Abstract
Objective Tested a family-based group problem-solving intervention, “Families Taking Control,” (FTC) to improve school functioning and health-related quality of life (HRQL) for children with sickle cell disease. Method Children and caregivers completed questionnaires assessing HRQL and school functioning and children completed performance-based measures of IQ and achievement at baseline and 6 months later. Families were randomized to the intervention (FTC, n = 42) or delayed intervention control (DIC, n = 41) group. FTC involved a full-day workshop followed by 3 booster calls. Results There were no differences between FTC completers (n = 24) and noncompleters (n = 18). FTC group (n = 24) and DIC group (n = 38) did not differ significantly on primary outcomes at follow-up: number of formal academic and disease-related accommodations, individualized education plan/504 service plan, school absences, school HRQL, or academic skills. Conclusions Although families found FTC to be acceptable, there were no intervention effects. Challenges of the trial and implications for future research are discussed.
Keywords: family-based intervention, randomized controlled trial, sickle cell disease
Sickle cell disease (SCD), a chronic genetic condition affecting hemoglobin, occurs in 1 in 365 African-American births and 1 in 1,200 Hispanic-American births in the United States (Hassell, 2010). SCD necessitates lifelong disease management and is associated with physical and psychosocial complications (Barakat, Lash, Lutz, & Nicolaou, 2006) related to medical, sociodemographic, and psychosocial factors (Kral, Brown, & Hynd, 2001). Effective behavioral interventions designed to improve management of physical complications (e.g., pain episodes; Dinges et al., 1997; Gil et al., 2001) and SCD knowledge (Asnani, Quimby, Bennett, & Francis, 2014) have resulted in improved adaptation and adherence to treatment. However, few published interventions directly target health-related quality of life (HRQL) in SCD.
Children with SCD are at risk for poor HRQL (Dampier et al., 2010), particularly in school functioning (Ladd, Valrie, & Walcott, 2014) owing to risk factors including neurocognitive sequelae of overt and silent strokes and disease-related school absences. Compared with healthy siblings, children with SCD score lower in academic attainment and achievement, particularly for those with a history of cerebral infarct (Schatz, Brown, Pascual, Hsu, & DeBaun, 2001). Even in the absence of stroke, children with SCD exhibit poorer neurocognitive functioning compared with classroom peers (Noll et al., 2001) and age-based norms (Van Der Land et al., 2014) with a host of environmental, disease, and psychosocial factors contributing to lower academic achievement beyond IQ (Smith, Patterson, Szabo, Tarazi, & Barakat, 2013).
Prior research has documented annual school absences as high as 20–40 days for children with SCD (Peterson, Palermo, Swift, Beebe, & Drotar, 2005; Shapiro et al., 1995), double the criteria for chronic absenteeism. SCD complications including unpredictable vaso-occlusive pain episodes, frequent medical visits, and susceptibility to infection may increase school absences. School absences are associated with lower academic achievement and graduation rates (Balfanz, Herzog, & Mac Iver, 2007) as well as lower school, emotional, and physical functioning (Dampier et al., 2010). Findings suggest complex relationships between SCD and school functioning, wherein a myriad of environmental and biological factors influence outcome.
Family functioning is supported as a resistance factor for medical and psychosocial outcomes for children with SCD (Barakat, Lutz, Nicolaou, & Lash, 2005; Burlew, Telfair, Colangelo, & Wright, 2000; Thompson, Gil, Burbach, Keith, & Kinney, 1993), and caregiver problem-solving ability moderates the relationship between disease complications and child HRQL (Barakat, Daniel, Smith, Robinson, & Patterson, 2014). However, few interventions in SCD have addressed the influence of the family. An important exception is Kaslow and colleagues’ family psychoeducation intervention for youth with SCD, which demonstrated higher scores on measures of disease knowledge in the intervention group compared with controls (Kaslow et al., 1997). Family-based interventions linked to positive outcomes frequently utilize problem-solving methods emphasizing shared family responsibility for practical, effective solutions to problems (D’Zurilla & Nezu, 1982) and target family cohesion and disease management as mechanisms to improved child outcomes (Wysocki et al., 2006). Family problem-solving interventions in pediatric diabetes (Wysocki et al., 2006), childhood traumatic brain injury (Wade, Walz, Carey, & Williams, 2009), asthma (Seid, Varni, Gidwani, Gelhard, & Slymen, 2010), and pediatric pain (Palermo, Law, Essner, Jessen-Fiddick, & Eccleston, 2014) have found significant improvements in psychosocial functioning (adherence, self-management, and HRQL, respectively). The pediatric literature also reports the potential efficacy of brief, family problem-solving methods (Kaslow et al., 2000; Kazak et al., 2004; Sahler et al., 2005). Although mechanisms of change have not been formally explored in prior studies, we hypothesized that by improving family problem-solving skills to address school-related challenges that child HRQL and academic outcomes may improve.
This study aimed to evaluate the efficacy of a family-based, group problem-solving intervention, Families Taking Control (FTC), to improve school functioning for school-age children with SCD compared with a delayed intervention control (DIC) group. We hypothesized that participation in this evidence-based intervention would improve school functioning for this at-risk group as assessed by school HRQL (rated by children and caregivers), school attendance, and access to school resources via formal education plans and accommodations compared with children in the DIC group. We utilized community-based participatory strategies such as focus groups and a peer patient navigator to improve intervention uptake owing to high rates of attrition in pediatric psychology (Karlson & Rapoff, 2009) and SCD-specific intervention trials (Barakat, Schwartz, Salamon, & Radcliffe, 2010; Kaslow et al., 1997). The study design and attrition affected the evaluation of the efficacy of FTC, thus we present our approach and difficulties encountered as a roadmap to improve future interventions for this population.
Methods
Participants
Children with SCD between the ages of 6 and 12, from primarily English-speaking families, receiving follow-up care at one of two comprehensive sickle cell clinics in children’s hospitals in a northeastern city were included. This age group was selected based on focus group input about the need for preventing educational challenges and the literature suggesting increased cognitive impact of SCD (Schatz, 2004) and greater likelihood of grade retention (Ladd et al., 2014) with age. Children with severe developmental delay or children/caregivers with severe psychopathology (i.e., substance abuse, bipolar disorder) that would adversely affect their ability to participate were excluded. Potential participants were identified through patient registries and approached at the hospital or prior to attending a SCD summer camp from 2009 to 2012.
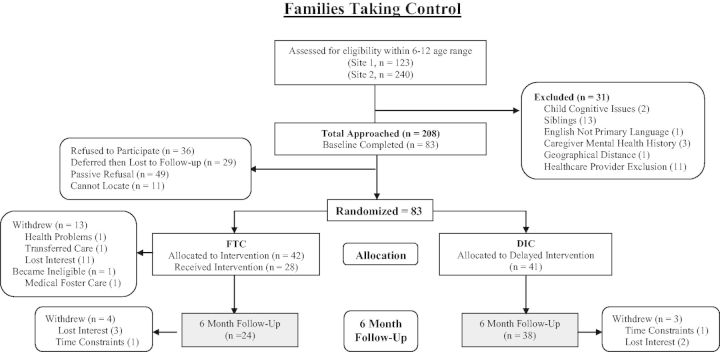
Of the 208 families approached, 36 refused (Figure 1). The most common reasons for refusal included schedule too busy (n = 13) and intervention viewed as not relevant to the child (n = 10). Of the 172 families who agreed to participate, 49 passively refused later (e.g., did not return phone calls) and 11 families were lost to follow-up (nonworking phone numbers and/or no-show to outpatient medical appointments). Eighty-three families were enrolled (FTC = 42, DIC = 41) and 61 families (FTC = 24; DIC = 38) completed Time 2 assessments (Table I).
Figure 1.
Consort diagram. FTC = Families Taking Control Intervention group; DIC = Delayed Intervention Control group.
Table I.
Sample Demographics
| Measure | FTC (n = 42) N (%) | DIC (n = 41) N (%) |
|---|---|---|
| Sex—male | 21 (50.00) | 21 (51.22) |
| Age [M (SD)] | 8.29 (2.12) | 8.66 (2.10) |
| Treatment site | ||
| Site 1 | 18 (42.86) | 10 (24.39) |
| Site 2 | 24 (57.14) | 31 (75.61) |
| Genotype | ||
| Less severe | 17 (40.48) | 15 (36.59) |
| Severe | 25 (59.52) | 26 (63.41) |
| Disease complications [M (SD)] | 5.88 (3.99) | 6.20 (4.43) |
| School type | ||
| Public | 25 (59.52) | 32 (78.05) |
| Private/Charter | 16 (38.10) | 9 (21.95) |
| Home schooling | 1 (2.38) | 0 |
| School district | ||
| Urban | 34 (80.95) | 40 (97.56) |
| Suburban | 8 (19.05) | 1 (2.44) |
| IQ [M (SD)] | 93.62 (12.07) | 93.24 (12.28) |
| Primary caregiver education | ||
| Some high school | 4 (9.52) | 5 (12.20) |
| High school graduate | 10 (23.81) | 12 (29.27) |
| Some college/vocational school | 17 (40.48) | 16 (39.02) |
| Bachelor’s degree | 9 (21.43) | 3 (7.32) |
| Professional/graduate school | 2 (4.76) | 5 (12.20) |
| Household annual income | ||
| $0–$833 | 2 (4.76) | 3 (7.32) |
| $834–$1,666 | 11 (26.19) | 10 (24.39) |
| $1,667–$2,499 | 7 (16.67) | 8 (19.51) |
| $2,500–$3,333 | 8 (19.05) | 16 (39.02) |
| $3,334–$4, 166 | 3 (7.14) | 0 |
| $4,167–$4,999 | 4 (9.52) | 1 (2.44) |
| $5,000–$5,833 | 3 (7.14) | 2 (4.88) |
| Over $5,834 | 4 (9.52) | 1 (2.44) |
| Intervention expectancy [M (SD)] | ||
| Child | 4.21 (0.69) | — |
| Caregiver | 4.22 (0.75) | — |
| SPSI [M (SD)] | 111.81 (11.48) | 106.71 (14.17) |
| Educational accommodations | ||
| 504 Plan | 6 (14.28) | 5 (12.19) |
| IEP | 5 (11.90) | 8 (19.51) |
Note. FTC = Families Taking Control Intervention group; DIC = Delayed Intervention Control group; SPSI = Social Problem Solving Inventory.
Measures
Medical Chart Review was completed by trained research assistants using a structured file review to collect genotype and disease complications. SCD genotype was dichotomized into more severe (HBSS and HBSβ0Thal) and less severe genotypes; this classification has been used in other studies of neurocognitive functioning in children with SCD (Smith et al., 2013). Medical complications were recorded and used to calculate a summary of SCD complications (Barakat et al., 2007, 2014; Casey, Brown, & Bakeman, 2000). Complications included number of pain episodes, lifetime history of stroke, diagnosis of asthma/reactive airway disease, acute chest episodes in the past year, and other SCD-related complications (e.g., iron overload, obstructive sleep apnea, avascular necrosis).
The Hematology/Oncology Psycho-Educational (HOPE) Needs Assessment (Peterson et al., 2005) is a 17-item caregiver report of child school functioning developed as a psychosocial screening measure for children with SCD. This intervention supported families in pursuing formal academic accommodations (i.e., 504 Plans and Individualized Education Plans [IEPs]). A 504 Plan, available to all children with SCD (Wright & Wright, 2008), allows accommodations related to disease management (i.e., pain medication, access to water) and missed school (i.e., extra set of books at home, excusing absences related to medical condition). An IEP is available to children with SCD in need of academic intervention (i.e., child with a history of stroke); although, some suggest that all children with SCD should have an IEP (Day & Chismark, 2006). Items used in this study identified the number of school accommodations, days absent in the past year, and the presence of an IEP or 504 Plan (yes/no). Days absent were reported categorically in 7-day increments and were significantly correlated with school reports of child absences (Excused absences rs = .68, p < .001; Total absences rs = .65, p < .001). School reports were not available for all participants; thus, caregiver report was used.
The Pediatric Quality of Life Inventory (PedsQL; Varni, Seid, & Rode, 1999) is a 23-item measure of HRQL. Children self-report and caregivers provide proxy-report regarding child problems with physical health, emotional functioning, social functioning, and school functioning over the past month on a 5-point Likert-type scale; higher scores indicate better HRQL. This scale has been validated in children with SCD (Panepinto, Pajewski, Foerster, & Hoffman, 2008). The school functioning subscale was used as a primary intervention outcome. Inter-item reliability for the school subscale was acceptable at Baseline (Child Baseline α = .71; Caregiver Baseline α = .70) and Time 2 (Child T2 α = .64; Caregiver T2 α = .79).
The Woodcock Johnson III (WJ-III) is an individually administered measure of achievement (Grenwelge, 2009). Children completed the Letter-Word Identification, Reading Fluency, Calculation, Math Fluency, Spelling, and Passage Comprehension subscales at Baseline (Form A) and Time 2 (Form B). The Academic Skills Composite Score was used as a primary intervention outcome. The WJ-III has been validated as a measure of academic functioning and is considered an acceptable measure for children with SCD (Daly, Kral, & Tarazi, 2011).
The Wechsler Abbreviated Scale of Intelligence (WASI; Weschler, 1999) is a brief reliable and valid measure of intelligence for individuals aged 6–89 years. The WASI Vocabulary and Matrix Reasoning subtests were administered at baseline, yielding a 2-subtest Full Scale IQ (FSIQ). FSIQ was examined as a potential covariate.
The Social Problem-Solving Inventory (SPSI) Revised Short Form (D’Zurilla & Nezu, 1982) is a self-report measure consisting of 25 items scored on a 5-point Likert-type scale assessing caregiver problem-solving style. The SPSI-Revised has been shown to be internally consistent and reliable (D'Zurilla & Nezu, 1990). Higher total scores indicate stronger problem-solving abilities. Subscale alphas ranged from .66 to .85. Total SPSI was examined as a covariate.
The Expectancy Form was created for the current study to assess caregiver and child expectations for the intervention after group randomization. A 10-item Feedback Form was also created to assess child and caregiver satisfaction with the intervention (e.g., “The content of the workshop was useful to my family.”). Both measures utilized a 5-point Likert-type scale, with higher scores indicating more positive expectations/feedback.
The Engagement Rating Form was created for the current study to assess the child and caregiver engagement in the material, competence using the problem-solving model, and level of commitment to using the problem-solving model. The primary interventionist rated the child and caregiver on a behaviorally based 1-5 scale, with higher scores indicating greater engagement, competence, and commitment through specific behaviors during the intervention and booster calls (i.e., could describe steps of problem-solving model).
Procedure
After institutional review board approval, eligible families were informed about the study by clinic staff and study team members and interested families scheduled a baseline assessment in the family’s home or during an outpatient visit. Caregivers and child participants provided consent/assent before assessments. Randomization (stratified by gender in blocks of 10) was concealed from the family and the study team until after completing the baseline assessment when an envelope with randomization status was opened and the family was informed of next steps. FTC families were invited to the next scheduled intervention, and DIC families were offered the intervention after a 6-month follow-up assessment (Time 2).
Intervention materials were reviewed by a focus group of parents of adolescents and young adults with SCD twice during intervention development. The focus group recommended that we: (1) work with families to implement educational accommodations before the child starts having school difficulties, (2) reduce the burden on families by holding the intervention on one weekend day, and (3) focus on family strengths (as opposed to identifying deficits). Based on focus group feedback, the FTC intervention was developed as a full-day (7-hr) weekend workshop at the hospital for children and their primary caregivers; school-age siblings were also invited to attend. Twenty workshops were held between August 2009 and April 2012. The manualized intervention was developed for the current study based on a problem-solving framework (Nezu, Nezu, Friedman, Faddis, & Houts, 1998; Wade et al., 2009). The workshop was divided into four sessions, lasting between 75 and 90 min (Table II). Following the intervention, the family had approximately three 30-min booster phone call sessions to provide support in implementing the problem-solving model by refining goals and trouble-shooting barriers.
Table II.
FTC Intervention Content and Strategies to Enhance Engagement Based on Literature and Focus Groups
| Session | Title | Format | Content | Strategies to enhance engagement |
|---|---|---|---|---|
| 1 | Introduction | Multi-family | Psychoeducation about SCD and introduction of the problem-solving model | Incorporate multiple families to offer social support and share experiences |
| Empower families with disease knowledge | ||||
| 2 | Applying problem-solving to school challenges | Caregiver | Providing information relevant to school resources, education programming, and education law as applied to children with SCD, and school-related problem-solving and role plays | Provide content on educational accommodations with a focus on specific challenges faced by families |
| Collaborative problem-solving to support caregivers in generating solutions | ||||
| Empower caregivers to advocate for child | ||||
| Child | Teaching school and social functioning related problem-solving using role plays | Include children and developmentally appropriate goals (e.g., improve communication with friends about SCD) | ||
| Support children in advocating for themselves | ||||
| 3 | Review session and solution generation | Multi-family | Multi-family game to review SCD information and the problem-solving model, establish a list of solutions to common challenges faced by families, and role-play practice with the model | Continue to provide disease management education in a multimedia format |
| Build family’s efficacy to solve challenges together | ||||
| Group solution generation | ||||
| 4 | Goal setting | Individual family | Each family paired with one interventionist for individual family sessions to identify goals and apply the model to current challenges | Develop rapport with individual interventionist |
| Set goals for all family members | ||||
| Post-workshop | Booster phone calls (3 × ∼30 min) | Individual family | Session 4 Interventionist followed manualized content to elicit the family’s challenges in meeting goal(s), generate potential solutions, identify barriers to implementation, and agree to a timeline for implementing solutions | Schedule based on family’s preference |
| Provide ongoing support to family to achieve goals (both caregiver and child goals) | ||||
| Reinforce material learned during the workshop and promote application to everyday life |
The interventionists were doctoral and master’s graduate students under live supervision from a licensed psychologist. All study staff received in-depth training in SCD, problem-solving therapy, and cultural considerations in working with African-American families and regular supervision (live and pre and post-intervention). Time 2 assessments were completed 6 months post-baseline (either in home or clinic), after finishing the workshop for FTC families. At all study visits, families were given parking passes or public transportation reimbursement, offered snacks and childcare, and compensated for their time after Time 2 assessments.
Owing to challenges with retention, a peer patient navigator was introduced 18 months into the trial to reduce barriers to participation. This navigator, a young adult with SCD, worked with families during clinic visits and by phone to identify benefits and barriers to study participation (i.e., transportation, childcare, understanding the purpose of the workshop) and offer solutions and support to bolster engagement. A protocol to guide the navigator in addressing barriers to engagement in the study and highlighting potential benefits for their families or other families with SCD was developed based on our prior research identifying such factors for families of children with SCD (Petterson et al., 2014). Based on the protocol, the navigator was introduced at recruitment to review the study information with families and then made contact with families at key points in the study procedures (prior to Baseline assessment and Time 2 assessment, prior to Intervention) to identify and address the perceived barriers and to promote perceived benefits to participation in the study.
Treatment Fidelity
To ensure consistency in intervention delivery, the study used a detailed treatment manual delivered by graduate students in clinical psychology, live supervision during all workshops, and weekly supervision with licensed psychologists (L.P.B., C.A.P., M.R.R.) for interventionists. Treatment protocol adherence was determined through review of audio-taped interventions by trained research assistants. The primary adherence rater checked 50% of intervention sessions for adherence, and a second adherence rater checked 25% of interventions sessions rated by the primary rater. Disagreements were reviewed with the study team and discussed until agreement was reached. The initial level of agreement between raters was acceptable (ICC = .79), total adherence was 95%. The majority of departures were related to practice with the problem-solving model and reflect flexibility with delivering a manualized intervention by tailoring content to the needs of the families present at the workshop.
Data Analysis
The FTC and DIC groups were compared on demographic factors (site, child age/gender, caregiver education, family income, school type), medical factors (genotype, disease complications), and baseline study variables (FSIQ, SPSI total score, HOPE Needs Assessment variables), using chi-square tests or t-tests in SAS 9.3. Study completers (those completing the Intervention and Time 2 assessment) and noncompleters were compared on the same variables and the WJ-III. To better understand the academic needs and services received by participants, baseline WJ-III and school accommodations were compared between caregivers with and without educational concerns using chi-square tests and t-tests. To evaluate the FTC intervention, analysis of covariance (ANCOVA) models were used to compare the FTC and DIC group on primary outcomes at Time 2 post-intervention (number of school accommodations, presence of an IEP or 504 service plan, school absences, school HRQL, or WJ-III academic skills), controlling for baseline values of the primary outcome. A p value of .05 was established for significance.
Results
Description of the Variables
Caregiver baseline data and child baseline academic functioning have been described previously (Smith, Patterson, Szabo, Tarazi, & Barakat, 2013). Description of sample variables is presented in Table I. At baseline, families reported slightly more than one school accommodation and school absences in the 0–7 days range for the FTC group and 8–14 range for the DIC group. Children and caregivers reported moderate school HRQL. Academic skills were in the average range for both groups. Caregivers reported strong problem-solving skills in both groups, with a trend for better problem-solving in the FTC group compared with the DIC group.
Across groups, approximately half of the sample endorsed concerns about their child’s academic functioning at baseline (n = 39; 47% of the sample: 38% FTC, 56% DIC). Academic functioning was significantly lower in the group endorsing school concerns (Concerns WJ Academic Skills M = 89.56, SD = 17.87; No Concerns M = 101.14, SD = 15.65; F(1, 81) = 9.90, p = .002). The children from concerned families were significantly more likely to have formal accommodations than those without concerns (χ2(1) = 4.54, p = .039), but rates of accommodations were low in both groups (33% of concerned families reported accommodations compared to 14 % of nonconcerned families). Concerned families were significantly more likely to have had their child tested for learning problems (χ2(1) = 5.40, p = . 026; 68% of concerned families reported testing vs. 39% of nonconcerned families).
Baseline Group Comparisons for Intervention Outcomes
At baseline, there were no differences between the FTC and DIC group on demographic variables, medical variables, school accommodations, school HRQL, or academic skills; thus, no covariates were included in the primary outcome analysis. The DIC group reported significantly more school absences than the FTC group (t(81) = −2.65, p = .009), and the FTC group reported slightly better social problem-solving (t(81) = 1.80, p = .075) and slightly fewer school concerns (χ2(1) = 2.69, p = .100) than the DIC group. There were more children in suburban schools in the FTC group compared to the DIC group (χ2(1) = 5.92, p = .029). Within the FTC group, there were no differences between completers (n = 28) and noncompleters (n = 14) on primary outcomes and other variables at baseline including expectancy, suggesting that completers well-represented patients randomized to FTC; thus, subsequent analyses used only completers from the FTC group. We repeated analyses using an intent-to-treat approach (assuming no change to outcomes for randomized participants lost to follow-up); there were no differences in results.
Evaluation of Primary Intervention Outcomes
Controlling for Time 1, the FTC and DIC groups did not differ on primary outcomes at Time 2 (Table III). DIC caregivers reported more academic concerns and school absences at Time 2, but these group differences did not reach significance when adjusting for baseline values. (Logistic Model of Academic Concerns could not converge; ANCOVA School Absences: F(1) = 3.56, p = .064). Post hoc power calculation indicated power of 0.61 to detect a medium effect (0.3–0.5).
Table III.
ANCOVA Comparing School Functioning Outcomes by Group Controlling for Time 1 Functioning
| Descriptive statistics [Mean (SD)] |
ANCOVA model |
|||||||
|---|---|---|---|---|---|---|---|---|
| School functioning outcomes | FTC T1 | FTC T2 | DIC T1 | DIC T2 | Overall R2 | Fa | pa | Effect size Cohen’s da |
| School accommodations | 1.22 (1.01) | 1.44 (1.35) | 1.15 (1.24) | 1.39 (1.17) | 0.19 | 0.08 | .775 | 0.04 |
| School absences | 0.73 (0.91) | 0.48 (0.77) | 1.22 (1.08) | 1.10 (1.03) | 0.25 | 3.56 | .064 | 0.66 |
| School HRQL child | 60.00 (24.40) | 60.40 (23.89) | 64.02 (21.62) | 64.60 (16.94) | 0.22 | 0.27 | .604 | 0.21 |
| School HRQL caregiver proxy | 57.14 (21.14) | 60.08 (18.80) | 61.22 (21.50) | 62.36 (22.68) | 0.42 | 0.00 | .966 | 0.11 |
| WJ academic skills | 96.98 (18.19) | 97.32 (19.05) | 94.39 (17.13) | 92.97 (17.42) | 0.80 | 1.61 | .209 | 0.24 |
| n (%) | n (%) | n (%) | n (%) | |||||
| School concernsb | 16 | 2 | 23 | 12 | NA | NA | NA | NA |
| (38.10%) | (9.09%) | (56.10%) | (31.58%) | |||||
aF values, p value, and effect size are for group as a predictor.
bThe logistic model controlling for time 1 functioning did not converge.
Workshop and Booster Call Session Satisfaction and Engagement
Participants rated the workshop as interesting (Caregiver M = 4.71, SD = 0.46; Child M = 4.71, SD = 0.85) and useful (Caregiver M = 4.36, SD = 0.68; Child M = 4.39, SD = 1.17). Most caregivers in the FTC group (93%) stated that the intervention workshop was worth their time. Interventionists rated children and caregivers as highly engaged in the workshop and booster calls (Caregivers: M = 4.28–4.43, Child: M = 3.00–3.86), competent in applying the problem-solving model (Caregivers: M = 3.96-4.31, Child: M = 3.00–4.00), and committed to using problem-solving (Caregivers: M = 3.95–4.34, Child: M = 2.86–3.64). Retention across the booster phone call sessions was strong. Of the 28 completers, 25 (89%) completed the first session, 24 (86%) the second session, and 21 (75%) the third session.
Discussion
This is one of the first published, family-based interventions for children with SCD targeting school functioning. The goal of the intervention was to improve caregiver problem-solving to address disease complications and pursue educational accommodations during the primary grades, and child problem-solving to improve teacher and peer communication. The current study found no significant group differences between the FTC and DIC groups on primary study outcomes at the 6-month follow-up: number of school accommodations, presence of an IEP or 504 service plan, school absences, school HRQL, or academic skills. Although there were trends for fewer school absences and fewer school concerns at Time 2 in the FTC group compared to the DIC group, these differences likely reflect group differences at baseline. We hypothesized that increasing caregiver problem-solving, with a focus on educational challenges faced by children with SCD, would improve school functioning, as prior research utilizing problem-solving therapy in pediatric psychology has yielded promising results (Palermo et al., 2014; Sahler et al., 2005; Seid et al., 2010; Wade et al., 2009; Wysocki et al., 2006). Given the risk for SCD to impact academic performance both directly [e.g., neurological complications (Schatz et al., 2001)] and indirectly [i.e., fatigue, missed school (Noll et al., 2001; Smith et al., 2013)], evidence-based educational interventions (Rathvon, 2008) and increased caregiver involvement in school (Jeynes, 2003) are needed in pediatric SCD. Strengths of the trial included novel intervention delivery methods (telemedicine), community participatory strategies, and the use of a peer patient navigator. Combining these strategies in a more comprehensive, systemic intervention integrating medical home, school, and family may result in improved school functioning.
We provide a deconstruction of our methodological approach as a roadmap for future trial design. The challenges of this trial included limited retention resulting in low-moderate power, threats to internal validity despite randomization (high problem-solving skills, fewer school concerns, and fewer school absences for the FTC group at baseline), and external factors including changes in Comprehensive Sickle Cell Center structure and funding during the data collection period. The 6-month follow-up timeframe was likely too short to see substantive changes in school outcomes, and the time of year of study participation may have affected school concerns and the family’s ability to act on specific recommendations (e.g., meet with teachers when school is not in session). Although parent problem-solving was targeted with this intervention, a direct measure of parental skill and/or competence in the school context (e.g., advocacy, engagement) would have facilitated a better understanding of intervention effects. Post-randomization attrition (i.e., families not completing the intervention after baseline and randomization) in the intervention group was also a significant problem, as noted in prior studies in SCD (Barakat et al., 2010; Kaslow et al., 1997). Such attrition may reflect family differences that impacted engagement. Ladd and colleagues have described family cohesion and achievement-orientation as protective against grade retention in children with SCD (Ladd et al., 2014). It is possible that families who attended the workshop were more achievement-oriented owing to their interest in a program designed to improve school performance, and in combination with better functioning at baseline, our ability to identify improvement in this group with limited power was hampered.
Aspects of the study design that were responsive to input from community stakeholders may have resulted in unforeseen challenges. Focus groups with families of adolescents with SCD provided important feedback on study materials and recruitment strategies. Informally, we met with Parent Advisory Groups at our SCD Center and through the local Sickle Cell Disease Association of America to increase stakeholder buy-in. In addition to changes to the study materials, study recommendations were to create a single-session workshop and to include all caregivers, regardless of presentation/perception of school difficulties. This strategy, however, may have resulted in some families receiving no intervention owing to the one-time time commitment rather than a partial dose. We also attempted to reduce the burden of multiple in-person sessions at the hospital by delivering intervention material by phone. Phone sessions demonstrated strong retention and engagement and such telehealth strategies may be a more effective means for future studies for intervention delivery with hard-to-engage samples. Including all comers may have resulted in more attrition, as we observed lower completion rates (though nonsignificant) in families with no school concerns (81% of families with concerns vs. 57% with no concerns). However, despite only half of the sample indicating school concerns at baseline, school HRQL was very low across the board and parents appeared to have significant difficulty getting their children academic accommodations. Only a third of patients had formal accommodations, despite 68% of children having undergone psychoeducational or neuropsychological evaluation. More concerning is that of 11 children in the study with a history of stroke, only two had a 504 Plan or IEP. Although targeting those with educational needs is a first step toward efficacious intervention development, as our focus groups suggested, it is important to offer interventions broadly because many caregivers do not possess the skills to secure accommodations and once the child is struggling, it may be too late to begin this complicated process.
A peer patient navigator (young adult with SCD) was introduced two-thirds of the way through the trial to reduce barriers to participation encountered in the initial enrollment phase. Navigators have been effective in improving screening for cancer among low-income and ethnic minority adults (Dohan & Schrag, 2005), but to our knowledge, navigators had not been applied in pediatric studies prior to this trial. After implementation of navigator support, 52% of families attended the intervention compared with 42% of families who had not worked with the navigator, and retention was improved for follow-up assessments. The navigator worked with families to identify perceived benefits and barriers of participation in the study according to a study protocol (Patterson et al., 2015). The most commonly identified benefits were meeting other families with SCD and learning more about the disease as benefits of participation. The most commonly addressed barriers were childcare and transportation to the intervention. Although we cannot fully test effectiveness and cost-effectiveness should be evaluated, use of navigators may be helpful in reducing post-randomization attrition.
The challenges of the current study are instructional to future intervention trials in pediatric SCD. To facilitate enrollment, acceptability, and retention, we utilized a parent focus group, a peer patient navigator, and telephone follow-up sessions. While these strategies resulted in intervention material acceptable to children and caregivers and potentially assisted with long-term retention, they were not enough to engage families from study initiation. Novel intervention delivery methods (i.e., telehealth, during clinic appointments, partnering with schools) reduced perceived burden and also allowed for longer-term work with an interventionist. In addition, future studies should utilize feedback based on screening measures of academic and psychosocial functioning to educate families about the need for intervention at baseline and thus enhance engagement and retention, perhaps with a motivational interviewing approach to increase the uptake of the intervention. Longer follow-up periods, measuring concrete family behaviors, are likely also necessary to allow time for families to implement the interventions and see changes in HRQL. Although we continue to see value in offering interventions to all children with SCD, targeting those with demonstrated educational needs and/or more severe disease risk may result in greater uptake of the intervention and increase the likelihood of seeing change with intervention.
Clinically, the difficulty navigating families through a preventative intervention suggests that they may need more systemic support to advocate for their children in the school setting. Universal neurocognitive screening for all patients with SCD, or at the very least for children with a history of stroke and/or abnormal imaging studies, is a first step toward ensuring that children who need more support are identified early. Such screening should be part of medical follow-up rather than an optional component to use only when children are having difficulties. Interventions proceeding from such screenings should be multidisciplinary: integrating the school, medical home (including hospital based teachers), and the family to ensure that educational accommodations are established, rather than leaving the burden on caregivers who may not possess the necessary skills to navigate complicated school systems alone.
Despite the pitfalls encountered in this trial, children with SCD are in great need of psychosocial interventions owing to the myriad of risk factors that threaten HRQL. Families were enthusiastic about the study throughout, but more is needed than enthusiasm to see clinically meaningful change. Enhancing engagement through stakeholder support and peer patient navigation are a strong starting point for future trials, and together with creative strategies to bring interventions to new settings, future research may find greater success.
Funding
NHLBI (U54 HL070585) to M.S. (PI), BTRP to LPB (PI); and NCMHD (1RC1MD004418) to L.P.B. (PI).
Conflicts of interest: None declared.
References
- Asnani M. R., Quimby K. R., Bennett N. R., Francis D. K. (2014). Interventions for patients and caregivers to improve knowledge of sickle cell disease and recognition of its related complications (Protocol). Cochrane Database of Systematic Reviews, 6. Art. No.: CD011175. doi: 10.1002/14651858.CD011175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balfanz R., Herzog L., Mac Iver D. J. (2007). Preventing student disengagement and keeping students on the graduation path in urban middle-grades schools: Early identification and effective interventions. Educational Psychologist , 42, 223–235. [Google Scholar]
- Barakat L. P., Daniel L. C., Smith K., Robinson M. R., Patterson C. A. (2014). Parental problem-solving abilities and the association of sickle cell disease complications with health-related quality of life for school-age children. Journal of Clinical Psychology in Medical Settings , 21, 56–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barakat L. P., Lash L. A., Lutz M. J., Nicolaou D. C. (2006). Psychosocial adaptation of children and adolescents with sickle cell disease. In Brown R. T. (Ed.), Comprehensive handbook of childhood cancer and sickle cell disease: A biopsychosocial approach (pp. 471–495). New York, NY: Oxford University Press. [Google Scholar]
- Barakat L. P., Lutz M., Nicolaou D., Lash L. (2005). Parental locus of control and family functioning in the quality of life of children with sickle cell disease. Journal of Clinical Psychology in Medical Settings , 12, 323–331. doi:10.1007/s10880-005-7818-9 [Google Scholar]
- Barakat L. P., Patterson C. A., Weinberger B. S., Simon K., Gonzalez E. R., Dampier C. (2007). A prospective study of the role of coping and family functioning in health outcomes for adolescents with sickle cell disease. Journal of Pediatric Hematology/Oncology , 29, 752–760. doi:710.1097/MPH.1090b1013e318157fdac [DOI] [PubMed] [Google Scholar]
- Barakat L. P., Schwartz L. A., Salamon K. S., Radcliffe J. (2010). A family-based randomized controlled trial of pain intervention for adolescents with sickle cell disease. Journal of Pediatric Hematology/Oncology , 32, 540–547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burlew K., Telfair J., Colangelo L., Wright E. C. (2000). Factors that influence adolescent adaptation to sickle cell disease. Journal of Pediatric Psychology , 25, 287–299. [DOI] [PubMed] [Google Scholar]
- Casey R., Brown R. T., Bakeman R. (2000). Predicting adjustment in children and adolescents with sickle cell disease: A test of the risk-resistance-adaptation model. Rehabilitation Psychology , 45, 155–178. [Google Scholar]
- D'Zurilla T. J., Nezu A. M. (1990). Development and preliminary evaluation of the Social Problem-Solving Inventory. Psychological Assessment: A Journal of Consulting and Clinical Psychology , 2, 156. [Google Scholar]
- D’Zurilla T. J., Nezu A. (Eds.) (1982). Social problem solving in adults (Vol. 1). New York, NY: Springer. [Google Scholar]
- Daly B., Kral M. C., Tarazi R. A. (2011). The role of neuropsychological evaluation in pediatric sickle cell disease. The Clinical Neuropsychologist , 25, 903–925. [DOI] [PubMed] [Google Scholar]
- Dampier C., Lieff S., LeBeau P., Rhee S., McMurray M., Rogers Z., Smith-Whitley K., Wang W.; Comprehensive Sickle Cell Centers (CSCC) Clinical Trial Consortium (CTC) (2010). Health-related quality of life in children with sickle cell disease: A report from the Comprehensive Sickle Cell Centers Clinical Trial Consortium. Pediatric Blood and Cancer , 55, 485–494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Day S., Chismark E. (2006). The cognitive and academic impact of sickle cell disease. The Journal of School Nursing , 22, 330–335. [DOI] [PubMed] [Google Scholar]
- Dinges D. F., Whitehouse W. G., Orne E. C., Bauer N. K., Bloom P. B., Carlin M. C., Bauer N. K., Gillen K. A., Shapiro B. S., Ohene-Frempong K., Dampier C., Orne M. T. (1997). Self-hypnosis training as an adjunctive treatment in the management of pain associated with sickle cell disease. International Journal of Clinical and Experimental Hypnosis , 45, 417–432. [DOI] [PubMed] [Google Scholar]
- Dohan D., Schrag D. (2005). Using navigators to improve care of underserved patients: Current practices and approaches. Cancer , 104, 848–855. [DOI] [PubMed] [Google Scholar]
- Gil K. M., Anthony K. K., Carson J. W., Redding-Lallinger R., Daeschner C. W., Ware R. E. (2001). Daily coping practice predicts treatment effects in children with sickle cell disease. Journal of Pediatric Psychology , 26, 163–173. [DOI] [PubMed] [Google Scholar]
- Grenwelge C. H. (2009). Test Review: Woodcock, RW, Schrank, FA, Mather, N., & McGrew, KS 2007). Woodcock-Johnson III Tests of Achievement, Form C/Brief Battery. Journal of Psychoeducational Assessment, 27, 345–350. [Google Scholar]
- Hassell K. L. (2010). Population estimates of sickle cell disease in the US. American Journal of Preventive Medicine , 38, S512–S521. [DOI] [PubMed] [Google Scholar]
- Jeynes W. H. (2003). A meta-analysis the effects of parental involvement on minority children’s academic achievement. Education and Urban Society , 35, 202–218. [Google Scholar]
- Karlson C. W., Rapoff M. A. (2009). Attrition in randomized controlled trials for pediatric chronic conditions. Journal of Pediatric Psychology , 34, 782–793. [DOI] [PubMed] [Google Scholar]
- Kaslow N. J., Collins M. H., Loundy M. R., Brown F., Hollins L. D., Eckman J. (1997). Empiricaly validated family intervetions for pediatric psychology: Sickle cell disease as an exemplar. Journal of Pediatric Psychology , 22, 213–227. [DOI] [PubMed] [Google Scholar]
- Kaslow N. J., Collins M. H., Rashid F. L., Baskin M. L., Griffith J. R., Hollins L., Eckman J. E. (2000). The efficacy of a pilot family psychoeducational intervention for pediatric sickle cell disease (SCD). Families, Systems, & Health, 18, 381–404. [Google Scholar]
- Kazak A. E., Alderfer M. A., Streisand R., Simms S., Rourke M. T., Barakat L. P., Gallagher P., Cnaan A. (2004). Treatment of posttraumatic stress symptoms in adolescent survivors of childhood cancer and their families: A randomized clinical trial. Journal of Family Psychology , 18, 493–504. [DOI] [PubMed] [Google Scholar]
- Kral M. C., Brown R. T., Hynd G. W. (2001). Neuropsychological aspects of pediatric sickle cell disease. Neuropsychology Review , 11, 179–196. [DOI] [PubMed] [Google Scholar]
- Ladd R. J., Valrie C. R., Walcott C. M. (2014). Risk and resilience factors for grade retention in youth with sickle cell disease. Pediatric Blood and Cancer , 61, 1252–1256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nezu A. M., Nezu C. M., Friedman S. H., Faddis S., Houts P. S. (1998). Helping cancer patients cope: A problem-solving approach. Washington, DC: American Psychological Association. [Google Scholar]
- Noll R. B., Stith L., Gartstein M. A., Ris M. D., Grueneich R., Vannatta K., Kalinyak K. (2001). Neuropsychological functioning of youths with sickle cell disease: Comparison with non-chronically ill peers. Journal of Pediatric Psychology , 26, 69–78. [DOI] [PubMed] [Google Scholar]
- Palermo T. M., Law E. F., Essner B., Jessen-Fiddick T., Eccleston C. (2014). Adaptation of problem-solving skills training (PSST) for parent caregivers of youth with chronic pain. Clinical Practice in Pediatric Psychology , 2, 212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panepinto J. A., Pajewski N. M., Foerster L. M., Hoffman R. G. (2008). The performance of the PedsQL generic core scales in children with sickle cell disease. Journal of Pediatric Hematology/Oncology , 30, 666–673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patterson C. A., Chavez V., Mondestin V., Deatrick J., Li Y., Barakat L. P. (2015). Clinical trial decision-making in pediatric sickle cell disease: A qualitative study of perceived benefits and barriers to participation. Journal of Pediatric Hematology/Oncology, 37, 412–422. Advance Access publication. doi:10.1097/MPH.0000000000000216 [DOI] [PubMed] [Google Scholar]
- Peterson C. C., Palermo T. M., Swift E., Beebe A., Drotar D. (2005). Assessment of psycho-educational needs in a clinical sample of children with sickle cell disease. Children's Health Care , 34, 133–148. [Google Scholar]
- Rathvon N. (2008). Effective school interventions: Evidence-based strategies for improving student outcomes. New York, NY: Guilford Press. [Google Scholar]
- Sahler O. J. Z., Fairclough D. L., Phipps S., Mulhern R. K., Dolgin M. J., Noll R. B., Katz E. R., Varni J. W., Copeland D. R., Butler R. W. (2005). Using problem-solving skills training to reduce negative affectivity in mothers of children with newly diagnosed cancer: Report of a multisite randomized trial. Journal of Consulting and Clinical Psychology , 73, 272. [DOI] [PubMed] [Google Scholar]
- Schatz J. (2004). Brief Report: Academic attainment in children with sickle cell disease. Journal of Pediatric Psychology , 29, 627–633. [DOI] [PubMed] [Google Scholar]
- Schatz J., Brown R., Pascual J., Hsu L., DeBaun M. (2001). Poor school and cognitive functioning with silent cerebral infarcts and sickle cell disease. Neurology , 56, 1109–1111. [DOI] [PubMed] [Google Scholar]
- Seid M., Varni J. W., Gidwani P., Gelhard L. R., Slymen D. J. (2010). Problem-solving skills training for vulnerable families of children with persistent asthma: Report of a randomized trial on health-related quality of life outcomes. Journal of Pediatric Psychology , 35, 1133–1143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shapiro B. S., Dinges D. F., Orne E. C., Bauer N., Reilly L. B., Whitehouse W. G., Ohene-Frempong K., Orne M. T. (1995). Home management of sickle cell-related pain in children and adolescents: Natural history and impact on school attendance. Pain , 61, 139–144. [DOI] [PubMed] [Google Scholar]
- Smith K. E., Patterson C. A., Szabo M. M., Tarazi R. A., Barakat L. P. (2013). Predictors of academic achievement for school-age children with sickle cell disease. Advances in School Mental Health Promotion , 6, 5–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson R. J., Gil K. M., Burbach D. J., Keith B. R., Kinney T. R. (1993). Psychological adjustment of mothers of children and adolescents with sickle cell disease: The role of stress, coping methods, and family functioning. Journal of Pediatric Psychology , 18, 549–559. [DOI] [PubMed] [Google Scholar]
- Van Der Land V., Hijmans C. T., de Ruiter M. A., Mutsaerts H. J., Cnossen M. H., Majoie C. B., Nederveen A. J., Grootenhuis M. A., Fijnvandraat K. (2014). Volume of white matter hyperintensities predicts neurocognitive functioning in children with sickle cell disease. Blood, 124, 2720–2720. [Google Scholar]
- Varni J. W., Seid M., Rode C. A. (1999). The PedsQL™: Measurement model for the pediatric quality of life inventory. Medical Care , 37, 126–139. [DOI] [PubMed] [Google Scholar]
- Wade S. L., Walz N. C., Carey J. C., Williams K. M. (2009). Brief report: Description of feasibility and satisfaction findings from an innovative online family problem-solving intervention for adolescents following traumatic brain injury. Journal of Pediatric Psychology , 34, 517–522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weschler D. (1999). Weschler abbreviated scale of intelligence (WASI). London: Psychological Corporation. [Google Scholar]
- Wright P., Wright P. (2008). Key differences between section 504, the ADA, and the IDEA. Wright's Law, Retrieved from http://www.wrightslaw.com/info/sec504.summ.rights.htm [Google Scholar]
- Wysocki T., Harris M. A., Buckloh L. M., Mertlich D., Lochrie A. S., Taylor A., Sadler M., Mauras N., White N. H. (2006). Effects of behavioral family systems therapy for diabetes on adolescents’ family relationships, treatment adherence, and metabolic control. Journal of Pediatric Psychology , 31, 928–938. [DOI] [PubMed] [Google Scholar]