Abstract
Rice false smut has become an increasingly serious fungal disease in rice (Oryza sativa L.) production worldwide. Ustilaginoidins are bis-naphtho-γ-pyrone mycotoxins previously isolated from the rice false smut balls (FSBs) infected by the pathogen Villosiclava virens in rice spikelets on panicles. To investigate the main ustilaginoidins and their distribution in rice FSBs, five main bis-naphtho-γ-pyrones, namely ustilaginoidins A (1), G (2), B (3), I (4) and C (5), were isolated and identified by NMR and high-resolution mass spectrometry as well as by comparison with the data in the literature. The rice FSBs at early, middle and late maturity stages were divided into their different parts and the contents of five main ustilaginoidins for each part were determined by HPLC analysis. The results revealed that the highest levels of ustilaginoidins were in late stage rice FSBs, followed by those at middle stage. Most ustilaginoidins, 96.4% of the total quantity, were distributed in the middle layer at early stage. However, ustilaginoidins were mainly distributed in the outer and middle layers at middle and late stages. Small amounts of ustilaginoidins A (1) and G (2) were found in the inner part of rice FSBs at each maturity stage. The contents of ustilaginoidins A (1) and G (2) without hydroxymethyl groups at C-2 and C-2’ of the γ-pyrone rings in rice FSBs were relatively high at early stage, while the contents of ustilaginoidins B (3), I (4), and C (5) with hydroxymethyl groups at C-2 or C-2’ were relatively high at late stage.
Keywords: ustilaginoidins, mycotoxin, phytotoxin, rice false smut balls, Villosiclava virens, Ustilaginoidea virens, HPLC
1. Introduction
Rice false smut, caused by Villosiclava virens (Nakata) Tanaka & Tanaka (anamorph: Ustilaginoidea virens Takahashi) [1], is one of the most destructive fungal diseases in many rice (Oryza sativa L.) cultivation areas over the past few years [2]. The infection of V. virens occurs in rice spikelets on panicles [3]. The fungus transforms grains into the ball-like colonies which we called false smut balls (FSBs). The color of rice FSBs gradually changes from white to yellow, then yellowish green, olive-green, and ultimately greenish-black during maturity [4,5]. Each mature FSB consists of dark-green chlamydospores (the outer layer), orange mycelia with immature chlamydospoes (the middle layer), white psudoparenchyma (the inner part) and glume. Rice false smut disease not only results in rice yield loss, but also contaminates rice grains and feed, and even more importantly, generates mycotoxins that are poisonous to humans and animals and creates concerns for food and feed safety [2,6].
It has been reported that rice FSBs and the false smut pathogen could produce two kinds of mycotoxins, namely ustiloxins and ustilaginoidins [7,8]. Ustiloxins are cyclic peptides which have been reported to have antimitotic activity by inhibiting microtubule assembly and cell skeleton formation of plant and animal cells [9,10,11,12,13,14,15]. Ustilaginoidins are bis-naphtho-γ-pyrone mycotoxins, and eighteen ustilaginoidins, namely isochaetochromin B2, ustilaginoidins A-P and E1, have been isolated so far [7,8,16]. They exhibit a variety of biological activities such as cytotoxic activity [17,18,19,20], antibacterial activity [8,21], inhibitory activity on HIV-1 integrase [22], phytotoxic activity [8,23,24], and inhibitory activity on triacylglycerol synthesis in mammalian cells [25].
The main ustiloxins (i.e., ustiloxins A and B) can be purified from rice FSBs by macroporous resins in combination with a hydrophilic C18 (ODS-AQ) column chromatography [26,27]. Moreover, ustiloxins can be detected by the methods of high-performance liquid chromatography (HPLC) [27,28], liquid chromatography-mass spectrometry (LC-MS) [27], and enzyme-linked immunosorbent assay (ELISA) [29,30]. To our knowledge, there are no reports on the identification of the main ustilaginoidins and their distribution in rice FSBs or on methods for detecting the contents of ustilaginoidins in samples. In this study, five main ustilaginoidins were isolated and identified from rice FSBs. The contents of the main ustilaginoidins in the samples were analyzed by HPLC. The distribution of ustilaginoidins as well as their contents in different parts (i.e., glume, outer layer, middle layer and inner part) of rice FSBs were also clarified. The results will provide the basis for isolation and analysis of the ustilagiloidins in rice samples, as well as for revealing metabolic pathways, physiological and ecological functions of the ustilaginoidins in rice FSBs.
2. Results and Discussion
2.1. Characterization and Analysis of the Main Ustilaginoidins in Rice FSBs
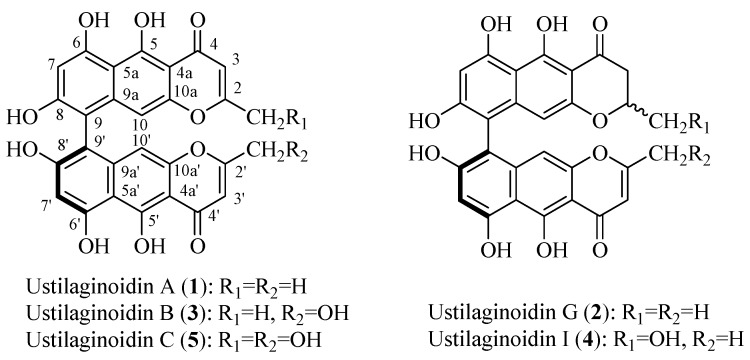
After repeated column chromatographic purification using Sephadex LH-20, preparative high-speed counter-current chromatography (HSCCC), and semi-preparative HPLC, the ethyl acetate extract of rice FSBs afforded five main compounds 1–5. After comparing their physicochemical and spectrometric data with those reported in the literature [8,16], they were identified as ustilaginoidins A (1), G (2), B (3), I (4) and C (5), whose structures are shown in Figure 1 and their UV absorption spectra are shown in Supplementary Figure S1.
Figure 1.
Chemical structures of the five main ustilaginoidins.
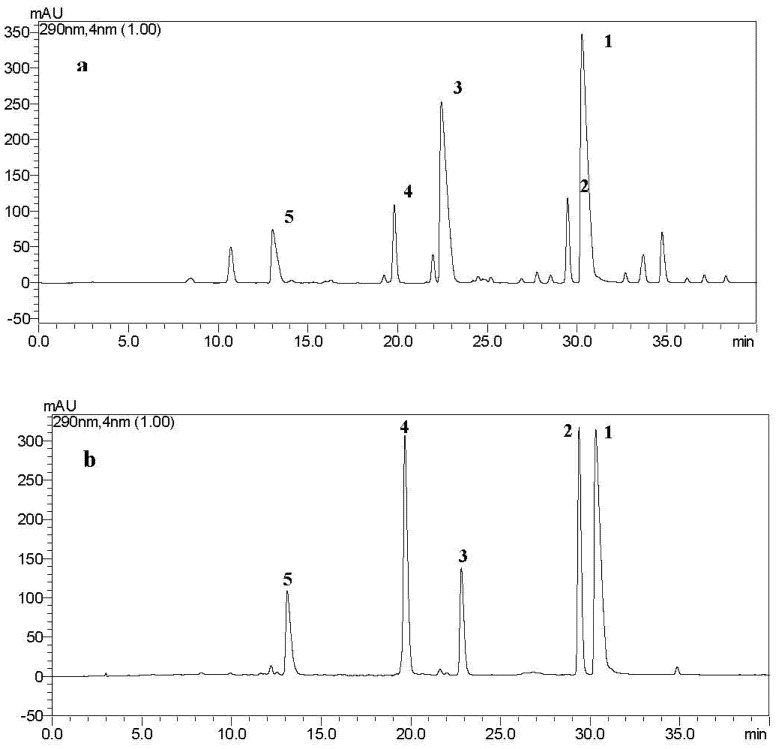
Figure 2 shows the HPLC profiles of the ethyl acetate extract and standard ustilaginoidins A (1), G (2), B (3), I (4) and C (5), respectively. The main ustilaginoidins A, G, B, I and C in rice FSBs were identified by comparison of their retention times with the standard ustilaginoidins as well as the UV absorption spectra. HPLC analysis was completed in 40 min.
Figure 2.
HPLC analysis of ustilaginoidins A (1), G (2), B (3), I (4) and C (5) in rice FSBs. (a) is the HPLC profile of the ethyl acetate extract from the mycelia. Arabic numerals 1–5 in the figure represent ustilaginoidins A, G, B, I and C, respectively; (b) is the HPLC profile of the standard ustilaginoidins A, G, B, I and C. The retention times of ustilaginoidins C, I, B, G and A were 13.10, 19.67, 22.82, 29.39 and 30.32 min, respectively.
Based on the above results, the five main ustilaginoidins were selected for quantitative analysis by HPLC. The linear equations for ustilaginoidins A, G, B, I and C are shown in Table 1. The results showed that the developed HPLC method had good linearity within the range of 0.03125–1.5 μg in the sample injected each time.
Table 1.
The linear equations of five main ustilaginoidins by HPLC analysis.
| Ustilaginoidin | Retention Time (min) | Linear equation Y = aX + b | Correlation coefficient (R2) |
|---|---|---|---|
| Ustilaginoidin A (1) | 30.32 | Y = 6156397.5754 X − 33957.4836 | 0.9995 |
| Ustilaginoidin G (2) | 29.39 | Y = 6279322.9760 X − 7659.1240 | 0.9985 |
| Ustilaginoidin B (3) | 22.82 | Y = 7087462.4058 X + 263979.1177 | 0.9996 |
| Ustilaginlodin I (4) | 19.67 | Y = 4858545.7034 X + 128004.6527 | 0.9986 |
| Ustilaginoidin C (5) | 13.10 | Y = 6189302.6749 X + 35291.4332 | 0.9993 |
Y is the peak area, X is the quantity (μg) of the sample injected each time, and R2 is the correlation coefficient.
2.2. Distribution of Main Ustilaginoidins in Rice FSBs
Table 2 shows the contents of the five main ustilaginoidins in different parts of rice FSBs at early, middle and late maturity stages. We considered the sum of the five main ustilaginoidins (i.e., ustilaginoidins A, G, B, I and C) as the total content of ustilaginoidins in each sample though there were some other minor ustilaginoidins detected in the crude extract (Figure 2A). With the increasing of maturity degree, the FSBs enlarged gradually. The total average dry weight for each FSB was 32.9, 97.7 and 151.3 mg, respectively. Meanwhile, the total ustilaginoidin quantity for each FSB increased with maturity degree as well, from 0.6 to 5.5 and finally to 11.8 mg, respectively. Moreover, we found that the ustilaginoidins were distributed mainly in the middle layer at early stage, accounting for 96.4% of the total ustilaginoidin quantity, whereas at the mid and late stages, the ustilaginoidins exhibited a distribution mostly in the outer and middle layers (Table 2).
Table 2.
Contents of main ustilaginoidins in rice FSBs at different maturity stages.
| Part of FSB | Average weight in each FSB (mg) | Ustilginoidin content (mg/g) | |||||
|---|---|---|---|---|---|---|---|
| Ustilginoidin A (1) | Ustilginoidin G (2) | Ustilginoidin B (3) | Ustilginoidin I (4) | Ustilginoidin C (5) | Total | ||
| Early stage | |||||||
| Outer layer | Nd | nd | nd | nd | nd | nd | nd |
| Middle layer | 5.7 ± 0.4 ef | 60.4 ± 5.8 a | 5.4 ± 0.1 c | 21.9 ± 2.6 c | 3.9 ± 0.3 c | 4.1 ± 0.5 d | 95.7 ± 9.2 b |
| Inner part | 22.9 ± 1.3 d | 0.7 ± 0.1 d | 0.2 ± 0.0 d | nd | nd | nd | 0.9 ± 0.1 c |
| Glume | 4.3 ± 0.6 f | nd | nd | nd | nd | nd | nd |
| Middle stage | |||||||
| Outer layer | 14.1 ± 3.0 de | 54.7 ± 1.8 ab | 6.3 ± 0.1 b | 33.9 ± 1.2 ab | 10.1 ± 0.5 b | 12.1 ± 0.8 a | 117.1 ± 3.9 a |
| Middle layer | 38.7 ± 7.5 c | 56.0 ± 5.9 ab | 8.7 ± 0.3 a | 21.7 ± 2.2 c | 5.9 ± 0.7 c | 5.4 ± 0.5 c | 97.4 ± 8.8 b |
| Inner part | 41.6 ± 5.8 bc | 0.6 ± 0.1 d | 0.4 ± 0.1 d | nd | nd | nd | 1.0 ± 0.2 c |
| Glume | 3.3 ± 0.6 f | nd | nd | nd | nd | nd | nd |
| Late stage | |||||||
| Outer layer | 49.3 ± 3.0 ab | 44.9 ± 5.5 c | 6.0 ± 1.0 bc | 36.8 ± 3.9 a | 15.1 ± 1.9 a | 12.5 ± 1.2 a | 115.4 ± 13.5 a |
| Middle layer | 54.7 ± 12.8 a | 54.0 ± 2.1 b | 9.3 ± 0.3 a | 29.8 ± 2.5 b | 8.7 ± 2.3 b | 9.0 ± 0.2 b | 110.8 ± 6.6 a |
| Inner part | 44.1 ± 6.3 bc | 1.2 ± 0.3 d | 0.6 ± 0.1 d | nd | nd | nd | 1.8 ± 0.4 c |
| Glume | 3.2 ± 0.2 f | nd | nd | nd | nd | nd | nd |
FSB: false smut ball; Each value represents the mean of triplicate ± standard deviations. Different letters indicate significant differences among different maturity sates in each column at p ≤ 0.05. nd: not detected.
The levels of ustilaginoidins in the inner part of the FSBs were significantly lower than those in the outer and middle layer at all stages (Table 2). However, the levels of ustilaginoidins in the inner parts increased with the maturity increase of rice FSBs. The contents were 0.9, 1.0 and 1.8 mg/g at early, middle and late stage, respectively.
When the chlamydospores emerged, the contents of ustilaginoidins B (3), I (4) and C (5) in the outer layer (33.9, 10.1 and 12.1 mg/g at middle stage, and 36.8, 15.1 and 12.5 mg/g at late stage, respectively) were significantly higher than those in the middle layer (21.7, 5.9 and 5.4 mg/g at middle stage, and 29.8, 8.7 and 9.0 mg/g at late stage, respectively), and contents of these three ustilaginoidins reached the maximum values at late stage. However, the contents of ustilaginoidins A (1) and G (2) at middle stage were almost equal to those at late stage. The total content of ustilaginoidins in the outer layer was higher than that in the middle layer.
The present study showed that total ustilaginoidin content maximized at late maturity stage (Table 2), and it increased significantly at each increased maturity degree, indicating that levels of ustilaginoidins depend on the maturity stage of rice grains. In addition, we found that though the content of each ustilaginoidin in inner part was very low, it increased gradually with maturity increases. Interestingly, the contents of ustilaginoidins B (3), I (4), and C (5) with relatively big polarity were low at early stage, and their contents increased significantly at late stage. By contrast, the contents of ustilaginoidins A (1) and G (2) with relatively small polarity were high at early stage, and their contents decreased at late stage (Table 2). This indicates that ustilaginoidin A (1) was oxidized at C-2 or C-2’ of the γ-pyrone rings into ustilaginoidins B (3) and C (5) containing one or two hydroxymethyl groups, and ustilaginoidin G (2) was oxidized at C-2’ into ustilaginoidin I (4), which suggests that both ustilaginoidins A (1) and G (2) should be the precursors of ustilaginoidins B (3), I (4), and C (5), a prediction that needs to be verified. By comparing the ustilaginoidins isolated from rice FSBs and pathogen fermentation cultures, 13 ustilaginoidins (i.e., ustilaginoidins A, D, E, F, G, E1, K-P, and isochaetochromin B2) isolated from the fermentation cultures of rice false smut pathogen were shown to be low-oxidative-degree ustilaginoidins without hydroxymethyl groups at C-2 or C-2’ [8]. Ustilaginoidins B, C, H, I and J, which had a high degree of oxidation with hydroxymethyl groups at C-2 or C-2’ of the γ-pyrone rings, were only found in rice FSBs [16]. In this study, ustilaginoidins B, I and C were found in high concentrations in the outer layer of the FSBs, indicating that these high-oxidative-degree ustilaginoidins may have important biological functions that need to be investigated in detail. The genomic data of V. virens indicated that some transcriptional genes involved in biosynthesis of the secondary metabolites including ustilaginoidins were highly enhanced during early infection and thus were speculated to play vital roles [31]. However, the interaction between V. virens and its host as well as the conversion process of the ustilaginoidins were still unclear.
3. Experimental Section
3.1. General
HR-ESI-MS spectra were recorded on a Bruker Apex IV FTMS instrument (Bruker Daltonics, Bremen, Germany). 1H and 13C-NMR spectra were measured on Bruker Avance 600 NMR spectrometers (1H at 600 MHz and 13C at 150 MHz) (Bruker BioSpin, Zurich, Switzerland). Chemical shifts were expressed in δ (ppm) relative to tetramethylsilane (TMS) as an internal standard. Preparative high-speed counter-current chromatography (HSCCC) was performed on a TBE-300B instrument (Tauto Biotech, Shanghai, China), equipped with three preparative coils, a polytetrafluoroethylene tube (2.6 mm in diameter, and total volume of 300 mL), and a 20-mL sample loop. The separation was carried out at 25 °C using a two-phase solvent system, at a flow rate of 3.2 mL/min, revolution speed of 800 rpm, and detection wavelength at 280 nm. Semi-preparative HPLC separation was carried out on a Lumtech instrument (Lumiere Tech. Ltd., Beijing, China) equipped with a K-501 pump (flow rate was 3 mL/min) and a K-2501 UV detector (detection was set at 290 nm), using a Luna-C18 column (250 mm × 10 mm i.d., 5 μm, Phenomenex Inc., Torrance, CA, USA). A Shimadzu Prominence LC-20A high-performance liquid chromatography system (Kyoto, Japan) was consisted of two LC-20AT solvent delivery units, an SIL-20A autosampler, an SPD-M20A photodiode array detector, a CBM-20Alite system controller, and a reversed-phase Luna C18 column (250 mm × 4.6 mm, 5 μm) (Phenomenex, Torrance, CA, USA). An electric heating constant temperature incubator was purchased from Tianjin Zhonghuan Experiment Electric Stove Co. Ltd. (Tianjin, China). An ultrasonic cleaner (KH-500E, Kunshan, China) was purchased from Kunshan Hechuang Ultrasonic Apparatus Co. Ltd.
Silica gel (200-300 mesh) for column chromatography was purchased from the Qingdao Marine Chemical Company (Qingdao, China). Sephadex LH-20 was purchased from Pharmacia Biotech, Sweden. All other chemicals and reagents were of analytical grade.
3.2. Rice False Smut Balls
The rice false smut balls (FSBs) were collected from Chengdu (104.1°E, 30.7°N) in the Sichuan Province of China in 2014. The materials were left to dry in shade at room temperature to a constant weight, and were then stored in sealed plastic bags at −20 °C until required.
The color of the rice FSBs at early stage was white to yellow, and the average weight for each ball was 32.9 mg. At middle stage, the average weight for each ball was about 97.7 mg, and the balls became yellowish green covered by a powder of dark-green chlamydospores. Lastly, the color of rice FSBs was greenish black, and the balls became much bigger (average weight for each ball was 151.3 mg) with cracks on the surface. The matured FSBs in rice plants as well as the whole balls at the early, middle and late stages, and their corresponding sections are shown in Figure 3.
Figure 3.
Rice false smut balls (FSBs) and their sections. (a) Matured FSBs in rice plants; (b) Rice FSBs collected from rice plants infected with false smut disease; (c) Whole FSB at early stage; (d) Whole FSB at middle stage; (e) Whole FSB at late stage; (f) Section of rice FSB at early stage. (g) Section of rice FSB at middle stage. (h) Section of rice FSB at late stage.
The rice FSBs at different maturity stages were carefully divided into the parts of chlamydospores (outer layer), mycelia with immature chlamydospores (middle layer), psudoparenchyma (inner part) and glume by tweezer and scalpel. All samples were weighed and ground, and then kept at 4 °C until analysis.
3.3. Extraction, Fractionation and Identification of the Ustilaginoidins
The dry and powdered FSBs (9.1 kg) were soaked in deionized water at room temperature three times (3 × 30 L, 48 h for each time). After filtration, the residue was soaked in ethanol at room temperature another three times (3 × 30 L, 48 h for each time). The ethanol filtrates were combined and concentrated in a vacuum to obtain a black gum substance which was suspended in water and extracted first with the equal volume of petroleum ether, then with ethyl acetate (EtOAc), and last with n-butanol for three repetitions. The combined EtOAc solution was concentrated to obtain the crude EtOAc extract (264.09 g) which was first chromatographed on a silica gel column eluted with a step gradient of CH2Cl2-EtOAc (1:0, 100:1, 10:1, 1:1, and 0:1, v/v) to yield two main fractions Fr.A (27.5 g) and Fr.B (43.5 g). Fr.A (27.5 g) was performed on high-speed counter-current chromatography (HSCCC) with a two-phase solvent system composed of n-hexane-ethyl acetate-methanol-water (6.5:3.5:5.0:5.0, v/v) to yield two peak fractions which were further purified by semi-preparative HPLC under a methanol:water volume ratio of 75:25 to obtain ustilaginoidins A (1, 11.5 mg) and G (2, 9.3 mg). Fr.B (10.0 g) was fractionated on a Sephadex LH-20 column eluted with CHCl3-CH3OH (1:1, v/v) to afford Fr.B1 (5.2 g) and Fr.B2 (3.6 g). Fr.B1 was performed on HSCCC with two-phase solvent system composed of n-hexane-ethyl acetate-methanol-water (4.0:5.0:5.0:6.0, v/v) to yield two peak fractions which were further purified by semi-preparative HPLC under a methanol:water volume ratio of 65:35 to obtain ustilaginoidins B (3, 10.0 mg) and I (4, 10.8 mg). Fr.B2 was performed on HSCCC with two-phase solvent system composed of n-hexane-ethyl acetate-methanol-water (3.0:5.0:4.0:6.7, v/v) to yield one peak fraction which was further purified by semi-preparative HPLC under a methanol:water volume ratio of 50:50 to obtain ustilaginoidin C (5, 4.6 mg).
All the purified compounds were isolated as red or yellow amorphous powder. The molecular formula C28H18O10 of ustilaginoidin A (1) was assigned by HR-ESI-MS, m/z 513.0831 [M − H]− (calculated for C28H17O10, 513.0827). Similarly, molecular formula C28H20O10 of ustilaginoidin G (2) was assigned by HR-ESI-MS, m/z 515.0987 [M − H]− (calculated for C28H19O10, 515.0984); molecular formula C28H18O11 of ustilaginoidin B (3) was assigned by HR-ESI-MS, m/z 531.0903 [M + H]+ (calculated for C28H19O11, 531.0922); molecular formula C28H20O11 of ustilaginoidin I (4) was assigned by HR-ESI-MS, m/z 533.1070 [M + H]+ (calculated for 533.1078); molecular formula C28H18O12 of ustilaginoidin C (5) was assigned by HR-ESI-MS, m/z 547.0856 [M + H]+ (calculated for C28H19O12, 547.0871). The 1H NMR (600 MHz) and 13C NMR (150 MHz) data of the compounds were shown in Supplementary Tables S1 and S2 of Supplementary Materials. All the data were consistent with those in literature [8,16].
3.4. HPLC Analysis of Main Ustilaginoidins in Rice FSBs
The powdery sample (10 mg) was weighed and then extracted with ethyl acetate for three times (3 × 1 mL, 20 min for each time) in an ultrasonic bath at room temperature. The ethyl acetate extract was concentrated by a rotary evaporator to dryness under vacuum at 28 °C. The obtained residue was dissolved in 3 mL of methanol. It was then filtered through a microporous filter (pore size, 0.22 μm) before analysis.
1 mg of the purified ustilaginoidin was dissolved in 1 mL of methanol to obtain the mother solution (1 mg/mL) which was further diluted into a series of concentrations of 150, 100, 50, 31.25, 25, 12.5, 6.25 and 3.125 μg/mL with methanol, and the diluted solutions were kept at 4 °C. Each solution was filtered and analyzed by an HPLC system eluted with a linear gradient of methanol from 50 to 100% (v/v) and water (containing 0.01% oxalic acid) from 50 to 0% (v/v) over 40 min at a flow rate of 1.0 mL/min. The temperature was maintained at 30 °C, UV detection at 290 nm, and the sample injection volume at 10 μL. The LC-solution multi-PDA workstation was employed to acquire and process chromatographic data.
3.5. Statistical Analysis
All tests were performed with three replications, and the results were represented by their mean values and the standard deviations (SD). Statistical analysis of the data was carried out using analysis of variance (one-way ANOVA) to detect significant differences by PROC ANOVA of SAS version 8.2 (SAS Institute, Cary, NC, USA). The term significant was used to denote the differences at p ≤ 0.05.
4. Conclusions
In this study, ustilaginoidins A (1), G (2), B (3), I (4) and C (5) as the main bis-naphtho-γ-pyrone mycotoxins were isolated and identified in the ethyl acetate extract from rice false smut balls (FSBs). The contents of the ustilaginoidins in rice FSBs increased with the increase of maturity degree. At early maturity stage, ustilaginoidins were mainly distributed in the middle layer with a proportion of 96.4%, while at middle and late stages, ustilaginoidins were mainly distributed in the middle and outer layer. Very low levels of ustilaginoidins were detected in the inner layer of rice FSBs at each maturity stage. The contents of ustilaginoidins A and G in rice FSBs, with a low degree of oxidation, were relatively high at early stage, while the contents of ustilaginoidins B, I, and C, with a high degree of oxidation, were relatively high at late stage. This indicated that both ustilaginoidins A and G should be the precursors of ustilaginoidins B, I, and C, something which needs to be verified. Isolation and structural identification of other ustilaginoidins from rice FSBs are in progress. The physiological and ecological functions of ustilaginoidins in rice FSBs need to be studied in detail.
Acknowledgments
This work was supported in part by the grants from the National Basic Research Program of China (2013CB127805), the Special Fund for Agro-Scientific Research in the Public Interest of China (201203037), and the National Natural Science Foundation of China (31271996 and 31471729).
Supplementary Materials
Supplementary materials can be accessed at: http://www.mdpi.com/2072-6651/7/10/4023/s1.
Author Contributions
Ligang Zhou and Guozhen Zhang conceived and designed the experiments. Jiajia Meng and Weibo Sun performed the experiments. Ziling Mao, Dan Xu and Xiaohan Wang contributed reagents and materials, and participated in the discussions. Daowan Lai, Weibo Sun and Shiqiong Lu identified the structures of the ustilaginoidins. Yang Liu and Daowan Lai revised the manuscript. Jiajia Meng, Weibo Sun and Ligang Zhou interpreted the data and wrote the paper. All authors read and approved the final manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Tanaka E., Ashizawa T., Sonoda R., Tanaka C. Villosiclava virens gen. nov., comb. nov., the teleomorph of Ustilaginoidea virens, the causal agent of rice false smut. Mycotaxon. 2008;106:491–501. [Google Scholar]
- 2.Guo X., Li Y., Fan J., Li L., Huang F., Wang W. Progress in the study of false smut disease in rice. J. Agric. Sci. Technol. 2012;2:1211–1217. [Google Scholar]
- 3.Ashizawa T., Takahashi M., Arai M., Arie T. Rice false smut pathogen, Ustilaginoidea virens, invades through small gap at the apex of a rice spikelet before heading. J. Gen. Plant Pathol. 2012;78:255–259. doi: 10.1007/s10327-012-0389-3. [DOI] [Google Scholar]
- 4.Tang Y., Jin J., Hu D., Yong M., Xu Y., He L. Elucidation of the infection process of Ustilaginoidea virens (teleomorph: Villosiclava virens) in rice spikelets. Plant Pathol. 2013;62:1–8. doi: 10.1111/j.1365-3059.2012.02629.x. [DOI] [Google Scholar]
- 5.Hu M., Luo L., Wang S., Liu Y., Li J. Infection processes of Ustilaginoidea virens during artificial inoculation of rice panicles. Eur. J. Plant Pathol. 2014;139:66–77. doi: 10.1007/s10658-013-0364-7. [DOI] [Google Scholar]
- 6.Zhou L., Lu S., Shan T., Wang P., Sun W., Chen Z., Wang S. Chemistry and biology of mycotoxins from rice false smut pathogen. In: Melborn B.J., Greene J.C., editors. Mycotoxins: Properties, Applications and Hazards. Nova Science Publishers; New York, NY, USA: 2012. pp. 109–130. [Google Scholar]
- 7.Lu S., Tian J., Sun W., Meng J., Wang X., Fu X., Wang A., Lai D., Liu Y., Zhou L. Bis-naphtho-γ-pyrones from fungi and their bioactivities. Molecules. 2014;19:7169–7188. doi: 10.3390/molecules19067169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lu S., Sun W., Meng J., Wang A., Wang X., Tian J., Fu X., Dai J., Liu Y., Lai D., et al. Bioactive bis-naphtho-γ-pyrones from rice false smut pathogen Ustilaginoidea virens. J. Agric. Food Chem. 2015;63:3501–3508. doi: 10.1021/acs.jafc.5b00694. [DOI] [PubMed] [Google Scholar]
- 9.Suwa M. Experimental Ustilaginoidea toxicosis. Igaku Chuo Zasshi. 1915;13:661–686. [Google Scholar]
- 10.Koiso Y., Natori M., Iwasaki S., Sato S., Sonoda R., Fujita Y., Yaegashi H., Sato Z. Ustiloxin: A phytotoxin and a mycotoxin from false smut balls on rice panicles. Tetrahedron Lett. 1992;33:4157–4160. doi: 10.1016/S0040-4039(00)74677-6. [DOI] [Google Scholar]
- 11.Koiso Y., Li Y., Iwasaki S., Hanaoka K., Kobayashi T., Sonoda R., Fujita Y., Yaegashi H., Sato Z. Ustiloxins, antimitotic cyclic peptides from false smut balls on rice panicles caused by Ustilaginoidea virens. J. Antibiot. 1994;47:765–773. doi: 10.7164/antibiotics.47.765. [DOI] [PubMed] [Google Scholar]
- 12.Nakamura K., Izumiyama N., Ohtsubo K., Koiso Y., Iwasaki S., Sonoda R., Fujita Y., Yaegashi H., Sato Z. “Lupinosis”-like lesions in mice caused by ustiloxin, produced by Ustilaginoieda virens: a morphological study. Nat. Toxins. 1994;2:22–28. doi: 10.1002/nt.2620020106. [DOI] [PubMed] [Google Scholar]
- 13.Luduena R.F., Roach M.C., Prasad V., Banerjee M., Koiso Y., Li Y., Iwasaki S. Interaction of ustiloxin A with bovine brain tubulin. Biochem. Pharmacol. 1994;47:1593–1599. doi: 10.1016/0006-2952(94)90537-1. [DOI] [PubMed] [Google Scholar]
- 14.Li Y., Koiso Y., Kobayashi H., Hashimoto Y., Iwasaki S. Ustiloxins, new antimitotic cyclic peptides: interaction with porcine brain tubulin. Biochem. Pharmacol. 1995;49:1367–1372. doi: 10.1016/0006-2952(95)00072-8. [DOI] [PubMed] [Google Scholar]
- 15.Morisaki N., Mitsui Y., Yamashita Y., Koiso Y., Shirai R., Hashimoto Y., Iwasaki S. Synthesis and anti-tubulin activity of ustiloxin D derivatives. J. Antibiot. 1998;51:423–427. doi: 10.7164/antibiotics.51.423. [DOI] [PubMed] [Google Scholar]
- 16.Koyama K., Natori S. Further characterization of seven bis(naphtho-γ-pyrone) congeners of ustilaginoidins, coloring matters of Claviceps virens (Ustilaginoidea virens) Chem. Pharm. Bull. 1988;36:146–152. doi: 10.1248/cpb.36.146. [DOI] [Google Scholar]
- 17.Matsumoto M., Minato H., Kondo E., Mitsugi T., Katagiri K. Cephalochromin, dihydroisoustilaginoidin A, and iso-ustilaginoidin A from Verticillium sp. K-113. J. Antibiot. 1975;28:602–604. doi: 10.7164/antibiotics.28.602. [DOI] [PubMed] [Google Scholar]
- 18.Tsuchiya T., Sekita S., Koyama K., Natori S., Takahashi A. Effect of chaetochromin A, chaetochromin D and ustilaginoidin A, bis (naphtho-γ-pyrone) derivatives, on the mouse embryo limb bud and midbrain cells in culture. Congenit. Anom. 1987;27:245–250. doi: 10.1111/j.1741-4520.1987.tb00707.x. [DOI] [Google Scholar]
- 19.Kawai K., Hisada K., Mori S., Nozawa Y., Koyama K., Natori S. The imparing effect of chaetochromin A and related mycotoxins on mitochondrial respiration. Proc. Jpn. Assoc. Mycotoxicol. 1991;33:25–29. doi: 10.2520/myco1975.1991.25. [DOI] [Google Scholar]
- 20.Koyama K., Ominato K., Natori S., Tashiro T., Tsuruo T. Cytotoxicity and antitumor activities of fungal bis(naphtho-γ-pyrone) derivatives. J. Pharmacobio-Dyn. 1998;11:630–635. doi: 10.1248/bpb1978.11.630. [DOI] [PubMed] [Google Scholar]
- 21.Kong X., Ma X., Xie Y., Cai S., Zhu T., Gu Q., Li D. Aromatic polyketides from a sponge-derived fungus Metarhizium anisopliae mxh-99 and their antitubercular activities. Arch. Pharm. Res. 2013;36:739–744. doi: 10.1007/s12272-013-0077-7. [DOI] [PubMed] [Google Scholar]
- 22.Singh S.B., Zink D.L., Bills G.F., Teran A., Silverman K.C., Lingham R.B., Felock P., Hazuda D.J. Four novel Bis-(naphtho-γ-pyrones) isolated from Fusarium species as inhibitors of HIV-1 integrase. Bioorg. Med. Chem. Lett. 2003;13:713–717. doi: 10.1016/S0960-894X(02)01057-0. [DOI] [PubMed] [Google Scholar]
- 23.Tanaka T., Okubo Y., Goto J. Toxicity of crops contaminated with ustilaginoidin (I) Nihon Chikusan Gakkai Kaihou. 1937;10:265–289. [Google Scholar]
- 24.Goto Y., Ookubo Y. Toxicity of crops contaminated with ustilaginoidin (II) Nihon Chikusan Gakkai Kaihou. 1938;11:183–192. [Google Scholar]
- 25.Ugaki N., Matsuda D., Yamazaki H., Nonaka K., Masuma R., Omura S., Tomoda H. New isochaetochromin, an inhibitor of triacylglycerol synthesis in mammalian cells, produced by Penicillium sp. FKI-4942. I. taxonomy, fermentation, isolation and biological properties. J. Antibiot. 2012;65:15–19. doi: 10.1038/ja.2011.105. [DOI] [PubMed] [Google Scholar]
- 26.Shan T., Sun W., Wang X., Fu X., Sun W., Zhou L. Purification of ustiloxins A and B from rice false smut balls by macroporous resins. Molecules. 2013;18:8181–8199. doi: 10.3390/molecules18078181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shan T., Sun W., Liu H., Gao S., Lu S., Wang M., Sun W., Chen Z., Wang S., Zhou L. Determination and analysis of ustiloxins A and B by LC-ESI-MS and HPLC in false smut balls of rice. Int. J. Mol. Sci. 2012;13:11275–11287. doi: 10.3390/ijms130911275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Miyazaki S., Matsumoto Y., Uchihara T., Morimoto K. High-performance liquid chromatographic determination of ustiloxin A in forage rice silage. J. Vet. Med. Sci. 2009;71:239–241. doi: 10.1292/jvms.71.239. [DOI] [PubMed] [Google Scholar]
- 29.Fu X., Wang X., Cui Y., Wang A., Lai D., Liu Y., Li Q.X., Wang B., Zhou L. A monoclonal antibody-based enzyme-linked immunosorbent assay for detection of ustiloxin A in rice false smut balls and rice samples. Food Chem. 2015;181:140–145. doi: 10.1016/j.foodchem.2015.02.068. [DOI] [PubMed] [Google Scholar]
- 30.Fu X., Wang A., Wang X., Lin F., He L., Lai D., Liu Y., Li Q.X., Zhou L., Wang B. Development of a monoclonal antibody-based icELISA for the detection of ustiloxin B in rice false smut balls and rice grains. Toxins. 2015;7:3481–3496. doi: 10.3390/toxins7093481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhang Y., Zhang K., Fang A., Han Y., Yang J., Xue M., Bao J., Hu D., Zhou B., Sun X., et al. Specific adaptation of Ustilaginoidea virens in occupying host florets revealed by comparative and functional genomics. Nat. Commun. 2014;5:3849. doi: 10.1038/ncomms4849. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.