Abstract
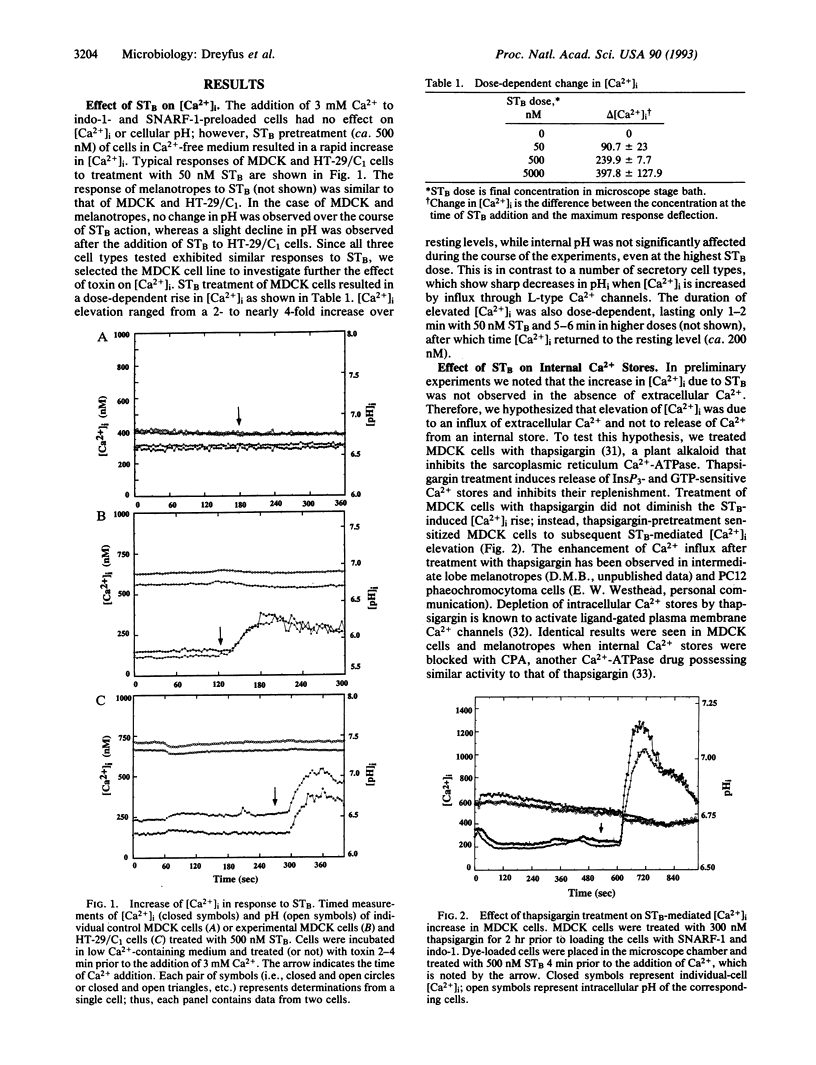
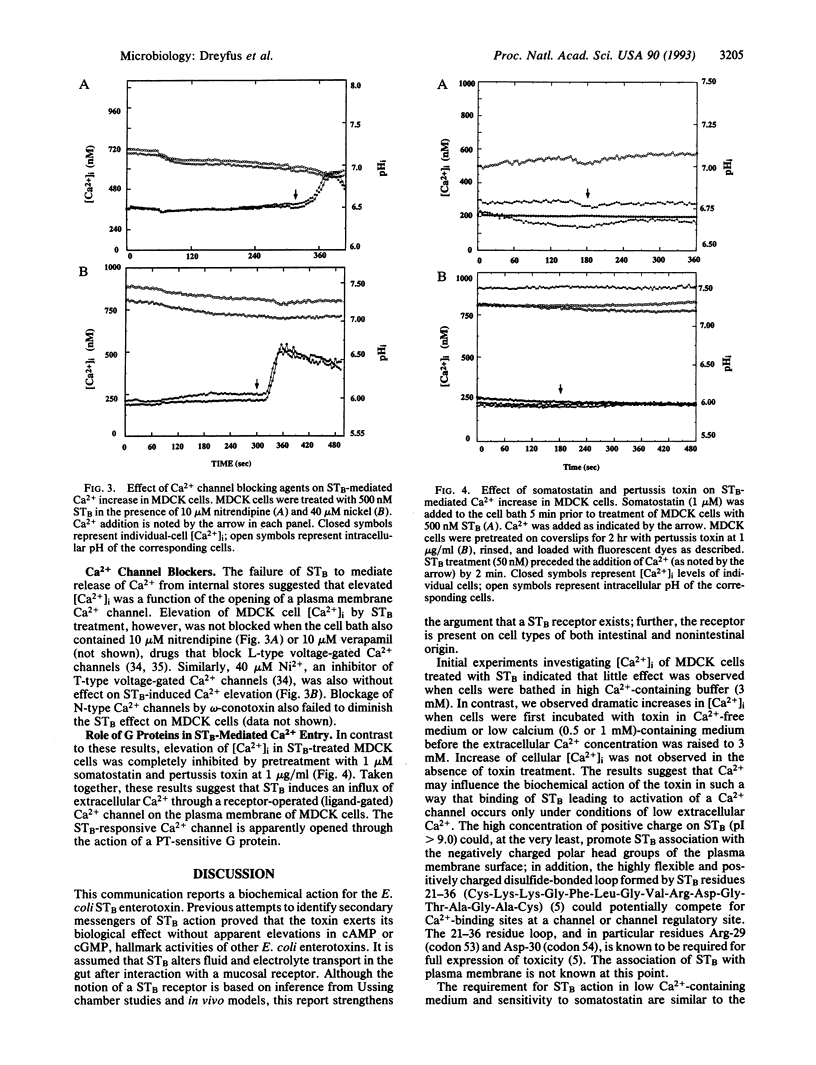
The heat-stable enterotoxin B (STB) of Escherichia coli is a 48-amino acid extracellular peptide that induces rapid fluid accumulation in animal intestinal models. Unlike other E. coli enterotoxins that elicit cAMP or cGMP responses in the gut [heat-labile toxin (LT) and heat-stable toxin A (STA), respectively], STB induces fluid loss by an undefined mechanism that is independent of cyclic nucleotide elevation. Here we studied the effects of STB on intracellular calcium concentration ([Ca2+]i), another known mediator of intestinal ion and fluid movement. Ca2+ and pH measurements were performed on different cell types including Madin-Darby canine kidney (MDCK), HT-29/C1 intestinal epithelial cells, and primary rat pituitary cells. Ca2+ and pH determinations were performed by simultaneous real-time fluorescence imaging at four emission wavelengths. This allowed dual imaging of the Ca(2+)- and pH-specific ratio dyes (indo-1 and SNARF-1, respectively). STB treatment induced a dose-dependent increase in [Ca2+]i with virtually no effect on internal pH in all of the cell types tested. STB-mediated [Ca2+]i elevation was not inhibited by drugs that block voltage-gated Ca2+ channels including nitrendipine, verapamil (L-type), omega-conotoxin (N-type), and Ni2+ (T-type). The increase in [Ca2+]i was dependent on a source of extracellular Ca2+ and was not affected by prior treatment of MDCK cells with thapsigargin or cyclopiazonic acid, agents that deplete and block internal Ca2+ stores. In contrast to these results, somatostatin and pertussis toxin pretreatment of MDCK cells completely blocked the STB-induced increase in [Ca2+]i. Taken together, these data suggest that STB opens a GTP-binding regulatory protein-linked receptor-operated Ca2+ channel in the plasma membrane. The nature of the STB-sensitive Ca2+ channel is presently under investigation.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abdel-Latif A. A. Calcium-mobilizing receptors, polyphosphoinositides, and the generation of second messengers. Pharmacol Rev. 1986 Sep;38(3):227–272. [PubMed] [Google Scholar]
- Bean B. P. Nitrendipine block of cardiac calcium channels: high-affinity binding to the inactivated state. Proc Natl Acad Sci U S A. 1984 Oct;81(20):6388–6392. doi: 10.1073/pnas.81.20.6388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birnbaumer L., Abramowitz J., Brown A. M. Receptor-effector coupling by G proteins. Biochim Biophys Acta. 1990 May 7;1031(2):163–224. doi: 10.1016/0304-4157(90)90007-y. [DOI] [PubMed] [Google Scholar]
- Burgess M. N., Bywater R. J., Cowley C. M., Mullan N. A., Newsome P. M. Biological evaluation of a methanol-soluble, heat-stable Escherichia coli enterotoxin in infant mice, pigs, rabbits, and calves. Infect Immun. 1978 Aug;21(2):526–531. doi: 10.1128/iai.21.2.526-531.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chinkers M., Garbers D. L. Signal transduction by guanylyl cyclases. Annu Rev Biochem. 1991;60:553–575. doi: 10.1146/annurev.bi.60.070191.003005. [DOI] [PubMed] [Google Scholar]
- Chronwall B. M., Bishop J. F., Gehlert D. R. Rat intermediate lobe in culture: a histological and biochemical characterization. Peptides. 1988;9 (Suppl 1):169–180. doi: 10.1016/0196-9781(88)90241-0. [DOI] [PubMed] [Google Scholar]
- Dean A. G., Ching Y. C., Williams R. G., Harden L. B. Test for Escherichia coli enterotoxin using infant mice: application in a study of diarrhea in children in Honolulu. J Infect Dis. 1972 Apr;125(4):407–411. doi: 10.1093/infdis/125.4.407. [DOI] [PubMed] [Google Scholar]
- Dreyfus L. A., Urban R. G., Whipp S. C., Slaughter C., Tachias K., Kupersztoch Y. M., Drefus L. A. Purification of the STB enterotoxin of Escherichia coli and the role of selected amino acids on its secretion, stability and toxicity. Mol Microbiol. 1992 Aug;6(16):2397–2406. doi: 10.1111/j.1365-2958.1992.tb01414.x. [DOI] [PubMed] [Google Scholar]
- Field M., Graf L. H., Jr, Laird W. J., Smith P. L. Heat-stable enterotoxin of Escherichia coli: in vitro effects on guanylate cyclase activity, cyclic GMP concentration, and ion transport in small intestine. Proc Natl Acad Sci U S A. 1978 Jun;75(6):2800–2804. doi: 10.1073/pnas.75.6.2800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frantz J. C., Jaso-Friedman L., Robertson D. C. Binding of Escherichia coli heat-stable enterotoxin to rat intestinal cells and brush border membranes. Infect Immun. 1984 Feb;43(2):622–630. doi: 10.1128/iai.43.2.622-630.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hitotsubashi S., Fujii Y., Yamanaka H., Okamoto K. Some properties of purified Escherichia coli heat-stable enterotoxin II. Infect Immun. 1992 Nov;60(11):4468–4474. doi: 10.1128/iai.60.11.4468-4474.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holz G. G., 4th, Rane S. G., Dunlap K. GTP-binding proteins mediate transmitter inhibition of voltage-dependent calcium channels. Nature. 1986 Feb 20;319(6055):670–672. doi: 10.1038/319670a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy D. J., Greenberg R. N., Dunn J. A., Abernathy R., Ryerse J. S., Guerrant R. L. Effects of Escherichia coli heat-stable enterotoxin STb on intestines of mice, rats, rabbits, and piglets. Infect Immun. 1984 Dec;46(3):639–643. doi: 10.1128/iai.46.3.639-643.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kupersztoch Y. M., Tachias K., Moomaw C. R., Dreyfus L. A., Urban R., Slaughter C., Whipp S. Secretion of methanol-insoluble heat-stable enterotoxin (STB): energy- and secA-dependent conversion of pre-STB to an intermediate indistinguishable from the extracellular toxin. J Bacteriol. 1990 May;172(5):2427–2432. doi: 10.1128/jb.172.5.2427-2432.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lattanzio F. A., Jr, Bartschat D. K. The effect of pH on rate constants, ion selectivity and thermodynamic properties of fluorescent calcium and magnesium indicators. Biochem Biophys Res Commun. 1991 May 31;177(1):184–191. doi: 10.1016/0006-291x(91)91966-g. [DOI] [PubMed] [Google Scholar]
- Lattanzio F. A., Jr The effects of pH and temperature on fluorescent calcium indicators as determined with Chelex-100 and EDTA buffer systems. Biochem Biophys Res Commun. 1990 Aug 31;171(1):102–108. doi: 10.1016/0006-291x(90)91362-v. [DOI] [PubMed] [Google Scholar]
- Martínez-Zaguilán R., Martínez G. M., Lattanzio F., Gillies R. J. Simultaneous measurement of intracellular pH and Ca2+ using the fluorescence of SNARF-1 and fura-2. Am J Physiol. 1991 Feb;260(2 Pt 1):C297–C307. doi: 10.1152/ajpcell.1991.260.2.C297. [DOI] [PubMed] [Google Scholar]
- Misfeldt D. S., Hamamoto S. T., Pitelka D. R. Transepithelial transport in cell culture. Proc Natl Acad Sci U S A. 1976 Apr;73(4):1212–1216. doi: 10.1073/pnas.73.4.1212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ransnäs L. A., Leiber D., Insel P. A. Inhibition of subunit dissociation and release of the stimulatory G-protein, Gs, by beta gamma-subunits and somatostatin in S49 lymphoma cell membranes. Biochem J. 1991 Dec 1;280(Pt 2):303–307. doi: 10.1042/bj2800303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson D. C., McDonel J. L., Dorner F. E. coli heat-labile enterotoxin. Pharmacol Ther. 1985;28(3):303–339. doi: 10.1016/0163-7258(85)90057-9. [DOI] [PubMed] [Google Scholar]
- Rose R., Whipp S. C., Moon H. W. Effects of Escherichia coli heat-stable enterotoxin b on small intestinal villi in pigs, rabbits, and lambs. Vet Pathol. 1987 Jan;24(1):71–79. doi: 10.1177/030098588702400112. [DOI] [PubMed] [Google Scholar]
- Scherübl H., Hescheler J., Schultz G., Kliemann D., Zink A., Ziegler R., Raue F. Inhibition of Ca(2+)-induced calcitonin secretion by somatostatin: roles of voltage dependent Ca2+ channels and G-proteins. Cell Signal. 1992 Jan;4(1):77–85. doi: 10.1016/0898-6568(92)90009-w. [DOI] [PubMed] [Google Scholar]
- Schramm M., Selinger Z. Message transmission: receptor controlled adenylate cyclase system. Science. 1984 Sep 21;225(4668):1350–1356. doi: 10.1126/science.6147897. [DOI] [PubMed] [Google Scholar]
- Seidler N. W., Jona I., Vegh M., Martonosi A. Cyclopiazonic acid is a specific inhibitor of the Ca2+-ATPase of sarcoplasmic reticulum. J Biol Chem. 1989 Oct 25;264(30):17816–17823. [PubMed] [Google Scholar]
- Seth S. D., Seth S. Calcium channels and calcium channel blockers. Indian J Physiol Pharmacol. 1991 Oct;35(4):217–231. [PubMed] [Google Scholar]
- Takao T., Hitouji T., Aimoto S., Shimonishi Y., Hara S., Takeda T., Takeda Y., Miwatani T. Amino acid sequence of a heat-stable enterotoxin isolated from enterotoxigenic Escherichia coli strain 18D. FEBS Lett. 1983 Feb 7;152(1):1–5. doi: 10.1016/0014-5793(83)80469-4. [DOI] [PubMed] [Google Scholar]
- Takemura H., Hughes A. R., Thastrup O., Putney J. W., Jr Activation of calcium entry by the tumor promoter thapsigargin in parotid acinar cells. Evidence that an intracellular calcium pool and not an inositol phosphate regulates calcium fluxes at the plasma membrane. J Biol Chem. 1989 Jul 25;264(21):12266–12271. [PubMed] [Google Scholar]
- Thastrup O., Cullen P. J., Drøbak B. K., Hanley M. R., Dawson A. P. Thapsigargin, a tumor promoter, discharges intracellular Ca2+ stores by specific inhibition of the endoplasmic reticulum Ca2(+)-ATPase. Proc Natl Acad Sci U S A. 1990 Apr;87(7):2466–2470. doi: 10.1073/pnas.87.7.2466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urban R. G., Pipper E. M., Dreyfus L. A., Whipp S. C. High-level production of Escherichia coli STb heat-stable enterotoxin and quantification by a direct enzyme-linked immunosorbent assay. J Clin Microbiol. 1990 Nov;28(11):2383–2388. doi: 10.1128/jcm.28.11.2383-2388.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weikel C. S., Grieco F. D., Reuben J., Myers L. L., Sack R. B. Human colonic epithelial cells, HT29/C1, treated with crude Bacteroides fragilis enterotoxin dramatically alter their morphology. Infect Immun. 1992 Feb;60(2):321–327. doi: 10.1128/iai.60.2.321-327.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weikel C. S., Nellans H. N., Guerrant R. L. In vivo and in vitro effects of a novel enterotoxin, STb, produced by Escherichia coli. J Infect Dis. 1986 May;153(5):893–901. doi: 10.1093/infdis/153.5.893. [DOI] [PubMed] [Google Scholar]
- Whipp S. C. Assay for enterotoxigenic Escherichia coli heat-stable toxin b in rats and mice. Infect Immun. 1990 Apr;58(4):930–934. doi: 10.1128/iai.58.4.930-934.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whipp S. C., Moseley S. L., Moon H. W. Microscopic alterations in jejunal epithelium of 3-week-old pigs induced by pig-specific, mouse-negative, heat-stable Escherichia coli enterotoxin. Am J Vet Res. 1986 Mar;47(3):615–618. [PubMed] [Google Scholar]
- Whipp S. C. Protease degradation of Escherichia coli heat-stable, mouse-negative, pig-positive enterotoxin. Infect Immun. 1987 Sep;55(9):2057–2060. doi: 10.1128/iai.55.9.2057-2060.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yatani A., Imoto Y., Codina J., Hamilton S. L., Brown A. M., Birnbaumer L. The stimulatory G protein of adenylyl cyclase, Gs, also stimulates dihydropyridine-sensitive Ca2+ channels. Evidence for direct regulation independent of phosphorylation by cAMP-dependent protein kinase or stimulation by a dihydropyridine agonist. J Biol Chem. 1988 Jul 15;263(20):9887–9895. [PubMed] [Google Scholar]



