. [DOI] [PubMed] [Google Scholar]
- Lysyk T. J. 1998. Relationships between temperature and life-history parameters of Stomoxys calcitrans (Diptera: Muscidae). J. Med. Entomol. 35: 107–119. [DOI] [PubMed] [Google Scholar]
- Meifert D. W., Patterson R. S., Whitfield T., Labrecque G. C., Weidhaas D. E. 1978. Unique attractant-toxicant system to control stable fly populations. J. Econ. Entomol. 71: 290–292. [DOI] [PubMed] [Google Scholar]
- Mihok S. 2002. The development of a multipurpose trap (the NZI) for tse-tse and other biting flies. Bull. Entomol. Res. 92: 385–403. [DOI] [PubMed] [Google Scholar]
- Mihok S., Kang ’Ethe E. K., Kamau G. K. 1995. Trials of traps and attractants for Stomoxys spp. (Diptera: Muscidae). J. Med. Entomol. 32: 283–289. [DOI] [PubMed] [Google Scholar]
- Rayaisse J.-B., Kröber T., McMullin A., Solano P., Mihok S., Guerin P. M. 2012. Standardizing visual control devices for tsetse flies: West African species Glossina tachinoides, G. palpalis gambiensis and G. morsitans submorsitans. PLoS Negl. Trop. Dis. 6: e1491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rugg D. 1982. Effectiveness of Williams traps in reducing the numbers of stable flies (Diptera: Muscidae). J. Econ. Entomol. 75: 857–859 [DOI] [PubMed] [Google Scholar]
- SAS 2012. SAS 9.3 help and documentation. SAS Institute, Cary, NC. [Google Scholar]
- Servicio Nacional de Salud Animal (SENASA). 2012. Informe Estadístico denuncias por Mosca del Establo. Informe anual Dirección Regional de Salud Animal Región Huetar Norte. MAG, Costa Rica. [Google Scholar]
- (SEPSA) Secretaria Ejecutiva de Planificacion Agropecuaria. 2009. Estadísticas del sector Agropecuario 2008. San José, Costa Rica. [Google Scholar]
- Solórzano J.-A., Treviño J., Hidalgo. E., Gomez Y., Blanco H., Apuy M., Gonzalez L., Memenes D. 2013. Manual de recomendaciones para el manejo de la mosca del establo Stomoxys calcitrans en el cultivo de piña. Memorias Taller manejo de rastrojos del cultivo de piña y plagas que afectan la competitividad 30, 31 Octubre y 01 de Noviembre 2012. Hotel Tilajari, Muelle de San Carlos. PITTA PIÑA. 1 era Edición. ISBN 978-9968-877-58-9. CANAPEP/FITTACORI. 32 p. [Google Scholar]
- Taylor D. B., Berkebile D. R. 2006. Comparative efficiency of six stable fly (Diptera: Muscidade) traps. J. Econ. Entomol. 90: 1414–1419. [DOI] [PubMed] [Google Scholar]
- Taylor D. B., Berkebile D. R. 2011. Phenology of stable fly (Diptera: Muscidae) larvae in round bale hay feeding sites in eastern Nebraska. Environ. Entomol. 40: 184–193. [DOI] [PubMed] [Google Scholar]
- Taylor D. B., Moon R. D., Mark D. R. 2012. Economic impact of Stable Fly (Diptera: Muscidae) on beef cattle production. J. Med. Entomol. 49: 198–209. [DOI] [PubMed] [Google Scholar]
- Vargas C., Solórzano J.-A. 2015. Biología y cría de la mosca del establo Stomoxys calcitrans Diptera Muscidae INTA. Costa Rica. Alcances Tecnológicos 8. [Google Scholar]
- Williams D. F. 1973. Sticky traps for sampling populations of Stomoxys calcitrans. J. Econ. Entomol. 66: 1279–1280. [DOI] [PubMed] [Google Scholar]