Abstract
The Asian bush mosquito (Aedes japonicus japonicus, Theobald 1901) is an invasive culicid species which originates in Asia but is nowadays present in northern America and Europe. It is a competent vector for several human disease pathogens. In addition to the public health threat, this invasive species may also be an ecological threat for native container-breeding mosquitoes which share a similar larval habitat. Therefore, it is of importance to gain knowledge on ecological and eco-toxicological features of the Asian bush mosquito. However, optimal laboratory feeding conditions have not yet been established. Standardized feeding methods will be needed in assessing the impact of insecticides or competitional strength of this species. To fill this gap, we performed experiments on food quality and quantity for Ae. j. japonicus larvae. We found out that the commercial fish food TetraMin (Tetra, Melle, Germany) in a dose of 10 mg per larva is the most suitable food tested. We also suggest a protocol with a feeding sequence of seven portions for all larval stages of this species.
Keywords: Asian bush mosquito, feeding, reserve substance, life cycle, water parameter
The Asian bush mosquito (Aedes japonicus japonicus, Theobald 1901) is an invasive species, which originates in Japan and Korea (Tanaka et al. 1979) but has invaded New Zealand, North America, and Europe (Kampen and Werner 2014). It was shown that this species is competent to several arboviruses such as West Nile virus (Sardelis and Turell 2001), Japanese encephalitis virus (Takashima and Rosen 1989), or St. Louis encephalitis virus (Sardelis et al. 2003) under laboratory conditions. A German population of the Asian bush mosquito could be experimentally infected with Japanese encephalitis virus, but this population was resistant to infection with West Nile virus (Huber et al. 2014). Besides the potential threat to public health, the Asian bush mosquito may play a role as nuisance mosquito (Medlock et al. 2012), and its spreading outside of its area of origin may have negative ecological impacts on the native mosquito fauna (Andreadis and Wolfe 2010). Because of these harmful effects, larvicide application to combat the Asian bush mosquito may be necessary.
For evaluating suitable insecticides and application procedures, a standardized feeding protocol for the respective mosquito species is a requirement because it was shown, for instance, that the toxicity of lambda-cyhalothrin on the Asian tiger mosquito (Aedes albopictus, Skuse 1894) depends on the nutrition (Kress et al. 2014) or that the toxins of Bacillus thuringiensis israelensis are less efficient when food particles are present as shown for Aedes aegypti L., 1762 (Ben-Dov et al. 2003). To our knowledge, there is no standardized feeding protocol for the Asian bush mosquito. It is, e.g., suggested in the World Health Organization guideline for the laboratory testing of larvicides to add food to bioassays when long exposures are expected; however, no food plan has been specified (World Health Organization [WHO] 2005). In addition, a standardized feeding protocol allows for comparisons of collected data among laboratories.
We examined the impact of six different food types (of animal, plant, and microbial origin) at equicalorical amount on the Asian bush mosquito in a life cycle experiment spanning all stages from the first larval stage to adults. In addition, we tested four quantities for the two food types performing best in the equicalorical feeding experiment. Based on the studied life cycle parameters (mortality, sex ratio, pupation and emergence time, and length of the R1 wing vein of adult females), the reserve substances lipid, glycogen and protein, and the water parameters (pH, conductivity, oxygen, ammonium, phosphate, biofilm formation measured as colony forming units, and remaining filterable matter), we suggest here a laboratory feeding protocol for the Asian bush mosquito.
Materials and Methods
Origin and Keeping of Asian Bush Mosquito
Eggs of the Asian bush mosquito were collected by depositing black plastic beakers with press board sticks in a garden area in Biberach (Baden), Germany. Oviposition sticks were collected and stored in closed plastic bags for 1 wk at 25°C, 90% humidity, and a photoperiod of 16:8 (L:D) h in a climate chamber (Flohr Instruments, Utrecht, The Netherlands). Larval hatch was initiated by placing the oviposition sticks in reverse osmosis dechlorinated water at 25°C, 90% humidity, and a photoperiod of 16:8 (L:D) h. Larvae hatched from at least four sticks from four different traps were pooled. For both, food quality and food quantity experiment, 30 larvae younger than 24 h were placed in 1-liter plastic beakers filled with 800 ml reverse osmosis dechlorinated water. The number of larvae per beaker was chosen because of the limited availability of biological material and because no competition was observed for 30 larvae per beaker (unpublished data). However, no experiments on the optimal crowding conditions or larval competition were made. Five biological replicates per food type or food amount were set up.
Food Quality Experiment
The food quality experiment was started on 2 November 2014 with eggs collected on 28 October 2014.
Calorimetric Measurements
Walnut (Juglans regia L.) fresh leaves and fallen leaves were collected on 5 October 2014 in Biberach (Baden). They were kept separately and dried for 7 d at 80°C in the dark together with fish foods TetraMin, TetraPhyll (both Tetra, Melle, Germany), chironomid larvae (Zoohaus Haindl, Frankfurt, Germany), and fresh baker’s yeast (Uniferm, Werne, Germany). Specific energy [J/g] of food types were measured in a C200 calorimeter (IKA, Staufen, Germany).
Feeding
Because the leaves of the calorimetric measurements were used up, walnut fresh leaves and walnut fallen leaves were collected again on 28 October 2014 from Biberach (Baden), Germany. They were dried for 2 d at 80°C in the dark. TetraMin, TetraPhyll, chironomid larvae, and the two types of walnut leaves were shredded in a ball mill (Pulverisette; Fritsch, Idar-Oberstein, Germany) and particles sieved through a 900 µm by 900 µm mesh net were used. They were kept at −20°C until further use to avoid putrefaction. The food types represent two animal-based (TetraMin, chironomid larvae), three plant-based (TetraPhyll, walnut fresh, and walnut fallen leaves), and one microbial (baker’s yeast) food. Walnut-fallen leaves were the main organic input of water containers found at the egg collection site. TetraMin, chironomid larvae, and TetraPhyll are commercially available fish food products which are easily accessible in sufficient amounts for laboratory breeding. Walnut fresh leaves were chosen as natural food source and because it has a better availability throughout the year compared with fallen leaves. It was differentiated between walnut fallen and fresh leaves because of their different chemical composition. Fresh baker’s yeast was solved in reverse osmosis dechlorinated water containing 0.5 g/liter vitamin C and 0.5 g/liter glucose, heated to 38°C for 1 h and subsequently fed. The baker’s yeast solution was chosen because it represents the egg hatch medium used by Kress et al. (2014) for the Asian tiger mosquito and is there also the first food source of the newly hatched larvae.
Experimental Setup
For the food quality experiment, 100 Joule per larva was equicalorically fed in seven portions: 10% on days 0, 2, 4, and 5 after onset of the experiment and 20% on days 7, 8, and 10. All foods were suspended in reverse osmosis dechlorinated water prior to feeding. The experiment was carried out at 25°C, 90% humidity, and a photoperiod of 16:8 (L:D) h in a climate chamber (Flohr Instruments).
Life Cycle Parameters
Pupae were removed and kept individually. Day of pupation and day of emergence were recorded. Adults were frozen to death after emergence. The sex was determined morphologically for each individual and percentage of females was calculated. Mortality was calculated for both sexes together and the length of the R1 wing vein of females was measured. The wing length of females corresponds positively with the total number of produced eggs (R2 = 0.35; Oliver and Howard 2005) and is therefore used as a fitness proxy.
Reserve Substances
Total contents of protein, lipid, and glycogen in individual adult mosquitoes (N = 10 per food type) of the food quality experiment were measured photometrically according to the methods of Bradford (1976, proteins), van Handel (1985a; glycogen), and van Handel (1985b; lipids). Single individuals were placed in 300 µl 2% sodium sulfate solution and were homogenized for 3 min at 30 rounds per second in a mixer mill (MM 400; Retsch, Haan, Germany). The sample was split: 100 µl were used for lipid and glycogen analysis and 200 µl homogenate was used for protein analysis. Bradford reagent (1.5 ml) was added in triplicate to 50 µl homogenate (protein detection); 1.6 ml 1:1-chloroform/methanol solution was added to the remaining 100 µl of the homogenate and centrifuged. The supernatant was separated from the pellet, and 600 µl water was added, centrifuged, and the bottom phase was mixed with 200 µl 95–98% sulfuric acid, and 5 ml vanillin reagent was added after cooling (lipid). The pellet was replenished to 5 ml with anthrone reagent and heated (glycogen). The values of the three reserve substances were transformed into size-specific caloric contents (SSCC; Timmermann and Briegel 1999). For this transformation, the caloric content was divided by the cubic length of the wing vein R1, which was measured under a microscope (SZX16) with attached camera (DP25) by means of a polygonal line in the labSense 1.3 software (all Olympus, Tokyo, Japan). Since newly eclosed and unfed adults were used for this analysis, their reserve constitution represents their larval biosynthesis. We also analyzed the protein, glycogen, and lipid contents of the foods to characterise their nutritional composition with the same methods.
Water Parameters
The water parameters pH, conductivity, and oxygen were measured with a sensor (WTW, Weilheim, Germany), and ammonium and phosphate were measured photometrically in triplicate (Hach Lange, Düsseldorf, Germany) for each beaker on the day when the last individual pupated or died. The number of colony forming heterotrophic microorganisms as potential indirect food source was assessed using the streak plate method with Reasoner’s 2A agar incubated at 35°C for 2 d. Filterable matter was measured by vacuum filtration through micro-glass fibre filters grade MG C (Munktell, Falun, Sweden).
Food Quantity Experiment
The food quantity experiment was set up on 4 August 2014 with eggs collected on 29 July 2014. Five different amounts (2.5, 5.0, 7.5, or 10.0 mg) of TetraMin or TetraPhyll were fed per larva. Feeding of sub-portions was scheduled: 10% on days 0, 2, 4, and 5 after onset of the experiment and 20% on days 7, 8, and 10. We took the publication by Müller et al. (2013) as orientation for food amounts and feeding sequence but modified the sequence in accordance to the life cycle characteristics of the Asian bush mosquito. The life cycle parameters mortality, the percentage of females, pupation time, emergence time, and length of the wing vein R1 were assessed.
Statistical Data Analysis
Mortality data were normalized and arcsine transformed for analysis of variance (ANOVA) analyses. Sex ratio was expressed as percentage of females. Pupation and emergence data are expressed as the point of time at which 50% of the larvae have pupated (PT50) and as point of time at which 50% of the imagines emerged (EmT50). To do so, nonlinear regressions using the least squares fitting method were computed with cumulative data of pupated respectively emerged individuals per day in Prism5 (GraphPad, La Jolla, CA). The deviation of female and male numbers from the 1:1 ratio was tested with two-tailed Chi-squared tests with α < 0.05. Nonparametric one-way ANOVAs with a Kruskal–Wallis test and a Dunns post-test were done in Prism5 (GraphPad) and used for all parameter comparisons. The influence of food type and food amount (food quantity experiment) on the variation of mortality, sex ratio, EmT50, and length of the R1 wing vein and the food type and sex on the amount of proteins, lipids, and glycogen (food quality experiment) were tested using a two-way ANOVA with Bonferroni post-test in Prism5. Principal component analysis for the analysis of the reserve substances was done in Past3 (Hammer et al. 2001).
Results
Food Quality Experiment
Life Cycle Parameters
The larval development was fastest in the group fed with TetraPhyll (PT50 = 10.14 d, EmT50 = 12.56 d; Table 1), followed by the groups fed with TetraMin and chironomid larvae. The longest individual larval development time (75 d) until pupation was found in two individuals fed with walnut fresh leaves. One died as pupa and one emerged on day 78 (female). In all treatments, more than 50% females emerged. Highest female percentages were found in the TetraMin group (70.43%) as well as in the group fed with chironomid larvae (68.61%; Table 1). The mean percentage of females was not significantly different between the six different food groups and the sex ratio did not differ significantly from an expected 1:1 ratio (df = 4, Chi-square values between 0.06 and 7.99, P values between 0.09 and 1.00). The mean mortality ranged from 5.33% (TetraPhyll) to 96.67% (baker’s yeast; Table 1). Compared with the groups fed with TetraPhyll, TetraMin, and chironomid larvae, the mortality of the group fed with baker’s yeast is significantly increased (P ≤ 0.05). The largest females with a mean R1 wing vein length of 3,624 µm emerged when fed as larvae with chironomid larvae (Table 1). When compared with this group, females fed as larvae with walnut leaves (fresh or fallen leaves) show significantly decreased (P ≤ 0.05) R1 lengths of 2,664 µm and 2,392 µm, respectively (Table 1).
Table 1.
Life cycle parameters of the food quality experiment
| Food type | PT50 (d) (95% CI) | EmT50 (d) (95% CI) | Percentage of females mean (min–max) | Mortality (%) mean (min–max) | Mean female R1 length (µm) (min–max) N = 10 |
|---|---|---|---|---|---|
| TetraMin | 10.41 | 12.66 | 70.43 | 7.33 | 3,492 |
| (10.09–10.72) | (12.65–12.68) | (58.62–78.26) | (0.00–23.33) | (3,340–3,589) | |
| TetraPhyll | 10.14 | 12.56 | 65.34 | 5.33 | 3,522 |
| (9.81–10.47) | (12.53–12.60) | (56.67–81.48) | (3.33–10.00) | (3,334–3,750) | |
| Chironomid | 11.02 | 13.33 | 68.61 | 10.00 | 3,624 |
| larvae | (10.74–11.31) | (13.24–13.43) | (55.17–88.46) | (3.33–20.00) | (3,541–3,785) |
| Walnut fresh | 15.67 | 19.66 | 58.40 | 16.67 | 2,664 |
| leaves | (14.74–16.60) | (19.17–20.15) | (57.14–60.00) | (6.67–36.67) | (2,323–3,073) |
| Walnut | 24.00 | 25.16 | 51.70 | 37.33 | 2,392 |
| fallen leaves | (23.36–24.65) | (24.55–25.76) | (40.00–75.00) | (23.33–50.00) | (2,238–2,732) |
| Baker’s yeast | 26.62 | unkn. | 53.33 | 96.67 | unkn. |
| (24.45–28.78) | (66.67–100.00) | (90.00–100.00) |
PT50: time at which 50% of the larvae had pupated; CI: confidence interval; EmT50: time at which 50% of the adults had emerged; unkn.: values were not calculated due to the high mortality in this food group; min: minimum; max: maximum.
Reserve Substances
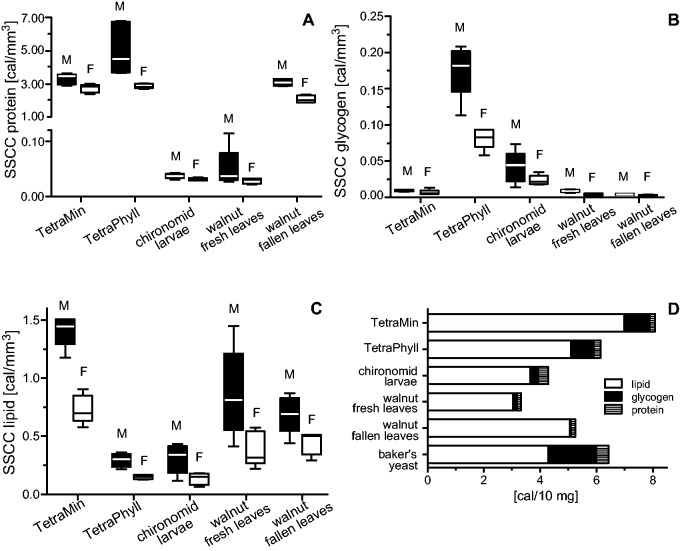
The SSCCs of proteins, glycogen, and lipids are generally lower in females than in males (Fig. 1A–C). The highest protein SSCC was found in adults fed as larvae with TetraPhyll (5.08 ± 1.57 cal/mm3 for males and 2.86 ± 0.14 cal/mm3 for females). The protein SSCCs for TetraMin and walnut fallen leaves were similar with 3.30 ± 0.32 cal/mm3 and 3.08 ± 0.21 cal/mm3 for males and 2.72 ± 0.26 cal/mm3 and 2.06 ± 0.22 cal/mm3 for females, respectively. Individuals with chironomid larvae and walnut fresh leaves as larval food showed only small protein SSCCs of 0.03 ± 0.004 cal/mm3 and 0.05 ± 0.04 cal/mm3 for males and 0.03 ± 0.003 cal/mm3 and 0.03 ± 0.005 cal/mm3 for females, respectively. When comparing food groups of the same sex, the SSCC protein contents of males and females fed with chironomid larvae and males fed with walnut fresh leaves are significantly (P ≤ 0.01) decreased compared with the TetraPhyll-fed individuals (Fig. 1A).
Fig. 1.
Reserve substances in individual adult mosquitoes (A–C) and protein, glycogen, and lipid content of foods (D) (food quality experiment). (A) Proteins; (B) glycogen; and (C) lipids. (A–C) M: males, F: females. Box plots with 5–95% whiskers are shown (N = 10). No mosquitoes fed with baker’s yeast were analyzed because of the lack of biological material due to the high mortality (Table 1). D: glycogen, lipid, and protein content of each food. Only means are shown (N = 3), SDs are named in the text.
The glycogen SSCCs decrease from individuals fed with TetraPhyll (males: 0.18 ± 0.04 cal/mm3, females: 0.08 ± 0.01 cal/mm3) over chironomid larvae (males: 0.04 ± 0.02 cal/mm3, females: 0.02 ± 0.01 cal/mm3) to TetraMin (males: 0.009 ± 0.001 cal/mm3, females: 0.006 ± 0.004 cal/mm3) to walnut fresh leaves (males: 0.009 ± 0.002 cal/mm3, females: 0.004 ± 0.001 cal/mm3) and walnut fallen leaves (males: 0.004 ± 0.001 cal/mm3, females: 0.003 ± 0.0005 cal/mm3) whereby the decrease of individuals of the same sex fed with TetraPhyll compared with both walnut food groups is significant (P ≤ 0.05; Fig. 1B).
The lipid SSCCs were highest in individuals fed with TetraMin (males: 1.41 ± 0.14 cal/mm3, females: 0.73 ± 0.12 cal/mm3). Medium lipid SSCCs were found in Asian bush mosquitoes fed with walnut fresh leaves (males: 0.87 ± 0.38 cal/mm3, females: 0.39 ± 0.15 cal/mm3) and with walnut fallen leaves (males: 0.68 ± 0.16 cal/mm3, females: 0.45 ± 0.11 cal/mm3). The lowest SSCCs were found in individuals fed with chironomid larvae (males: 0.31 ± 0.13 cal/mm3, females: 0.14 ± 0.05 cal/mm3) and TetraPhyll (males: 0.30 ± 0.05 cal/mm3, females: 0.15 ± 0.02 cal/mm3). The chironomid larvae-fed males contain significantly (P ≤ 0.05) less lipid then TetraMin-fed males (Fig. 1C).
A principle component analysis shows that protein (eigenvalue 3.21) accounts for 95.59%, lipid for 4.37% (eigenvalue 0.15), and glycogen for 0.04% (eigenvalue: <0.01) of the total variance. The highest SSCCs were also found for protein, followed by lipid and glycogen. However, the different fish foods contain mainly lipids (Fig. 1C). TetraMin shows the highest amount of calories, followed by baker’s yeast and TetraPhyll (Fig. 1C).
Ten-milligram TetraMin consists of 0.19 ± 0.06 cal proteins, 6.99 ± 0.16 cal lipids, and 0.90 ± 0.01 cal glycogen (total: 2.69 ± 3.24 cal); 10-mg TetraPhyll contains 0.25 ± 0.003 cal proteins, 5.10 ± 0.07 cal lipids, and 0.80 ± 0.01 cal glycogen (total: 2.05 ± 2.30 cal); 10-mg chironomid larvae has 0.35 ± 0.05 cal proteins, 3.65 ± 0.03 cal lipids, and 0.29 ± 0.003 cal glycogen (total: 1.43 ± 1.67 cal); 10-mg walnut fresh leaves contain 0.14 ± 0.05 cal protein, 3.03 ± 0.03 cal lipids, and 0.14 ± 0.01 cal glycogen (total: 1.11 ± 1.44 cal); 10-mg walnut fallen leaves shows a protein content of 0.14 ± 0.05 cal, 5.06 ± 0.11 cal lipids, and 0.04 ± 0.001 cal glycogen (total: 1.75 ± 2.48 cal); and 10-mg baker’s yeast contain 0.44 ± 0.05 cal proteins, 4.28 ± 0.09 cal lipids, and 1.7 ± 0.003 cal glycogen (total: 2.14 ± 1.70 cal) (Fig. 1C). When testing the influence of food type (df = 4; Fprotein = 116.50, Fglycogen = 132.40, Flipid = 40.14) and sex (df = 1; Fprotein = 26.59, Fglycogen = 33.96, Flipid = 46.99) on the amounts of protein, glycogen, and lipid, both parameters and their interaction (df = 4; Fprotein = 7.55, Fglycogen = 18.40, Flipid = 5.36) significantly (all P values ≤ 0.006) account for the variation of all three reserve substances.
Water Parameters
The mean pH measured at the end of the experiment ranged from 6.63 for TetraMin to 7.65 for chironomid larvae (Table 2) but was not significantly different for the different food types. The conductivity showed lowest values for walnut fresh leaves (37.97 µS/cm) and fallen leaves (26.96 µS/cm) as well as baker’s yeast (26.51 µS/cm) (Table 2). The conductivity of the chironomid larvae food regime was significantly increased when compared with the three aforementioned food types (102.7 µS/cm; P ≤ 0.05).
Table 2.
Water parameters measured at the end of the food quality experiment
| Food type | pH | Conductivity (µS/cm) | Oxygen (%) | Total PO43− (mg/liter) | NH4+ (mg/liter) | CFU × 106 (ml −1) | FM × 10−5 (g/ml) |
|---|---|---|---|---|---|---|---|
| TetraMin | 6.63 ± 0.53 | 66.92 ± 1.92 | 42.78 ± 6.27 | 4.35 ± 0.41 | 5.96 ± 0.30 | 1.80 ± 3.22 | 5.70 ± 1.41 |
| TetraPhyll | 6.71 ± 0.45 | 71.30 ± 5.97 | 43.19 ± 16.16 | 4.27 ± 0.57 | 6.66 ± 0.65 | 3.56 ± 5.68 | 5.15 ± 2.46 |
| Chironomid larvae | 6.89 ± 0.59 | 102.7 ± 4.57 | 53.70 ± 26.75 | 3.56 ± 0.25 | 10.49 ± 0.62 | 3.60 ± 5.51 | 8.35 ± 2.22 |
| Walnut fresh leaves | 7.35 ± 0.22 | 37.97 ± 7.77 | 91.86 ± 3.67 | 0.83 ± 0.27 | 1.09 ± 0.19 | 0.02 ± 0.04 | 7.43 ± 4.20 |
| Walnut fallen leaves | 7.65 ± 0.73 | 26.96 ± 8.60 | 92.94 ± 6.05 | 0.51 ± 0.18 | 0.75 ± 0.38 | 5.12 ± 1.09 | 10.68 ± 4.69 |
| Baker’s yeast | 6.91 ± 0.96 | 26.51 ± 9.83 | 77.49 ± 25.93 | 1.58 ± 0.58 | 2.04 ± 1.11 | 9.28 ± 7.93 | 4.84 ± 4.10 |
CFU, colony forming units; FM, filterable matter. Mean values ± SDs are shown.
The oxygen saturation showed the lowermost values for TetraMin and TetraPhyll (42.78% and 43.19%, respectively) and the highest for walnut fresh (91.86%) and fallen leaves (92.94%). The oxygen of TetraMin compared with walnut fallen leaves was significantly decreased (P ≤ 0.05).
The total phosphate content was the highest for TetraMin (4.35 mg/liter) and TetraPhyll (4.27 mg/liter). Compared with these two food types, the remaining water of the set up with walnut fresh leaves showed a significantly (P ≤ 0.05) decreased phosphate content (0.83 mg/liter) and the set up with walnut fallen leaves a very significantly (P ≤ 0.01) decreased total phosphate content of 0.51 mg/liter (Table 2).
Ammonium of 10.49 mg/liter was found in the water of the food treatment with chironomid larvae, 6.66 mg/liter and 5.96 mg/liter were measured in TetraPhyll and TetraMin food treatments, respectively. Baker’s yeast food treatment showed an ammonium amount of 2.04 mg/liter and the food treatments with walnut fresh leaves (1.09 mg/liter) and fallen leaves (0.75 mg/liter) showed fewest ammonium in the water (Table 2). The ammonium content of water of the food treatment with chironomid larvae was extremely significantly (P ≤ 0.001) increased when compared with the food treatment with walnut fallen leaves, very significantly (P ≤ 0.01) increased compared with the food treatment with walnut fresh leaves and significantly increased in comparison with baker’s yeast as food treatment (P ≤ 0.05). In addition, the ammonium content of the food treatment with TetraPhyll compared with the one with walnut fallen leaves was significantly increased (P ≤ 0.05).
The number of colony forming units was lowest in the beakers treated with walnut fresh leaves (0.02 by 106 per ml) and highest in baker’s yeast food treatment (9.28 by 106 per ml). The difference between these two food types was very significant (P ≤ 0.01). There was no significant difference between the filterable matters weighted. Their values ranged between 4.84 by 10−5 g/ ml and 10.68 by 10−5 g/ ml (Table 2).
Food Quantity Experiment
Two-way ANOVA revealed that the interactive effect of food type (TetraMin or TetraPhyll) and food amount (2.5, 5.0, 7.5 and 10.0 mg per larva) accounts for most variation of the mortality among food groups (df = 3, F = 2.90, P = 0.04) but food type (df = 1, F = 0.14, P = 0.71) and amount (df = 3, F = 0.81, P = 0.50) showed no significant effect. The percentage of females was not influenced by food type (df = 1, F = 2.50, P = 0.12), food amount (df = 3, F = 0.23, P = 0.87), and their interaction (df = 3, F = 0.93, P = 0.44). The variation of EmT50 significantly depends on the food type (df = 1, F = 21.99, P < 0.0001) and the food amount (df = 3, F = 99.14, P < 0.0001), but the interaction has no significant effect (df = 3, F01.86, P = 0.16). The mean values of these life cycle parameters can be found in Table 3. The mean R1 length for males increases from 2,669 ± 37 µm for 2.5 mg TetraMin per larva to 2,864 ± 66 µm for 5.0 mg TetraMin per larva to 2,946 ± 73 µm for 7.5 mg TetraMin per larva. For 10.0 mg TetraMin per larva, the mean R1 length of males decreases to 2,924 ± 52 µm. For females, the mean R1 length increases with increasing amount of TetraMin per larva: 3,278 ± 81 µm for 2.5 mg, 3,658 ± 104 µm for 5.0 mg, 3,716 ± 54 for 7.5 mg, and 3,810 ± 160 µm for 10.0 mg (Fig. 2A). Asian bush mosquitoes fed with TetraPhyll have generally a smaller R1 wing length compared with TetraMin-fed individuals except for the smallest food amount. The male mean R1 length for 2.5 mg TetraPhyll per larva is 2,651 ± 167 µm, for 5.0 mg TetraPhyll per larva 2,751 ± 99 µm, for 7.5 mg TetraPhyll per larva 2,666 ± 111 µm, and for 10.0 mg TetraPhyll per larva 2,797 ± 47 µm (Fig. 2A). The largest females were found when feeding with 5.0 mg TetraPhyll per larva (3,632 ± 102 µm), followed by 10.0 mg per larva (3,599 ± 33 µm) and 7.5 mg TetraPhyll per larva (3,561 ± 44 µm). The smallest female mean R1 length was found when fed with the smallest food amount (3,360 ± 205 µm) (Fig. 2A). Male and female R1 lengths are affected by food type, food amount and their interaction (test statistics in Fig. 2B).
Table 3.
Life cycle parameters of the food quantity experiment with the Asian bush mosquito
| Food type | Food amount (mg/larva) | EmT50 (d) (95% CI) | Percentage of females mean (min–max) | Mortality (%) mean (min–max) |
|---|---|---|---|---|
| TetraMin | 2.5 | 13.78 (13.66–13.90) | 57.84 (45.83–74.07) | 11.33 (0.00–20.00) |
| 5.0 | 11.56 (11.54–11.59) | 56.07 (46.43–66.67) | 12.00 (6.67–16.67) | |
| 7.5 | 11.33 (11.32–11.35) | 50.56 (33.33–67.86) | 4.67 (0.00–20.00) | |
| 10.0 | 10.90 (10.87–10.93) | 57.04 (50.00–67.86) | 5.33 (3.33–6.67) | |
| TetraPhyll | 2.5 | 12.61 (12.45–12.76) | 46.55 (33.33–62.50) | 3.33 (0.00–10.00) |
| 5.0 | 10.93 (10.90–10.96) | 49.95 (37.04–64.29) | 9.34 (6.67–16.67) | |
| 7.5 | 10.61 (10.61–10.62) | 53.15 (40.00–64.29) | 8.67 (0.00–23.33) | |
| 10.0 | 10.46 (10.31–10.62) | 53.05 (44.83–50.09) | 15.33 (3.33–26.67) |
EmT50: time at which 50% of the adults had emerged; CI: confidence interval; min: minimum; max: maximum.
Fig. 2.
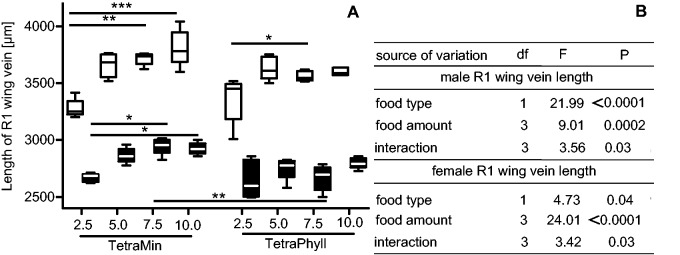
Length of the R1 wing vein (quantitative food experiment). (A) Effects of food type and food quantity on the length of the R1 wing vein. White bars: females, black bars: males. Significance levels due to a nonparametric one–way ANOVA with Kruskal–Wallis test and Dunns post–test (α = 0.05): *P ≤ 0.05, **P ≤ 0.01, ***P ≤ 0.001. (B) Test statistic values for a two-way ANOVA (α = 0.05) for male and female R1 length variation sourced by food type and food quantity.
Discussion
Based on the life cycle parameters (Table 1), the larval development of the Asian bush mosquito is fastest when fed with TetraMin and TetraPhyll. Feeding with these two food types also resulted in the lowest mortality (Table 1). However, the largest adult females were found in the cohort fed with chironomid larvae (Table 1). We found that protein accounts for most of the variation of all studied reserve substances (see Results—Reserve substances). Protein occurs mostly in structural components of the mosquito body (Timmermann and Briegel 1999) and correlates positively with the body size in unfed mosquito adults (Timmermann and Briegel 1996). Mosquitoes can take up proteins with the larval diet or during blood meals. Since males do not feed on blood, larval diet is the only protein source (Van Handel 1984).
The highest protein contents were found in adults fed with TetraMin and TetraPhyll. TetraMin-fed individuals also showed the highest lipid content and TetraPhyll-fed individuals the highest glycogen content (Fig. 1). Glycogen was found in fourth-instar larvae of Ae. aegypti in lesser contents in the fat body and in parts of the nervous system but is mainly deposited in the muscles (Wigglesworth 1942), where it functions as energy source (Steele 1982). Lipid is stored as droplets of the fat body especially in form of triglycerides. In insects, lipid is mobilized to directly or indirectly fuel flight, during starvation or immune response as well as during embryogenesis (Arrese and Soulages 2010). The doubled lipid contents of males fed with TetraMin compared with females was also shown by Timmermann and Briegel (1999) for Ae. aegypti. It is not known why males seem to require increased lipid storage.
None of the assessed water parameters seem to influence the life cycle parameters. For all food types, a microbial film formed (analyzed as colony forming units) which is an additional larval food source. However, no significant difference between the two best performing food types TetraMin and TetraPhyll was seen. The same is true for the filterable matter, which was chosen as measure for dietary leftovers. Altogether, the advantages for the cohorts feeding with TetraMin and TetraPhyll were the short developmental times, the low mortalities, the high protein contents, and the high caloric contents of the food itself. Therefore, they were chosen for the quantitative food experiment.
The quantitative food experiment showed for both food types that the emergence time decreases with increasing food amount. TetraPhyll performs slightly better when comparing the EmT50 values. Sex ratios were balanced for both food types and amounts (Table 3). The mortality decreased with increasing food amount for TetraMin-fed individuals and increased for TetraPhyll. The adults fed as larvae with TetraMin were generally bigger than the ones fed with TetraPhyll (Fig. 2). We decided for 10 mg TetraMin per larva as optimal food of the tested ones because the differences to TetraPhyll in regard to the PT50 and EmT50 values was only minimal and we weighted the total caloric content of the food, the protein content of the adult mosquitoes, and the bigger individuals as most important parameters. Hoshino et al. (2010) report that TetraMin is also a suitable food source for the maintenance of early instar larvae of the Asian bush mosquito but they use a different fish food for late instar. They also schedule the food amount on the larval stage. However, they do not feed per larva but per day (Hoshino et al. 2010).
For the Asian bush mosquito, we modified the feeding sequences proposed for the Asian tiger mosquito (Müller et al. 2013). We found out that the Asian bush mosquito has a 1 day-longer larval development time than the Asian tiger mosquito at 25°C (unpublished data compared with Müller et al. 2013). The last food portion for the Asian bush mosquito was therefore scheduled 1 day later (day 10 instead of day 9) to allow for a sufficient food intake on the day directly before pupation. Williges et al. (2008) present a larval diet for the laboratory colonization of the Asian bush mosquito, which is based on Purina Lab Diet and the amount of food depends on the larval stage. They do not study different food sources in their study and they do not adjust the food amount to the number of larvae. However, their study shows that there may be further suitable food sources for this species. A study on intra- and interspecific competition of larvae of the Asian bush mosquito and the North American rock pool mosquito Aedes atropalpus points out how important food regime and other experimental factors are for laboratory competition experiments (Armistead et al. 2008). Our study is the first to provide results on different food types and food quantities for Ae. japonicus to help to avoid experimental stress. However, knowledge on parameters such as temperature (Reiskind and Janairo 2015), larval densities (Timmermann and Briegel 1993, Armistead et al. 2008), water surface area (Wynn and Paradise 2001), and water depth (Timmermann and Briegel 1993, Wynn and Paradise 2001) is prerequisite for the successful establishment of bioassays for the Asian bush mosquito, but assessment of these parameters was not subject of this study.
We present a detailed plan (Table 4) for the feeding of the Asian bush mosquito as it is applied in our laboratory at 25°C, although minor facility-specific modifications may be necessary. TetraMin is grinded and sieved to sort out the particles with desired size, e.g., 560–900 µm. The needed amount is weighted in and suspended in the larval medium (here: reverse osmosis dechlorinated water). The food solution is pipetted into the experimental vessel by means of a plastic Pasteur pipet. To enable experiments with different larval densities, we show a scheme expressing the food per larva. The total amount of food per larva is 10 mg divided into seven subportions to avoid mold formation and decrease the number of (partial) water changes. Ten percent of the total food amount is fed on days 0, 2, 4, and 5 (which corresponds to 1 mg per larva) and 20% are fed on days 7, 8, and 10 (2 mg per larva). An example for the food amount for 150 larvae is given in Table 4. Partial water changes may be necessary to avoid putrefaction or algal growth at other temperatures.
Table 4.
Feeding schedule for the Asian bush mosquito with 10 mg TetraMin per larva
| Day | Food per larva (%) | Food per larva (mg) | Food for 150 larvae (g) |
|---|---|---|---|
| 0 | 10 | 1 | 0.15 |
| 2 | 10 | 1 | 0.15 |
| 4 | 10 | 1 | 0.15 |
| 5 | 10 | 1 | 0.15 |
| 7 | 20 | 2 | 0.30 |
| 8 | 20 | 2 | 0.30 |
| 10 | 20 | 2 | 0.30 |
Acknowledgments
We thank A. Swonkow for technical assistance, S. Gallus for logistic support, and J. Oehlmann for fruitful discussions and technical support during the experiments. This work was supported by the Hessian Centre on Climate Change (FZK) of the Hessian Agency for Environment and Geology (HLUG) and by the Rhineland-Palatinate Centre of Excellence for Climate Change Impacts. M.P., R.M., and U.K. were partly supported by the research funding programme “LOEWE-Landes-Offensive zur Entwicklung Wissenschaftlich-ökonomischer Exzellenz” of Hesse’s Ministry of Higher Education, Research, and the Arts. This study was conducted at the Senckenberg Biodiversity and Climate Research Centre Frankfurt (BiK-F).
References Cited
- Andreadis T. G., Wolfe R. J. 2010. Evidence of reduction of native mosquitoes with increased expansion of invasive Ochlerotatus japonicus japonicus (Diptera: Culicidae) in the Northeastern United States. J. Med. Entomol. 47: 43–52. [DOI] [PubMed] [Google Scholar]
- Armistead J. S., Nishimura N., Escher R. L., Lounibos L. P. 2008. Larval competition between Aedes japonicus and Aedes atropalpus (Diptera: Culicidae) in simulated rock pools. J. Vector Ecol. 33: 238–246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arrese E. L., Soulages J. L. 2010. Insect fat body: energy, metabolism, and regulation. Ann. Rev. Entomol. 55: 207–225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben-Dov E., Saxena D., Wang Q., Manasherob R., Boussiba S., Zaritsky A. 2003. Ingested particles reduce susceptibility of insect larvae to Bacillus thuringiensis. J. Appl. Entomol. 127: 146–152. [Google Scholar]
- Bradford M. M. 1976. A rapid and sensitive method for the quantification of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem 72: 248–254. [DOI] [PubMed] [Google Scholar]
- Hammer O. D., Harper A., Ryan P. D. 2001. PAST: paleontological statistics software package for education and data analysis. Palaeontol. Electron. 4: 1–9. [Google Scholar]
- Hoshino K., Isawa H., Tsuda Y., Kobayashi M. 2010. Laboratory colonization of Aedes japonicus japonicus (Diptera: Culicidae) collected in Narita, Japan and the biological properties of the established colony. Jpn. J. Infect. Dis. 63: 401–404. [PubMed] [Google Scholar]
- Huber K., Jansen S., Leggewie M., Badusche M., Schmidt-Chanasit J., Becker N., Tannich E., Becker S. C. 2014. Aedes japonicus japonicus (Diptera: Culicidae) from Germany have vector competence for Japan encephalitis virus but are refractory to infection with West Nile virus. Parasitol. Res. 113: 3195–3199. [DOI] [PubMed] [Google Scholar]
- Kampen H., Werner D. 2014. Out of the bush: the Asian bush mosquito Aedes japonicus japonicus (Theobald, 1901) (Diptera, Culicidae) becomes invasive. Parasite. Vector. 7: 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kress A., Kuch U., Oehlmann J., Müller R. 2014. Impact of temperature and nutrition on the toxicity of the insecticide lambda-cyhalothrin in full-lifecycle tests with the target mosquito species Aedes albopictus and Culex pipiens. J. Pest Sci. 87: 739–750. [Google Scholar]
- Medlock J. M., Hansford K. M., Schaffner F., Versteirt V., Hendrickx G., Zeller H., Bortel W. Van. 2012. A review of invasive mosquitoes in Europe: ecology, public health risks, and control options. Vector Borne Zoonotic Dis. 12: 435–447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller R., Knautz T., Völker J., Kress A., Kuch U., Oehlmann J. 2013. Appropriate larval food quality and quantity for Aedes albopictus (Diptera: Culidicae). J. Med. Entomol. 50: 668–673. [DOI] [PubMed] [Google Scholar]
- Oliver J., Howard J. J. 2005. Fecundity of naturally blood-fed Ochlerotatus japonicus. J. Med. Entomol. 42: 254–259. [DOI] [PubMed] [Google Scholar]
- Reiskind M. H., Janairo M. S. 2015. Late-instar behaviour of Aedes aegypti (Diptera: Culidicae) larvae in different thermal and nutritive environments. J. Med. Entomol. 52: 789–796. [DOI] [PubMed] [Google Scholar]
- Sardelis M. R., Turell M. J. 2001. Ochlerotatus j. japonicus in Frederick County, Maryland: discovery, distribution, and vector competence for West Nile virus. J. Am. Mosq. Control Assoc. 17: 137–141. [PubMed] [Google Scholar]
- Sardelis M. R., Turrell M. J., Andre R. G. 2003. Experimental transmission of St. Louis encephalitis virus by Ochlerotatus j. japonicus. J. Am. Mosq. Control Assoc. 19: 159–162. [PubMed] [Google Scholar]
- Steele J. E. 1982. Glycogen phosphorylase in insects. Insect Biochem. 12: 131–147. [Google Scholar]
- Takashima I., Rosen L. 1989. Horizontal and vertical transmission of Japanese encephalitis virus by Aedes japonicus. J. Med. Entomol. 26: 454–458. [DOI] [PubMed] [Google Scholar]
- Tanaka K., Mizusawa K., Saugstad E. S. 1979. A revision of the adult and larval mosquitoes of Japan (including the Ryukyu Archipelago and the Ogasawara Islands) and Korea (Diptera: Culicidae). Contrib. Am. Entomol. Inst. 16: 1–987. [Google Scholar]
- Timmermann S. E., Briegel H. 1993. Water depth and larval density affect development and accumulation of reserves in laboratory populations of mosquitoes. Bull. Soc. Vector Ecol. 18: 174–187. [Google Scholar]
- Timmermann S. E., Briegel H. 1996. Effect of plant, fungal and animal diets on mosquito development. Entomol. Exp. Appl. 80: 173–176. [Google Scholar]
- Timmermann S. E., Briegel H. 1999. Larval growth and biosynthesis of reserves in mosquitoes. J. Insect Physiol. 45: 461–470. [DOI] [PubMed] [Google Scholar]
- Van Handel E. 1984. Metabolism of nutrients in the adult mosquito. Mosq. News 44: 573–579. [Google Scholar]
- Van Handel E. 1985a. Rapid determination of glycogen and sugars in mosquitoes. J. Am. Mosq. Control Assoc. 1: 299–301. [PubMed] [Google Scholar]
- Van Handel E. 1985b. Rapid determination of total lipids in mosquitoes. J. Am. Mosq. Control Assoc. 1: 302–303. [PubMed] [Google Scholar]
- (WHO) World Health Organization. 2005. Guidelines for laboratory and field testing of mosquito larvicides. WHO reference number WHO/CDS/WHOPES/GCDPP/2005.13. WHO, Geneva. [Google Scholar]
- Wigglesworth V. B. 1942. The storage of protein, fat, glycogen and uric acid in the fat body and other tissues of mosquito larvae. J. Exp. Biol. 19: 56–77. [Google Scholar]
- Williges E., Farajollahi A., Scott J. J., McCuiston L. J., Crans W. J., Gaugler R. 2008. Laboratory colonization of Aedes japonicus japonicus. J. Am. Mosq. Control Assoc. 24: 591–593. [DOI] [PubMed] [Google Scholar]
- Wynn G., Paradise C. J. 2001. Effects of microcosm scaling and food resources on growth and survival of larval Culex pipiens. BMC Ecol. 1: 3. [DOI] [PMC free article] [PubMed] [Google Scholar]