We characterized long-term microbiologic responses to early antipseudomonal therapy for new Pseudomonas aeruginosa infection in patients with cystic fibrosis. There were no differences in long-term clinical outcomes between those who achieved sustained P. aeruginosa eradication and those who did not.
Keywords: Pseudomonas infections, Pseudomonas aeruginosa, cystic fibrosis, eradication therapy, treatment outcomes
Abstract
Background. Pseudomonas aeruginosa (Pa) is the most important pathogen infecting the airways in individuals with cystic fibrosis. A key question is whether children with newly acquired Pa infection who are able to achieve sustained eradication after early antipseudomonal therapy demonstrate improved long-term health outcomes compared with those who are unable to achieve a sustained microbiologic response.
Methods. This cohort study utilized observational follow-up data on children participating in the Early Pseudomonas Infection Control trial who received standardized therapy for newly acquired Pa. Sustained eradicators were defined as those who maintained Pa-negative cultures for 12 months after initial antipseudomonal therapy. Associations between eradication status and outcomes were assessed.
Results. Of the 249 trial participants included in the study, 172 (69%) achieved sustained eradication of Pa during the trial (sustained eradicators). Over the median 5-year follow-up, sustained eradicators had a 74% reduced risk of developing chronic Pa (hazard ratio [HR], 0.26; 95% confidence interval [CI], .17–.40) and a 57% reduced risk of mucoidy (HR, 0.43; 95% CI, .25–.73) compared with nonsustained eradicators. Sustained eradicators had significantly less anti-Pa antibiotic usage during follow-up compared with nonsustained eradicators. There was no association between eradication status and clinical outcomes including rate of exacerbation and lung function decline.
Conclusions. This is the first study to quantify the long-term durability of microbiological response associated with early antipseudomonal therapy, demonstrating the critical importance of optimizing antipseudomonal therapies during early Pa infection. The clinical impact of failure to achieve sustained Pa eradication remains unclear, however, and may be confounded by anti-Pa antibiotic usage.
Clinical Trials Registration. NCT00097773.
(See the Editorial Commentary by Zemanick and Laguna on pages 716–8.)
Pseudomonas aeruginosa (Pa) is perhaps the most important pathogen infecting the airways of individuals with cystic fibrosis (CF) [1, 2]. Early antibiotic treatment of newly acquired Pa, regardless of the presence of accompanying symptoms, is now considered standard of care to rapidly clear the pathogen and delay establishment of chronic infection [1, 3, 4]. However, the long-term impact of early antipseudomonal antibiotics on the progression of disease remains unclear. The majority of studies to date have focused on the comparison of clinical outcomes between those newly vs never infected with Pa [2, 5–9]; however, there is now a need to shift our efforts and determine the long-term clinical impact of current antipseudomonal treatment approaches for new Pa. A key clinical question is whether individuals with new Pa who are able to successfully achieve sustained Pa eradication after early antipseudomonal therapy at the time of new Pa acquisition demonstrate improved outcomes compared with those unable to achieve sustained eradication.
Consistent data across recent multicenter studies suggest that approximately one-third of children with CF and newly acquired Pa will fail to achieve sustained Pa eradication over a 1- to 2-year period after initial antipseudomonal therapy [10, 11], and yet there are limited data over a long duration of follow-up to quantify how their clinical progression of disease is affected. The Early Pseudomonas Infection Control (EPIC) clinical trial randomized children aged 1–12 years with newly acquired Pa to assess the efficacy of more aggressive vs less aggressive antipseudomonal therapy, and demonstrated no significant differences in outcomes between treatment regimens [10]. These children have been closely monitored for >5 years in an ongoing observational study, providing a unique opportunity to investigate the long-term impact of sustained Pa eradication [12].
The objectives of this study were to characterize Pa recurrence patterns among a contemporary cohort treated for newly acquired Pa and to compare those who achieved sustained eradication during the clinical trial with those who did not in terms of long-term microbiologic and clinical outcomes after completion of the clinical trial. We hypothesized that children who achieved sustained Pa eradication had a reduced risk of chronic Pa infection and mucoid Pa acquisition, reduced pulmonary exacerbation rates, and less rapid pulmonary function decline compared with participants who were unable to achieve sustained Pa eradication.
METHODS
Study Cohort and Definition of Sustained Eradication at Trial Completion
The cohort was comprised of participants from the EPIC Clinical Trial (ClinicalTrials.gov identifier NCT00097773) [10] who consented for follow-up in the EPIC observational study [12]. Key eligibility criteria and treatment received during the trial are shown in Figure 1 and have been previously described [12]. Participants were defined as “sustained eradicators” upon completion of the 18-month clinical trial if all quarterly cultures obtained during the preceding 12 months were free of Pa (Leeds definition of “Pa-free”) [13]. This study was approved by the Institutional Review Board at Seattle Children's Hospital, Seattle, Washington.
Figure 1.
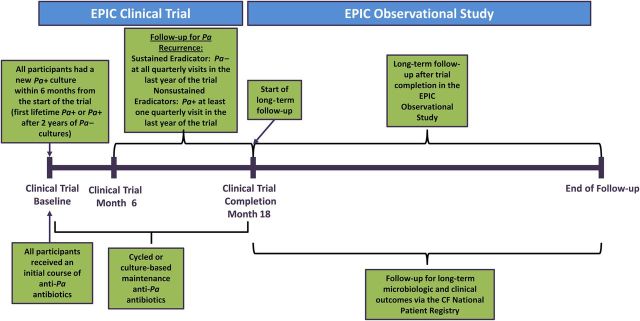
Overview of study design. Children with cystic fibrosis (CF) aged 1–12 years with newly identified Pseudomonas aeruginosa (Pa) infection within 6 months of enrollment were eligible for the trial. Newly identified Pa was defined as the first lifetime documented Pa-positive culture or a Pa-positive culture after a 2-year absence of Pa culture positivity (requiring at least 1 culture per year). For children aged 12–15 months, at least 1 Pa-positive culture since birth was required. Other eligibility criteria have been previously reported [12]. During the first quarter of the study, all children received initial Pa eradication treatment consisting of tobramycin inhalation solution, with half of the children randomly assigned to a concurrent course of oral ciprofloxacin and half to oral placebo. Children were randomized to 1 of 2 maintenance treatment strategies: (1) cycled therapy—treatment provided in quarterly cycles regardless of findings from scheduled quarterly respiratory cultures; or (2) culture-based therapy—treatment only in response to identification of Pa from quarterly cultures. Sustained Pa eradication was based on the Leeds definition of “Pa-free” [13] and required participants to have been Pa culture negative for at least 12 months prior to the completion of the trial (based on cultures obtained quarterly, with the average number of cultures for the cohort in the 12 months of the trial equal to 4.7). The assessment period for defining sustained Pa eradication allowed up to 6 prior months of intensive antipseudomonal therapy to achieve initial Pa eradication and was defined during the maintenance therapy phase of the trial. Long term follow-up was provided via the Early Pseudomonas Infection Control (EPIC) observational study and the CF National Patient Registry.
Microbiologic and Clinical Outcomes After Trial Completion
Respiratory cultures during follow-up were performed at site clinical microbiology laboratories as part of standard clinical care, with results recorded in the Cystic Fibrosis Foundation National Patient Registry (CFFNPR). Microbiologic endpoints included time to first and second Pa recurrence and time to chronic Pa, defined as the third quarter in which a Pa-positive culture was observed within a 2-year period, which included retrospectively assessing cultures during the clinical trial period as applicable for earlier cultures during the follow-up period [14]. Time to mucoidy and resistant Pa were defined, respectively, as the first quarter in which a mucoid or resistant Pa culture was recorded in the CFFNPR since trial completion.
Clinical outcomes included antibiotic usage and rate of pulmonary exacerbations as defined by the physician and requiring home intravenous antibiotics or hospitalization. Exacerbations were counted as unique events if separated by at least 21 days. Antibiotic therapy received during the follow-up period was recorded at each clinical care episode. The forced expiratory volume in 1 second (FEV1) was recorded among those old enough to perform spirometry and expressed as a percentage of predicted [15, 16].
Statistical Methods
Multivariable regression models included eradication status as the primary predictor, with a priori adjustment for age at completion of the trial, lifetime Pa history (never infected vs a history of Pa positivity >2 years prior to the start of the trial), and treatment received during the trial (cycled vs culture-based therapy). Further details can be found in the Supplementary Data.
RESULTS
Overview of the Study Cohort at Trial Completion
Of the 304 participants randomized into the EPIC trial, 249 (82%) completed the trial and continued in the EPIC observational study. Of these, 172 (69%) achieved sustained eradication of Pa during the trial (“sustained eradicators”) and 77 (31%) failed to achieve sustained Pa eradication and had at least 1 recurrence of Pa within the last year of the trial (“nonsustained eradicators”).
The mean age of the cohort at the end of the trial was 7.2 years, and average FEV1% predicted was 97.7% (Table 1). No clinical characteristics significantly distinguished the sustained eradicators and nonsustained eradicators. The 55 trial participants not included in our study due to lack of follow-up data were younger than those in our cohort and had slightly lower FEV1% predicted (Supplementary Table E1). Consistent with results from the trial [10], more participants on cycled therapy achieved sustained eradication (93/172 [54%]) compared with those on culture-based therapy (29/77 [38%]) (16% difference; 95% confidence interval [CI], 3%–29%); however, the majority of participants in the culture-based therapy group responded to subsequent antibiotic therapy so that the prevalence of positive Pa cultures at the end of the trial was the same between the cycled and culture-based groups (10% vs 11%, respectively).
Table 1.
Study Cohort Characteristics
| Characteristic | Nonsustained Eradicator (n = 77) | Sustained Eradicator (n = 172) | Total (N = 249) | P Value |
|---|---|---|---|---|
| Sex | ||||
| Female | 40 (51.9%) | 86 (50.0%) | 126 (50.6%) | .776 |
| Age at trial completion, y | ||||
| Mean (SD) | 7.6 (4.1) | 7.0 (3.3) | 7.2 (3.6) | .308 |
| Age at trial completion | .079 | |||
| <6 y | 35 (45.5%) | 79 (45.9%) | 114 (45.8%) | |
| 6 to <10 y | 17 (22.1%) | 57 (33.1%) | 74 (29.7%) | |
| ≥10 y | 25 (32.5%) | 36 (20.9%) | 61 (24.5%) | |
| Race | .443 | |||
| White | 75 (97.4%) | 163 (94.8%) | 238 (95.6%) | |
| Hispanic | 1 (1.3%) | 1 (0.6%) | 2 (0.8%) | |
| African American | … | 5 (2.9%) | 5 (2.0%) | |
| Unknown/other | 1 (1.3%) | 3 (1.7%) | 4 (1.6%) | |
| Genotype | .928 | |||
| F508 del homozygous | 42 (54.5%) | 88 (51.2%) | 130 (52.2%) | |
| F508 del heterozygous | 28 (36.4%) | 64 (37.2%) | 92 (36.9%) | |
| Othera | 4 (5.2%) | 12 (7.0%) | 16 (6.4%) | |
| Unknown | 3 (3.9%) | 8 (4.7%) | 11 (4.4%) | |
| FEV1% predicted at trial completion | ||||
| No. | 46 | 106 | 152 | |
| Mean (SD) | 98.0 (18.4) | 97.6 (15.9) | 97.7 (16.6) | .895 |
| FEV1% predicted distribution at trial completion | .708 | |||
| <75% | 3 (3.9%) | 8 (4.7%) | 11 (4.4%) | |
| 75% to <100% | 21 (27.3%) | 55 (32.0%) | 76 (30.5%) | |
| ≥100% | 22 (28.6%) | 43 (25.0%) | 65 (26.1%) | |
| Pa history prior to trial enrollment | .236 | |||
| No lifetime history of Pa positivity | 56 (72.7%) | 112 (65.1%) | 168 (67.5%) | |
| Lifetime history of Pa positivity | 21 (27.3%) | 60 (34.9%) | 81 (32.5%) | |
| Treatment regimen received during the trial | .017 | |||
| Cycled therapy | 29 (37.7%) | 93 (54.1%) | 122 (49.0%) | |
| Culture-based therapy | 48 (62.3%) | 79 (45.9%) | 127 (51.0%) | |
| Oral treatment (in addition to TIS) received during the trial | .394 | |||
| Placebo | 43 (55.8%) | 86 (50.0%) | 129 (51.8%) | |
| Ciprofloxacin | 34 (44.2%) | 86 (50.0%) | 120 (48.2%) | |
Data are presented as No. (%) unless otherwise specified.
Abbreviations: FEV1, forced expiratory volume at 1 second; Pa, Pseudomonas aeruginosa; SD, standard deviation; TIS, tobramycin inhalation solution.
a Other refers to participants with 2 non-F508 del CFTR mutations.
Pa Recurrence Patterns After Completion of the Trial
The median follow-up time of the study cohort after completion of the study was 5.0 years (range, 0.3–6.6 years), with sustained eradicators having a slightly greater median follow-up time than nonsustained eradicators (5.1 vs 4.9 years, respectively). Overall, adherence to quarterly cultures during follow-up was high in this cohort, with 85% of 3995 total follow-up quarters having ≥1 culture result available; culture frequency was comparable between sustained eradicators and nonsustained eradicators (85% vs 84% of follow-up quarters with ≥1 culture result, respectively).
Over the follow-up period, 65 of 77 (84%) of the nonsustained eradicators vs 103 of 172 (60%) of the sustained eradicators experienced ≥1 Pa recurrence (Table 2). The median time to first Pa recurrence was 3.5 years among the sustained eradicators vs only 1 year among the nonsustained eradicators (a 58% reduction: hazard ratio [HR], 0.42; 95% CI, .3–.57). Treatment received during the trial was not significantly associated with Pa recurrence during follow-up.
Table 2.
Time to First and Second Pseudomonas aeruginosa Recurrence After Completion of the Clinical Trial, by Eradication Status
| Recurrence | Nonsustained Eradicator (n = 77) | Sustained Eradicator (n = 172) | Overall (N = 249) | Difference or HR (95% CI) |
|---|---|---|---|---|
| First Pa recurrence | ||||
| Participants with at least 1 Pa recurrence, No. (%) | 65 (84.4) | 103 (59.9) | 168 (67.5) | Difference, 24.5% (12.5%–34.4%) |
| Median time, y, to first recurrence (95% CI) | 1.0 (.75–1.75) | 3.5 (2.75–4.75) | 2.75 (2.50–3.50) | HR, 0.42 (.30– .57) |
| Second Pa recurrence | ||||
| Participants with at least 2 Pa recurrences, No. (%)a | 50 (76.9) | 57 (55.3) | 107 (63.7) | Difference, 21.6% (6.8%–34.5%) |
| Median time, y, to second recurrence since first recurrence (95% CI) | 0.75 (.5–1.5) | 2.00 (1.25–3.5) | 1.50 (1.0–2.0) | HR, 0.55 (.38–.81) |
Abbreviations: CI, confidence interval; HR, hazard ratio; Pa, Pseudomonas aeruginosa.
a Denominator is the number of subjects with at least 1 Pa recurrence.
Among those with a recurrence of Pa, 55% of the 103 sustained eradicators and 77% of the 65 nonsustained eradicators had a second recurrence of Pa during the follow-up period. Among those with a first Pa recurrence, the median time to second recurrence was 2.0 and 0.75 years for the sustained and nonsustained eradicators, respectively (HR, 0.55; 95% CI, .38–.81; Table 2).
Association Between Sustained Eradication and the Development of Chronic and Mucoid Pa
Whereas 56% of the 77 nonsustained eradicators developed chronic Pa infection during follow-up after a median of 3.25 years, only 23% of the 172 sustained eradicators developed chronic Pa infection (33% difference; 95% CI, 20%–45%). As shown in Table 3 and Figure 2A, sustained eradication during the trial was associated with a 74% reduced risk of development of chronic Pa infection (HR, 0.26; 95% CI, .17–.40), even after adjustment for potential confounders. Increasing age and lifetime history of Pa positivity were significantly associated with the risk of development of chronic Pa infection. Interestingly, use of cycled therapy during the trial was also associated with increased risk of the development of chronic Pa infection during the follow-up period.
Table 3.
Results From Univariate Unadjusted and Multivariable Adjusted Cox Proportional Hazards Models for the Association Between Eradication Status and Time to Chronic and Mucoid Pseudomonas aeruginosa
| Variable | HR | 95% CI | P Value |
|---|---|---|---|
| Time to chronic Pa (unadjusted) | |||
| Nonsustained eradicator | … | … | … |
| Sustained eradicator | 0.26 | (.17–.40) | <.001 |
| Time to chronic Pa (adjusted multivariable model) | |||
| Nonsustained eradicator | … | … | … |
| Sustained eradicator | 0.21 | (.13–.33) | <.001 |
| Age at trial completion | |||
| <6 y | … | … | … |
| 6 to <10 y | 1.31 | (.73–2.36) | .359 |
| ≥10 y | 1.78 | (1.00–3.19) | .052 |
| Pa history prior to trial enrollment | |||
| No lifetime history of Pa positivity | … | … | … |
| Lifetime history of Pa positivity | 1.69 | (1.01–2.82) | .046 |
| Treatment regimen received during the trial | |||
| Culture-based therapy | … | … | … |
| Cycled therapy | 1.60 | (1.02–2.53) | .042 |
| Time to mucoid Pa (unadjusted) | |||
| Nonsustained eradicator | … | … | … |
| Sustained eradicator | 0.43 | (.25–.73) | .002 |
| Time to mucoid Pa (adjusted multivariable model) | |||
| Nonsustained eradicator | … | … | … |
| Sustained eradicator | 0.36 | (.20–.63) | <.001 |
| Age at trial completion | |||
| <6 y | … | … | … |
| 6 to <10 y | 1.84 | (.84–4.00) | .126 |
| ≥10 y | 3.26 | (1.52–6.98) | .002 |
| Pa history prior to trial enrollment | |||
| No lifetime history of Pa positivity | … | … | … |
| Lifetime history of Pa positivity | 1.96 | (1.06–3.63) | .032 |
| Treatment regimen received during the trial | |||
| Culture-based therapy | … | … | … |
| Cycled therapy | 1.30 | (.75–2.26) | .345 |
Abbreviations: CI, confidence interval; HR, hazard ratio; Pa, Pseudomonas aeruginosa.
Figure 2.
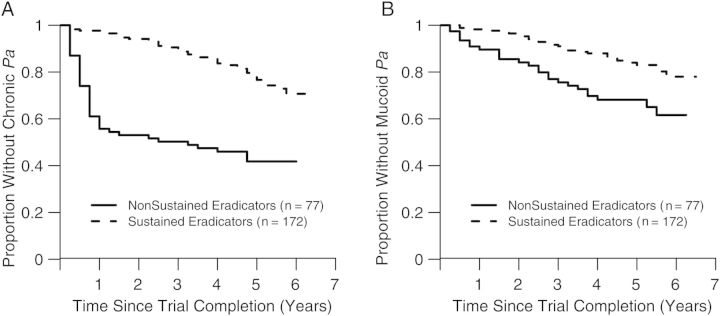
Kaplan–Meier plots of time to chronic Pseudomonas aeruginosa (Pa) infection (A) and time to mucoid Pa infection (B) after completion of the clinical trial, by eradication status.
Over the course of follow-up, 33% of the 77 nonsustained eradicators vs 17% of the sustained eradicators presented with mucoid Pa (16% difference, 95% CI, 4%–28%). Sustained eradication was associated with a 57% reduced risk of the appearance of mucoid Pa over the follow-up period (HR, 0.43; 95% CI, .25–.73; Figure 2B). In multivariable modeling, increased age and lifetime history of Pa positivity were also associated with increased risk of mucoid Pa, although use of cycled therapy during the trial was not (Table 3). Of the 168 participants with Pa recurrence, only 25 (15%) had at least 1 Pa isolate recorded as resistant to either aminoglycosides, quinolones, or β-lactams during the follow-up period, and these participants were comparably distributed by eradication status and use of cycled or culture-based therapy during the trial.
Association Between Eradication and Long-term Clinical Outcomes
Overall, sustained eradicators had 50%–60% less usage of inhaled antibiotics and oral quinolones over the follow-up period compared with nonsustained eradicators, and comparable rates of macrolide usage (Supplementary Table E2). A total of 102 (59%) sustained eradicators vs 60 (78%) nonsustained eradicators recorded use of chronic inhaled antibiotics during the follow-up period, with median times to use of 3.61 and 0.79 years, respectively (HR, 0.52; 95% CI, .38–.72).
Of the 172 sustained eradicators, 48% had ≥1 pulmonary exacerbation during the follow-up period compared with 45% of the 77 nonsustained eradicators; event rates were comparable between sustained eradicators and nonsustained eradicators (rate ratio, 0.86; 95% CI, .54–1.34; Table 4). In multivariable regression modeling, female sex was significantly associated with exacerbation rate. Multivariable modeling did not include FEV1% predicted due to a significant proportion of children too young for spirometry at the beginning of the follow-up period (38% of the cohort); however, among participants with spirometry, similar available findings were observed (Supplementary Table E3). Additional sensitivity analyses were performed to assess risk of exacerbations among nonsustained eradicators with more frequent recurrence of Pa during the trial, and although there was a trend toward an increased risk of exacerbations in this subcohort compared with the sustained eradicators, this result was not statistically significant (Supplementary Table E4).
Table 4.
Association Between Eradication Status and Rate of Physician-Defined Pulmonary Exacerbations Requiring Intravenous Antibiotics or Hospitalization During the Follow-up Period
| Rate Ratio | 95% CI | P Value | |
|---|---|---|---|
| Exacerbation rate (unadjusted) | |||
| Nonsustained eradicator | … | … | … |
| Sustained eradicator | 0.86 | (.54–1.34) | .501 |
| Exacerbation rate (adjusted multivariable model) | |||
| Intercept | 0.16 | (.09–.28) | <.001 |
| Nonsustained eradicator | … | … | … |
| Sustained eradicator | 0.85 | (.53–1.35) | .492 |
| Sex | |||
| Male | … | … | … |
| Female | 1.82 | (1.19–2.80) | .005 |
| Age at trial completion | |||
| <6 y | … | … | … |
| 6 to <10 y | 1.28 | (.77–2.15) | .355 |
| ≥10 y | 1.29 | (.74–2.28) | .387 |
| Pa history prior to trial enrollment | |||
| No lifetime history of Pa positivity | … | … | … |
| Lifetime history of Pa positivity | 1.38 | (.86–2.22) | .206 |
| Treatment regimen received during the trial | |||
| Culture-based therapy | … | … | … |
| Cycled therapy | 1.37 | (.90–2.10) | .139 |
Abbreviations: CI, confidence interval; Pa, Pseudomonas aeruginosa.
Pulmonary function remained remarkably stable over the follow-up period, and the average rate of change in FEV1% predicted in the study cohort was only −0.1% per year (95% CI, −.8% to .7%). After adjusting for potential confounders, the rate of change in FEV1% predicted did not significantly differ between sustained eradicators (0.60% average increase per year; 95% CI, −2.18% to 3.39%) and nonsustained eradicators (−0.41% average decrease per year; 95% CI, −1% to .2%).
DISCUSSION
Our study represents the largest prospective study to date assessing long-term microbiologic and clinical outcomes among a cohort receiving standardized antipseudomonal therapy for newly acquired Pa. Prior studies have reported robust microbiologic response to early antipseudomonal therapy [3], and in the largest and longest eradication studies, EPIC [10] and ELITE [11], approximately 70% of children remained Pa culture negative in response to therapy for at least 18 to 27 months, respectively. Our study extends these results to show that for the majority of patients who are able to achieve sustained eradication as defined by Pa culture negativity throughout the final 12 months of the EPIC trial, the median time to subsequent Pa recurrence was an additional 3.5 years. Moreover, only 23% of those who achieved sustained eradication developed chronic infection over a median 5-year follow-up period, and only 17% developed mucoid Pa. Whereas prior studies have demonstrated a delay in chronic and mucoid Pa infection with early antipseudomonal therapy in comparison to no therapy [17–19], our study demonstrates a significantly increased risk of chronic and mucoid Pa among children who fail to achieve sustained eradication despite receiving antipseudomonal therapy. Although there is increased relative risk of these poor microbiologic outcomes among nonsustained eradicators, it is important to note that the absolute risk specifically for mucoid Pa remains low in this subcohort, with only 33% developing mucoid Pa during the median 5-year follow-up period.
In the original EPIC trial, slightly more participants who received cycled therapy achieved sustained eradication compared with those who received culture-based therapy. Our current study shows that those who received cycled therapy were at increased risk for the development of chronic infection. This suggests the potential that cycled antipseudomonal therapy may have been suppressing Pa culture positivity during the trial in some children, and because there was no evidence suggesting that these children remained on cycled therapy after the trial, that cessation of this therapy may have increased their risk for Pa recurrence. Thus, there remains no evidence for the benefit of cycled therapy over culture-based therapy with respect to long-term microbiologic outcomes.
The long-term health impact of new Pa acquisition remains unclear, particularly in the era of early antipseudomonal therapy. Those who failed to achieve sustained eradication had strikingly greater antibiotic usage than sustained eradicators, which may have confounded any comparisons between these groups. Overall, our study cohort remained relatively healthy, with approximately half of our cohort not experiencing a single pulmonary exacerbation requiring intravenous antibiotics or hospitalization during the median 5-year period, and the average rate of decline in FEV1 in the cohort was only −0.1% per year. Importantly, there were no differences in clinical outcomes between those who achieved sustained eradication vs those who did not. There are several potential explanations for this finding. First, as evidenced in placebo-controlled clinical trials for established Pa infection [20, 21] that demonstrate improved clinical efficacy with administration of antipseudomonal therapy, it is possible that antipseudomonal therapy administered during early and intermittent infection is also having a positive impact on outcomes such as exacerbations and lung function that keep the nonsustained eradicators from declining in health despite recurrence of Pa. Second, contemporary cohorts such as the one in this study may be managing their Pa infection better because of advancing improvements in the management of CF, including better nutritional management and the adoption of several new therapies into the standard of care [21–25]. Last, as opposed to explanations focused on the host, it is possible that Pa itself is less pathogenic during initial and intermittent infection [9], and only has significant detrimental impact when chronic infection is established and upon increasing bacterial density in the airways.
Our findings of a lack of association between microbiologic response to early eradication therapy and clinical outcomes may also be explained by factors related to our study design. The median follow-up time of our cohort was 5 years; this may not have been a long enough period to assess outcomes related to the transition to chronic and mucoid Pa. Our study was also restricted to the definition of exacerbation recorded in the CFFNPR, representing more severe events requiring intravenous antibiotics or hospitalization. It is possible that there is a more measurable impact of sustained eradication on more mild exacerbations treated with oral or inhaled antibiotics. Although limited data collection was available in the follow-up study to quantify this, a suggestion that sustained eradication was associated with reduced frequency of more mild respiratory events is reflected in the significantly reduced rates of antibiotic usage among the sustained eradicators vs the nonsustained eradicators. Lack of sensitive outcome measures to capture decline in health in children with CF additionally remains a critical problem, and it is likely that FEV1 is not a sensitive measure. Although one study has suggested that individuals who have cleared Pa after initial therapy have poorer lung function than those who had never been infected [8], recent studies have suggested that lung function does not deteriorate after new Pa acquisition in the presence of antipseudomonal treatment [5, 9]. Other studies have shown that the decline in lung function occurs only after acquisition of mucoid Pa [26].
There are several notable limitations to our study, including the restriction of our investigation to a cohort of children newly diagnosed with Pa. Additionally, there are no standardized definitions of eradication, and further work is needed to determine whether a duration of sustained eradication longer than assessed in this study is associated with better clinical outcomes. Our study relies heavily on accuracy of culture results, and although this was controlled during the clinical trial [12], this was not the case during the observational follow-up period. However, a recent audit was conducted that found excellent (98.9%) concurrence between microbiologic results recorded in the CFFNPR vs electronic medical records [27]. Second, it is possible that sampling approaches utilizing oropharyngeal swabs and sputum with traditional culture methods are not sufficiently sensitive to accurately characterize early stages of Pa infection in either the lower or upper airways, and thus are unable to detect underlying infection despite culture negativity for Pa [28, 29]. This approach remains the standard of care in the United States, however, and the prognostic ability of more sensitive monitoring of infection using serology to predict initial Pa infection, Pa recurrence, and treatment failure has not yet been demonstrated [30, 31].
In summary, children with sustained eradication after antipseudomonal therapy for newly acquired Pa have significantly better long-term microbiologic outcomes than those unable to achieve sustained eradication, including longer times to chronic and mucoid Pa. The lack of benefit of cycled therapy compared with culture-based therapy on long-term clinical and microbiologic outcomes continues to support current recommendations for use of culture-based therapy as an effective approach for management of early Pa infection [4]. The comparability of clinical outcomes between those who achieved sustained eradication vs those who did not suggests that the management of this entire cohort with the current standard of care has been successful in maintaining clinical stability with respect to lung function decline and pulmonary exacerbations requiring intravenous antibiotics. Despite this, those who fail to achieve sustained eradication are at greater risk of chronic and mucoid Pa, and although it is unclear how these key microbiologic endpoints impact subsequent clinical outcomes in an era of aggressive early antipseudomonal therapy, it is imperative that efforts continue to focus on improving eradication success through novel therapeutic approaches.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online (http://cid.oxfordjournals.org). Supplementary materials consist of data provided by the author that are published to benefit the reader. The posted materials are not copyedited. The contents of all supplementary data are the sole responsibility of the authors. Questions or messages regarding errors should be addressed to the author.
Notes
Acknowledgments. The authors gratefully acknowledge the children and families with cystic fibrosis who participated in the Early Pseudomonas Infection Control clinical trial and observational study.
Financial support. This work was supported by >the National Heart, Lung, and Blood Institute and the >National Institute of Diabetes and Digestive and Kidney Diseases (grant number >U01-HL080310>); Cystic Fibrosis Foundation Therapeutics (CFFT; grant numbers EPIC0K0 and OBSERV04K0); and an investigator-initiated grant from Novartis Pharmaceuticals.
Potential conflicts of interest. N. M.-H. receives grant support from CFFT and the >National Institutes of Health (>NIH), and is the principal investigator of investigator-initiated grants supported by Novartis and Pharmaxis, for which she receives salary support. M. R. receives grant support from CFFT, >NIH, and Vertex Pharmaceuticals. R. L. G. receives grant support from CFFT and >NIH; a limited portion of his institutional salary is also supported by industry-sponsored trials within the CFF Therapeutics Development Network (CFTDN) including Vertex Pharmaceuticals. B. W. R. receives grant support from CFFT and >NIH, and has received support from the following companies through her role as Director of the CFTDN Coordinating Center: Apartia, Bayer Healthcare AG, Bristol-Myers Squibb, Eli Lilly, Genentech, Gilead Sciences, Grifols Therapeutics, Hall Bioscience, Insmed Incorporated, Kalobios, Rempex Pharmaceuticals, N30 Pharmaceuticals, LLC, Nikan Pharmaceuticals, Nordmark, Novartis Phamaceuticals Corporation, Pulmatrix, Savara Pharmaceuticals, Talecris, Vectura Ltd, Vertex Pharmaceuticals, 12th Man Technologies, Achaogen, Celtaxys, Cornerstone Therapeutics, Aptalis Pharma, Breathe Easy, Ltd, CSL Behring LLC, INC Research, Pharmaxis Ltd, PumoFlow, and Respira Therapeutics. G. Z. R.-B. receives grant support from CFFT, and a limited portion of his institutional salary is also supported by industry-sponsored trials within the CF Foundation Therapeutics Development Network (Vertex Pharmaceuticals, Savara Pharmaceuticals, Gilead Sciences, and PTC Therapeutics). M. K. and V. T. receive grant support from Novartis and CFFT. J. E. receives grant support from CFFT.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Doring G, Flume P, Heijerman H, Elborn JS. Treatment of lung infection in patients with cystic fibrosis: current and future strategies. J Cyst Fibros 2012; 11:461–79. [DOI] [PubMed] [Google Scholar]
- 2.Emerson J, Rosenfeld M, McNamara S, Ramsey B, Gibson RL. Pseudomonas aeruginosa and other predictors of mortality and morbidity in young children with cystic fibrosis. Pediatr Pulmonol 2002; 34:91–100. [DOI] [PubMed] [Google Scholar]
- 3.Hewer SC, Smyth AR. Antibiotic strategies for eradicating Pseudomonas aeruginosa in people with cystic fibrosis. Cochrane Database Syst Rev 2009; 4:CD004197. [DOI] [PubMed] [Google Scholar]
- 4.Mogayzel PJ, Jr, Naureckas ET, Roginson KA, et al. Cystic fibrosis pulmonary guideline: pharmacologic approaches to prevention and eradication of initial Pseudomonas aeruginosa infection. Ann Am Thorac Soc 2014; 11:1640–50. [DOI] [PubMed] [Google Scholar]
- 5.Amin R, Lam M, Dupuis A, Ratjen F. The effect of early Pseudomonas aeruginosa treatment on lung function in pediatric cystic fibrosis. Pediatr Pulmonol 2011; 46:554–8. [DOI] [PubMed] [Google Scholar]
- 6.Hudson VL, Wielinski CL, Regelmann WE. Prognostic implications of initial oropharyngeal bacterial flora in patients with cystic fibrosis diagnosed before the age of two years. J Pediatr 1993; 122:854–60. [DOI] [PubMed] [Google Scholar]
- 7.Kosorok MR, Zeng L, West SE, et al. Acceleration of lung disease in children with cystic fibrosis after Pseudomonas aeruginosa acquisition. Pediatr Pulmonol 2001; 32:277–87. [DOI] [PubMed] [Google Scholar]
- 8.Kozlowska WJ, Bush A, Wade A, et al. Lung function from infancy to the preschool years after clinical diagnosis of cystic fibrosis. Am J Respir Crit Care Med 2008; 178:42–9. [DOI] [PubMed] [Google Scholar]
- 9.Zemanick ET, Emerson J, Thompson V, et al. Clinical outcomes after initial Pseudomonas acquisition in cystic fibrosis. Pediatr Pulmonol 2015; 50:42–8. [DOI] [PubMed] [Google Scholar]
- 10.Treggiari MM, Retsch-Bogart G, Mayer-Hamblett N, et al. Comparative efficacy and safety of 4 randomized regimens to treat early Pseudomonas aeruginosa infection in children with cystic fibrosis. Arch Pediatr Adolesc Med 2011; 165:847–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ratjen F, Munck A, Kho P, Angyalosi G. Treatment of early Pseudomonas aeruginosa infection in patients with cystic fibrosis: the ELITE trial. Thorax 2010; 65:286–91. [DOI] [PubMed] [Google Scholar]
- 12.Treggiari MM, Rosenfeld M, Mayer-Hamblett N, et al. Early anti-pseudomonal acquisition in young patients with cystic fibrosis: rationale and design of the EPIC clinical trial and observational study. Contemp Clin Trials 2009; 30:256–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lee TW, Brownlee KG, Denton M, Littlewood JM, Conway SP. Reduction in prevalence of chronic Pseudomonas aeruginosa infection at a regional pediatric cystic fibrosis center. Pediatr Pulmonol 2004; 37:104–10. [DOI] [PubMed] [Google Scholar]
- 14.Mayer-Hamblett N, Rosenfeld M, Gibson RL, et al. Pseudomonas aeruginosa in vitro phenotypes distinguish cystic fibrosis infection stages and outcomes. Am J Respir Crit Care Med 2014; 190:289–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang X, Dockery DW, Wypij D, Fay ME, Ferris BG., Jr Pulmonary function between 6 and 18 years of age. Pediatr Pulmonol 1993; 15:75–88. [DOI] [PubMed] [Google Scholar]
- 16.Hankinson JL, Odencrantz JR, Fedan KB. Spirometric reference values from a sample of the general U.S. population. Am J Respir Crit Care Med 1999; 159:179–87. [DOI] [PubMed] [Google Scholar]
- 17.Frederiksen B, Koch C, Hoiby N. Antibiotic treatment of initial colonization with Pseudomonas aeruginosa postpones chronic infection and prevents deterioration of pulmonary function in cystic fibrosis. Pediatr Pulmonol 1997; 23:330–5. [DOI] [PubMed] [Google Scholar]
- 18.Hansen CR, Pressler T, Hoiby N. Early aggressive eradication therapy for intermittent Pseudomonas aeruginosa airway colonization in cystic fibrosis patients: 15 years experience. J Cyst Fibros 2008; 7:523–30. [DOI] [PubMed] [Google Scholar]
- 19.Valerius NH, Koch C, Hoiby N. Prevention of chronic Pseudomonas aeruginosa colonisation in cystic fibrosis by early treatment. Lancet 1991; 338:725–6. [DOI] [PubMed] [Google Scholar]
- 20.Ramsey BW, Pepe MS, Quan JM, et al. Intermittent administration of inhaled tobramycin in patients with cystic fibrosis. Cystic Fibrosis Inhaled Tobramycin Study Group. N Engl J Med 1999; 340:23–30. [DOI] [PubMed] [Google Scholar]
- 21.McCoy KS, Quittner AL, Oermann CM, Gibson RL, Retsch-Bogart GZ, Montgomery AB. Inhaled aztreonam lysine for chronic airway Pseudomonas aeruginosa in cystic fibrosis. Am J Respir Crit Care Med 2008; 178:921–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Saiman L, Anstead M, Mayer-Hamblett N, et al. Effect of azithromycin on pulmonary function in patients with cystic fibrosis uninfected with Pseudomonas aeruginosa: a randomized controlled trial. JAMA 2010; 303:1707–15. [DOI] [PubMed] [Google Scholar]
- 23.Saiman L, Marshall BC, Mayer-Hamblett N, et al. Azithromycin in patients with cystic fibrosis chronically infected with Pseudomonas aeruginosa: a randomized controlled trial. JAMA 2003; 290:1749–56. [DOI] [PubMed] [Google Scholar]
- 24.Elkins MR, Robinson M, Rose BR, et al. A controlled trial of long-term inhaled hypertonic saline in patients with cystic fibrosis. N Engl J Med 2006; 354:229–40. [DOI] [PubMed] [Google Scholar]
- 25.Retsch-Bogart GZ, Burns JL, Otto KL, et al. A phase 2 study of aztreonam lysine for inhalation to treat patients with cystic fibrosis and Pseudomonas aeruginosa infection. Pediatr Pulmonol 2008; 43:47–58. [DOI] [PubMed] [Google Scholar]
- 26.Li Z, Kosorok MR, Farrell PM, et al. Longitudinal development of mucoid Pseudomonas aeruginosa infection and lung disease progression in children with cystic fibrosis. JAMA 2005; 293:581–8. [DOI] [PubMed] [Google Scholar]
- 27.Knapp EA, Salsgiver E, Fink A, Sewall A, Marshall BC, Saiman L. Trends in respiratory microbiology of people with cystic fibrosis in the United States, 2006–2012 (Abstract). Pediatr Pulmonol 2014; (suppl 38):317.24678058 [Google Scholar]
- 28.Hansen SK, Rau MH, Johansen HK, et al. Evolution and diversification of Pseudomonas aeruginosa in the paranasal sinuses of cystic fibrosis children have implications for chronic lung infection. ISME J 2012; 6:31–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rosenfeld M, Emerson J, Accurso F, et al. Diagnostic accuracy of oropharyngeal cultures in infants and young children with cystic fibrosis. Pediatr Pulmonol 1999; 28:321–8. [DOI] [PubMed] [Google Scholar]
- 30.Anstead M, Heltshe SL, Khan U, et al. Pseudomonas aeruginosa serology and risk for re-isolation in the EPIC trial. J Cyst Fibros 2013; 12:147–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Daines C, VanDeVanter D, Khan U, et al. Serology as a diagnostic tool for predicting initial Pseudomonas aeruginosa acquisition in children with cystic fibrosis. J Cyst Fibros 2014; 13:542–9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


