Abstract
To explore grazing effects on carbon fluxes in alpine meadow ecosystems, we used a paired eddy-covariance (EC) system to measure carbon fluxes in adjacent fenced (FM) and grazed (GM) meadows on the Tibetan plateau. Gross primary productivity (GPP) and ecosystem respiration (Re) were greater at GM than FM for the first two years of fencing. In the third year, the productivity at FM increased to a level similar to the GM site. The higher productivity at GM was mainly caused by its higher photosynthetic capacity. Grazing exclusion did not increase carbon sequestration capacity for this alpine grassland system. The higher optimal photosynthetic temperature and the weakened ecosystem response to climatic factors at GM may help to facilitate the adaption of alpine meadow ecosystems to changing climate.
Land use and associated management practices can affect ecosystem nutrient, carbon and water cycling by acting on plant photosynthesis, respiration and energy partitioning1,2,3. Grazing is the most ubiquitous land use practice for managed grassland ecosystems4,5.It exerts strong influences on ecosystem carbon budget, energy balance, and ecosystem function6,7. Knowledge of the responses of carbon fluxes to grazing is essential for developing sustainable management plans, coping with consequences of climate change, and mitigating related effects8,9.
Alpine meadows comprise the representative vegetation on the plateau and cover about 35% of its total area10. After thousand-years of years of grazing in these meadows, the practice has become an indispensable agriculture activity for the Tibetan grassland in terms of both regional sustainable development and culture. Over time, the Tibetan grassland has adapted to the effects of both natural climatic variations and anthropogenic disturbances caused by grazing11. In past decades, however, overgrazing has resulted in environmental deterioration and even desertification in the alpine grassland ecosystem, consequently generating considerable quantities of carbon efflux10,12.
It is still far from clear how grazing acts on biogeochemical cycling of the Tibetan grassland, and whether grazing depresses or stimulates grassland productivity. Grazing effects vary with stocking densities and grazing period length3. In general, grazing leads to decreased leaf area, in turn lowered ecosystem carbon acquisition. However, older leaves in ungrazed grasslands may possess lower ecosystem carbon fixation ability12,13. Grazing also substantially modifies canopy structure and land surface energy balance, altering microclimates in such aspects as soil temperature and moisture and potentially facilitating more rapid plant growth14,15. In contrast, slowed nitrogen dynamics under long-term grazing can negatively influence plant growth and soil microbial activities16. All these changes have the potential to alter the magnitude of ecosystem carbon uptake and emission4,17.
The Tibetan Plateau ecosystem is a relatively fragile system and is sensitive to climate changes18. It acts as a critical “first response region” to climate change in China and East Asia19. Climatic factors and grassland management both have strong influences on the seasonal and inter-annual dynamics of carbon fluxes3,7.Grazing mediates the relationships between ecosystem function and carbon flux variability by means of altered canopy structure and plant physiology4. Changes in ecosystem function can dominate the inter-annual variability of carbon fluxes20 and determine the ecosystem adaptability to climate change4. Thus, quantifying grazing effects on carbon dioxide uptake and emissions of alpine grassland systems is critical for understanding their role in global climate change.
To effectively monitor grazing effects on carbon flux, the eddy covariance technique is a reliable source of in situ measurements with its capability of automatic and high-frequency measurements of gas and energy fluxes. There have been few prior studies that have used the eddy covariance technique on paired treatments of grazing-nongrazing12,21, especially for actively managed grasslands22,23. These research gaps constrain our capacity to adequately address grazing disturbance effects. In particular, knowledge about grazing impacts on carbon fluxes is necessary to scale up carbon budget from measured sites to larger terrestrial entities24. To this end, in this study we focused on carbon fluxes of alpine meadow ecosystem under grazed and ungrazed treatments in the same environmental setting to examine how carbon fluxes respond to grazing in alpine meadows on the Tibetan Plateau. Our main research questions were: 1) Does light-intensity grazing could maintain higher ecosystem productivity compared with short-term fencing in these alpine meadows? and 2) How does grazing affect the responses of carbon fluxes to climatic variations?
Data and Methods
Site description
Our study site is located in the core zone of the Northern Tibetan Plateau, where a typical alpine Kobresia pygmaea meadow is distributed. The region belongs to the plateau subfrigid monsoon climate, with no absolute frost-free period throughout the year. The soil freezing period is from October to next May, and the annual mean air temperature is −1.9 °C. The mean annual rainfall is 380 mm, with 80% falling between June and September. The annual average sunlight exceeds 2886 h. The soil is classified as meadow soil with sandy loam. The vegetation is dominated by Kobresia pygmaea, accompanied by Potentilla bifurca, Potentilla saundersiana, Leontopodium pusillum, and Carex moorcroftii.
The grazing exclusion treatment meadow (FM, 31.643686°N, 92.009683°E, 4598 m a.s.l.) has been fenced since October 2011 and lies in the same watershed with the grazed treatment meadow (GM, 31.648722°N, 92.007208°E, 4607 m a.s.l.), which ensures that the two treatment sites experience similar local climates. The topographic map around the paired eddy covariance towers is shown in Fig. 1. Prior to the grazing exclusion treatment, vegetation and climates were quite similar between fenced and grazed sites. They exhibited no significant net ecosystem exchange (NEE) differences (P = 0.997) and had relatively homogeneous vegetation, dominated by Kobresia pygmaea (>65%). Before the experiment was established, both GM and FM was grazed using a common local grazing intensity of <0.5 sheep and <1.2 yak unit ha−1 half-y−1 for over 20 years, and the same grazing intensity maintained during the experiment at GM. Under the atmospheric neutral stability assumption, the footprint analysis25 showed that approximately 95% and 97% of the measured scalar fluxes originated from the FM and GM towers, respectively.
Figure 1. Area relief map of the study site based on a Digital Elevation Model (DEM) with 100 m equidistance lines.
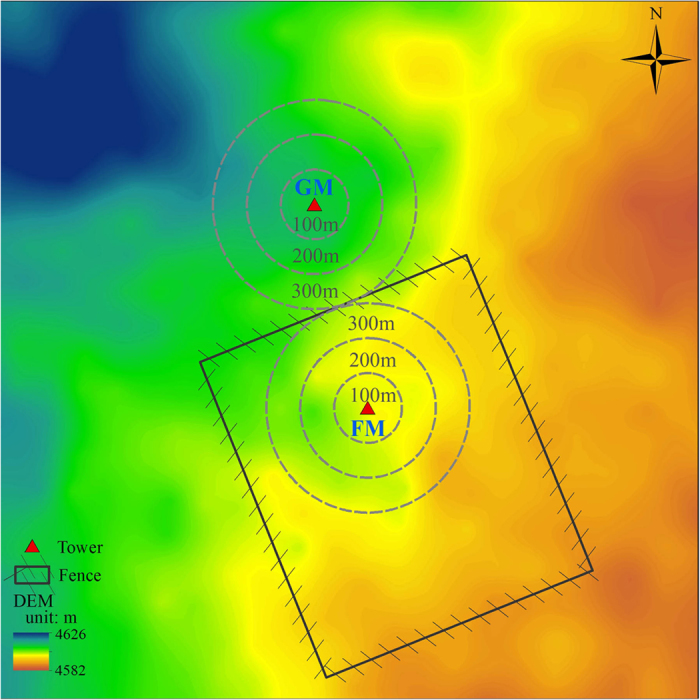
The paired towers are located in the fenced and grazed meadows, respectively, with 600 m distance between two towers.
Experimental measurements
The open-path eddy covariance (OPEC) system was used to measure carbon and water vapor fluxes at 2.3 m above the ground. The OPEC system consists of a 3-D sonic anemometer (Model CSAT-3, Campbell Scientific Inc., Logan, UT, USA) to measure three-dimensional wind speed and temperature fluctuations, and an infrared gas analyzer (Model LI-7500A, Li-cor Inc., Lincoln, NE, USA) to measure CO2 and water vapor densities. All signals were sampled at a frequency of 10 Hz. The CO2 and H2O fluxes were calculated and recorded at 30 min intervals by a CR3000 datalogger (Model CR3000, Campbell Scientific).
The meteorological conditions, including solar radiation, net radiation (Rn) and photosynthetically active radiation (PAR), were observed at 1.5 m height above the ground using a four-component net radiometer (Model CNR-1, Kipp&Zonen, Netherlands) and a quantum sensor (LI190SB, Li-cor Inc.). Air temperature (Ta) and relative humidity (RH) were measured at 1.8 m height (Model HMP45C, Vaisala Inc., Helsinki, Finland). Soil temperatures (Ts) and soil water contents (SWC) were recorded at four depths (0.05, 0.1, 0.2 and 0.5 m) with thermometers (109-L, Campbell Scientific) and TDR probes (Model CS616-L, Campbell Scientific), respectively. The above meteorological data were summarized to half-hour intervals, except precipitation (PPT), which was measured continuously by the tipping bucket rain gauge (TE525MM-L, Campbell Scientific). All meteorological data were recorded using a CR1000 datalogger (Model CR1000, Campbell Scientific).
Replicate samples (n = 10) for aboveground biomass were collected during June—August from 2012 to 2014 once a month by clipping vegetation of 0.5 × 0.5 m2 quadrats within a radius of 250 m around each observation tower.
Data processing
The analyses were based on half-hour means of CO2 fluxes from 2012 to 2014. The flux data were first aligned with the coordinate system of mean wind direction using the three-dimensional rotation26,27, then corrected for bias caused by air density variation due to heat and water vapor fluctuations using the Webb, Pearman and Leuning density correction (WPL), a correction for the effects of air density fluctuations28. Anomalous or spurious values caused by interference from rain, extreme cloud cover, dew, hoarfrost, or birds were excluded from the analysis.
The CO2 flux measured by the eddy covariance technique represents the net ecosystem CO2 exchange (NEE), which is equivalent to the net ecosystem productivity (NEP) multiplied by −1. The NEE was partitioned into gross primary productivity (GPP) and ecosystem respiration (Re)29. The daytime NEE was assumed to be valid for our analysis when photosynthetically active radiation (PAR) was greater than 1 μmol m−2 s−1. In calculating nighttime NEE, PAR was always less than 1 μmol m−2 s−1.To avoid possible flux underestimation under stable conditions during the night, effects of friction velocity u* were examined statistically. This analysis determined that the threshold values of u* were 0.16 m s−1 for fenced and 0.17 m s−1 for grazed meadows in 2012, 0.11 m s−1 for fenced and 0.13 m s−1 for grazed meadows in 2013 and 0.11 m s−1 for fenced and 0.14 m s−1 for grazed meadows in 2014, respectively. The negative nighttime NEE were excluded from our analysis. All the missing data for each 30 min were filled using the methods of linear fitting, nonlinear fitting and mean diurnal variations27,30. The number of missing observations, filtered values and the total observations were counted (Table 1). The energy balance closure was evaluated based on half-hourly observations using the energy balance ratio (EBR).
Table 1. Number of half-hourly observations obtained from eddy flux measurements.
| Fenced |
Grazed |
|||||||
|---|---|---|---|---|---|---|---|---|
| missing data | filtered data | total data | EBR | missing data | filtered data | total data | EBR | |
| 2012 | 7597 | 6356 | 17568 | 0.85 | 8517 | 5187 | 17568 | 0.86 |
| 2013 | 7182 | 6351 | 17520 | 0.83 | 8117 | 6327 | 17520 | 0.77 |
| 2014 | 5483 | 6666 | 17520 | 0.91 | 4137 | 7131 | 17520 | 0.89 |
*EBR is energy balance ratio.
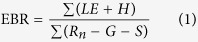 |
Where LE represents the latent heat flux, H represents sensible heat flux, Rn represents net radiation, G represents soil heat flux, and S represents canopy heat storage. The closer EBR is to 1, the better the energy balance closure.
To investigate grazing affects, we used data from 2012 to 2013 when the grazing effect was strongest (The differences between FM and GM were significant, P < 0.01). We used the Michaelis–Menten equation31 to calculate the ecosystem light response:
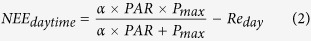 |
where the unit of NEEdaytime and PAR are mg CO2 m−2 s−1 and μmol m−2 s−1, respectively; α (mgCO2 μmolPhoton−1) is the apparent quantum yield or the initial slope of the light response curve; Pmax (mgCO2 m−2 s−1) is the maximum apparent photosynthetic capacity of the canopy; and Reday (mgCO2 m−2 s−1) is the ecosystem respiration during daytime.
Statistical analysis
To determine the driving factors on carbon fluxes, stepwise multiple linear regression analysis was employed. First, we conducted a comprehensive analysis that used Grazing (value = 0 at FM; value = 1 at GM), Rn, Ta, Ts, SWC and VPD as independent variables and NEP, Re and GPP as separate dependent variable. Second, to explore the relative contribution of each factor to carbon fluxes under the grazing or non-grazing treatment, we conducted partial correlation analysis of carbon fluxes (NEP, Re and GPP) against climatic factors (Rn, Ta, Ts, SWC and VPD) for FM and GM sites separately. By comparing the two response patterns (with and without grazing being included), we can simultaneously obtain knowledge about how grazing affects carbon flux response to climate change.
The one-way analysis of variance (ANOVA) was used to examine differences of carbon fluxes and environmental variables between the fenced and grazed sites. We used OriginPro 8 SR0 software to perform all the statistical analysis and the level of significance was set at 0.05.
Results
Characteristics of environmental variables and biotic environment
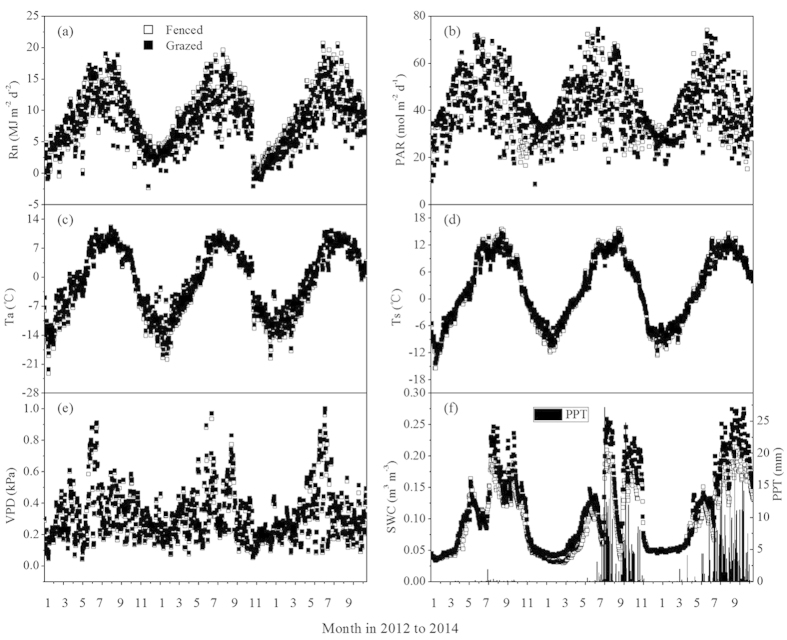
The net radiation (Rn), photosynthetically active radiation (PAR), air temperature (Ta), soil temperature (Ts), and vapor pressure deficit (VPD) all exhibited unimodal dynamics at both FM and GM within a year. The annual total radiations were similar between FM and GM (Fig. 2(a,b)). The average daily Ta was higher at GM than that at FM (Fig. 2(c), P = 0.048). The daily mean Ts was higher at FM than that at GM in growing season (P = 0.024), while the daily mean Ts was higher at GM in non-growing season (Fig. 2(d), P < 0.01). Both Ta and Ts reached their peaks from early July through late August at the two sites. The VPD was always lower at FM than that at GM across the three years (Fig. 2(e), P < 0.01). The mean SWC was lower at FM than at GM (P < 0.01) and the seasonal variations of SWC showed strong correspondence to rain events throughout each growing season (Fig. 2(f), P < 0.01). A severe drought in August 2013 resulted in a continuous decrease of SWC from early August until September (Fig. 2(f)) and a VPD peak in August (Fig. 2(e)).
Figure 2. Monthly change in daily net radiation (Rn, (a)), photosynthetically active radiation (PAR, (b)), air temperature (Ta, (c)), soil temperature (Ts, (d)) at 5 cm depth, vapor pressure deficit (VPD, (e)), and soil volumetric water content (SWC, f) at 5 cm depth for the fenced and grazed alpine meadows, 2012–2014.
Precipitation (PPT) data were available since June 2013.
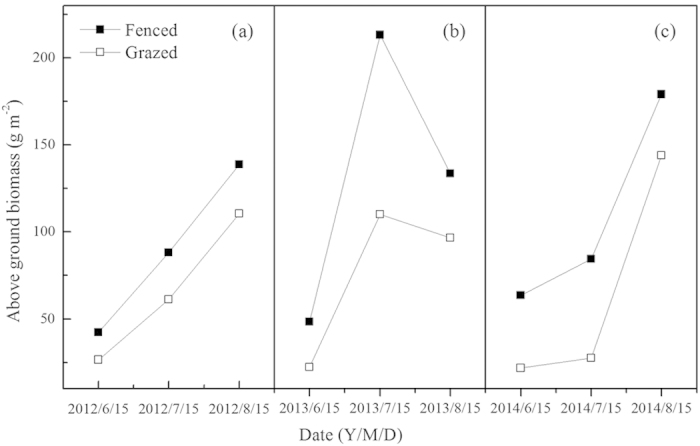
Both fenced and grazed grasslands achieved their maximum aboveground biomass in August 2012 and 2014 (Fig. 3(a,c)). The maximum aboveground biomass was 139 and 110 g m−2 for FM and GM in 2012, and 188 and 160 g m−2 in 2014, respectively. In 2013, an earlier precipitation peak arrived in July and resulted in an earlier biomass peak (Fig. 2(f)), with a maximum biomass values of 213 and 110 g m−2 for FM and GM, respectively. The biomass decreased sharply in August due to evident water stress in the alpine meadows.
Figure 3. Variations of aboveground biomass in peak season for the fenced and grazed alpine meadows for year (a) 2012, (b) 2013, and (c) 2014.
Daily and monthly dynamics of carbon fluxes
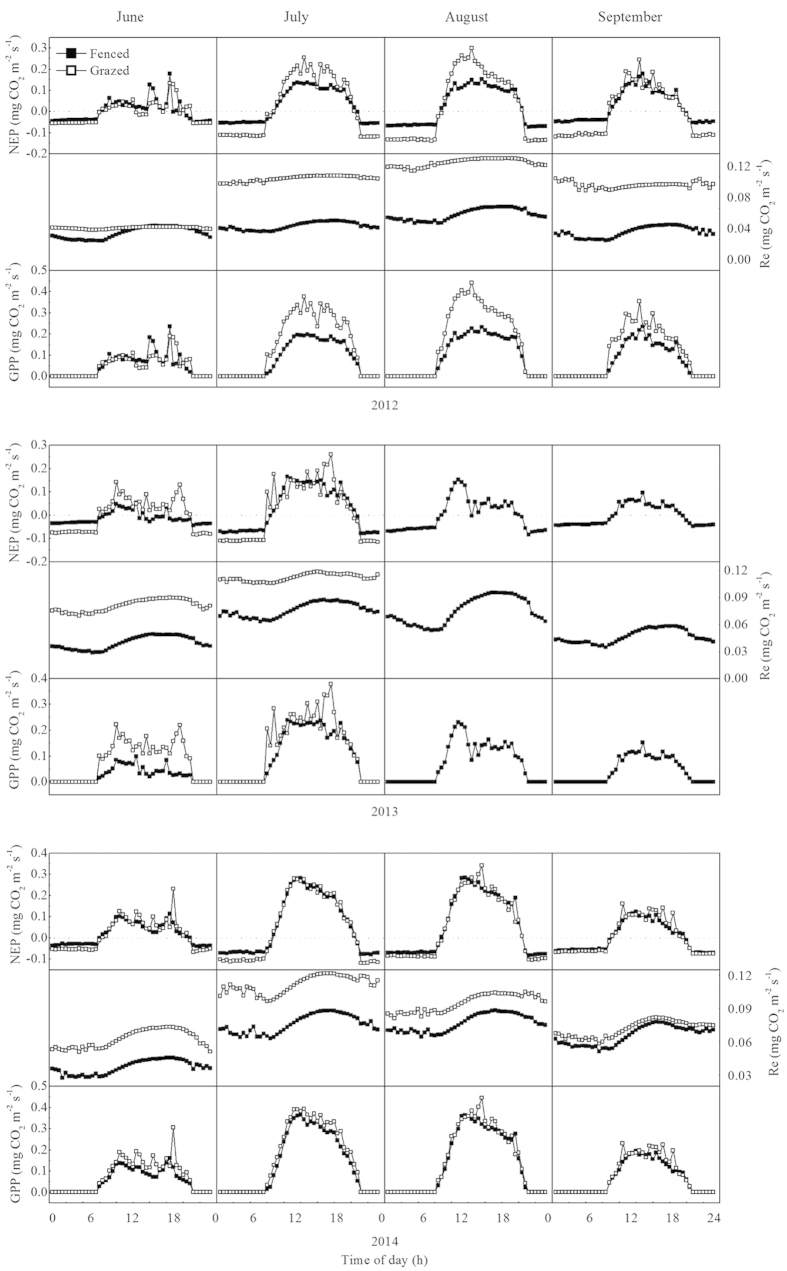
The daily dynamics of carbon fluxes (NEP, Re, and GPP) varied substantially between FM and GM within the growing seasons from 2012 to 2014 (Fig. 4). The GM had higher carbon flux peak values than those of FM, but their differences decreased throughout the experiment. The largest daily carbon fluxes in the two treatments were recorded for August in 2012 and 2014, and July in 2013. The NEP values were 0.152 and 0.299 mgCO2 m−2 s−1 for FM and GM in 2012, 0.285 and 0.342 mgCO2 m−2 s−1for FM and GM in 2014, and 0.166 and 0.260 mgCO2 m−2 s−1 for FM and GM in 2013. These peaks occurred simultaneously with the maximum biomass (Fig. 3). The NEP and GPP were greater before noon than in the afternoon, especially in August 2013—a severe drought month, leading to an asymmetrical distribution of NEP and GPP around noon. The daily maximum of NEP and GPP occurred at 11:00, with values of 0.152 and 0.229 mgCO2 m−2 s−1, respectively. The daily maximum Re occurred around 16:00–17:00, and the values were higher at GM than at FM throughout each growing season. Both ecosystems showed net carbon gains in July and August and neutral values in June and September. However, the net carbon sink turned to a weak carbon source in August 2013, with a NEP value of −1.64 gC m−2 in that month.
Figure 4. Daily variations of the monthly means of carbon fluxes (net ecosystem productivity (NEP), ecosystem respiration (Re), and gross primary productivity (GPP)) at the fenced and grazed meadows from June to September.
The 30 min data shown are means for all days in each month for the two ecosystems. Flux data were absent at the grazed meadow in August and September 2013.
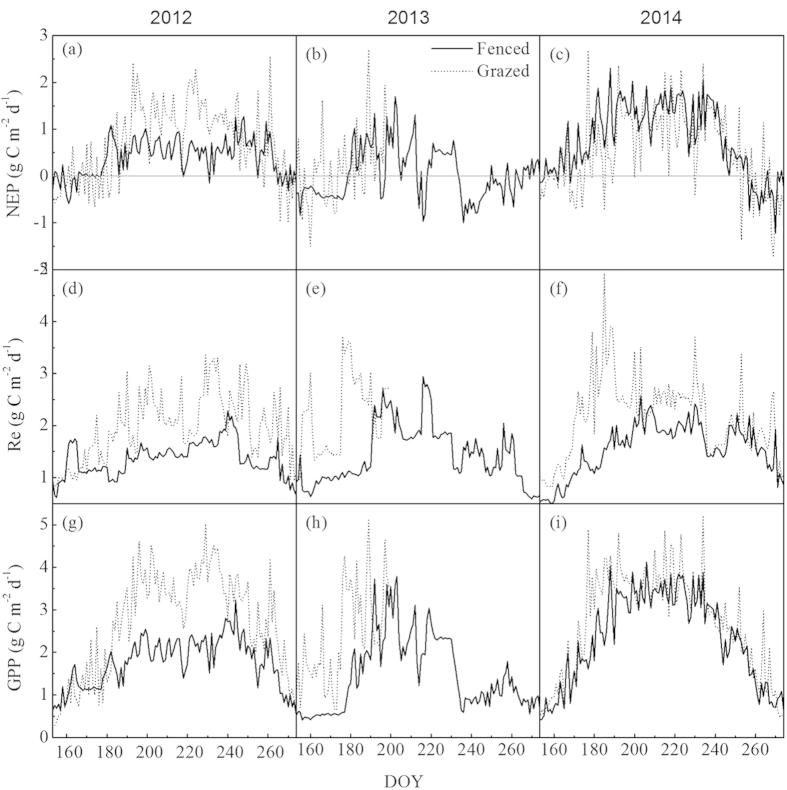
During the growing seasons from 2012 to 2014 (Fig. 5), the means of NEP were 0.39, 0.04, 0.79 gC m−2 d−1 for FM and 0.72, 0.19, 0.55 gC m−2 d−1 for GM, respectively. The means of Re were 1.36, 1.43, 1.55 gC m−2 d−1 for FM and 1.96, 2.19, 2.16 gC m−2 d−1 for GM, respectively. The means of GPP were 1.75, 1.47, 2.34 gC m−2 d−1 for FM and 2.68, 2.38, 2.71 gC m−2 d−1 for GM, respectively. One thing to note is that the carbon flux means at GM in 2013 were calculated using the data from DOY 153 to 200. Generally, carbon fluxes at GM were greater than those at FM in 2012. The NEP and GPP rebounded rapidly in the following two years, while recoveries of Re were not obvious. The carbon flux differences between FM and GM were obvious in 2012 and 2013 (P < 0.01), which provided an opportunity to investigate grazing effects on this alpine ecosystem.
Figure 5. Daily variations of NEP, Re, and GPP for the fenced and grazed meadows during the growing season from 2012 to 2014.
Comparison of photosynthetic capacity between FM and GM
Solar radiations were very strong at both sites (Fig. 2(a,b)). The NEE increased with light intensity and reached its maximum around 1500 μmol m−2 s−1 of PAR, then leveled off (Fig. 6). The α was 0.000358 mgCO2μmolPhoton−1 for the FM grassland and 0.000531 mgCO2μmolPhoton−1 for the GM grassland, respectively. The Pmax was 0.21 mgCO2 m−2 s−1 and 0.27 mgCO2 m−2 s−1 for the FM and GM grasslands, respectively.
Figure 6. Relationship between net ecosystem CO2 exchange (NEE) and light intensity at the fenced and grazed meadows during DOY 180–250, 2012–2013.
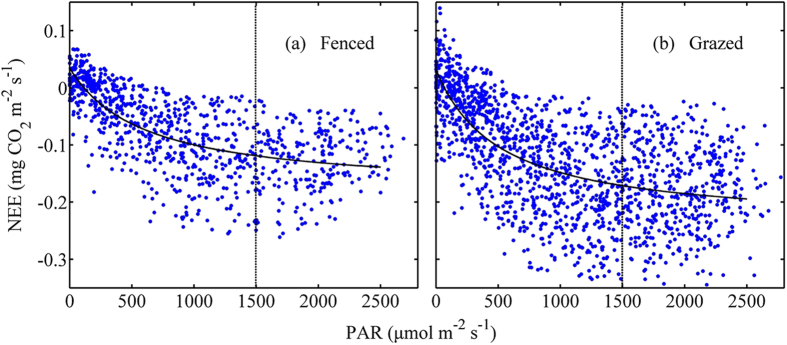
The curves are fitted results using Eq. (1) based on the observed data (nFenced = 902, nGrazed = 1565). The observed NEE were separated into two sections with a threshold of 1500 μmol m−2 s−1.
Under any environment group (Table 2), α and Pmax at the GM grasslands were greater than those at FM, indicating a greater carbon uptake capacity by the prior one. The α increased, but Pmax followed a flat trend along rising Ta at FM. At GM, both α and Pmax increased with rising Ta. The optimum temperatures were 10–13 °C and ~13 °C at FM and GM, respectively.
Table 2. Estimated coefficients describing the rectangular hyperbolic responses of daytime NEE to incident PAR (equation (2)) and corresponding average meteorological conditions under different air temperature (Ta), soil water content (SWC), and vapor pressure deficit (VPD) levels during the vegetation active periods of DOY 180–250 in the fenced meadow (FM) and grazed meadow (GM) from 2012 to 2013.
| α | Pmax | Reday | Ta | VPD | Ts | SWC | R2 | P | data volume | ||
|---|---|---|---|---|---|---|---|---|---|---|---|
| (mgCO2 μmolPhoton−1) | (mgCO2 m−2 s−1) | (°C) | (kPa) | (°C) | (m3 m−3) | ||||||
| Ta < 7 | Fenced | 0.000195 | 0.23 | 0.019 | 5.79 | 0.22 | 6.25 | 0.16 | 0.66 | <0.01 | 215 |
| Grazed | 0.000326 | 0.25 | 0.011 | 5.62 | 0.20 | 7.13 | 0.18 | 0.58 | <0.01 | 250 | |
| 7 ≤ Ta < 10 | Fenced | 0.000380 | 0.25 | 0.034 | 8.50 | 0.40 | 10.62 | 0.16 | 0.42 | <0.01 | 214 |
| Grazed | 0.000804 | 0.29 | 0.044 | 8.30 | 0.34 | 9.18 | 0.19 | 0.41 | <0.01 | 384 | |
| 10 ≤ Ta < 13 | Fenced | 0.000667 | 0.24 | 0.074 | 11.48 | 0.55 | 13.95 | 0.16 | 0.49 | <0.01 | 268 |
| Grazed | 0.000941 | 0.35 | 0.099 | 11.26 | 0.51 | 11.46 | 0.19 | 0.41 | <0.01 | 479 | |
| Ta ≥ 13 | Fenced | 0.000629 | 0.23 | 0.084 | 14.50 | 0.84 | 17.12 | 0.17 | 0.40 | <0.01 | 205 |
| Grazed | 0.000947 | 0.34 | 0.125 | 14.59 | 0.85 | 15.27 | 0.19 | 0.39 | <0.01 | 457 | |
| SWC < 0.13 | Fenced | 0.000278 | 0.22 | 0.036 | 8.66 | 0.46 | 12.28 | 0.11 | 0.40 | <0.01 | 168 |
| Grazed | 0.000444 | 0.24 | 0.021 | 9.19 | 0.36 | 10.13 | 0.12 | 0.33 | <0.01 | 82 | |
| 0.13 ≤ SWC < 0.18 | Fenced | 0.000350 | 0.23 | 0.036 | 9.46 | 0.54 | 11.93 | 0.16 | 0.51 | <0.01 | 478 |
| Grazed | 0.000482 | 0.31 | 0.037 | 10.05 | 0.56 | 11.68 | 0.16 | 0.46 | <0.01 | 602 | |
| 0.18 ≤ SWC < 0.23 | Fenced | 0.000500 | 0.20 | 0.034 | 10.88 | 0.44 | 12.30 | 0.19 | 0.53 | <0.01 | 256 |
| Grazed | 0.000557 | 0.27 | 0.035 | 10.86 | 0.53 | 11.27 | 0.21 | 0.44 | <0.01 | 586 | |
| SWC ≥ 0.23 | Fenced | — | — | — | — | — | — | — | — | — | — |
| Grazed | 0.000748 | 0.22 | 0.024 | 11.04 | 0.45 | 11.02 | 0.24 | 0.40 | <0.01 | 295 | |
| VPD < 0.3 | Fenced | 0.000277 | 0.26 | 0.023 | 5.57 | 0.17 | 7.62 | 0.16 | 0.53 | <0.01 | 270 |
| Grazed | 0.000484 | 0.29 | 0.017 | 6.52 | 0.18 | 8.24 | 0.19 | 0.45 | <0.01 | 425 | |
| 0.3 ≤ VPD < 0.6 | Fenced | 0.000574 | 0.25 | 0.065 | 10.19 | 0.44 | 11.63 | 0.16 | 0.42 | <0.01 | 316 |
| Grazed | 0.001204 | 0.36 | 0.115 | 10.47 | 0.45 | 10.49 | 0.20 | 0.38 | <0.01 | 578 | |
| 0.6 ≤ VPD < 0.9 | Fenced | 0.000800 | 0.27 | 0.104 | 12.12 | 0.75 | 15.42 | 0.16 | 0.46 | <0.01 | 222 |
| Grazed | 0.001054 | 0.38 | 0.144 | 12.54 | 0.72 | 13.23 | 0.19 | 0.41 | <0.01 | 357 | |
| VPD ≥ 0.9 | Fenced | 0.000485 | 0.23 | 0.079 | 14.32 | 1.03 | 18.73 | 0.15 | 0.54 | <0.01 | 94 |
| Grazed | 0.000777 | 0.33 | 0.113 | 15.26 | 1.05 | 16.73 | 0.18 | 0.40 | <0.01 | 205 | |
Eq. (2) was used to fit the regression coefficients based on the observed data.
The value of α increased with enhanced SWC at both FM and GM. Across the two treatment grasslands, Pmax was greatest when SWC was in the range between 0.13 and 0.18 m3 m−3. The maximum α in GM and FM occurred in the VPD range between 0.3 and 0.6 and between 0.6 and 0.9, respectively, with a greater value for the prior treatment than for the latter one. Both ecosystems achieved their maximum Pmax when VPD fell in the range between 0.6 and 0.9 kPa, and Pmax of GM was 29% greater than that of FM.
Drivers of carbon fluxes
We analyzed the relationships between carbon fluxes (i.e., NEP, Re and GPP) and their potential drivers (Grazed, Rn, Ta, Ts, SWC and VPD) using multiple linear stepwise regression (Table 3). In this alpine ecosystem, grazing had the strongest influence on Re, and the Rn had the strongest influence on NEP and GPP. Grazing was the second most important driver of GPP.
Table 3. The results of multiple linear stepwise regression analysis of carbon fluxes (NEP, Re and GPP) against the potential drivers (Grazed, Rn, Ta, Ts, SWC and VPD) during the active vegetation period from 2012 to 2014.
| regression equation | F | P | Grazed | Rn | Ta | Ts | SWC | VPD |
|---|---|---|---|---|---|---|---|---|
| NEP = 0.038–0.01Grazed + 0.0003Rn + 0.008Ta–0.005Ts + 0.23SWC–0.081VPD | 624.1 | <0.01 | −0.05** | 0.53** | 0.14** | −0.11** | 0.09** | −0.12** |
| Re = 0.104 + 0.051Grazed + 0.000006Rn + 0.001Ta–0.0004Ts–0.26SWC + 0.012VPD | 707.8 | <0.01 | 0.63** | 0.05** | 0.03* | 0.03* | −0.30** | 0.06** |
| GPP = 0.138 + 0.04Grazed + 0.0003Rn + 0.008Ta–0.004Ts–0.067VPD | 789.2 | <0.01 | 0.21** | 0.52** | 0.14** | −0.10** | — | −0.10** |
*indicates that the correlation is significant at the 0.05 level;
**indicates that the correlation is significant at the 0.01 level
The Grazed, Rn, Ta, Ts, SWC and VPD columns represent the partial correlation coefficients between carbon fluxes and each climatic factor, respectively.
The partial correlation analysis of carbon fluxes against climatic factors was conducted for the fenced and grazed meadow separately, to differentiate the role of grazing from climatic factors (Table 4). Rn was the main driver on NEP and GPP in both ecosystems. Re was mainly controlled by Ts at FM and by SWC at GM. In general, grazing weakened the correlations of GPP and NEP with climatic factors. Also, grazing weakened the correlations of Re with Rn, Ta and Ts, but enhanced the correlation of Re with SWC and VPD.
Table 4. The partial correlation coefficients between carbon fluxes and climatic factors during the active vegetation periods from 2012 to 2014 at FM (Fenced)and GM (Grazed).
| regression equation | F | P | Rn | Ta | Ts | SWC | VPD |
|---|---|---|---|---|---|---|---|
| Fenced: NEP = −0.009 + 0.0003Rn + 0.011Ta–0.007Ts + 0.46SWC–0.085VPD | 350.2 | <0.01 | 0.538** | 0.205** | −0.153** | 0.134** | −0.128** |
| Grazed: NEP = 0.056 + 0.0003Rn + 0.004Ta–0.004Ts + 0.155SWC–0.061VPD | 411.2 | <0.01 | 0.511** | 0.076** | −0.079** | 0.067** | −0.093** |
| Fenced: Re = 0.051 + 0.00001Rn + 0.001Ta + 0.002Ts–0.053SWC–0.006VPD | 272.6 | <0.01 | 0.112** | 0.061** | 0.299** | −0.086** | −0.049* |
| Grazed: Re = 0.191–0.002Ts–0.329SWC + 0.034VPD | 245.4 | <0.01 | — | — | −0.141** | −0.375** | 0.201** |
| Fenced: GPP = 0.043 + 0.0003Rn + 0.012Ta–0.004Ts + 0.407SWC–0.091VPD | 338.5 | <0.01 | 0.529** | 0.204** | −0.092** | 0.113** | −0.129** |
| Grazed: GPP = 0.246 + 0.0003Rn + 0.004Ta–0.005Ts–0.173SWC–0.027VPD | 411.9 | <0.01 | 0.498** | 0.072** | −0.112** | −0.072** | −0.04* |
*indicates that the correlation is significant at the 0.05 level.
**indicates that the correlation is significant at the 0.01 level.
Discussions
Response of carbon budget to grazing in the alpine meadow
The daily amplitudes of NEP at FM are within the range observed for grassland ecosystems with similar low leaf area index on the Tibetan Plateau32,33. A wider range of temporal variation has been identified at GM than at FM, though it has been fenced only for three years. This result indicates more carbon uptake in daytime and more carbon emission in nighttime at GM than at FM, which is in accord with similar studies conducted in tallgrass prairies in US12 and desert steppe on the Mongolian Plateau21. The wider daily NEP amplitude at GM during growing seasons emphasizes the importance of including anthropogenic activities disturbances in future carbon flux modeling work.
The grazed meadow acted as a stronger carbon sink than the fenced meadow in the first two years of fencing. This result shows that lightly-grazed alpine meadows can contribute more to atmospheric carbon uptake than similar meadows where grazing is excluded12,21. Similar findings have been reported in mixed prairie in Nevada, USA34,35. The higher GPP and Re at GM than that at FM were also in accord with previous studies36 conducted for shortgrass steppe in northeastern Colorado, US.
The short-term fencing decreased ecosystem productivity. However, the ecosystem productivity of FM increased over time and reached a level almost equal to the GM site in the third year of fencing. Possible reasons might be that the ecosystem had adapted to the new environment37, and that grazing intensity in this alpine ecosystem is light. This experiment provided a way to judge the grazing intensity of a grazed grassland ecosystem. It also shows that in overgrazed areas, fencing would be a good method to facilitate ecosystem recovery.
Response of photosynthetic capacity to grazing in various environmental conditions
Ecosystem gross photosynthesis is driven by biotic and abiotic factors, as well as their interactions30,30,38. The higher ecosystem carbon sequestration potential at GM than at FM might be caused by a temporary negative response of the latter ecosystem to non-grazing. More suitable microclimate at GM might be another reason21. Temperature is a critical environmental factor for plant growth on the Tibetan Plateau30. The alpine plants have been acclimated to the long-term low temperature conditions39 by developing photosynthetic and other related organisms optimal for low temperatures30. The higher optimum temperature for carbon uptake at GM than that at FM indicates that light-intensity grazing might improve alpine meadow ecosystem productivity under global warming.
Carbon assimilation was significantly reduced in both ecosystems when Ta < 7 °C. Both α and Pmax increased with Ta at GM, while Pmaxalmost leveled off along increased temperature at FM. This is the direct result of greater carbon uptake at GM than FM during the active growing season in the first two years of fencing. Grazing also indirectly affected carbon uptake by changing Ts4. Reduced biomass of litter and dead standing plants caused by grazing can weaken the greenhouse effect of canopy, and maintain suitable temperature and light conditions for plant growth.
Generally, water deficiency causes decreased net carbon uptake and internal leaf CO2 concentrations through adjusting leaf stomata40. In this study, α increased with SWC in both treatments, which was consistent with other studies41,42. The GM had higher SWC than FM due to reduced evapotranspiration caused by grazing13. Therefore, the GM had a greater potential for carbon sequestration. The maximum Pmax appeared when SWC fell in the rang within 0.13–0.18, not at the point of maximum SWC, which indicates that moisture was not a limiting factor in this alpine meadow. The higher α and Pmax for GM than for FM might be related to the grazed meadow’s more physiologically active leaves and higher single-leaf photosynthetic capacity21 under the situation of reduced green net primary productivity (GNPP) and leaf area index (LAI) caused by grazing14.
Normally, there is a reduction in carbon uptake at higher VPD because of stomatal closure under drought conditions43. In this study, the lowest carbon uptake was not observed when VPD was greater than 0.9. On the contrary, both ecosystems had lower carbon sequestration under when VPD was lower than 0.3 because of low temperature. This indicated that Ta, not VPD was the major factor regulating ecosystem carbon uptake of this alpine meadow during active growing seasons.
Grazing effects on the alpine meadow ecosystem productivity
Grazing can increase ecosystem photosynthesis and respiration. Light-intensity grazing will stimulate development of new leaves, whose higher photosynthetic capacity can compensate for reduced photosynthesis caused by thinned leaves12. In addition, grazing can improve soil fertility by increasing available nitrogen, which generates another avenue for enhancing ecosystem productivity44 and ecosystem respiration. The reason is that respiration is limited by supplies of carbohydrates fixed through photosynthesis30. Animal waste decomposition releases a large amount of CO2, which exerts a significant influence on ecosystem respiration. The improved microclimates at GM45,46 and influences of animal saliva on grass growth47 may also be responsible for increased carbon fluxes. For example, an open canopy allows for more adequate sunshine at GM, consequently prolonging carbon uptake time during thedaytime21. In contrast, the denser shading at FM can reduce carbon fluxes48,49.
The stepwise multiple linear regression analysis revealed the importance of grazing in controlling the carbon budget of this alpine meadow within three years since fencing. The absolute response magnitude of carbon fluxes to climate change should increase with plant biomass4. By reducing aboveground biomass, grazing lessened the strength of flux—climate correlations. It means that grazing weakened the response of carbon fluxes to climatic factors3,4, and light-intensity grazing might thus improve adaptation of this alpine ecosystem to changed climates50.
Additional Information
How to cite this article: Zhang, T. et al. Light-intensity grazing improves alpine meadow productivity and adaption to climate change on the Tibetan Plateau. Sci. Rep. 5, 15949; doi: 10.1038/srep15949 (2015).
Acknowledgments
This work was supported by the 973 Program (2013CB956302) of the Ministry of Science and Technology of China and One Hundred Talent Plan, Chinese Academy of Sciences. We thank Xianjin Zhu, Ke Huang, Yaojie Liu, Jian Tao, Yanbin Jiang, and Li Tian for their valuable comments and suggestions.
Footnotes
Author Contributions T.Z., Y.J.Z., G.R.Y. and X.Z.Z. conceived and designed the experiments. T.Z., Y.J.Z., X.Y., J.T.Z. and J.S.W. performed the experiments. T.Z., Y.J.Z., M.J.X. and S.L.N. analyzed the data. T.Z., Y.J.Z., M.C.W. and M.J.X. wrote the paper and edited the manuscript. All authors read and approved the final manuscript.
References
- Groisman P. Y. et al. The northern Eurasia earth sicence partnership. B. Am. Meteorol. Soc. 90, 671–688 (2009). [Google Scholar]
- Tan Z., Liu S., Tieszen L. L. & Tachie-Obeng E. Simulated dynamics of carbon stocks driven by changes in land use, management and climate in a tropical moist ecosystem of Ghana. Agric., Ecosyst. Environ. 130, 171–176, 10.1016/j.agee.2009.01.004 (2009). [DOI] [Google Scholar]
- Peichl M., Carton O. & Kiely G. Management and climate effects on carbon dioxide and energy exchanges in a maritime grassland. Agric., Ecosyst. Environ. 158, 132–146 (2012). [Google Scholar]
- Polley H. W., Frank A. B., Sanabria J. & Phillips R. L. Interannual variability in carbon dioxide fluxes and flux-climate relationships on grazed and ungrazed northern mixed-grass prairie. Global Change Biol. 14, 1620–1632, 10.1111/j.1365-2486.2008.01599.x (2008). [DOI] [Google Scholar]
- Eaton J. M., McGoff N. M., Byrne K. A., Leahy P. & Kiely G. Land cover change and soil organic carbon stocks in the Republic of Ireland 1851–2000. Clim. Change 91, 317–334 (2008). [Google Scholar]
- Zeeman M. J. et al. Management and climate impacts on net CO2 fluxes and carbon budgets of three grasslands along an elevational gradient in Switzerland. Agr. Forest Meteorol. 150, 519–530, 10.1016/j.agrformet.2010.01.011 (2010). [DOI] [Google Scholar]
- Schmitt M., Bahn M., Wohlfahrt G., Tappeiner U. & Cernusca A. Land use affects the net ecosystem CO2 exchange and its components in mountain grasslands. Biogeosciences 7, 2297–2309 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niu S. et al. Seasonal hysteresis of net ecosystem exchange in response to temperature change: patterns and causes. Global Change Biol 17, 3102–3114, 10.1111/j.1365-2486.2011.02459.x (2011). [DOI] [Google Scholar]
- Prescher A.-K., Grünwald T. & Bernhofer C. Land use regulates carbon budgets in eastern Germany: From NEE to NBP. Agr. Forest Meteorol 150, 1016–1025, 10.1016/j.agrformet.2010.03.008 (2010). [DOI] [Google Scholar]
- Cao G. et al. Grazing intensity alters soil respiration in an alpine meadow on the Tibetan plateau. Soil Biol. Biochem 36, 237–243 (2004). [Google Scholar]
- Chen B. et al. The impact of climate change and anthropogenic activities on alpine grassland over the Qinghai-Tibet Plateau. Agr. Forest Meteorol 189, 11–18, 10.1016/j.agrformet.2014.01.002 (2014). [DOI] [Google Scholar]
- Owensby C. E., Ham J. M. & Auen L. M. Fluxes of CO2 from grazed and ungrazed tallgrass prairie. Rangeland Ecol. Manag. 59, 111–127, 10.2111/05-116r2.1 (2006). [DOI] [Google Scholar]
- Bremer D. J., Auen L. M., Ham J. M. & Owensby C. E. Evapotranspiration in a Prairie Ecosystem. Agron. J. 93, 338–348 (2001). [Google Scholar]
- Shao C., Chen J., Li L. & Zhang L. Ecosystem responses to mowing manipulations in an arid Inner Mongolia steppe: An energy perspective. J. Arid Environ. 82, 1–10 (2012). [Google Scholar]
- Klein J. A., Harte J. & Zhao X.-Q. Dynamic and complex microclimate responses to warming and grazing manipulations. Global Change Biol. 11, 1440–1451, 10.1111/j.1365-2486.2005.00994.x (2005). [DOI] [Google Scholar]
- Enriquez A. S., Chimner R. A. & Cremona M. V. Long-term grazing negatively affects nitrogen dynamics in Northern Patagonian wet meadows. J. Arid Environ. 109, 1–5, 10.1016/j.jaridenv.2014.04.012 (2014). [DOI] [Google Scholar]
- McSherry M. E. & Ritchie M. E. Effects of grazing on grassland soil carbon: a global review. Global Change Biol. 19, 1347–1357, 10.1111/gcb.12144 (2013). [DOI] [PubMed] [Google Scholar]
- Zhang G., Zhang Y., Dong J. & Xiao X. Green-up dates in the Tibetan Plateau have continuously advanced from 1982 to 2011. PNAS 110, 4309–4314 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li C. Q. & Tang M. C. The climate change of Qinghai-Xizang plateau and its neighborhood in the recent 30 years. Plateau Meteorol. 4, 332–341 (1988). [Google Scholar]
- Marcolla B. et al. Climatic controls and ecosystem responses drive the inter-annual variability of the net ecosystem exchange of an alpine meadow. Agr. Forest Meteorol. 151, 1233–1243, 10.1016/j.agrformet.2011.04.015 (2011). [DOI] [Google Scholar]
- Shao C., Chen J. & Li L. Grazing alters the biophysical regulation of carbon fluxes in a desert steppe. Environ. Res. Lett. 8, 025012 (2013). [Google Scholar]
- Kato T. & Tang Y. Spatial variability and major controlling factors of CO2sink strength in Asian terrestrial ecosystems: evidence from eddy covariance data. Global Change Biol. 14, 2333–2348, 10.1111/j.1365-2486.2008.01646.x (2008). [DOI] [Google Scholar]
- Baldocchi D. et al. FLUXNET: A new tool to study the temporal and spatial variability of ecosystem-scale carbon dioxide, water vapor, and energy flux densities. B. Am. Meteorol. Soc. 82, 2415–2434, 10.1175/1520-0477(2001). [DOI] [Google Scholar]
- Yuan W. et al. Global estimates of evapotranspiration and gross primary production based on MODIS and global meteorology data. Remote Sens. Environ. 114, 1416–1431, 10.1016/j.rse.2010.01.022 (2010). [DOI] [Google Scholar]
- Stannard D. I. A theoretically based determination of Bowen-ratio fetch requirements. Bound-Lay Meteorol. 83, 375–406 (1997). [Google Scholar]
- Wilczak J. M., Oncley S. P. & Stage S. A. Sonic anemometer tilt correction algorithms. Bound-Lay Meteorol. 99, 127–150, 10.1023/a:1018966204465 (2001). [DOI] [Google Scholar]
- Falge E. et al. Gap filling strategies for defensible annual sums of net ecosystem exchange. Agr. Forest Meteorol. 107, 43–69, 10.1016/s0168-1923(00)00225-2 (2001). [DOI] [Google Scholar]
- Webb E. K., Pearman G. I. & Leuning R. Correction of flux measurements for density effects due to heat and water—vapor transfer. Q. J. Roy. Meteor. Soc. 106, 85–100 (1980). [Google Scholar]
- Reichstein M. et al. On the separation of net ecosystem exchange into assimilation and ecosystem respiration: review and improved algorithm. Global Change Biol. 11, 1424–1439 (2005). [Google Scholar]
- Fu Y. L. et al. Depression of net ecosystem CO2 exchange in semi-arid Leymus chinensis steppe and alpine shrub. Agr. Forest Meteorol. 137, 234–244, 10.1016/j.agrformet.2006.02.009 (2006). [DOI] [Google Scholar]
- Michaelis L. & Menten M. L. Die kinetik der invertinwirkung. Biochem. Z 49, 333–369 (1913). [Google Scholar]
- Shi P. L. et al. Net ecosystem CO2 exchange and controlling factors in a steppe - Kobresia meadow on the Tibetan Plateau. Sci. China Ser. D-Earth Sci. 49, 207–218, 10.1007/s11430-006-8207-4 (2006). [DOI] [Google Scholar]
- Kato T. et al. Carbon dioxide exchange between the atmosphere and an alpine meadow ecosystem on the Qinghai–Tibetan Plateau, China. Agr. Forest Meteorol. 124, 121–134 (2004). [Google Scholar]
- Frank A. B. & Dugas W. A. Carbon dioxide fluxes over a northern, semiarid, mixed-grass prairie. Agr. Forest Meteorol. 108, 317–326 (2001). [Google Scholar]
- Frank A. B. Carbon dioxide fluxes over a grazed prairie and seeded pasture in the Northern Great Plains. Environ. Pollut. 116, 397–403, 10.1016/s0269-7491(01)00216-0 (2002). [DOI] [PubMed] [Google Scholar]
- LeCain D. R., Morgan J. A., Schuman G. E., Reeder J. D. & Hart R. H. Carbon exchange and species composition of grazed pastures and exclosures in the shortgrass steppe of Colorado. Agric., Ecosyst. Environ. 93, 421–435 (2002). [Google Scholar]
- Misson L. et al. Functional changes in the control of carbon fluxes after 3 years of increased drought in a Mediterranean evergreen forest? Global Change Biol. 16, 2461–2475 (2010). [Google Scholar]
- Law B. E. et al. Environmental controls over carbon dioxide and water vapor exchange of terrestrial vegetation. Agr. Forest Meteorol. 113, 97–120 (2002). [Google Scholar]
- Cui X. et al. Photosynthetic depression in relation to plant architecture in two alpine herbaceous species. Environ. Exp. Bot. 50, 125–135 (2003). [Google Scholar]
- Farquhar G. D., Schulze E. D. & Kuppers M. Responses to humidity by stomata of Nicotiana glauca L. and Corylus avellana L. are consistent with the optimization of carbon dioxide uptake with respect to water loss. Funct. Plant Biol. 7, 315–327 (1980). [Google Scholar]
- Li S. G. et al. Net ecosystem carbon dioxide exchange over grazed steppe in central Mongolia. Global Change Biol. 11, 1941–1955 (2005). [Google Scholar]
- Wang Z., Xiao X. & Yan X. Modeling gross primary production of maize cropland and degraded grassland in northeastern China. Agr. Forest Meteorol. 150, 1160–1167, 10.1016/j.agrformet.2010.04.015 (2010). [DOI] [Google Scholar]
- Chen J. et al. Biophysical controls of carbon flows in three successional Douglas-fir stands based on eddy-covariance measurements. Tree Physiol 22, 169–177 (2002). [DOI] [PubMed] [Google Scholar]
- Lin Y. et al. Grazing intensity affected spatial patterns of vegetation and soil fertility in a desert steppe. Agric., Ecosyst. Environ. 138, 282–292 (2010). [Google Scholar]
- Xu L. K. & Baldocchi D. D. Seasonal variation in carbon dioxide exchange over a Mediterranean annual grassland in California. Agr. Forest Meteorol. 123, 79–96, 10.1016/j.agrformet.2003.10.004 (2004). [DOI] [Google Scholar]
- Zhang W. L. et al. Biophysical regulations of carbon fluxes of a steppe and a cultivated cropland in semiarid Inner Mongolia. Agr. Forest Meteorol. 146, 216–229, 10.1016/j.agrformet.2007.06.002 (2007). [DOI] [Google Scholar]
- Liu J. et al. Plants can benefit from herbivory: stimulatory effects of sheep saliva on growth of Leymus chinensis. PLoS One 7, e29259, 10.1371/journal.pone.0029259 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craine J. M., Wedin D. A. & Chapin Iii F. S. Predominance of ecophysiological controls on soil CO2 flux in a Minnesota grassland. Plant Soil 207, 77–86 (1999). [Google Scholar]
- Bremer D. J., Ham J. M., Owensby C. E. & Knapp A. K. Responses of soil respiration to clipping and grazing in a tallgrass prairie. J. Environ. Qual. 27, 1539–1548 (1998). [Google Scholar]
- Reichstein M. et al. Climate extremes and the carbon cycle. Nature 500, 287–295, 10.1038/nature12350 (2013). [DOI] [PubMed] [Google Scholar]