Abstract
It is crucial to define risk factors that contribute to host invasion by Staphylococcus aureus. Here, we demonstrate that the chromosomally encoded EDIN-B isoform from S. aureus contributes to the onset of bacteremia during the course of pneumonia. Deletion of edinB in a European lineage community-acquired methicillin resistant S. aureus (CA-MRSA) strain (ST80-MRSA-IV) dramatically decreased the frequency and magnitude of bacteremia in mice suffering from pneumonia. This deletion had no effect on the bacterial burden in both blood circulation and lung tissues. Re-expression of wild-type EDIN-B, unlike the catalytically inactive mutant EDIN-R185E, restored the invasive characteristics of ST80-MRSA-IV.
Keywords: bacteremia, virulence factor, toxins, methicillin-resistant Staphylococcus aureus
1. Introduction
Staphylococcus aureus is emerging as a major etiological agent of bacteremia and invasive infections. Bacteremia occurring during the course of pneumonia provoked both an increase of morbidity and mortality [1,2]. S. aureus produces an impressive arsenal of diverse virulence factors [3]; however with the exception of host cell adhesion molecules, little is known regarding the factors that promote bacterial translocation across epithelial and endothelial barriers [4,5].
Several pathogenic strains of S. aureus, particularly those of the European community-acquired methicillin-resistant S. aureus (MRSA) lineage (ST80-MRSA-IV) and others [6], express the epidermal cell differentiation inhibitor (EDIN) or EDIN-like exotoxins [7,8,9]. EDIN exotoxins belong to the family of C3 exoenzymes from Clostridium botulinum [7,8,9,10]. This group of factors catalyzes the ADP-ribosylation and inactivation of the small GTPase RhoA [7,11,12] and, to a lesser extent other Rho proteins [13]. Unlike other EDIN-like factors, EDIN-B is encoded on the chromosome [9,14].
The actin cytoskeleton and upstream regulators, notably the Rho GTPases, are common targets of major virulence factors expressed by highly pathogenic bacteria [10,15,16]. These small GTPases of the Rho protein family are essential proteins owing to their capacity to control the actin cytoskeleton, and therefore, the architecture of cells and tissues [15,17]. Cell biology approaches have revealed that ADP-ribosyltransferases targeting RhoA contribute to notch intercellular junctions in the epithelia, promote infection of gut tissues, as well as the formation of transcellular tunnels in endothelial cells, thereby compromising host barriers [5,15,18,19]. The in vivo relevance of EDIN factors during infection remains largely unappreciated.
Here, we constructed an edinB deletion mutant in a well-characterized strain of the ST80-MRSA-IV lineage and complemented strains to study the effect of EDIN activity in a mouse model of pneumonia.
2. Results
2.1. edinB Promotes Translocation of S. aureus into the Bloodstream During Pneumonia
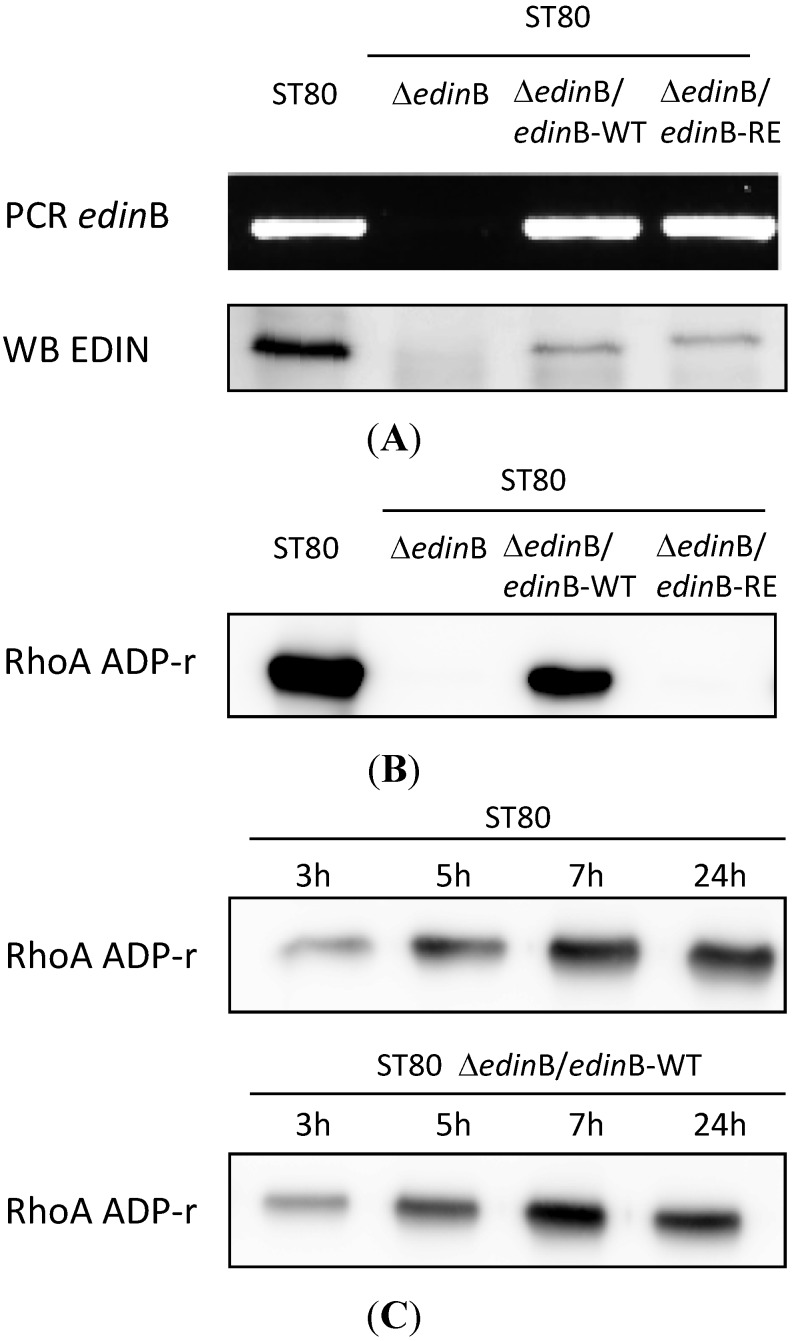
We deleted the edinB gene from WT ST80-MRSA-IV strain to generate the ΔedinB isogenic strain (ΔedinB) (Figure 1A). Moreover, we generated expression plasmids for EDINB wild-type (EDIN-WT) and the catalytically inactive mutant R185E (EDIN-RE). We complemented the ΔedinB strain with each plasmid to generate ΔedinB/edinB-WT and ΔedinB/edinB-RE strains (Figure 1A). The presence or absence of the edinB-encoding gene and toxin expression was verified in the different strains by PCR and immunoblotting (Figure 1A). Next, we tested the ADP-ribosylation activity of EDIN associated with the supernatant of the different strains. This confirmed the production of an ADP-ribosyltransferase activity targeting RhoA by WT ST80-MRSA-IV strain (ST80) and the ΔedinB/edinB-WT strains opposite to ΔedinB and ΔedinB/edinB-RE strains (Figure 1B). In parallel, we measured an increase of the RhoA ADP-ribosylation activity in the supernatant of EDIN-B producing strains as a function of time (Figure 1C). Altogether these data validated at the genetic and functional levels the deletion and complementation of EDIN-B in the different strains.
Figure 1.
Characterization of the ST80 and ST80 derived strains. (A) Genetic and functional analysis of the different strains. PCR and Western blot (WB) showing the presence or absence of edinB and EDIN-B in the WT strain ST80 SCCmecIV PVL + MRSA (ST80), the isogenic strain deleted from edinB (ΔedinB) and the ΔedinB strain complemented with expression plasmids encoding EDIN-B WT (ΔedinB/edinB-WT) or the catalytically inactive mutant R185E (ΔedinB/edinB-RE); (B,C) Detection of the ADP-ribosyltransferase activity targeting RhoA in the supernatant of ST80 stains grown 24 h (B) or 3, 5, 7, and 24 h (C). The biotinylated form of ADP-ribosylated RhoA was revealed with peroxidase-coupled streptavidin.
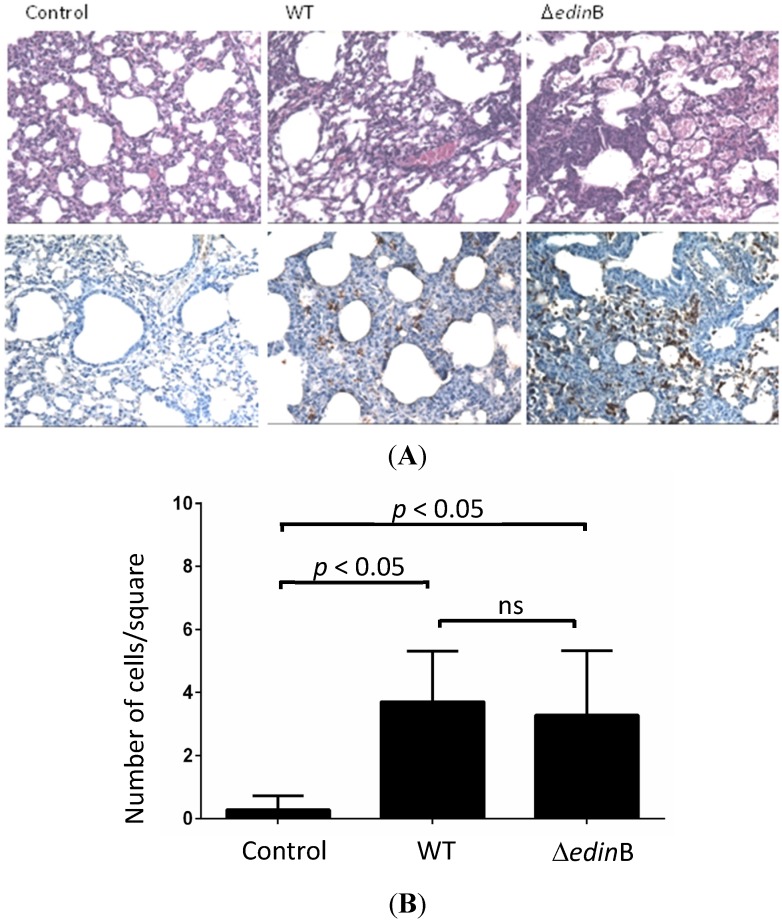
We then triggered pneumonia in mice with both the WT and ΔedinB strains. Pathological examination of the lung tissues showed inflammatory infiltrates largely confined within interalveolar walls mainly by a mononuclear inflammatory infiltrate composed of macrophages and lymphocytes, with a few neutrophils. Some bronchioalveolar air spaces were filled with rare alveolar macrophages and neutrophils (Figure 2A). Immunohistochemical analysis confirmed the presence of bacteria in lung tissues of both WT and ΔedinB infected mice (Figure 2A). Quantification of inflammatory infiltrates showed no significant difference between WT and ΔedinB strains as opposed to the control condition (Figure 2B).
Figure 2.
Histopathological examination. (A) Histopathological examination of the lung tissues of control, WT- and ΔedinB-infected mice showing thickened alveolar walls heavily infiltrated with immune cells (higher panels), as well as an immunohistochemical demonstration of bacteria within inflammatory infiltrates (lower panels). Magnification 40×; (B) Quantification of inflammatory infiltrates. Cells were scored manually using the ImageJ plugin cell counter. Cells were counted on image squares of 88 mm × 88 mm (n = 25 squares from 3 different mice; p < 0.05 or ns, not significant).
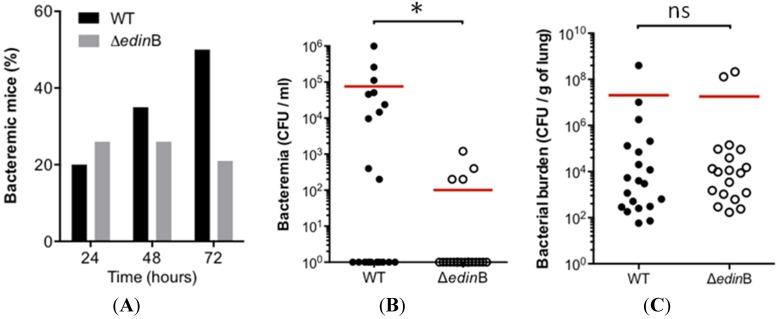
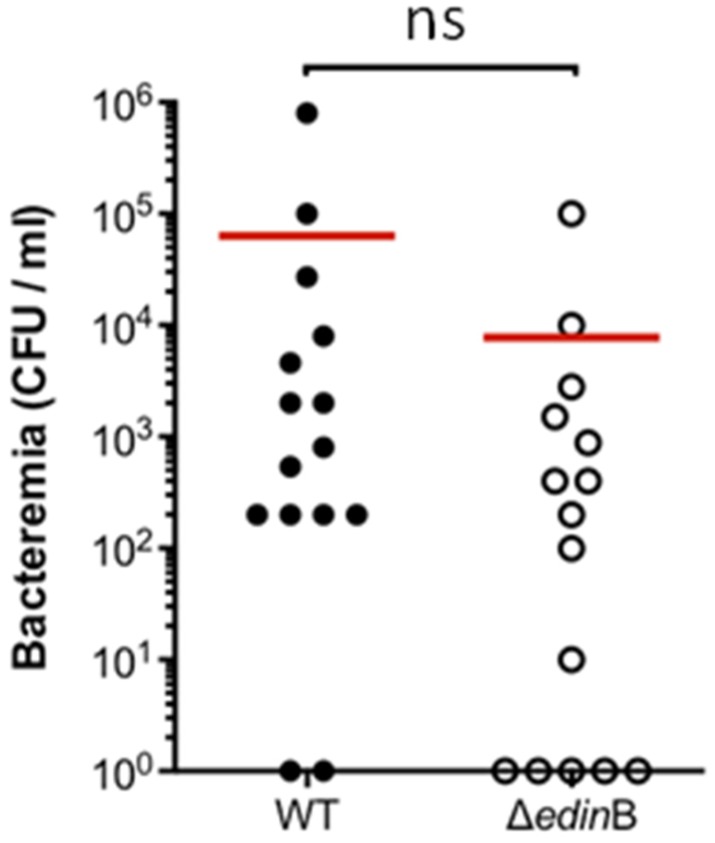
In parallel we monitored the occurrence and magnitude of bacteremia during the first 72 h of pneumonia (Figure 3A). In the WT strain-infected mice (n = 20), we measured a progressive increase in the occurrence of bacteremia, reaching 50% of the population at 72 h post-inoculation. In the ΔedinB-infected mice (n = 19), the percentage of concurrent bacteremia remained stable over the study period, reaching no more than 26% of the infected animals (Figure 3A). We went on to assess whether this higher occurrence of bacteremic animals in WT infected mice might be associated with higher bacterial burden in the blood. Indeed, we measured that bacteremic animals infected with the WT strain had a higher bacterial load in the blood of 7.6 × 104 CFU/mL compared to 1 × 102 CFU/mL for those infected with the ΔedinB strain (p = 0.0159) (Figure 3B). In contrast, we recorded no significant differences of bacterial burden in the lung tissues between both groups of animals (Figure 3C). Altogether, these results demonstrate that both the frequency and magnitude of bacteremia increase as a function of the presence of edinB.
Figure 3.
Role of edinB in pneumonia and bacteremia triggered by ST80-MRSA-IV. (A) Histogram showing the percentage of bacteremic mice after intranasal inoculation of 8 × 109 CFU of the WT and ΔedinB strains (20 and 19 animals, respectively); (B) Groups of mice were infected by intranasal inoculation of 8 × 109 CFU of the WT and ΔedinB strains for 72 h (n = 20 and 19, respectively). Dots represent the CFU values in the blood for each animal; red bars show the mean values; * p = 0.0159; (C) Groups of mice (n = 20 and 19, respectively) were infected by intranasal inoculation of 8 × 109 CFU of the WT and ΔedinB strains for 72 h. Dots represent the CFU values in the lung tissues for each animal; red bars show the mean values (ns, not significant).
Taken together these data revealed the critical role of EDIN-B in bacterial translocation into the bloodstream.
2.2. EDIN-B Activity Promotes the Translocation of S. aureus into the Bloodstream during Pneumonia
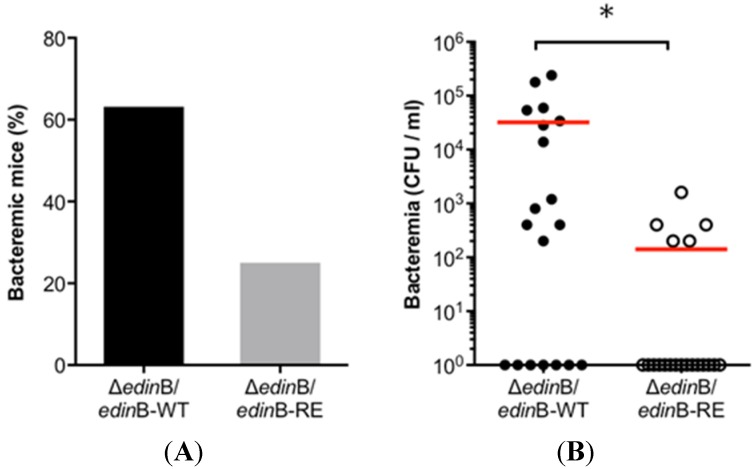
Next, we investigated the link between this newly described virulence property of EDIN-B and its enzymatic activity. To this aim, we generated the ΔedinB/edinB-WT and ΔedinB/edinB-RE strains. We triggered pneumonia in groups of mice with each strain. Of note, we measured a stability of both plasmids during the first 48 h of infection (not shown). We observed that the percentage of bacteremic animals reached 63% in the group of animals infected with ΔedinB/edinB-WT (n = 19), compared to 25% with ΔedinB/edinB-RE infected mice (n = 20) (Figure 4A). In parallel, we measured a dramatic increase of bacterial burden in the blood of animals infected with ΔedinB/edinB-WT (3.2 × 104 CFU/mL) compared to ΔedinB/edinB-RE (1.4 × 102 CFU/mL) (p = 0.0035) (Figure 4B).
Figure 4.
EDIN-B activity promotes bacterial translocation into the bloodstream during pneumonia. Groups of mice were infected for 48 h by intranasal inoculation of 9 × 109 CFU of the ΔedinB/edinB-WT or ΔedinB/edinB-RE strains (n = 19 and n = 20, respectively). (A) Histogram showing the percentage of bacteremic mice; (B) Dots represent the CFU values in the blood for each animal; red bars show the mean values (* p = 0.035).
These results show that the catalytically active EDIN-B potentiates the capacity of S. aureus to translocate into the bloodstream.
2.3. EDIN-B Activity and the Bacterial Persistence in the Blood
Finally, we aimed at determining whether the presence of edinB might favor bacterial persistence in the bloodstream. Two groups of mice (n = 15) were injected intravenously with either WT or the ΔedinB strain. We recorded the bacterial burden in the blood at 48 h of infection. This analysis revealed no significant difference between the bacterial burdens in both groups of infected animals (Figure 5).
Figure 5.
Measure of bacterial persistence in the bloodstream. Groups of mice (n = 15 each) were infected by intravenous inoculation of 3 × 107 CFU of the WT and ΔedinB strains. Dots represent the CFU values in the bloodstream for each animal at 48 h post infection; red bars show the mean values; ns, not significant.
Expression of EDIN-B does not affect bacterial persistence in the blood per se.
3. Discussion
Hospital- and community-acquired methicillin-resistant S. aureus is an increasingly recognized etiology of pneumonia and presents diagnostic and therapeutic challenges. In particular, the pejorative evolution of this type of infection into deadly bacteremia has clinical relevance [1,2]. Here, we report that ST80-MRSA-IV expressing EDIN-B produced a high occurrence of bacteremia in animals suffering from pneumonia. In a mouse model of infection, we demonstrate the critical role of EDIN-B activity in the translocation of bacteria from the lung tissues to the bloodstream, without excluding a contributing role of additional virulence factors of S. aureus. We also found that edinB does not promote detectable bacterial persistence in the lung tissues or in the blood. These results deserve further attention to confirm a similar role in humans given that human cells are sensitive to EDIN cytotoxicity. Collectively, our data point f a major role of EDIN-B as a virulence factor of methicillin-resistant S. aureus ST80 and for a possible implication of EDIN in pneumonia-induced bacteremia.
EDIN belongs to a large family of exoenzymes that target the small GTPase RhoA for inactivation [7,10]. The small GTPase RhoA is an essential regulator of actomyosin cytoskeleton contractility, thereby controlling cell adhesion, division, and migration [20]. We did not record a difference in bacterial persistence in the lung tissues or in the bloodstream, most likely excluding a role for EDIN at the level of immune cell effectors. Our data rather favor the hypothesis of a role for EDIN at the level of the progressive translocation of the bacteria from the lung tissues to the bloodstream. Indeed, by promoting the assembly of contractile actin cables, the small GTPase RhoA exerts a critical role in the control of barrier function of epithelia and endothelia that is a hotspot of targeting by bacterial virulence factors [5,15,18,20]. In contrast, when we injected bacteria in the bloodstream, we did not detect a specific role for EDIN-B in promoting their extravasation to the lung tissues (data not shown). Instead, we provide compelling evidence that EDIN-B activity promotes the translocation of bacteria from the lung mucosa to the blood. This breaching of host barriers may occur via different scenarios, such as a higher capacity of the bacteria to transcytose or to reduce the cohesion of the lung epithelial and endothelial barriers [19]. This second scenario is in agreement with cell biology approaches that have firmly established that RhoA inactivation contributes to notch intercellular junctions in epithelia [15,18]. More recent studies also documented the capacity of EDIN factors to induce the formation of transcellular tunnels in endothelial cells [19]. Consistent with these findings, we also observed a requirement of EDIN-B in the induction of macroapertures during endothelial cell infection (Figure S1). Further studies will have to clarify whether EDIN-B promotes the translocation of bacteria through two host barriers in the lung successively by intercellular and transcellular pathways.
Infection by pathogenic strains of S. aureus involves the deployment of a large arsenal of virulence factors [3,4]. Despite this diversity, we identified a critical role for EDIN in promoting bacterial dissemination. This work represents the first demonstration that EDIN-B is a virulence factor and thus fulfills the molecular Koch’s postulates. This study also reveals a major role of EDIN in promoting the translocation of bacteria from the mucosal environment to the blood circulation. These data extend previous findings suggesting that RhoA ADP-ribosyltransferase activity promotes the metastatic dissemination of S. aureus via a hematogenous route in mice [15,21] and that in humans, edin-positive strains of S. aureus are found in deep seated infections, such as high-grade diabetic foot ulcerations associated with bacteremia [6,22].
Our study in mice provides a genetic demonstration of the invasive virulence function of EDIN activity.
4. Experimental Section
4.1. Ethics Statement
The mice were maintained and handled in strict accordance with the recommendations for the ethical evaluation of experiments using laboratory animals and with the European guidelines 86/609/CEE. This protocol was approved by the ethics committee for animal experimentation (CIEPAL AZUR/N°28) and by the French Ministère de l’Education Nationale, de l’Enseignement Supérieur et de la Recherche under number 02330.01.
4.2. Bacterial Strains and DNA Constructs
HT20020209-LUG1799 is a minimally passaged ST80 SCCmecIV PVL + MRSA that was originally isolated in France [23] and that is referred to as WT in this study. All strains of S. aureus belonging to this lineage, such as HT20020209-LUG1799, contain edinB only [24]. Deposit in process to EMBL and http://www.ebi.ac.uk/ena. The isogenic ΔedinB mutant (LUG1840) was obtained by allelic replacement mutagenesis in LUG1799 using the pMAD plasmid as previously described [25], except the tetracycline resistance gene was replaced by a kanamycin resistance gene from the plasmid PCR 2.1 (Invitrogen, Carlsbad, CA, USA). The primers used to amplify the upstream and downstream regions of edinB were as follows: edin1117′, edin2165, edin2872, and edin3795 (Table S1). The primers used to verify the deletion of edinB were edinB-1 and edinB-2 (Table S1, Figure 1A). Replacement in the LUG1799 strain of the edin gene by the kanamycin cassette was confirmed by PCR using primers edin1099-Kcr4 and Kcr3-edin3893 (Table S1, data not shown). LUG1840 mutants were also complemented with either edinB-WT (ΔedinB/edinB-WT) or edinB-RE, which encodes for the catalytically inactive form of EDIN-B (ΔedinB/edinB-RE). The coding region of edinB was amplified by PCR using genomic DNA from LUG1799 and the primers edinB-F and edinB-R (Table S1). The BamHI-PstI PCR product was cloned into the vector pMK4-pPROT as described previously [26]. The resulting plasmid pMK4-pPROT-edinB was maintained in Escherichia coli Top10 (Invitrogen, Carlsbad, CA, USA), subcloned into S. aureus RN4220 and then transferred into LUG1840 mutants by electroporation. The EDIN-B R185E mutation was introduced by changing AGA to GAA in the coding sequence of edinB in pMK4-pPROT-edinB using a QuikChange Lightning Site-Directed Mutagenesis Kit (Agilent Technologies, Les Ulis, France). The recombinant plasmids were verified by sequencing. The bacterial strains were grown in Luria-Bertani medium alone or supplemented with kanamycin (50 µg/mL) or chloramphenicol (20 µg/mL) at 37 °C. We verified that all strains had identical growth properties (data not shown).
4.3. Biochemical Assays and Products
For immunoblotting, serum anti-EDIN was raised using standard rabbit immunization protocols (Agro-Bio, La Ferté Saint Aubin, France). Primary antibodies were revealed using goat anti-rabbit horseradish peroxidase-conjugated secondary antibodies (Dako, Glostrup, Denmark), followed by chemiluminescence detection ECL (GE Healthcare, Little Chalfont, UK). For RhoA ADP-ribosylation assays, each reaction was performed in a total volume of 50 μL, 30 min at 37 °C with 0.8 μg of RhoA, 10 μM biotinylated NAD+ (R & D Systems) and 1 μL of supernatant in 50 mM Tris-HCl (pH 7.5), 0.1 mM DTT, 1 mM MgCl2, 100 mM NaCl and complete protease inhibitors (Roche, Manheim, Germany). The reaction was stopped by adding 5× Laemmli buffer (Sigma, St Louis, MO, USA) and by boiling the samples for 5 min. The samples were further subjected to SDS-PAGE and transferred to PVDF membranes. The biotin-ADP-ribosylated proteins were visualized with peroxidase-coupled streptavidin (Sigma, St Louis, MO, USA) in a subsequent chemiluminescence reaction.
4.4. Murine Model of Pneumonia and Bacteremia
Six to eight-week-old female BALB/c mice were purchased from Janvier Laboratory (Le Genest St. Isle, France). Before infection, the bacterial strains were grown in LB medium with shaking for 16 h. The cultures were centrifuged, washed three times in phosphate-buffered saline (PBS; Gibco, Grand Island, NE, USA) and diluted in PBS to achieve the desired inoculum. Pneumonia was triggered by a 20-μL injection in each nostril for a total inoculum of approximately 1010 bacteria/mouse in animals anesthetized by intraperitoneal injection of xylazine (10 mg/kg) and ketamine (90 mg/kg). The bacterial burden in the blood was assessed after 5 µL of blood was collected, diluted in PBS and spread on LB agar plates with the corresponding antibiotic. The colony forming units (CFUs) were counted at 24 h after incubation at 37 °C. At the indicated period, the mice were euthanized, and the lungs were collected, weighed and homogenized in PBS using a Precellys 24 homogenizer before CFU enumeration. For bacteremia, the mice were directly infected with approximately 107 bacteria/mouse by intravenous injection of 100 µL of inoculum in the tail vein. The level of bacterial burden was recorded as indicated above.
4.5. Histopathology
The lungs from control and infected mice were collected, fixed in formalin and embedded in paraffin. Consecutive 3-µm paraffin sections were stained with hematoxylin-eosin-saffron (HES). The presence of bacteria within lung tissues was visualized using rabbit anti-S. aureus primary antibody (Eurogentec, Angers, France) and goat secondary anti-rabbit HRP antibody (Dako, Glostrup, Denmark).
4.6. Cell Culture and Immunofluorescence
Human umbilical vein endothelial cells (HUVECs, Promocell) were cultured in SFM medium (Invitrogen), supplemented with 20% Fetal Calf Serum (Invitrogen), 20 ng/mL fibroblast growth factor, 10 ng/mL epidermal growth factor, 0.01 U/mL penicillin, 10 ng/mL streptomycin and 1 µg/mL heparin (Sigma) For bacterial infection assays, 25,000 cells per well were seeded on gelatin coated (0.4% w/v in H2O) glass coverslips in 12-well plates 24 h before infection. After infection, cells were fixed in 4% formaldehyde (Sigma) in PBS. Actin filaments were labeled using 1 µg/mL FITC-conjugated phalloidin (Sigma). Bacteria were labeled with rabbit serum anti-S. aureus (Eurogentec). Texas Red anti-rabbit IgG antibody (Molecular Probes, ThermoFisher Scientific, Waltham, MA, USA). Immunosignals were analyzed with a DM5500 microscope (Leica, Wetzlar, Germany) with a ×63 lens.
4.7. Statistical Analysis
Statistical analyses were performed using PRISM V 5.0b (GraphPad Software, San Diego, CA, USA). For the pairwise analysis, a Mann-Whitney non-parametric test was performed; p-values <0.05 were considered statistically significant.
Acknowledgments
The authors greatly acknowledge the C3M animal room and MicorBio cell imaging facilities. This work was supported by funding from the INSERM and CNRS, from the university of Nice Sophia-Antipolis, the university of Lyon and the Ecole Normale Supérieure of Lyon, as well as by funding from the «Investments for the Future» LABEX SIGNALIFE: program reference #ANR-11-LABX-0028-01, ANR TransEndotheliaTunnel and by a fellowship from IHU Méditerranée infection to J.C.
Supplementary Materials
Supplementary materials can be accessed at: http://www.mdpi.com/2072-6651/7/10/4131/s1.
Author Contributions
François Vandenesch and Emmanuel Lemichez conceived and designed the experiments; Johan Courjon, Patrick Munro, Yvonne Benito, Coralie Bouchiat, Anne Doye, Hubert Lepidi, and Eric Ghigo performed the experiments; Laurent Boyer, Orane Visvikis, Patrick Munro, Jean-Philippe Lavigne, and Emmanuel Lemichez analyzed the data; Patrick Munro and Emmanuel Lemichez wrote the paper.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Schreiber M.P., Chan C.M., Shorr A.F. Bacteremia in Staphylococcus aureus pneumonia: Outcomes and epidemiology. J. Crit. Care. 2011;26:395–401. doi: 10.1016/j.jcrc.2010.09.002. [DOI] [PubMed] [Google Scholar]
- 2.Otter J.A., French G.L. Molecular epidemiology of community-associated meticillin-resistant Staphylococcus aureus in Europe. Lancet Infect. Dis. 2010;10:227–239. doi: 10.1016/S1473-3099(10)70053-0. [DOI] [PubMed] [Google Scholar]
- 3.Lowy F.D. How Staphylococcus aureus adapts to its host. N. Engl. J. Med. 2011;364:1987–1990. doi: 10.1056/NEJMp1100251. [DOI] [PubMed] [Google Scholar]
- 4.Edwards A.M., Massey R.C. How does Staphylococcus aureus escape the bloodstream? Trends Microbiol. 2011;19:184–190. doi: 10.1016/j.tim.2010.12.005. [DOI] [PubMed] [Google Scholar]
- 5.Edwards L.A., O’Neill C., Furman M.A., Hicks S., Torrente F., Pérez-Machado M., Wellington E.M., Phillips A.D., Murch S.H. Enterotoxin-producing staphylococci cause intestinal inflammation by a combination of direct epithelial cytopathy and superantigen-mediated T-cell activation. Inflamm. Bowel Dis. 2012;18:624–640. doi: 10.1002/ibd.21852. [DOI] [PubMed] [Google Scholar]
- 6.Messad N., Landraud L., Canivet B., Lina G., Richard J.L., Sotto A., Lavigne J.P., Lemichez E., French Study Group on the Diabetic Foot Distribution of edin in Staphylococcus aureus isolated from diabetic foot ulcers. Clin. Microbiol. Infect. 2013;19:875–880. doi: 10.1111/1469-0691.12084. [DOI] [PubMed] [Google Scholar]
- 7.Sugai M., Chen C.H., Wu H.C. Bacterial ADP-ribosyltransferase with a substrate specificity of the rho protein disassembles the Golgi apparatus in Vero cells and mimics the action of brefeldin A. Proc. Natl. Acad. Sci. USA. 1992;89:8903–8907. doi: 10.1073/pnas.89.19.8903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yamaguchi T., Hayashi T., Takami H., Ohnishi M., Murata T., Nakayama K., Asakawa K., Ohara M., Komatsuzawa H., Sugai M. Complete nucleotide sequence of a Staphylococcus aureus exfoliative toxin B plasmid and identification of a novel ADP-ribosyltransferase, EDIN-C. Infect. Immun. 2001;69:7760–7771. doi: 10.1128/IAI.69.12.7760-7771.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yamaguchi T., Nishifuji K., Sasaki M., Fudaba Y., Aepfelbacher M., Takata T., Ohara M., Komatsuzawa H., Amagai M., Sugai M. Identification of the Staphylococcus aureus etd pathogenicity island which encodes a novel exfoliative toxin, ETD, and EDIN-B. Infect. Immun. 2002;70:5835–5845. doi: 10.1128/IAI.70.10.5835-5845.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Aktories K. Bacterial protein toxins that modify host regulatory GTPases. Nat. Rev. Microbiol. 2011;9:487–498. doi: 10.1038/nrmicro2592. [DOI] [PubMed] [Google Scholar]
- 11.Chardin P., Boquet P., Madaule P., Popoff M.R., Rubin E.J., Gill D.M. The mammalian G protein rhoC is ADP-ribosylated by Clostridium botulinum exoenzyme C3 and affects actin microfilaments in Vero cells. EMBO J. 1989;8:1087–1092. doi: 10.1002/j.1460-2075.1989.tb03477.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Paterson H.F., Self A.J., Garrett M.D., Just I., Aktories K., Hall A. Microinjection of recombinant p21rho induces rapid changes in cell morphology. J. Cell Biol. 1990;111:1001–1007. doi: 10.1083/jcb.111.3.1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wilde C., Vogelsgesang M., Aktories K. Rho-specific Bacillus cereus ADP-ribosyltransferase C3cer cloning and characterization. Biochemistry. 2003;42:9694–9702. doi: 10.1021/bi034583b. [DOI] [PubMed] [Google Scholar]
- 14.Franke G.C., Bockenholt A., Sugai M., Rohde H., Aepfelbacher M. Epidemiology, variable genetic organization and regulation of the EDIN-B toxin in Staphylococcus aureus from bacteraemic patients. Microbiology. 2010;156:860–872. doi: 10.1099/mic.0.030304-0. [DOI] [PubMed] [Google Scholar]
- 15.Lemichez E., Aktories K. Hijacking of Rho GTPases during bacterial infection. Exp. Cell Res. 2013;319:2329–2336. doi: 10.1016/j.yexcr.2013.04.021. [DOI] [PubMed] [Google Scholar]
- 16.Aktories K., Lang A.E., Schwan C., Mannherz H.G. Actin as target for modification by bacterial protein toxins. FEBS J. 2011;278:4526–4543. doi: 10.1111/j.1742-4658.2011.08113.x. [DOI] [PubMed] [Google Scholar]
- 17.Jaffe A.B., Hall A. RHO GTPases: Biochemistry and Biology. Annu. Rev. Cell Dev. Biol. 2005;21:247–269. doi: 10.1146/annurev.cellbio.21.020604.150721. [DOI] [PubMed] [Google Scholar]
- 18.Nusrat A., Giry M., Turner J.R., Colgan S.P., Parkos C.A., Carnes D., Lemichez E., Boquet P., Madara J.L. Rho protein regulates tight junctions and perijunctional actin organization in polarized epithelia. Proc. Natl. Acad. Sci. USA. 1995;92:10629–10633. doi: 10.1073/pnas.92.23.10629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lemichez E., Gonzalez-Rodriguez D., Bassereau P., Brochard-Wyart F. Transcellular tunnel dynamics: Control of cellular dewetting by actomyosin contractility and I-BAR proteins. Biol. Cell. 2012;105:109–117. doi: 10.1111/boc.201200063. [DOI] [PubMed] [Google Scholar]
- 20.Heasman S.J., Ridley A.J. Mammalian Rho GTPases: New insights into their functions from in vivo studies. Nat. Rev. Mol. Cell Biol. 2008;9:690–701. doi: 10.1038/nrm2476. [DOI] [PubMed] [Google Scholar]
- 21.Munro P., Benchetrit M., Nahori M.A., Stefani C., Clément R., Michiels J.F., Landraud L., Dussurget O., Lemichez E. Staphylococcus aureus EDIN toxin promotes formation of infection foci in a mouse model of bacteremia. Infect. Immun. 2010;78:3404–3411. doi: 10.1128/IAI.00319-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Munro P., Clement R., Lavigne J.P., Pulcini C., Lemichez E., Landraud L. High prevalence of edin-C encoding RhoA-targeting toxin in clinical isolates of Staphylococcus aureus. Eur. J. Clin. Microbiol. Infect. Dis. 2011;30:965–972. doi: 10.1007/s10096-011-1181-6. [DOI] [PubMed] [Google Scholar]
- 23.Perret M., Badiou C., Lina G., Burbaud S., Benito Y., Bes M., Cottin V., Couzon F., Juruj C., Dauwalder O., et al. Cross-talk between Staphylococcus aureus leukocidins-intoxicated macrophages and lung epithelial cells triggers chemokine secretion in an inflammasome-dependent manner. Cell. Microbiol. 2012;14:1019–1036. doi: 10.1111/j.1462-5822.2012.01772.x. [DOI] [PubMed] [Google Scholar]
- 24.Stegger M., Wirth T., Andersen P.S., Skov R.L., de Grassi A., Simões P.M., Tristan A., Petersen A., Aziz M., Kiil K., et al. Origin and evolution of European community-acquired methicillin-resistant Staphylococcus aureus. mBio. 2014;5 doi: 10.1128/mBio.01044-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Labandeira-Rey M., Couzon F., Boisset S., Brown E.L., Bes M., Benito Y., Barbu E.M., Vazquez V., Höök M., Etienne J., et al. Staphylococcus aureus Panton-Valentine leukocidin causes necrotizing pneumonia. Science. 2007;315:1130–1133. doi: 10.1126/science.1137165. [DOI] [PubMed] [Google Scholar]
- 26.Boyer L., Doye A., Rolando M., Flatau G., Munro P., Gounon P., Clément R., Pulcini C., Popoff M.R., Mettouchi A., et al. Induction of transient macroapertures in endothelial cells through RhoA inhibition by Staphylococcus aureus factors. J. Cell Biol. 2006;173:809–819. doi: 10.1083/jcb.200509009. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.