Abstract
Objective We examined prospective connections among parental depressive symptoms, family dysfunction, and eosinophil activity in children with asthma. Methods 81 children with asthma and their parents completed two laboratory visits across a 1-year period. At baseline and 1 year later, parents reported about their depressive symptoms and family dysfunction. We collected peripheral blood in children to measure eosinophil counts and eosinophil cationic protein. Following visits, children recorded their asthma symptoms for 2 weeks. Results After controlling for demographic and biomedical covariates, a significant T1 × T2 Family Dysfunction interaction emerged, suggesting that the links between family dysfunction at T1 and eosinophil counts and activity at T2 depended on family functioning at T2. Parental depressive symptoms were unrelated to eosinophil activity and asthma symptoms. Conclusions These findings suggest that improvements in family functioning are associated with decreases in eosinophil activity, which may contribute to inflammatory processes that affect airway function.
Keywords: asthma; eosinophils, eosinophil cationic protein; family dysfunction; inflammation; parental depression
Asthma, a chronic respiratory disorder that results from inflammation and obstruction of the airways, affects over 7 million children in the United States (Bloom, Cohen, & Freeman, 2012). Despite improvements in treatment for managing the symptoms of asthma, almost three-quarters of a million emergency room visits for children in the United States each year are owing to asthma complications (Centers for Disease Control and Prevention, 2009). Further, asthma is one of the leading causes of pediatric hospitalization and school absenteeism. Asthma is a complex multifactorial disease, characterized by reversible airway hyperactivity and obstruction that develops in response to allergens, pollutants, irritants, and other stimuli (Busse & Lemanske, 2001). The immune system plays a key role in many cases of asthma, launching exaggerated responses to stimuli that cause mucus production, airway constriction, and difficulties in breathing.
In recent years, researchers have documented associations between psychosocial factors and the expression of asthma symptoms (Marshall, 2004; Wright, 2008). Notably, some of the most reliable psychosocial predictors of asthma expression are family-related stressors (Chen & Schreier, 2008; Kaugars, Klinnert, & Bender, 2004). For example, Shalowitz, Berry, Quinn, and Wolf (2001) found that parents of children with high asthma morbidity had a greater number of life stressors and were more likely to be depressed compared with parents of children who were low in asthma morbidity. These findings suggest a role for family-related stressors in the expression and exacerbation of asthma symptoms.
More recent studies have begun to shed light on the immunologic mechanisms by which these effects could occur (see Chen & Miller, 2007, for a review). Following exposure to allergens and irritants, individuals with asthma experience a cascade of immune responses, ultimately leading to inflammation, constriction, and obstruction of the airways. Upon sensing a foreign object (e.g., pollen, mold spores, pet dander, viruses), immune cells release chemical messengers known as cytokines. Of particular relevance in asthma are cytokines released by T helper cells, such as interleukin (IL)-4, IL-5, and IL-13. These messengers recruit other cells to the site of the foreign object (e.g., airways) and activate those cells to eliminate and neutralize the object. In patients with asthma, this response is often exaggerated and prolonged, resulting in the excessive production of mucus and tightening of the airways, which along with other processes result in classic disease symptoms, like coughing, wheezing, and shortness of breath.
Evidence suggests that psychosocial stressors engender changes in children’s immune systems that play a role in triggering exaggerated responses to asthma stimuli. For example, Kang and colleagues (1997) found that college students with asthma showed heightened in vitro stimulated leukocyte production of IL-5 during exam periods. Chronic family stress has been associated with higher in vitro stimulated leukocyte IL-5 and IL-13 production in children with asthma (Chen et al., 2006). Further, Schreier and Chen (2010) identified that a lack of family routines predicted greater in vitro stimulated leukocyte IL-13 production in children across a 1.5-year period. Finally, in a study of 2-year-old children, parental stress was prospectively associated with greater in vitro production of the inflammatory cytokine tumor necrosis factor-α, as well as higher levels of allergic inflammatory activity (Wright et al., 2004). These findings suggest that stressors, particularly those occurring within the family, may accentuate cytokine responses to asthma triggers.
Eosinophils are one of the cell types that cytokines recruit to the airways following exposure to asthma stimuli. Preliminary evidence suggests that psychosocial stressors can accentuate this process. One study exposed college students with mild asthma to an inhaled antigen, and measured eosinophils in sputum afterward (Liu et al., 2002). The antigen-induced increase in sputum eosinophils was more pronounced during a high-stress (i.e., final exams) versus low-stress period (with no exams). Eosinophils promote airway inflammation and protect against pathogens by releasing cytotoxic substances, such as eosinophil cationic protein (ECP). ECP, in turn, can irritate and damage the epithelial cells that line the airways, and by doing so amplify and prolong airway inflammation (Tomassini et al., 1996; Venge, 2004). Some initial evidence suggests that family-related stressors relate to ECP levels in asthma patients. For example, Wolf, Miller, and Chen (2008) found that parental depression was associated with an increase in children’s ECP over 6 months. Similarly, Miller, Gaudin, Zysk, and Chen (2009) found that, to the extent that children with asthma felt unsupported by parents, they had greater ECP.
This evidence suggests that stressors within the family environment—at least at a single time point—are related to higher eosinophil activity. Families can experience dramatic variations over time in functioning, however (Cox & Paley, 1997; Gottman, 1991), and it remains unclear how changing conditions affect eosinophil activity or counts. Further, previous studies have focused mostly on specific aspects of individual family members’ functioning (e.g., parental depression, chronic stress) that presumably spill over into the broader family emotional climate. But families function as systems in ways that are more complex than the functioning of each family member in isolation (Cox & Paley, 1997). For example, the impact of parental depression on children’s health may be offset within a family that maintains open communication and works together to create a safe, stable home. In contrast, some families are hostile, unsupportive, and stressful—irrespective of the mental health of individual family members—and this aspect of the family climate may not be captured in individual-level assessments.
To date, however, most research on family-related stressors and children’s asthma-related immune responses has focused on one type of risk factor within the family context (e.g., depressive symptoms). In the present study, we took a family systems approach to explore how changes in parents’ depressive symptoms and general family dysfunction may be associated with changes in children’s eosinophil activity and asthma symptoms over a 1-year period, when also accounting for demographic characteristics, asthma severity controls, and medication usage. We hypothesized that family dysfunction and parental depressive symptoms at Time 1 would predict eosinophil activity and symptom severity at Time 2. We further hypothesized that a significant T1 × T2 Family Dysfunction interaction would emerge, such that the links among T1 family dysfunction and T2 eosinophil activity and asthma symptoms would be shaped by T2 family functioning. In other words, as family functioning improved over the year, we expected T1 family functioning to be unrelated to or negatively associated with eosinophil activity and symptom reports. In contrast, if family dysfunction was evident at T2, we expected family dysfunction at T1 to be positively associated with eosinophil activity and asthma symptoms. We hypothesized that the same pattern would emerge with reports of parental depressive symptoms across the year, in that links between T1 parental depression and T2 eosinophil activity and asthma symptoms would depend on T2 measures of parental depression.
Finally, we conducted a series of exploratory hypotheses to consider effects of seasonality and an alternative hypothesis about the directionality of the proposed links among family dysfunction, eosinophil activity, and asthma symptoms. Parenting a child with a chronic disease can be stressful, and it may be that when children experience exacerbations in asthma symptoms, their parents struggle to maintain a stable family climate. We examined whether asthma-related factors shape family dysfunction and parental depressive symptoms over time.
Method
Participants
Participants included 81 English-speaking children (57 boys and 24 girls) and their parents from Vancouver, British Columbia who took part in a larger longitudinal study of 121 children with asthma (Chen et al., 2006; Miller et al., 2009; Schreier & Chen, 2010) and who had complete data on the measures in the study. Families were recruited through advertisements at schools, physician offices, and local newspapers. Interested families were prescreened and were determined to be eligible for the study if they had a child between 9 and 18 years old (Mage = 12.6, SD = 2.63) who had been diagnosed with asthma by a physician and who did not have a history of major psychiatric illness or other chronic medical illnesses. Fewer than 5% of eligible families declined to enroll in the study. Laboratory visits were conducted when children were medically stable, had not experienced any upper-respiratory illnesses in the prior 4 weeks, and had not taken any oral steroids in the prior 2 weeks. Children were mainly of European (62.8%) or Asian (26.4%) descent, lived with their parent(s), and largely came from married families (74.3%). All procedures were approved by the university’s institutional review board. Parents provided informed consent and children provided informed assent before each laboratory visit.
As part of the larger study, families visited the research center every 6 months over 2 years, for a total of five visits. In this report, we focus on predictors and outcomes assessed at the first and third study visits, separated by 1 year, because parents completed measures of family functioning only at these visits. For the sake of simplicity, we refer to the visits as Time 1 (T1, baseline) and Time 2 (T2, one year later). Of the 121 families who participated at Time 1 (T1), 101 families participated at Time 2 (T2). The sample size was reduced from 101 to 81 due to technical problems with blood collection and eosinophil measurement in a subset of youth. The present sample did not differ from the larger sample at T1 on children’s age, socioeconomic status (SES), race, parental depression, ECP, eosinophil counts, symptom reports, medication usage, or asthma severity (all p values > .14). Families who were included in the analyses were more likely to have a male child than a female child, compared with families not included in the analyses (who were equally male and female), χ2 (1, N = 121) = 7.75, p = .005. In addition, families in the analyses (M = 1.75, SD = .36) reported marginally fewer problems with family functioning at T1 than families who were not included in the analyses (M = 1.86, SD = .33), t(118) = 1.72, p = .09.
Measures
Family Functioning
At T1 and T2, parents completed the General Functioning Scale of the Family Assessment Device (FAD; Epstein, Baldwin, & Bishop, 1983; Miller, Ryan, Keitner, Bishop, & Epstein, 2000). This subscale includes 12 items that assess overall family functioning (αs = .82 at T1, .86 at T2), including overall family support (e.g., “In times of crisis we can turn to each other for support”), expression of emotion (e.g., “We can express feelings to each other”), and family decision-making (e.g., “We are able to make decisions about how to solve problems”). Parents completed the items using a 4-point Likert-type scale, with responses ranging from 1 (strongly agree) to 4 (strongly disagree). Higher scores indicate more dysfunction in the family. The FAD has demonstrated internal consistency and test–retest reliability (Byles, Byrne, Boyle, & Offord, 1988; Epstein et al., 1983). In the present study, family dysfunction scores were similar to averages from a nonclinical sample (Epstein et al., 1983) and were correlated across the 1-year period (r = .66, p < .001).
Parental Depressive Symptoms
At T1 and T2, parents completed the widely used Center for Epidemiological Studies Depression (CES-D) scale (Radloff, 1977). This 20-item measure assesses the frequency of depressive symptoms over the past 1 week, with scores ranging from 0 (none of the time or rarely; less than 1 day) to 3 (most or all of the time; 5–7 days). Scores are summed, such that higher scores indicate greater depressive symptoms (αs = .89 at T1, .90 at T2). Scores of 16 or greater indicate clinically significant levels of depressive symptoms, which characterized 18.5% of the sample at T1 and 16.0% of the sample at T2.
Eosinophil Counts and Activity
At each visit, we collected peripheral blood to measure eosinophil counts and activity. Eosinophil counts reflect the number of cells in circulation, which correlates with the number of eosinophils in the airways (Niimi et al., 1998; Venge, 2004). ECP, in contrast, reflects the activity level of the activated eosinophils in circulation. Greater eosinophil counts and ECP are indicative of a heightened inflammatory state (Venge, 2004). Counts were obtained from whole blood samples analyzed as part of an automated five-part differential on a Bayer Advia 70 hematology system (Diamond Diagnostics, Holiston, MA). For ECP, venous blood was collected into Serum Separator Tubes and, per manufacturer instructions, incubated for 90 min at room temperature, during which time-activated eosinophils released ECP. This procedure captures ECP already circulating in peripheral blood in addition to ECP released during the incubation period. Serum was frozen until the end of the study, at which point ECP concentrations were measured in batch using the ImmunoCAP system (ImmunoCAP®; Phadia AB, Uppsala, Sweden) with reagents from Somagen (Edmonton, Alberta, Canada).
Asthma Symptom Diary Reports
Following each laboratory visit, participants were asked to record their symptom severity for 2 weeks using a validated asthma symptom diary procedure (CAMP Research Group, 1999). After waking and before bedtime, children rated how bad four asthma symptoms were on scale of 0 (none) to 4 (really bad), resulting in 112 ratings over the 2-week period. Symptoms included coughing from asthma, wheezing, chest tightness/chest pain, and shortness of breath. Symptom reports were averaged across the 2-week period to yield a summary score of 2-week asthma symptom severity (αs = .98 at T1 and T2). Participants used automated time stampers that printed the current date and time to ensure that entries were being made at the appropriate time, and they received $25 after returning their completed diaries to the laboratory. Participants completed the majority of the items across the 2-week period (95% completion rate T1 and 83% completion rate at T2).
Family SES
Parents reported on their own level of education and (if applicable) their spouse’s level of education. We created an index of family SES that was based on the highest level of educational attainment between the two parents. Most parents (85.2%) reported at least some college education.
Asthma Severity and Treatment
We considered the possibility that asthma severity or treatment could act as confounds, inflating any association of family dysfunction with eosinophil activity. Asthma severity was calculated at T1 by the third author using an algorithm from the National Asthma Education and Prevention Program/Expert Panel Report 2 Guidelines (Bacharier et al., 2004). This algorithm uses reports of symptom frequency and medication use to categorize people into one of one of four categories, including: mild intermittent asthma (n = 13), mild persistent asthma (n = 31), moderate persistent asthma (n = 22), and severe persistent asthma (n = 15). Participants brought all asthma medications to the laboratory, and parents were asked to report how many days in the past 2 weeks their children had taken each medication (responses could range from 0 to 14 days). For analysis, medications were grouped into the two most common forms of asthma treatment, including inhaled corticosteroids and beta-agonists.
Data Analytic Approach
We tested the role of family dysfunction and parental depressive symptoms at T1 in predicting eosinophil counts, ECP, and asthma symptoms. All models controlled for T1 eosinophil values or symptom severity. We also included T1 × T2 Family Dysfunction and T1 × T2 Parental Depressive Symptoms interaction terms to examine how the associations between T1 functioning and T2 outcomes change in response to subsequent assessments of the family emotional climate. In this way, our models examine whether changes in family functioning are associated with changes in eosinophils, ECP, and asthma symptoms during the same period. All models adjusted for demographic controls, including SES, age, gender, and race, as well as asthma-related controls, including asthma severity (measured at T1), inhaled corticosteroid medication use (measured at T1 and T2), and beta-agonist use (measured at T2). Significant interactions were probed at ± 1 SD from the mean, following standard practices outlined by Aiken and West (1991).
Results
Descriptive Statistics
Descriptive statistics and Pearson correlations among the variables in the present study are provided in Table I. No gender differences emerged in reports of family income, family dysfunction, asthma severity, inflammatory markers, medication use, or diary reports of symptoms. White families were higher in educational attainment compared with minority families, t(79) = 2.29, p = .025. At baseline, minority children had greater eosinophil counts compared with White children, t(72) = 2.21, p = .03. No other race differences emerged, however.
Table I.
Descriptive Statistics and Intercorrelations Among Principal Variables
| Variable | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | 16 | 17 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1. Age | – | −.06 | −.05 | .26* | .04 | −.13 | −.02 | −.12 | .04 | .08 | .09 | −.03 | .00 | .23* | .09 | −.15 | −.15 |
| 2. Gender | – | −.01 | −.06 | .11 | .02 | −.11 | .13 | −.01 | −.04 | −.03 | −.06 | −.02 | −.16 | −.06 | .19† | .04 | |
| 3. Race | – | −.24* | .07 | .08 | .06 | .15 | .01 | .16 | .11 | .14 | .15 | −.05 | −.06 | −.13 | −.04 | ||
| 4. SES | – | .00 | −.22* | −.16 | −.35** | −.18 | −.04 | −.03 | −.12 | −.19† | −.13 | .16 | −.04 | −.07 | |||
| 5. T1 Asthma Severity | – | .03 | −.07 | .23* | −.16 | .30** | .12 | −.01 | .08 | −.12 | .10 | .07 | .07 | ||||
| 6. T1 Parental Depression | – | .39*** | −.08 | .07 | .11 | .49*** | .40*** | −.13 | .01 | .08 | .20† | .23* | |||||
| 7. T1 Family Functioning | – | −.05 | .12 | .09 | .19† | .66*** | −.17 | .19† | −.04 | −.21† | −.06 | ||||||
| 8. T1 Eosinophils | – | .34** | .02 | .05 | −.11 | .58*** | .32** | −.16 | .13 | .10 | |||||||
| 9. T1 ECP | – | −.10 | .04 | .03 | .43*** | .42*** | −.11 | −.10 | .04 | ||||||||
| 10. T1 Symptoms | – | .11 | .08 | −.05 | −.01 | .48*** | −.12 | −.03 | |||||||||
| 11. T2 Parental Depression | – | .30** | −.05 | .05 | .04 | .20† | .24* | ||||||||||
| 12. T2 Family Functioning | – | .18 | .18 | −.05 | −.19† | −.02 | |||||||||||
| 13. T2 Eosinophils | – | .59*** | −.09 | .05 | .08 | ||||||||||||
| 14. T2 ECP | – | −.12 | −.05 | .08 | |||||||||||||
| 15. T2 Symptoms | – | −.04 | .02 | ||||||||||||||
| 16. T2 Corticosteroid Use | – | .84*** | |||||||||||||||
| 17. T2 Beta Agonist Use | − | ||||||||||||||||
| Mean | 12.6 | .67 | .37 | 2.54 | 2.48 | 9.19 | 1.75 | .37 | 17.8 | .34 | 9.32 | 1.78 | .35 | 24.6 | .26 | 4.12 | 4.36 |
| SD | 2.6 | .47 | .49 | 1.01 | .98 | 7.74 | .36 | .28 | 18.2 | .46 | 8.24 | .41 | .28 | 21.0 | .37 | 5.90 | 5.74 |
Note. Gender coded as 0 = female, 1 = male. Race coded as 0 = White, 1 = minority.
†p < .10. *p < .05. **p < .01. ***p < .001.
Principal Analyses
Family Functioning and Eosinophil Counts
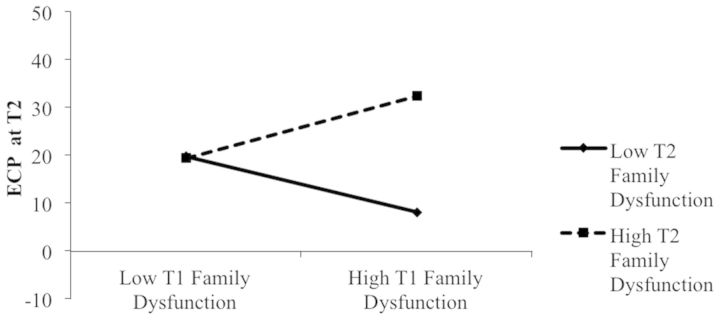
Although no main effect of T1 family dysfunction or parental depressive symptoms emerged in the prediction of T2 eosinophil counts, there was a significant T1 × T2 Family Dysfunction interaction. Post hoc probing of this interaction at 1 SD below the mean of T2 family functioning revealed a significant negative association between T1 family dysfunction problems and T2 eosinophil counts (simple slope = .60, t = 2.61, p = .01). When parents reported good family functioning at T2, family dysfunction at T1 was negatively associated with children’s eosinophil counts at T2 (see Figure 1). For children whose parents reported values 1 SD above the mean on T2 family dysfunction, no significant link emerged between T1 family dysfunction and T2 eosinophil counts.
Figure 1.
Prediction of eosinophil counts at T2 as a function of T1 and T2 family functioning. Regression analysis controls for T1 eosinophil counts, demographic controls, asthma severity, and medication usage.
Unexpectedly, as shown in Figure 1, children with consistently low levels of family dysfunction had the greatest eosinophil counts at T2. We examined this finding in more detail by comparing the asthma severity ratings of children from the five highest and five lowest family dysfunction families. Notably, children from families with the lowest levels of family dysfunction had marginally greater asthma severity ratings (M = 3.4, SD = .89) than children from families with the most family dysfunction (M = 2.4, SD = .55), t(8) = 2.13, p = .066. Thus, it may be that these children with consistently low family dysfunction have elevated eosinophil counts due to their comparatively more severe asthma status. As we note in the discussion, the low dysfunction may reflect parental efforts to shield a sick child from additional stress.
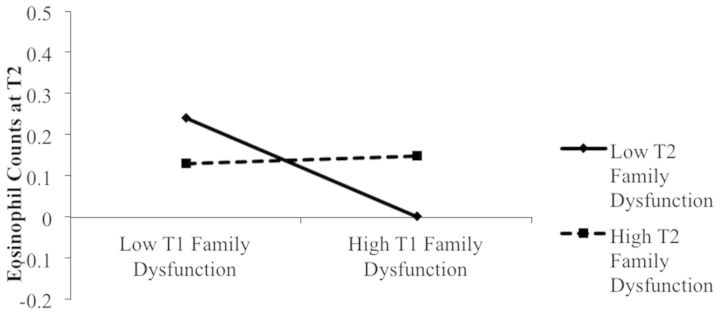
Family Functioning and ECP
No main effect of T1 family dysfunction or parental depressive symptoms emerged in predicting T2 ECP. However, a significant T1 × T2 Family Dysfunction interaction emerged (see Table II and Figure 2). As was the case with eosinophil counts, when values of T2 family dysfunction were 1 SD below the mean, family dysfunction at T1 was negatively associated with children’s ECP at T2 (simple slope = −41.4, t = 2.30, p = .02). Examination of values 1 SD above the mean on T2 family dysfunction revealed a positive association between family dysfunction at T1 and ECP at T2 (simple slope = 43.4, t = 2.33, p = .02). In other words, when parents reported elevated family dysfunction at T2, there was a positive association between T1 family dysfunction and T2 ECP.
Table II.
Predicting T2 Eosinophil Counts, ECP, and Diary Symptom Reports
| Model |
||||||
|---|---|---|---|---|---|---|
| Eosinophil counts |
ECP |
Diary symptom reports |
||||
| B (SE) | ß | B (SE) | ß | B (SE) | ß | |
| SES | −.01 (.03) | −.03 | −3.00 (2.25) | −.14 | .04 (.04) | .12 |
| Age | .01 (.01) | .09 | 2.29 (.83)** | .29 | .00 (.02) | −.03 |
| Gender | −.06 (.06) | −.10 | −7.29 (5.01) | −.15 | −.02 (.10) | −.02 |
| Race | .01 (.06) | .01 | −6.64 (4.55) | −.16 | −.14 (.09) | −.18 |
| T1 Asthma Severity | −.04 (.03) | −.13 | −3.58 (2.32) | −.17 | .01 (.05) | .02 |
| T1 Inhaled Corticosteroid Use | .00 (.01) | .00 | .53 (.38) | .15 | −.01 (.01) | −.20 |
| T2 Inhaled Corticosteroid Use | −.01 (.01) | −.24 | −.22 (.76) | −.06 | −.01 (.01) | −.22 |
| T2 Beta Agonist Use | .01 (.01) | .21 | .41 (.74) | .11 | .01 (.01) | .19 |
| Baseline Measure | .58 (.11)*** | .59 | .44 (.12)*** | .39 | .43 (.10)*** | .51 |
| T1 Parental Depression | .00 (.01) | .02 | −.38 (.37) | −.14 | .01 (.01) | .20 |
| T1 Family Dysfunction | −.15 (.11) | −.20 | .97 (8.63) | .02 | −.01 (.18) | −.01 |
| T2 Parental Depression | .00 (.00) | −.06 | −.06 (.33) | −.02 | .00 (.01) | −.05 |
| T2 Family Dysfunction | .04 (.10) | .07 | 16.74 (8.37)* | .32 | −.19 (.17) | −.20 |
| T1 × T2 Parental Depression | .00 (.00) | .06 | .02 (.03) | .10 | .00 (.00) | −.03 |
| T1 × T2 Family Dysfunction | .44 (.20)* | .25 | 42.4 (16.18)* | .30 | .08 (.34) | .03 |
| R2/adjusted R2 | .44/.31 | .42/.28 | .34/.17 | |||
Note. Gender coded as 0 = female, 1 = male. Race coded as 0 = White, 1 = minority.
†p < .10. *p < .05. **p < .01. ***p < .001.
Figure 2.
Prediction of ECP at T2 as a function of T1 and T2 family functioning. Regression analysis controls for T1 ECP, demographic controls, asthma severity, and medication usage.
Family Functioning and Asthma Symptom Severity
We examined the role of family dysfunction and parental depressive symptoms in predicting subsequent asthma symptom reports across a 2-week period (see Table II). Family dysfunction and parental depressive symptoms at T1 were unrelated to children’s symptom reports at T2.
Exploratory Analyses
We examined whether seasonality influenced eosinophil counts, ECP, and asthma symptoms; no significant effects emerged, however. Finally, to examine whether asthma-related factors predict the family emotional climate, we tested T1 asthma symptoms and eosinophil activity as predictors of T2 family dysfunction and parental depressive symptoms in a series of separate regressions. After controlling for demographic covariates and baseline family functioning or depressive symptoms, eosinophil activity and asthma symptoms at T1 were unrelated to family dysfunction and parental depressive symptoms at T2 (all p values > .24).
Discussion
The present study adds to research on family stressors and biological processes involved with asthma expression. Our findings suggest that changes in family dysfunction are associated with changes in eosinophil counts and ECP over a 1-year period. Children who had high levels of family dysfunction at T1 but low levels of dysfunction at T2 had the lowest eosinophil counts and ECP at T2. Additional findings revealed that children who experienced elevated family dysfunction at T1 and T2 had the highest levels of ECP at T2. This pattern of findings suggests that efforts to examine how the family emotional climate predicts subsequent eosinophil activity should consider the ways in which the family climate changes over time. These findings suggest that changes in the family are accompanied by changes in immunologic processes that underlie asthma expression.
Unexpectedly, the interaction effect depicted in Figure 1 suggests that eosinophil counts were comparable among children who experienced persistently high- or low-quality family functioning. It is unclear why this pattern emerged, although examination of the asthma severity ratings of these children may provide some insight into this pattern. In particular, compared with children with the highest levels of family dysfunction, children with the lowest levels of family dysfunction across T1 and T2 were rated as having marginally more severe. It may be that high eosinophil counts can emerge for a variety of reasons, including severe asthma or chronic family dysfunction. Some parents may try to shield their children from chaotic family experiences when their children have severe asthma symptoms. It is possible that asthma severity has a nonlinear effect on eosinophil counts. With a larger sample size, we would be able to test for quadratic and cubic trends, but these formal tests are beyond the scope of the present study. This pattern did not emerge for the prediction of ECP, however, so it is possible that this effect emerged by chance.
Exploratory analyses revealed that asthma symptoms and eosinophil activity were not predictive of later family functioning, which provides some preliminary evidence about the directionality of these effects. Of course, given that we have two waves of correlational data, we can only speculate about whether family processes exert a causal effect in modulating changes in children’s eosinophil activity. Interventions that aim to improve family functioning will provide insight into whether family experiences have a direct influence on these immunologic processes.
Unexpectedly, we found no evidence for the role of parental depressive symptoms as a predictor of children’s eosinophil activity or asthma morbidity. Notably, however, parental reports of depressive symptoms and family functioning were significantly correlated (r = .39 at T1 and r = .30 at T2, p values < .001). It may be that the negative effects of parental depression are embedded within the effects observed in relation to family functioning. Surprisingly, however, when we tested the regression models without the measures of family functioning included as predictors, parental depressive symptoms were still unrelated to eosinophil counts and ECP, suggesting that parental depressive symptoms may not relate to eosinophils in this sample. Although the CES-D is one of the most frequently used measures of depressive symptoms, several studies have questioned whether this measure overestimates the prevalence of depression (e.g., Santor, Zuroff, Ramsay, Cervantes, & Palacios, 1995). Other measures of parental depression (e.g., interviews) may be better suited for identifying changes in eosinophils over time. Further, given that parental symptoms were not associated with children’s eosinophil activity, it may be important to examine other biologic mechanisms, such as vagal cholinergic mechanisms, that could link parental depressive symptoms to children’s asthma symptoms.
Interestingly, the T1 × T2 family functioning interaction effects that emerged in the prediction of eosinophil counts and ECP were not significant in the prediction of children’s diary reports of asthma symptoms. It may be that changes in children’s diary reports of asthma symptoms are more likely to result when major life changes or stressful events occur. Our measure of family functioning included a range of items about support, emotions, and decision-making, but none of the items assessed major problems in the family (e.g., abuse, neglect). Further, few families reported high levels of difficulties within the family, and this restricted range may have impeded our ability to identify links to asthma symptoms.
Several limitations will be important to address in future research. First, future studies can improve on our measurement of family functioning in a couple ways. For example, more frequent measurement of family functioning will better capture the variability in families’ functioning to examine whether subtle variations predict variations in eosinophil activity and asthma symptoms. More frequent sampling would also allow for examination of whether seasonality shapes the extent to which changes in family stressors shape changes in eosinophil counts and ECP. Further, we relied on parental reports of family functioning, which may be only modestly related to day-to-day interactions within the family, particularly if parents are reluctant to endorse items that reflect strained family functioning. Future studies should incorporate behavioral observations of families, which may capture another dimension of the family emotional climate. Nevertheless, our findings are notable in that changes in parental perceptions of the family predicted changes in children’s eosinophil activity over the course of a year.
Additional limitations included our reliance on self-reports of medication usage and daily symptoms. Some studies suggest that informant reports of medication usage may be inflated (Bender et al., 2000). More accurate measures of medication usage (e.g., electronic measures of metered dose inhaler use, canister weights) may result in stronger links to eosinophil activity. Similarly, diary reports were unrelated to eosinophil activity at T1 and T2, so it will be important to examine other indicators of asthma symptoms in future studies. Missing diary report data may have hindered our ability to detect links between family functioning and asthma symptoms, particularly for children who were experiencing an increase in breathing difficulties.
Another important area for future research will be to examine possible buffers of problems within the family. Our findings suggest that improvements in family functioning can shape the extent to which prior estimates of family functioning are associated with subsequent eosinophil activity. But not all families will be able to improve their family functioning, and so it is important to identify other opportunities for shielding children from the negative consequences associated with a chaotic family. For example, if children have a parent who is dependable and available for support, despite the broader context of a chaotic and stressful family, then children may show fewer exacerbations in inflammatory responses relevant for asthma. Alternatively, factors outside the family, such as a close friendship or a supportive group within the community, may allow children to experience some support and stability in their lives, which may result in improvements in their response to asthma stimuli. Examinations of possible buffers will help to clarify the extent to which family experiences negatively affect children’s biologic processes that shape children’s asthma, as well as whether the consequences of these negative experiences can be dampened through other support systems.
Although our sample reflected the demographic characteristics of the city from which it was drawn, the sample included only White and Asian children, largely from two-parent, middle class families. It will be important for future studies to examine the connections between family functioning and eosinophil activity in larger and more diverse samples, especially given that asthma morbidity and mortality rates are higher in low SES and minority families (Akinbami, Moorman, Garbe, & Sondik, 2009). Additionally, in the present study, we did not include measures of environmental exposures, like allergens, pollutants, and irritants, which also contribute to eosinophil activity and asthma symptoms. It is possible that these exposures may serve as a mediating mechanism to explain how family experiences shape immunologic processes that underlie asthma expression. Similarly, examination of other mediators, such as medication use, will help shed light on the mechanisms that explain how family experiences come to shape eosinophil activity. Medication use was not associated with eosinophil activity or asthma symptoms in the present study, but it is possible that other studies with more refined measures will be better suited to examine its role as a possible mediator.
In summary, our findings highlight the role that families play in the biologic processes that underlie asthma. These findings can help inform interventions that are designed to improve children’s asthma. For example, efforts to improve family stability and communication may result in better compliance with medication regimens, or may reduce children’s environmental exposures to environmental triggers that exacerbate asthma symptoms. Our findings suggest that family functioning difficulties are associated with the mobilization and activation of eosinophils, and improvements in family functioning can bring about changes in eosinophil activity.
Funding
This research was supported by National Institutes of Health (NIH) grants HL073975, HL108723, and HD076563.
Conflicts of interest: None declared.
References
- Aiken L. S., West S. G. (1991). Multiple regression: Testing and interpreting interactions. Thousand Oaks, CA: Sage. [Google Scholar]
- Akinbami L. J., Moorman J. E., Garbe P. L., Sondik E. J. (2009). Status of childhood asthma in the United States, 1980−2007. Pediatrics, 123, S131–S145. doi:10.1542/peds.2008-2233C [DOI] [PubMed] [Google Scholar]
- Bacharier L. B., Strunk R. C., Mauger D., White D., Lemanske R. F., Sorkness C. A. (2004). Classifying asthma severity in children: Mismatch between symptoms, medication use, and lung function. American Journal of Respiratory and Critical Care Medicine, 170, 426–432. doi:10.1164/rccm.200308-1178OC [DOI] [PubMed] [Google Scholar]
- Bender B., Wamboldt F. S., O’Connor S. L., Rand C., Szefler S., Milgrom H., Wamboldt M. Z. (2000). Measurement of children’s asthma medication adherence by self report, mother report, canister weight, and Doser CT. Annals of Allergy, Asthma, and Immunology , .85 , 416–421. doi:10.1016/S1081-1206(10)62557-4 [DOI] [PubMed] [Google Scholar]
- Bloom B., Cohen R. A., Freeman G. (2012). Summary health statistics for U.S. children: National Health Interview Survey, 2011. National Center for Health Statistics. Vital Health Statistics 10, 1–180. [PubMed] [Google Scholar]
- Busse W. W., Lemanske R. F. (2001). Asthma. New England Journal of Medicine, 344, 350–362. doi:10.1056/NEJM200102013440507 [DOI] [PubMed] [Google Scholar]
- Byles J., Byrne C., Boyle M., Offord D. (1988). Ontario Child Health Study – Reliability and validity of the general functioning subscale of the McMaster Family Assessment Device. Family Process, 27, 97–104. [DOI] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention. (2009). National Hospital Ambulatory Medical Care Survey. Hyattsville, MD: US Department of Health and Human Services, Centers for Disease Control and Prevention. [Google Scholar]
- Chen E., Hanson M. D., Paterson L. Q., Griffin M. J., Walker H. A., Miller G. E. (2006). Socioeconomic status and inflammatory processes in childhood asthma: The role of psychological stress. Journal of Allergy and Clinical Immunology, 117, 1014–1020. doi:10.1016/j.jaci.2006.01.036 [DOI] [PubMed] [Google Scholar]
- Chen E., Miller G. E. (2007). Stress and inflammation in exacerbations of asthma. Brain, Behavior, and Immunity , .21 , 993–999. doi:10.1016/j.bbi.2007.03.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen E., Schreier H. M. C. (2008). Does the social environment contribute to asthma? Immunology and Allergy Clinics of North America, 28, 649–664. doi:10.1016/j.iac.2008.03.007 [DOI] [PubMed] [Google Scholar]
- Childhood Asthma Management Program (CAMP) Research Group. (1999). The Childhood Asthma Management Program (CAMP): Design rationale, and methods. Controlled Clinical Trials, 20, 91–120. [PubMed] [Google Scholar]
- Cox M. J., Paley B. (1997). Families as systems. Annual Review of Psychology, 48, 243–267. doi:10.1146/annurev.psych.48.1.243 [DOI] [PubMed] [Google Scholar]
- Epstein N. B., Baldwin L., Bishop D. S. (1983). The McMaster Family Assessment Device. Journal of Marital and Family Therapy, 9, 171–180. [Google Scholar]
- Gottman J. M. (1991). Chaos and regulated change in family interaction. In Cowan P., Hetherington E. M. (Eds.), New directions in family research: Transition and change (pp. 247–272). Hillsdale, NJ: Erlbaum. [Google Scholar]
- Kang D. H., Coe C. L., McCarthy D. O., Jarjour N. N., Kelly E. A., Rodriguez R. R., Busse W. W. (1997). Cytokine profiles of stimulated blood lymphocytes in asthmatic and healthy adolescents across the school year. Journal of Interferon and Cytokine Research, 17, 481–487. [DOI] [PubMed] [Google Scholar]
- Kaugars A. S., Klinnert M. D., Bender B. G. (2004). Family influences on pediatric asthma. Journal of Pediatric Psychology, 29, 475–491. doi:10.1093/jpepsy/jsh051 [DOI] [PubMed] [Google Scholar]
- Liu L. Y., Coe C. L., Swenson C. A., Kelly E. A., Kita H., Busse W. W. (2002). School examinations enhance airway inflammation to antigen challenge. American Journal of Respiratory and Critical Care Medicine, 165, 1062–1067. doi:10.1164/ajrccm.165.8.2109065 [DOI] [PubMed] [Google Scholar]
- Marshall G. D. (2004). Neuroendocrine mechanisms of immune dysregulation: Applications to allergy and asthma. Annals of Allergy, Asthma and Immunology, 93, S11–S17. doi:10.1016/S1081-1206(10)61482-2 [DOI] [PubMed] [Google Scholar]
- Miller G. E., Gaudin A., Zysk E., Chen E. (2009). Parental support and cytokine activity in childhood asthma: The role of glucocorticoid sensitivity. Journal of Allergy and Clinical Immunology, 123, 824–830. doi:10.1016/j.jaci.2008.12.019 [DOI] [PubMed] [Google Scholar]
- Miller I. W., Ryan C. E., Keitner G. I., Bishop D. S., Epstein N. B. (2000). The McMaster approach to families: Theory, assessment, treatment and research. Journal of Family Therapy, 22, 168–189. [Google Scholar]
- Niimi A., Amitani R., Suzuki K., Tanaka E., Murayama T., Kuze F. (1998). Serum eosinophil cationic protein as a marker of eosinophilic inflammation in asthma. Clinical and Experimental Allergy, 28, 233–240. doi:10.1046/j.1365-2222.1998.00217.x [DOI] [PubMed] [Google Scholar]
- Radloff L. S. (1977). The CES-D scale: A self-report depression scale for research in the general population. Applied Psychological Measurement, 1, 385–401. doi:10.1177/014662167700100306 [Google Scholar]
- Santor D. A., Zuroff D. C., Ramsay J. O., Cervantes P., Palacios J. (1995). Examining scale discriminability in the BDI and CES-D as a function of depression severity. Psychological Assessment, 7, 131–139. doi:10.1037/1040-3590.7.2.131 [Google Scholar]
- Schreier H. M. C., Chen E. (2010). Longitudinal relationships between family routines and biological profiles among youth with asthma. Health Psychology, 29, 82–90. doi:10.1037/a0018311 [DOI] [PubMed] [Google Scholar]
- Shalowitz M. U., Berry C. A., Quinn K. A., Wolf R. L. (2001). The relationship of life stressors and maternal depression to pediatric asthma morbidity in a subspecialty practice. Ambulatory Pediatrics, 1, 185–193. doi:10.1367/1539-4409(2001)001<0185:TROLSA>2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Tomassini M., Magrini L., De Petrillo G., Adriani E., Bonini S., Balsano F., Bonini S. (1996). Serum levels of eosinophil cationic protein in allergic diseases and natural allergen exposure. Journal of Allergy and Clinical Immunology, 97, 1350–1355. doi:10.1016/S0091-6749(96)70204-X [DOI] [PubMed] [Google Scholar]
- Venge P. (2004). Monitoring the allergic inflammation. Allergy, 59, 26–32. doi:10.1046/j.1398-9995.2003.00386.x [DOI] [PubMed] [Google Scholar]
- Wolf J. M., Miller G. E., Chen E. (2008). Parent psychological states predict changes in inflammatory markers in children with asthma and healthy children. Brain, Behavior, and Immunity, 22, 433–441. doi:10.1016/j.bbi.2007.10.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright R. J. (2008). Exploring biopsychosocial influences on asthma expression in both the family and community context. American Journal of Respiratory and Critical Care Medicine, 177, 129–130. doi:10.1164/rccm.200710-1526ED [DOI] [PubMed] [Google Scholar]
- Wright R. J., Finn P., Contreras J. P., Cohen S., Wright R. O., Staudenmayer J., Wand M., Perkins D., Weiss S. T., Gold D. R. (2004). Chronic caregiver stress and IgE expression, allergen-induced proliferation, and cytokine profiles in a birth cohort predisposed to atopy. Journal of Allergy and Clinical Immunology, 113, 1051–1057. [DOI] [PubMed] [Google Scholar]