Abstract
As part of an ongoing research project on the pollination networks in European heathlands, the objective of this study was to assess the insect visitor guild on Calluna vulgaris (L.) Hull (Ericaceae). We focused the study on a region renowned for its largely well-preserved heathlands, the Cévennes National Park, Southern France. In 2013, flower visitors were observed over 3 d per site, in four heathland sites at mont Lozère. Honeybees (Apis mellifera L.) were the main visitors (62–88% of total visitors). Besides honeybees, a high diversity of visitors was detected with 57 different species identified (42 Diptera and 15 Hymenoptera). Hoverflies (Syrphidae, Diptera) visitors were abundant and diverse, especially individuals belonging to the genera Eristalis and Episyrphus. The reported diversity of visitors was probably due to the preservation of large heathland areas at mont Lozère and to the generalist pollination system of C. vulgaris.
RESUME. Cette étude fait partie d’un projet de recherche en cours sur les réseaux de pollinisation dans les landes européennes. Son objectif est d’évaluer la guilde des insectes visiteurs de Calluna vulgaris (L.) Hull (Ericaceae). Cette étude se déroule dans une région réputée pour ses landes globalement bien préservées: le Parc natinal des Cévennes, situé dans le sud de la France. En 2013, les insectes visiteurs ont été observés durant trois jours par site, dans quatre sites au mont Lozère. Les abeilles domestiques (A. mellifera L.) sont les visiteurs principaux (62–88% du nombre total de visiteurs). Outre les abeilles domestiques, une diversité importante de visiteurs est constatée: 57 espèces ont été identifiées (42 appartenant à l'ordre des Diptères et 15 à l'ordre des Hyménoptères). Les syrphes (Syrphidae, Diptera) sont abondants et diversifiés, en particulier les genres Eristalis et Episyrphus. La diversité de visiteurs observée peut certainement s’expliquer par la préservation de grandes étendues de landes au mont Lozère et par le caractère généraliste de la pollinisation de la callune.
Keywords: pollination network, Cévennes National Park, heather, Syrphidae, Apidae
Pollinators are declining (Patiny et al. 2009, Potts et al. 2010, Brown 2011). The loss of insect species and subsequent homogenization could significantly alter the stability of pollination networks and further threaten the maintenance of plant communities and global diversity (Memmott et al. 2004, Hadley and Betts 2012). The main causes of the current loss of pollinators are related to anthropogenic activities (Brown and Paxton 2009). Among these causes, habitat loss, fragmentation, and disturbance modify the landscape matrix (Goulson et al. 2008, Kleijn and Raemakers 2008, Winfree et al. 2009). Throughout Europe, habitat loss and fragmentation especially impact open habitats like dry grasslands, fallow lands, or heathlands (Steffan-Dewenter and Tscharntke 1999, Rasmont et al. 2005). The flowering species in these biotopes are crucial for insect pollinators, which depend on floral resources for feeding (Mayer et al. 2012, Somme et al. 2014). Floral resources are composed of nectar and/or pollen. Pollen represents the source of proteins, amino acids, lipids (mainly sterols) as well as of some vitamins. Nectar constitutes mainly the resource in sugars (Goulson 2010).
In Europe, ancestral extensive agricultural practices have created original open habitats, hosting a high biodiversity (Diemont et al. 2013). Heathlands, mainly originating from sheep and cattle grazing, consist of oligotrophic biotopes and are dominated by ericaceous dwarf shrubs (Gimingham 1972, Webb 1998). These heathlands have largely been destroyed or fragmented over the last two centuries (Gimingham 1972, Aerts and Heil 1993, Webb 1998, Piessens and Hermy 2006) and were converted into agricultural or afforested areas (e.g., pine plantations in the Cévennes region, Parc National des Cévennes [PNC] 2007). In Europe, a densely populated continent, nature conservation priorities focus on these “historical” biotopes. The Natura2000 network considers several open biotopes of high priority (calcareous grasslands, saline marshes, and wet and dry heathlands, Diemont et al. 2013). Among these open habitats, heathlands remain poorly studied (Mahy et al. 1998, Mayer et al. 2012, Diemont et al. 2013). Therefore, little is known over the current diversity and abundance of the local wild insect pollinators. Yet, heathlands host several specialist pollinators, particularly sensitive to decline, which are dependent on the floral resources of ericaceous species (Goulson et al. 2005, Mayer et al. 2012). For instance, the solitary bee Andrena lapponica Zetterstedt collects pollen mainly from Vaccinium species. Heathlands also constitute the exclusive habitat for Bombus monticola Smith and Bombus jonellus Kirby, the latter being a decreasing bumblebee species (Rasmont et al. 1993). Little is known about other insect pollinators besides the fact that syrphid flies are increasingly recognized as efficient pollinators of numerous plant species (Goulson and Wright 1998, Jacquemart et al. 2007, Sarthou 2011, Inouye et al. 2015). Pollination efficiency of Syrphidae varies among species, and large species (like Sericomya and Eristalis) are considered as valuable pollinators (Mahy et al. 1998, Jacquemart et al. 2007).
Calluna vulgaris (L.) Hull (Ericaceae), the heather, usually constitutes the dominant species in dry heathlands (Diemont et al. 2013). The species is a small, evergreen shrub (50–100 cm in height). The small flowers, which measure 5–8 mm in length, are tetramerous and grouped in racemes. The flowering period extends from July to September according to the location of the heathlands (Gimingham 1960). The species is pollinated by insects, although wind is also reported to be a pollinating agent (Mahy et al. 1998). The species is crucial for insects at the end of the season, since it represents large stands of flowers and offers large quantities of pollen and nectar (Mahy and Jacquemart 1998, Vanderplanck et al. 2014). Compared with several Ericaceae species, which present poridical anthers and force visitors to vibrate the anthers to release pollen (e.g., buzz-pollination, Jacquemart 2003), the anthers of C. vulgaris present longitudinal splits that allow an easy access to pollen to a large diversity of insects. Only a few species are able to perform buzzing, in particular bumblebees (Buchmann et al. 1983). Since C. vulgaris is an emblematic plant species in heathlands at mont Lozère and little is known about the current diversity and abundance of wild pollinators, the main objective of this study was to investigate the visitor guild of C. vulgaris.
This study is part of an ongoing larger research project whose goal is to compare pollinator guilds and their abundances in different European heathlands to establish guidelines for heathland restoration and management. In mont Lozère, heathlands remain large and in a relatively good conservation status. We therefore hypothesize that these heathlands will maintain a higher diversity and abundance of insect visitors relative to smaller size remnants. (Diemont et al. 2013).
Materials and Methods
The study took place at mont Lozère (Languedoc-Roussillon, France). This granite massif is located along the southeastern margin of Massif Central and is included in the central part of the Cévennes National Park. The mont Lozère presents a steep relief (highest peak at 1,699 m) and mountainous climatic conditions. Biotopes of this area are influenced by the Atlantic Ocean in the Western part and by the Mediterranean Sea in its Eastern part. Such climatic influences generate diversified biotopes, which shelter unique flora and fauna, including several endemic species (PNC 2007). Because of a local rapid human demographic growth in the middle of the 19th century, land-use modifications induced important changes to the vegetation cover, which led to huge soil erosion and catastrophic floods in the valleys. To counteract soil erosion, ambitious tree plantation programs (particularly with Pinus sylvestris) took place at mont Lozère at the end of the 19th century. The abandonment of agro-pastoral practices in the 20th century strengthened scrub encroachment dynamics, further reducing heathland cover (Lhuillier 2000). Nowadays, Calluna heathlands still cover about 1,550 ha at mont Lozère, due to the management of these biotopes through extensive cattle and sheep grazing linked to transhumance (seasonal move to summer pastures, Parc National des Cévennes [PNC] 2012). These heathlands belong to the Genisto pilosae-Vaccinion uliginosi Br.- Bl.1926 alliance (Boissier 2002).
Observations were conducted in 2013 in four sites (Table 1) ranging between 1,290 and 1,530 m a.s.l. Flower visitors were recorded during peak of flowering of C. vulgaris over 3 consecutive days per site. Observations were conducted for 20 min every hour between 8.30 a.m. and 5.30 p.m. between August 22 and September 3. All insect individuals foraging on C. vulgaris were collected with an insect net on a 10 m2 plot of continuous Calluna shrub cover. Because of apiary activities in the region, honeybees largely dominated the insect guild. At each site, two people collected the insect visitors, including honeybees, on the first day of observation. On days 2 and 3, one people collected the insect visitors, excluding honeybees. After the 20-min period, the insects were identified and released. Several individuals per insect morphotype were killed and caught for further identification (Table 3).
Table 1.
Characteristics of the four sites of C. vulgaris at mont Lozère
| Population | Municipality | Coordinates | Altitude (m) | Flowering plant diversity | Forest cover (%) | Date of insect surveys |
|---|---|---|---|---|---|---|
| Barrandon | Saint-Etienne-du-Valdonnez | 44° 27′17.95″ N 3° 36′59.81″ E | 1,387 | 10 | 90 | 1 September 2013 |
| 2 September 2013 | ||||||
| 3 September 2013 | ||||||
| Bleymard | Mas-d’Orcières | 44° 26′36.28″ N 3° 44′46.71″ E | 1,490 | 7 | 70 | 22 August 2013 |
| 23 August 2013 | ||||||
| 24 August 2013 | ||||||
| Finiels | Mas-d’Orcières | 44° 25′43.93″ N 3° 45′51.93″ E | 1,530 | 11 | 30 | 25 August 2013 |
| 26 August 2013 | ||||||
| 27 August 2013 | ||||||
| Villeneuve | Pont-de-Montvert | 44° 22′32.42″ N 3° 47′29.04″ E | 1,290 | 15 | 30 | 29 August 2013 |
| 30 August 2013 | ||||||
| 31 August 2013 |
Flowering plant diversity refers to the number of entomophilous plant species flowering within a 50 -m radius around the insect observation plots. Forest cover refers to the percentage of forest cover in a 1,000 m radius from the centre of the site.
Table 3.
Insect individuals collected on C. vulgaris in the four study sites
| Order | Family | Species | Barrandon | Bleymard | Finiels | Villeneuve |
|---|---|---|---|---|---|---|
| Hymenoptera | Andrenidae | Andrena flavipes (Panzer 1799) | X | |||
| Andrena spp. | X | |||||
| Apidae | Bombus lapidarius (L. 1758) | X | ||||
| Bombus quadricolor (Lepeletier 1832) | X | |||||
| Bombus soroeensis proteus (F. 1793) | X | X | X | X | ||
| Bombus soroeensis soroeensis (F. 1793) | X | X | X | |||
| Bombus sylvarum (L. 1761) | X | X | ||||
| Xylocopa violacea (L. 1758) | X | |||||
| Colletidae | Colletes hederae (Schmidt and Westrich 1993) | X | ||||
| Halictidae | Halictus rubicundus (Christ 1791) | X | X | |||
| Lasioglossum cfr. malachurum (Kirby 1802) | X | |||||
| Lasioglossum sp. | X | X | ||||
| Ichneumonidae | Ichneumoninae sp. | X | ||||
| Vespidae | Polistes sp. | X | ||||
| Diptera | Conopidae | Myopa fasciata (Meigen 1804) | X | X | ||
| Myopa variegata (Meigen 1804) | X | |||||
| Empididae | Empis serotina (Loew 1867) | X | ||||
| Muscidae | Musca autumnalis (De Geer 1776) | X | ||||
| Syrphidae | Callicera aurata (Rossi 1790) | X | ||||
| Chrysotoxum arcuatum (L. 1758) | X | X | ||||
| Chrysotoxum festivum (L. 1758) | X | |||||
| Didea fasciata (Macquart 1834) | X | |||||
| Didea intermedia (Loew 1854) | X | |||||
| Epistrophe euchroma (Kowarz 1885) | X | X | ||||
| Episyrphus balteatus (De Geer 1776) | X | X | X | |||
| Eristalis horticola (De Geer 1776) | X | X | X | |||
| Eristalis nemorum (L. 1758) | X | |||||
| Eristalis pratorum (Meigen 1822) | X | X | X | |||
| Eristalis tenax (L. 1758) | X | |||||
| Eupeodes luniger (Meigen 1822) | X | X | ||||
| Helophilus pendulus (L. 1758) | X | |||||
| Platycheirus albimanus (F. 1781) | X | X | ||||
| S. pyrastri (L. 1758) | X | X | ||||
| S. pyrastri var. unicolor (Curtis 1834) | X | |||||
| Scaeva selenitica (Meigen 1822) | X | X | X | |||
| Se. silentis (Harris 1776) | X | X | ||||
| Sphaerophoria interrupta (F. 1805) | X | |||||
| Sphaerophoria ruepelli (Wiedemann 1830) | X | |||||
| Sphaerophoria scripta (L. 1758) | X | |||||
| Syritta pipiens (L. 1758) | X | X | ||||
| Syrphus ribesii (L. 1758) | X | |||||
| Sy. vitripennis (Meigen 1822) | X | X | X | |||
| Volucella pellucens (L. 1758) | X | |||||
| Xanthandrus comtus (Harris 1780) | X | |||||
| Tachinidae | Gymnosoma rotundatum (L. 1758) | X | ||||
| Therevidae | Thereva nobilitata (F. 1775) | X |
For individual belonging to Ichneumonidae family, the identification was not possible beyond the subfamily level.
Flowering plant surveys following the Braun-Blanquet method were made on 100 m2 plot each site in the vicinity of the plot of insect visitor surveys (Table 2). The flowering plant diversity per site represented the total number of entomophilous species in flower at the time of the survey. A higher number of flowering species was recorded at Villeneuve site compared with the three other sites (15 vs. 7–11). The high number was due to the proximity of meadows, whereas the other sites were situated in a landscape matrix including only coniferous forests and heathlands within a radius of 500 m.
Table 2.
Abundance of flowering plant species in the four study sites at mont Lozère on a 100 m2 plot per site
| Family | Species | Barrandon | Bleymard | Finiels | Villeneuve |
|---|---|---|---|---|---|
| Asteraceae | Achillea millefolium L. | + | 1 | ||
| Hieracium gr. glaucinum | 1 | 3 | 1 | ||
| Hieracium pilosella L. | 1 | 1 | |||
| Serratula tinctoria L. | + | + | |||
| Campanulaceae | Campanula rotundifolia L. | 1 | 1 | 1 | |
| Jasione laevis Lam. | + | ||||
| Jasione montana L. | + | + | 1 | ||
| Caryophyllaceae | Dianthus sylvaticus Hoppe ex Willd. | 1 | + | + | |
| Dipsacaceae | Succisa pratensis Moench | + | |||
| Ericaceae | Calluna vulgaris Hull. | 4 | 4 | 4 | 3 |
| Fabaceae | Genista pilosa L. | 1 | 2 | 1 | 3 |
| Lotus corniculatus L | 1 | + | + | ||
| Lamiaceae | Betonica officinalis L. | + | |||
| Thymus praecox ssp. polytichus Jalas | 1 | ||||
| Orobranchaceae | Euphrasia spp. | + | |||
| Rosaceae | Alchemilla saxatilis Buser | + | 1 | 2 | 1 |
| Rubiaceae | Galium verum L. | 1 | 1 | 1 | 1 |
| Scrophulariaceae | Linaria repens L. | + | + | ||
| Linaria spp. | + | ||||
| Violaceae | Viola canina L. | + | + | 1 |
Plant species abundance was assessed using Braun-Blanquet scale: +, sparse individuals (<10 individuals); 1, cover < 5%; 2; cover 5–25%; 3, cover 25–50%; 4, cover 50–75%; 5, cover >75%.
For statistical analyses, we grouped insect visitors in four groups: two groups for Hymenoptera, honeybees (Apis mellifera) and other Hymenoptera; and two groups for Diptera, hoverflies (Syrphidae) and other Diptera. Pearson correlation tests were used to determine whether the abundances of visitor groups and flowering plant diversity were statistically correlated.
Results
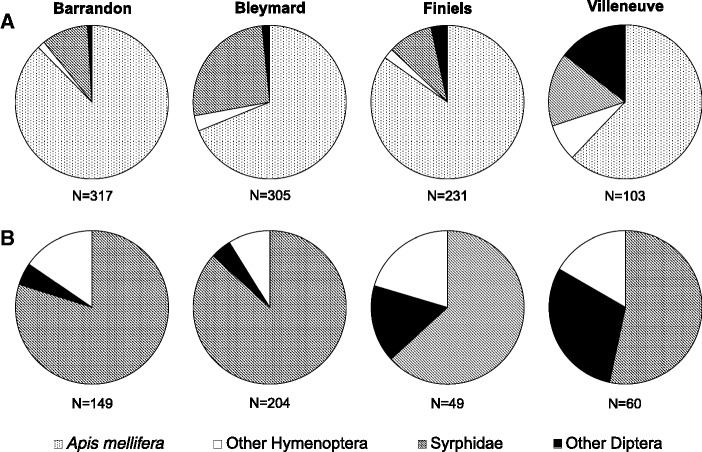
In total, 57 species of insect visitors were observed, including 42 Diptera and 15 Hymenoptera species (Table 3). Honeybees were the main visitors representing 76% (range 62–88%) of the observed visitors over all sites (Fig. 1A). Besides honeybees, 462 insect individuals were collected on C. vulgaris. Most of them belonged to the Diptera order (84%), which mainly consisted of Syrphidae and more specifically to the Eristalis genus. Individuals from this genus represented more than 40% of Syrphidae. Individuals from the Hymenoptera order only represented 16% of the visitors, among which 7% were bumblebees (Fig. 1B).
Fig. 1.
Relative abundances of the insect visitor groups on C. vulgaris in the four study sites during August 2013. The abundance of visitor group was calculated as the ratio between the numbers of insects of each group against the total numbers of insect visitors observed during the 3 d. (A) Relative abundance of visitor groups on day 1 for each site including A. mellifera individuals. (B) Relative abundance of visitor groups on 3 d excluding A. mellifera. Other Diptera refers to Diptera species, except Syrphidae, and Other Hymenoptera refers to Hymenoptera species, except A. mellifera.
The abundance of Hymenoptera visitors (“other Hymenoptera” group, excluding honeybees) was positively correlated to flowering plant diversity, namely the number of plant species flowering around the observation plots (Pearson correlation, r = 0.973, P = 0.027, Table 2). Villeneuve and Finiels 15 and 11 flowering species, respectively, presented the highest abundance of “other Hymenoptera” visitors (Fig. 1B).
Discussion
The number of insect species observed on the flowers of C. vulgaris in our study (57 species) confirmed its generalist pollination system and supported results from previous studies from The Netherlands (61 species, Beijerinck 1940) or Belgium (50 species, Mahy et al. 1998, Bacchetta 2014). This generalist system might be explained by the access to floral resources (pollen and nectar). C. vulgaris has an open small sized (<6 mm) corolla allowing easy access to nectar.
Fourteen new insect species belonging to the Syrphidae family were added to a list of 104 species identified by Sarthou et al. (2010) in Lozère. Callicera aurata Rossi, Chrysotoxum festivum L., Didea intermedia Loew, Epistrophe euchroma Kowarz, Eristalis pratorum Meigen, Metasyrphus luniger Meigen, Scaeva pyrastri L., Scaeva pyrastri var. unicolor L., Scaeva selenitica Meigen, Sericomyia silentis Harris, Sphaerophoria menthastri L., Syrphus vitripennis Meigen, Volucella pellucens L., and Xanthandrus comtus Harris have never been observed before in the department of Lozère. Some of the insect species observed, such as S. pyrastri, Se. silentis, or Sy. vitripennis, have already been reported to visit C. vulgaris flowers in Belgium (Mahy et al. 1998).
Honeybees dominated the visitor guild. The ubiquitous beekeeping at mont Lozère (Lehébel-Péron 2012) might explain their abundance. Hives are installed mainly in late summer, during the flowering period of the heather. Heather honey is still appreciated in several European countries (Lehébel-Péron 2012). Honeybees might negatively impact wild pollinator populations. We only observed 33 bumblebee individuals, whereas Hymenoptera, especially bumblebees, are considered as the main pollinators of C. vulgaris in Germany (Knuth 1908, Jeunieaux 2014), Belgium (Mahy et al. 1998; Bacchetta 2014), The Netherlands (Beijerinck 1940), the United Kingdom (Willis and Burkill 1895, Gimingham 1960). Bumblebee population dynamics could be affected by emerging infectious diseases transmitted by honeybees (Fürst et al. 2014). Honeybees compete with wild pollinators for access to floral resources. Competition effects on native pollinators have been demonstrated in several countries where honeybees were introduced (Australia [Goulson 2003, Paini 2004] and the United States [Thomson 2004]), but few convincing results are available for countries where honeybees are native (Steffan-Dewenter and Tscharntke 2000). The low abundance of bumblebees at mont Lozère may be explained by the colony phenology as the production of workers may be reduced in the fall when new queens emerge (Iserbyt and Rasmont 2012). Further investigations on the insect community and particularly on bumblebees, within the mont Lozère area over several years will extent the existing list of pollinators.
Our study further highlighted the influence of flowering plant diversity on the abundance and diversity of visitors (Nicolson 2007, Munidasa and Toquenaga 2010, Jha and Kremen 2012). The abundance of Hymenoptera, except honeybees, was positively linked to the abundance and diversity present in the vicinity of the study sites (e.g., Genista pilosa and Campanula rotundifolia). Bumblebee and solitary bee species usually collect pollen and nectar from several plant species (i.e. polylectism, Michener 2007). Besides the relative abundance and density of a plant species in the surrounding of the bee nest, the composition of its pollen and nectar have been reported to influence the foraging behavior of bees since they rely exclusively on these floral resources for larvae feeding (Vanderplanck et al. 2014, Somme et al. 2015, Moquet et al. 2015).
Acknowledgments
We are grateful to A. Lehébel-Péron and M. Milor for site information and C. Robin and C. Mallet for the accommodation of C. D. at Mont Lozère and S. Francoisse for field assistance. Many thanks are due to B. Righetti, N. Vereecken, P. Grootaert, and C. Martens for assistance in insect identification, F. Hopkins and P. Lhoir for plant identification. We thank the Cévennes National Park for allowing us to sample plants and insect individuals. We also thank two anonymous reviewers for their constructive comments on the manuscript. This work was supported by Belgian Fund for Scientific Research (FNRS contract 2.4613.12) and the Université catholique de Louvain (FSR project). This work is part of a project focusing on the availability of floral resources in European heathlands and their impacts on pollinator diversity, abundance, and behavior (L. Moquet, Ph.D. thesis). This study constituted the master thesis of C. Descamps.
References Cited
- Aerts R., Heil G. W. 1993. Heathlands: patterns and processes in a changing environment. Kluwer, Dordrecht, The Netherlands. [Google Scholar]
- Beijerinck W. 1940. Calluna: a monograph on the scotch heather. Verhandelingen Akademie Wetenschappen Amsterdam 38: 1–180. [Google Scholar]
- Bacchetta R. 2014. Impacts de la taille et de la densité florale des populations de Calluna vulgaris (L.) Hull sur la diversité, l'abondance et la diète de sa guilde de pollinisateurs au plateau des Tailles (Ardenne, Belgique). M.S. Thesis, Université catholique de Louvain, Louvain-la-Neuve, Belgique. [Google Scholar]
- Boissier J. M. 2002. Catalogue des stations forestières et para-forestières des Hautes Cévennes siliceuses: un outil pour une gestion forestière durable. Forêt méditerranéenne 4: 368–374. [Google Scholar]
- Brown M. J. 2011. Conservation: the trouble with bumblebees. Nature 469: 169–170. [DOI] [PubMed] [Google Scholar]
- Brown M. J., Paxton R. J. 2009. The conservation of bees: a global perspective. Apidologie 40: 410–416. [Google Scholar]
- Buchmann S. L., Jones C. E., Little R. J. 1983. Buzz pollination in Angiosperms, pp. 73–113. In Jones C. E., Little R. J. (eds.), Handbook of experimental pollination biology. Van Nostrand Reinhold, New York, USA. [Google Scholar]
- Diemont W. H., Heijman W. J., Siepel H., Webb N. 2013. Economy and ecology of heathlands. KNNV Publishing, Zeist, The Netherlands. [Google Scholar]
- Fürst M. A., McMahon D. P., Osborne J. L., Paxton R. J., Brown M. J. 2014. Disease associations between honeybees and bumblebees as a threat to wild pollinators. Nature 506: 364–366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gimingham C. H. 1960. Calluna Salisb. J. Ecol. 48: 455–483. [Google Scholar]
- Gimingham C. H. 1972. Ecology of heathlands. Chapman & Hall, London, UK. [Google Scholar]
- Goulson D. 2003. Effects of introduced bees on native ecosystems. Annu Rev. Ecol. Evol. Syst. 34: 1–26. [Google Scholar]
- Goulson D. 2010. Bumblebees: behaviour, ecology, and conservation, 2nd ed Oxford University Press, NY. [Google Scholar]
- Goulson D., Wright N. P. 1998. Flower constancy in the hoverflies Episyrphus balteatus (Degeer) and Syrphus ribesii (L.) (Syrphidae). Behav. Ecol. 9: 213–219. [Google Scholar]
- Goulson D., Hanley M., Darvill B., Ellis J., Knight M. 2005. Causes of rarity in bumblebees. Biol. Cons. 122: 1–8. [Google Scholar]
- Goulson D., Lye G. C., Darvill B. 2008. Decline and conservation of bumblebees. Annu. Rev. Entomol. 53: 191–208. [DOI] [PubMed] [Google Scholar]
- Hadley A., Betts M. 2012. The effects of landscape fragmentation on pollination dynamics: Absence of evidence not evidence of absence. Biol. Rev. 87: 526–544. [DOI] [PubMed] [Google Scholar]
- Inouye D., Larson B., Ssymank A., Kevan P. 2015. Flies and flowers III: ecology of foraging and pollination. J. Pollinat. Ecol. 16: 115–133. [Google Scholar]
- Iserbyt S., Rasmont P. 2012. The effect of climatic variation on abundance and diversity of bumblebees: a ten years survey in a mountain hotspot. Annales de la Société Entomologique de France (N.S.) 48: 261–273. [Google Scholar]
- Jacquemart A. L. 2003. Floral traits of Belgian Ericaceae species: are they good indicators to assess the breeding systems? Belg. J. Bot. 136: 154–164. [Google Scholar]
- Jacquemart A. L., Gillet C., Cawoy V. 2007. Floral visitors and the importance of honey bee on buckwheat (Fagopyrum esculentum Moench) in central Belgium. J. Hortic. Sci. Biotechnol. 82: 104–108. [Google Scholar]
- Jeunieaux A. 2014. Étude de l'abondance, de la diversité et de la diète pollinique des visiteurs associés à Calluna vulgaris dans la Lande de Lunebourg (Nord de l’Allemagne). M.S. Thesis, Université catholique de Louvain, Louvain-la-Neuve, Belgique. [Google Scholar]
- Jha S., Kremen C. 2012. Resource diversity and landscape-level homogeneity drive native bee foraging. Proc. Nat. Acad. Sci. USA 110: 555–558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleijn D., Raemakers I. 2008. A retrospective analysis of pollen host plant use by stable and declining bumblebee species. Ecology 89: 1811–1823. [DOI] [PubMed] [Google Scholar]
- Knuth P. 1908. Handbook of flower pollination, vol. 2 Clarendon Press, Oxford, U.K. [Google Scholar]
- Lehébel-Péron A. 2012. Activité apicole du massif du mont Lozère: état des connaissances. M.S. Thesis, Parc national des Cévennes, Centre d’écologie fonctionnelle et évolutive CEFE-CNRS, Montpellier, France. [Google Scholar]
- Lhuillier S. 2000. Evolution des formations végétales sur le mont Lozère et le Bougès nord entre 1970 et 1999 à partir des photographies aériennes. M.S. Thesis, Université de Montpellier II, Montpellier, France. [Google Scholar]
- Mahy G., De Sloover J., Jacquemart A. L. 1998. The generalist pollination system and reproductive success of Calluna vulgaris in the upper Ardenne. Can. J. Bot. 76: 1843–1851. [Google Scholar]
- Mahy G., Jacquemart A. L. 1998. Mating system of Calluna vulgaris: self sterility and outcrossing estimations. Can. J. Bot. 76: 37–42. [Google Scholar]
- Mayer C., Michez D., Chyzy A., Bredat E., Moerman R., Jacquemart A. L. 2012. The abundance and pollen foraging behaviour of bumble bees in relation to population size of whortleberry (Vaccinium uliginosum). PLoS One 7: e50353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Memmott J., Waser N. M., Price M. V. 2004. Tolerance of pollination networks to species extinctions. Proc. R. Soc. Lond. Ser. B Biol. Sci. 271: 2605–2611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michener C. D. 2007. The bees of the world. Johns Hopkins University Press, Baltimore, MA. [Google Scholar]
- Moquet L., Mayer C., Michez D., Wathelet B., Jacquemart A. L. 2015. Early spring floral foraging resources for pollinators in wet heathlands in Belgium. J. Ins. Cons. in press. [Google Scholar]
- Munidasa D. T., Toquenaga Y. 2010. Do pollen diets vary among adjacent bumble bee colonies? Ecol. Res. 25: 639–646. [Google Scholar]
- Nicolson W. S. 2007. Nectar consumers, pp. 289–342. In Nicolson S. W., Nepi M., Pacini E. (eds.), Nectaries and nectar, Springer, The Netherlands. [Google Scholar]
- Paini D. R. 2004. Impact of the introduced honey bee (Apis mellifera) (Hymenoptera: Apidae) on native bees: a review. Austral. Ecol. 29: 399–407. [Google Scholar]
- Patiny S., Rasmont P., Michez D. 2009. A survey and review of the status of wild bees in the west-palaearctic region. Apidologie 40: 313–331. [Google Scholar]
- Piessens K., Hermy M. 2006. Does the heathland flora in north-western Belgium show an extinction debt? Biol. Conserv. 132: 382–394. [Google Scholar]
- (PNC) Parc National des Cévennes . 2007. Guide du naturaliste: causses - cévennes, à la découverte des milieux naturels du Parc national des Cévennes. Libris, Grenoble, France. [Google Scholar]
- (PNC) Parc National des Cévennes . 2012. Eléments d’état des lieux pour l’élaboration de la charte. Parc national des Cévennes, France. [Google Scholar]
- Potts S. G., Biesmeijer J. C., Kremen C., Neumann P., Schweiger O., Kunin W. E. 2010. Global pollinator declines: trends, impacts and drivers. Trends. Ecol. Evol. 25: 345–353. [DOI] [PubMed] [Google Scholar]
- Rasmont P., Mersch P. 1988. Première estimation de la dérive faunique chez les bourdons de la Belgique (Hymenoptera: Apidae). Ann. Soc. R. Zool. Belg. 118: 141–147. [Google Scholar]
- Rasmont P., Leclercq J., Jacob-Remacle A., Pauly A., Gaspar C. 1993. The faunistic drift of Apoidea in Belgium, pp. 65–87. In Proceedings of an EC workshop, Bees for pollination, 2–3 March 1992, UE, Brussels, Belgium. [Google Scholar]
- Rasmont P., Pauly A., Terzo M., Patiny S., Michez D., Iserbyt S., Barbier Y., Haubruge E. 2005. The survey of wild bees (Hymenoptera, Apoidea) in Belgium and France. Food and Agriculture Organisation, Rome, Italy. [Google Scholar]
- Sarthou J. P., Fromage P., Genet B., Vinauger A., Heintz W., Monteil C. 2010. SYRFID vol.4: Syrphidae of France, interactive data. (http://syrfid.ensat.fr/index.php). [Google Scholar]
- Sarthou V. 2011. Diversité des Syrphidae en grandes cultures et intérêt entomologique, pp. 15–18. In Proceedings, Colloque de restitution du programme CASDAR: les entomophages en grandes cultures: diversité, service rendu et potentialités des habitats, 17 November 2011, Paris, France. [Google Scholar]
- Somme L., Mayer C., Jacquemart A. L. 2014. Multilevel spatial structure impacts on the pollination services of, Comarum palustre (Rosaceae). PLoS One 9: e99295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somme L., Vanderplanck M., Michez D., Lombaerde I., Moerman R., Wathelet B., Wattiez R., Lognay G., Jacquemart A. L. 2015. Pollen and nectar quality drive major and minor floral choices of bumble bees. Apidologie 46:92–106. [Google Scholar]
- Steffan-Dewenter I., Tscharntke T. 1999. Effects of habitat isolation on pollinator communities and seed set. Oecologia 121: 432–440. [DOI] [PubMed] [Google Scholar]
- Steffan-Dewenter I., Tscharntke T. 2000. Resource overlap and possible competition between honey bees and wild bees in Central Europe. Oecologia 122: 288–296. [DOI] [PubMed] [Google Scholar]
- Thomson D. 2004. Competitive interactions between the invasive European honey bee and native bumblebees. Ecology 85: 458–470. [Google Scholar]
- Vanderplanck M., Moerman R., Rasmont P., Lognay G., Wathelet B., Wattiez R., Michez D. 2014. How does pollen chemistry impact development and feeding behaviour of polylectic bees? PLoS One 9: e86209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webb N. R. 1998. The traditional management of European heathlands. J. Appl. Ecol. 35: 987–990. [Google Scholar]
- Willis J. C., Burkill I. H. 1895. Flowers and insects in Great Britain. Ann. Bot. 17: 539–571. [Google Scholar]
- Winfree R., Aguilar R., Vázquez D. P., LeBuhn G., Aizen M. A. 2009. A meta-analysis of bees’ responses to anthropogenic disturbance. Ecology 90: 2068–2076. [DOI] [PubMed] [Google Scholar]