Abstract
A facile hydrothermal approach has been developed to prepare defective TiO2−x nanocrystals using Ti(III)-salt as a precursor and L-ascorbic acid as reductant and structure direction agent. The prepared TiO2−x nanocrystals are composed of a highly crystallized TiO2 core and a disordered TiO2−x outer layer, possessing high surface area, controlled oxygen vacancy concentration and tunable bandgap via simply adjusting the amount of added L-ascorbic acid. The defective TiO2−x shows high photocatalytic efficiency in methylene blue and phenol degradation as well as in hydrogen evolution under visible light, underlining the significance of the present strategy for structural and bandgap manipulation in TiO2-based photocatalysis.
TiO2 is one of extensively studied photocatalytic materials due to its excellent physicochemical properties as well as its earth abundance, nontoxicity and stability1,2,3,4. However, the large band gap (3.2 eV for anatase) limits its utilization of sunlight and thus its practical applications in many important fields such as photocatalytic hydrogen evolution5, environmental remediation6 and solar energy conversion7. To overcome this barrier, great efforts have been devoted to engineer TiO2′s band gap from a variety of aspects in order to approach a high photoactivity under visible light irradiation. Introducing dopants is one of the most intensively investigated strategies to enhance its visible light utilization. Initially, metal ions were used as dopants to introduce states into the TiO2 band gap8,9,10, but the subsequential problems such as thermal instability, increased carrier recombination centers11,12, and the need for an expensive ion-implantation facility significantly limit its applications. Nonmetal elements was also adopted13,14, if doped under the right conditions, can effectively narrow band gap of TiO2 and improve its visible-light absorption. Compared to other elements, nitrogen doping is thought to be the most useful choice to enhance its visible-light photocatalytic activity15. But unfortunately, further studies find that the activity of N-TiO2 for visible-light induced hydrogen evolution is quite low16.
Recently, the intrinsic defects in TiO2 matrix such as oxygen vacancy (Vo) and Ti3+ have been proved to trigger the visible-light activity of TiO217,18,19,20,21,22,23,24. Chen et al.17 have reported that hydrogen thermal treatment of TiO2 nanoparticles can generate an amorphous layer near the surface to form defective black TiO2−x nanoparticles. Such defective black TiO2−x nanoparticles display excellent photoactivity and stability in photocatalytic hydrogen generation. Theoretical calculations demonstrate that high vacancy concentration could induce a vacancy band of electronic states just below the conduction band and narrow the band gap to 1.0 eV. Except its optical and electronic properties, the performance of defective TiO2−x also depends largely on its morphological and structural properties25,26,27, like its surface area28, particle size and pore structure29. However, to the best of our knowledge, rare work has been reported on controlling optical and morphological structure properties simultaneously for defective TiO2−x. Herein, we report a facile hydrothermal approach to produce defective TiO2−x nanocrystals with high surface area and tailoring band gap using TiCl3 as a precursor and L-ascorbic acid as reductant and structure direction agent.
Results
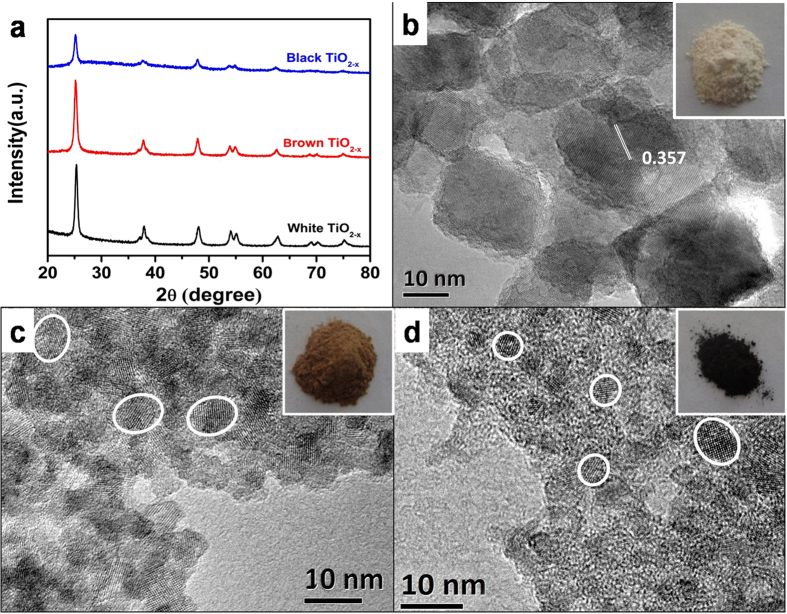
The obtained defective TiO2−x samples display diffraction peaks at 25.4°, 37.9°, 48.1° and 53.1° in the XRD patterns, indicating that as synthesized TiO2−x is in a pure anatase phase (Fig. 1a). It suggests that the presence of L-ascorbic acid does not influence the crystal structure of TiO2−x. TEM analysis with the obtained defective TiO2−x samples shows that the particle size of highly crystallized TiO2 core gradually decreases from 50 nm to 10 nm (Figs. 1b-1d) as increasing the amount of L-ascorbic acid from 0, 0.3 to 0.7 g in 80 mL solution. The crystal lattices of TiO2 core can be clearly identified in all the samples, indicating that the TiO2 core is highly crystallized. Since the particles are overlapped, the disordered TiO2−x outer layer cannot be distinguished in TEM, but its presence can be indirectly confirmed via further sintering of the defective black TiO2−x at 500 °C in N2 atmosphere considering that high concentration of vacancy could significantly reduce the melting temperature of TiO225,26. The sintered TiO2−x exhibits a nanoscroll structure with several layers of TiO2−x sheets and the interlayer spacing is 0.76 nm (see Fig. S1a for details). EDX measurement (Fig. S1b) confirms that the TiO2−x nanoscroll is only composed of Ti (56.4 at%) and O (28.8 at%) elements, implying the presence of defective TiO2−x layer surrounding the TiO2 core. It is worth to mention, the defective TiO2−x nanosheet is a fascinating structure to apply in Li-ion battery and photocatalysis for its ultrahigh cycle rate and high efficiency30,31. This report provides a new and simple method for preparing the defective TiO2−x nanosheets.
Figure 1. XRD patterns (a), TEM images (white TiO2−x(b), brown TiO2−x(c), black TiO2−x(d)), of the defective TiO2−x nanocrystals.
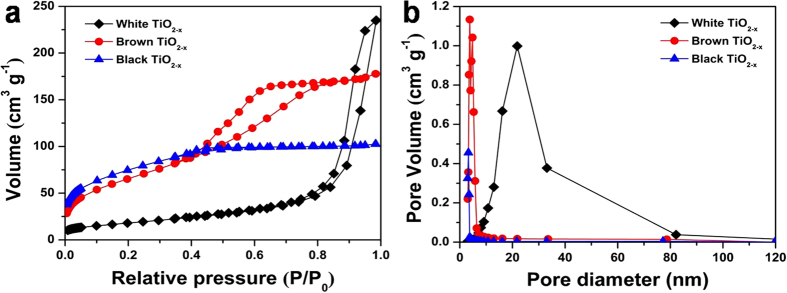
The N2 adsorption–desorption isotherms (Fig. 2a) of defective TiO2−x samples exhibit a typical type-IV isotherm with a distinct hysteretic loop, indicating mesoporous features. The average pore size (Fig. 2b) decreases with increasing the amount of added L-ascorbic acid, while the corresponding BET surface area (Tab. S1) dramatically increases from 64.56 m2 g−1 (white TiO2−x), 188.75 m2 g−1 (brown TiO2−x) to 263.95 m2 g−1 (black TiO2−x). Therefore, the presence of L-ascorbic acid molecules significantly affects the pore structure and surface area of the obtained TiO2−x samples. The porous structure makes the defective TiO2−x materials suitable for photocatalysis application because of their abundant porous channels.
Figure 2. N2 adsorption–desorption isotherms (a) and pore size distribution (b) of the defective TiO2−x nanocrystals.
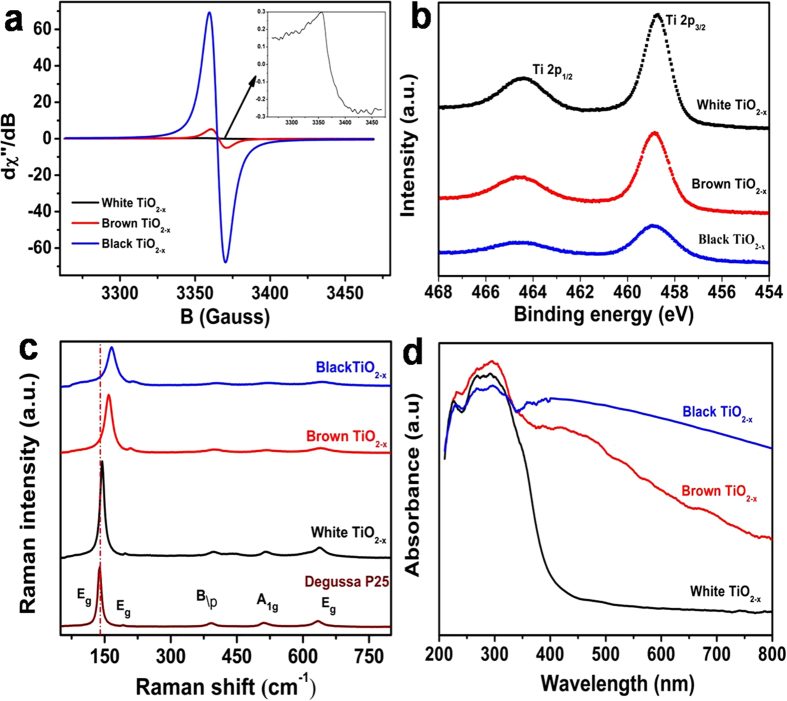
Electron paramagnetic resonance (EPR) measurements were conducted at room temperature to verify the presence of high concentration Vo. As shown in Fig. 3a, all the defective TiO2−x samples show a very strong EPR signal at g-value of 2.003 which indicates the significant presence of Vo. As discussed previously, the EPR signal appearing at g-value of 2.003 is caused by electrons trapped on surface Vo32,33. However, the representative signal of Ti3+ usually appearing at g ≈ 1.9434,35 is not shown here, which suggests the absence of rhombic Ti3+ in defective TiO2−x samples. Furthermore, the EPR signals of brown and black TiO2−x show a great enhancement in the intensity, which indicates their substantially increased Vo concentrations due to the increase in the amount of added L-ascorbic acid. Therefore, the defects in the colorful TiO2−x are present as Vo instead of Ti3+. X-ray photoelectron spectroscopy (XPS) characterization of the white, brown and black TiO2−x samples (Fig. 3b) shows only Ti2p1/2 and Ti2p3/2 peaks with slightly difference but no Ti3+ peaks appeared, which further confirms that Ti3+ does not exist in the defective TiO2−x nanocrystals. Structural properties of the obtained TiO2−x samples were further examined by measuring Raman scattering. For comparison, P25 Degussa was also analyzed. As shown in Fig. 3c, the six (3Eg + 2B1g + A1g) Raman-active modes of anatase phase with frequencies at 144, 197, 399, 515, 519 (superimposed with the 515 cm–1 band), and 639 cm–1 were detected in all investigated samples. Compared with P25, the defective TiO2−x nanocrystals display a varying degree of blue-shift in Raman bands (Eg from 139 to 144, 153 and 164 cm−1, respectively), which indicates that the original symmetry of TiO2 lattice is broken down due to the disordered TiO2−x layer formed by introducing of L-ascorbic acid36. In addition, the peak shift of defective TiO2−x gradually increases along with the darkening color, suggesting the increase in the Vo concentration. This result further supports that the Vo concentration could be controlled by the amount of L-ascorbic acid directly. The optical property of all the defective TiO2−xpowder samples was characterized by UV-visible diffuse reflectance spectra (DRS), as shown in Fig. 3d. The white TiO2−xexhibits a strong absorption in the UV range, while the brown and black TiO2−x samples display a broad absorption over the entire UV-vis wavelength range investigated. Furthermore, the black TiO2−x sample shows even higher absorption of UV-vis light than the brown TiO2−x sample which further confirms the assumption that the high concentration of Vo is capable of generating a new vacancy band locating just below the conduction band edge of pure TiO2.
Figure 3. EPR (a), XPS (b), Raman (c), and DRS (d) profiles of the defective TiO2−x nanocrystals.
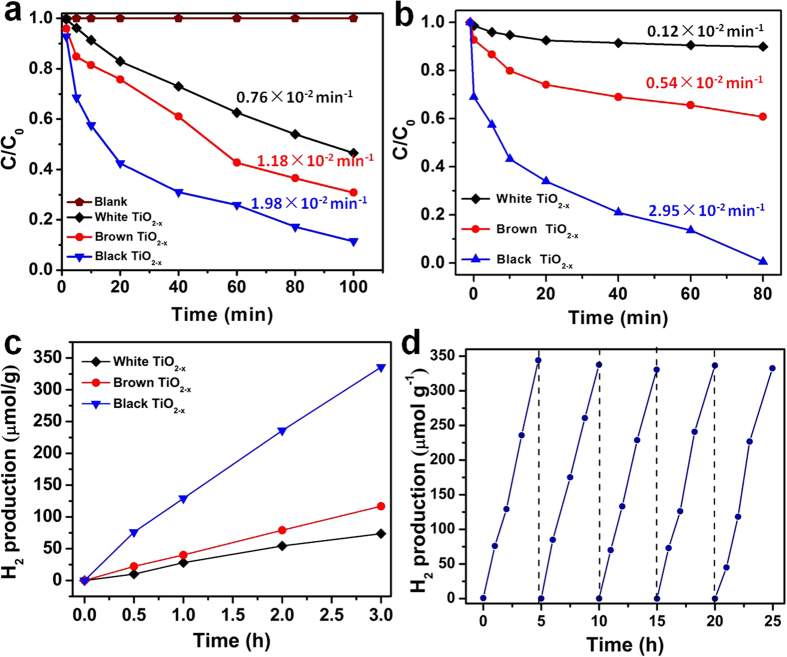
The photocatalytic degradation of MB (20 mg/L) and phenol (10 mg/L) was performed using 0.5 g/L of the as-synthesized white, brown and black TiO2−x powders under a 300 W Xenon lamp with UV cut-off filter (λ > 420 nm). As shown in Figs. 4a-4b, the black TiO2−x exhibits the highest efficiency in both MB and phenol degradation. The kinetic reaction rate of MB degradation at black TiO2−x is 1.98 × 10−2 min−1, while it is 1.18 × 10−2 min−1 at the brown TiO2−x, and 0.76 × 10−2 min−1 at the white TiO2−x. Similar improvement was also observed for phenol degradation, the kinetic rate at black TiO2−xis 2.95 × 10−2 min−1, which is about 25.41 times greater than that at white TiO2−x. The phenol molecules could be totally decomposed at black TiO2−x nanocrystals under visible light irradiation in about 80 min. Moreover, the photocatalytic activity of the defective TiO2−x was also tested for hydrogen generation under visible light (Fig. 4c). All the catalysts were loaded with 0.6 wt% Pt, and methanol was used as sacrificial agent. The black TiO2−x nanocrystals show a much higher photocatalytic activity with a hydrogen evolution rate of 116.7 μmol g−1 h−1 compared to the white TiO2 (20.6 μmol g−1 h−1) and brown TiO2−x (38.9 μmol g−1 h−1) under visible light irradiation in the presence of 20 mg/L photocatalyst. The cycling test results of the visible-light driven photocatalytic activity of black TiO2−x nanocrystals for hydrogen evolution as a function of time during a 25-hour testing period are shown in Fig. 4d. No noticeable decrease in H2 production rate for black TiO2−x in 5 cycle tests were observed within the test period, indicating good stability of the black TiO2−x nanocrystals in the photocatalytic production of hydrogen from water under visible light. The photocatalysis results confirm that the presence of defective TiO2−x outer layer with well-developed porosity is the key factor leading to improved photoactivity. The high Vo concentration in the defective TiO2−x nanocrystals enhances the absorption of visible light and thereby the generation of charge carriers, which are further transformed into abundant active species of ·O2− and ·OH to degrade the pollutants and split water to produce H2 under the visible light irradiation. The high surface area and rich pore structure increase the collision possibility of the pollutant molecules with catalyst surface and the adsorbed active radicals. These factors are responsible for the enhancement of photocatalytic activity of defective TiO2−x nanocrystals for pollutants degradation and hydrogen evolution. This is further confirmed by the measurement of photocurrent densities with the defective TiO2−x nanocrystals photoanodes at a constant potential of 0 V (vs Ag/AgCl) under visible light (Figs. 5 and 6). The photocurrent density of black TiO2−x is the highest in all the samples, which is almost 10 times of that of white TiO2−x. Thus, the high concentration of Vo defects gives rise to high visible-light induced photo-electron transformation and results in high efficiency in photocatalytic activity.
Figure 4. Degradation of MB (a) and phenol (b), hydrogen evolution (c) and cycle test (d) of the defective TiO2−x nanocrystals under visible light (>420 nm).
Figure 5. Photocurrent (I: white TiO2−x; II: brown TiO2−x; III: black TiO2−x) of the defective TiO2−x nanocrystals under visible light (>420 nm).
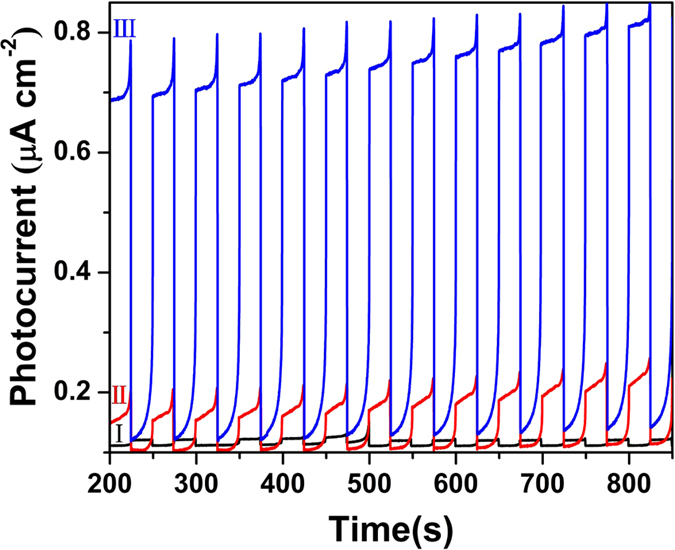
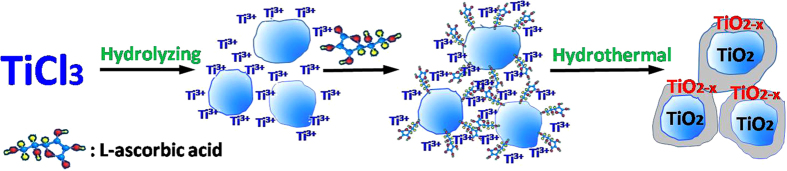
Figure 6. Schematic diagram for the formation of defective TiO2−x nanocrystals.
Discussion
Due to its unique optical property and superior visible-light-driven photocatalytic activity, defective TiO2−x, has attracted plenty of attentions recently. But most of the reports focused on tailoring the photochemical properties, rare study is reported on its structure and morphology. To the best of our knowledge, the highest surface area of defective TiO2−x reported was near 90 m2/g37. In this report, we presented a novel method for fabricating defective TiO2−x nanocrystals with tunable bandgap and high surface area using Ti(III)-salt as a precursor and L-ascorbic acid as reductant and structure direction agent. The formation of defective TiO2−x nanocrystals is schematically shown in Fig. 5. During hydrolysis, the -TiOH2+ was formed first (Eq. 1) and oxidized by the dissolved oxygen to the Ti(IV)-oxo species (Eq. 2)38, and L-ascorbic acid molecules were adsorbed on the initial particle surface through Ti-O-C bond, while the excessive Ti3+ was diffused in the interspace of L-ascorbic acid molecules. The Ti(IV)-oxo species is assumed to be an intermediate between TiO2+ and TiO2, consisting of partially dehydrated polymeric Ti(IV)hydroxide39. Following hydrothermal process, Ti(IV)-oxo was transferred to highly crystallized TiO2 (Eq. 3). If without the presence of L-ascorbic acid molecules, the excessive Ti3+ would react with the dissolved oxygen molecules in the solution to grow into TiO2 crystals. But the chemical adsorption of L-ascorbic acid inhibited the diffusion of dissolved oxygen molecules to the TiO2 core surface, and hindered the growth of TiO2 crystals40,41. Since the oxidation process suffered from the insufficient supply of oxygen, after removing the adsorbed L-ascorbic acid, Vo was produced outside of the TiO2 core20. Therefore, a defective, nonstoichiometric TiO2−x layer was formed surround the TiO2 core with rich oxygen vacancies. In addition, abundant pore structures were formed in the defective TiO2−x layer after removing the adsorbed L-ascorbic acid, evidencing its critic role as a structure direction agent.
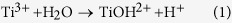 |
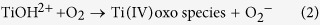 |
 |
In summary, a facile hydrothermal approach has been developed for preparing defective TiO2−x nanocrystals with TiCl3 as a precursor. L-ascorbic acid plays critical role in controlling the morphology and bandgap structure towards engineering the prepared defective TiO2. The defects in the defective TiO2−x nanocrystals proved to be of Vo, while the Vo concentration and band gap of the defective TiO2−x nanocrystals could be easily tailored by varying the introduced amount of L-ascorbic acid. Comparing with the white and brown defective TiO2−x, the black TiO2−x shows much higher surface area and efficiency in degradation of organic pollutants (MB and phenol) and hydrogen evolution under visible light irradiation. The present work provides an alternative approach for fabricating defective TiO2−x nanocrystal photocatalysts with controllable band gap and morphological structure for environmental remediation and solar fuel generation.
Method
Materials synthesis
For the preparation of reduced TiO2 nanocrystals, different amounts of L-ascorbic acid (0, 0.3 g and 0.7 g) were added to 70 mL DI water and stirred for 10 min at RT. Subsequently, 3.1 mL of TiCl3 was added and a purple solution was formed. Then, NaOH solution (1 mol/L) was added to raise the pH to 4. After stirring for another 30 min at RT, the mixture was transferred to a 100 mL Teflon-lined stainless steel autoclave and heated at 180 °C for 12 h. The obtained precipitates were collected by centrifugation, rinsed with water and ethanol for several times. After drying at 80 °C for overnight, the defective TiO2−x samples were labeled according to its color as white, brown and black TiO2−x.
Characterization
X-ray diffraction (XRD) patterns of the samples were collected on Bruker D8 Advance powder diffractometer over scattering angles from 20° to 80° using Cu Kα radiation. Transmission electron microscopy (TEM) characterization was performed on a JEOL-JEM 2100 electron microscope. Optical property was examined by UV−Visible diffuse reflectance spectrophotometer (DRS) (Shimadzu SolidSpec-3700DUV). The electron paramagnetic resonance (EPR) spectra were characterized with Bruker E500 Spectrophotometer. X-ray photoelectron spectra (XPS) of the samples were measured using a Kratos Analytical AMICUS XPS instrument.
Methylene blue (MB) and phenol degradation
40 mL MB (20 mg/L) solution or phenol solution (10 mg/L) was placed in a 50 mL quartz photoreactor. The photocatalyst (0.5 g/L) was dispersed into the solution at neutral pH. In order to attain adsorption-desorption equilibrium, the solution was stirred in dark for 40 min. The solution was then irradiated by a 300 W Xenon lamp with UV cut-off filter (λ > 420 nm) at RT. Samples were taken at given time interval to test the concentration of MB and phenol. The concentration of MB was measured by UV‒visible spectrophotometer (UV-1800, Shimadzu). The phenol concentration was determined by Thermo Fisher Ultra 3000 HPLC equipped with a 25 cm × 4.6 mm Cosmosil C18 column.
Photocatalytic hydrogen evolution
The photocatalytic reactions of H2 evolution were carried out in a closed gas circulation system with an external-irradiation type of a glass reactor. The light source was a 300 W Xenon lamp with UV cut-off filter (λ > 420 nm). The co-catalyst Pt was loaded by an in-situ photodeposition method. The 0.6 wt% of Pt-loaded catalyst (25 mg) was dispersed with a magnetic stirrer in a methanol aqueous solution (10 mL of CH3OH and 90 mL of H2O). The evolved gas including H2 was analyzed using an online gas chromatograph (7890A, Agilent) equipped with a thermal conductivity detector (TCD).
Photocurrent measurement
The photocurrent was performed with an electrochemical instrument CHI660E using a three-electrode system. The samples (0.1 g) were loaded on conductive surface of ITO glass and 0.5 M Na2SO4 solution was used as electrolyte. 300 W Xenon lamp equipped with UV cut‒off filter (λ > 420 nm) was used as light source, and standard calomel electrode (SCE) as reference electrode, Pt slice as counter electrode.
Additional Information
How to cite this article: Wajid Shah, M. et al. Facile Synthesis of Defective TiO2-x Nanocrystals with High Surface Area and Tailoring Bandgap for Visible-light Photocatalysis. Sci. Rep. 5, 15804; doi: 10.1038/srep15804 (2015).
Supplementary Material
Acknowledgments
This work was supported by the National Nature Science Foundation of China (Grant No. 21173261), the “Western Light” Program of Chinese Academy of Sciences (XBBS201410), the CAS/SAFEA International Partnership Program for Creative Research Teams, the CAS “Western Action Plan” (KGZD-EW-502), and the High-Technology Research & Development Project of Xinjiang Uyghur Autonomous Region (201415110).
Footnotes
Author Contributions M.W.S. and Y.Z. performed the experiments and wrote the main manuscript text. C.W. guided the whole work. X.F., J.Z., Y.L. made contribution for discussions and critical revision of the manuscript. J.Z. also tested the BET measurement, and S.A. assisted the photocurrent measurements. All authors reviewed the manuscript.
References
- Fujishima A. & Honda K. Electrochemical Photolysis of Water at a SemiconductorElectrode. Nature 238, 37–38 (1972). [DOI] [PubMed] [Google Scholar]
- Chen X. & Mao S. S. Titanium dioxide nanomaterials: synthesis, properties,modifications, and applications. Chem. Rev. 107, 2891–2959 (2007). [DOI] [PubMed] [Google Scholar]
- Hoffmann M. R., Martin S. T. & Bahnemannt D. W. Environmental Applications of Semiconductor Photocatalysis. Chem. Rev. 95, 69–96 (1995). [Google Scholar]
- Yang D. et al. An Efficient Photocatalyst Structure: TiO2(B) Nanofibers with a Shell of Anatase Nanocrystals. J. Am. Chem. Soc. 131, 17885–17893 (2009). [DOI] [PubMed] [Google Scholar]
- Manivannan A. & Wu N. Solar Hydrogen Generation by a CdS-Au-TiO2 Sandwich Nanorod Array Enhanced with Au Nanoparticle as Electron Relay and Plasmonic Photosensitizer. J. Am. Chem. Soc. 136, 8438–8449 (2014). [DOI] [PubMed] [Google Scholar]
- Alvaro M., Aprile C., Benitez M., Carbonell E. & García H. Photocatalytic Activity of Structured Mesoporous TiO2 Materials. J.Phys. Chem. B 110, 6661–6665 (2006). [DOI] [PubMed] [Google Scholar]
- Zhang J., Bang J., Tang C. & Kamat P. V. Tailored TiO2-SrTiO3 Heterostructure Nanotube Arrays for Improved Photoelectrochemical Performance. ACS Nano 4, 387–395 (2010). [DOI] [PubMed] [Google Scholar]
- Li J. et al. Synthesis, Characterization, and Visible Light Activity of New Nanoparticle Photocatalysts Based on Silver, Carbon, and Sulfur-doped TiO2. J. Colloid Interface Sci. 311, 514–522 (2007). [DOI] [PubMed] [Google Scholar]
- Valero J. M., Obregón S. & Colón G. Active Site Considerations on the Photocatalytic H2 Evolution Performance of Cu-Doped TiO2 Obtained by Different Doping Methods. ACS Catal. 4, 3320–3329 (2014). [Google Scholar]
- Hamedani H. A., Allam N. K., Garmestani H. & El-Sayed M. A. Electrochemical Fabrication of Strontium-Doped TiO2 Nanotube Array Electrodes and Investigation of Their Photoelectrochemical Properties. J. Phys. Chem. C 115, 13480–13486 (2011). [Google Scholar]
- Zheng Z. et al. Facile in situ Synthesis of Visible-light Plasmonic Photocatalysts M@TiO2 (M=Au, Pt, Ag) and Evaluation of Their Photocatalytic Oxidation of Benzene to Phenol. J. Mater. Chem. 21, 9079–9087 (2011). [Google Scholar]
- Xu P. et al. I2-Hydrosol-Seeded Growth of (I2)n-C-Codoped Meso/Nanoporous TiO2 for Visible Light-Driven Photocatalysis. J. Phys. Chem. C 114, 9510–9517 (2010). [Google Scholar]
- Toro C. & Buriak J. M. F– Doping on TiO2 Provided Important Insights into Photocatalysis. Chem. Mater. 27, 1443–1444 (2015). [Google Scholar]
- Wang F., Jiang Y., Gautam A., Li Y. & Amal R. Exploring the Origin of Enhanced Activity and Reaction Pathway for Photocatalytic H2 Production on Au/B-TiO2 Catalysts. ACS Catal. 4, 1451–1457 (2014). [Google Scholar]
- Asahi R., Morikawa T., Ohwaki T., Aoki K. & Taga Y. Visible-light photocatalysis in nitrogen-doped titanium oxides. Science 293, 269–271 (2001). [DOI] [PubMed] [Google Scholar]
- Gopal N. O., Lo H. H. & Ke S. C. Chemical State and Environment of Boron Dopant in B,N-Codoped Anatase TiO2 Nanoparticles: An Avenue for Probing Diamagnetic Dopants in TiO2 by Electron Paramagnetic Resonance Spectroscopy. J. Am. Chem. Soc. 130, 2760–2761 (2008). [DOI] [PubMed] [Google Scholar]
- Chen X., Liu L., Yu Y. P. & Mao S. S. Increasing Solar Absorption for Photocatalysis with Black Hydrogenated Titanium Dioxide Nanocrystals. Science 331, 746–750 (2011). [DOI] [PubMed] [Google Scholar]
- Zuo F. et al. Self-Doped Ti3+ Enhanced Photocatalyst for Hydrogen Production under Visible Light. J. Am. Chem. Soc. 132, 11856–11857 (2010). [DOI] [PubMed] [Google Scholar]
- Wendt S. et al. The Role of Interstitial Sites in the Ti3d Defect State in the Band Gap of Titania. Science 320, 1755–1759 (2008). [DOI] [PubMed] [Google Scholar]
- Chen C. C., Say W. C., Hsieh S. J. & Diau E. W. A Mechanism for the Formation of Annealed Compact Oxide Layers at the Interface Between Anodic Titania Nanotube Arrays and Ti Foil. Appl. Phys. A95, 889–898 (2009). [Google Scholar]
- Su J. et al. Porous Titania with Heavily Self-Doped Ti3+ for Specific Sensing of CO at Room Temperature. Inorg. Chem. 52, 5924–5930 (2013). [DOI] [PubMed] [Google Scholar]
- Xia T., Zhang Y., Murowchick J. & Chen X. Vacuum-treated titanium dioxide nanocrystals: Optical properties, surface disorder, oxygen vacancy, and photocatalytic activities. Catal. Today 225, 2–9 (2014). [Google Scholar]
- Liu L. & Chen X. Titanium Dioxide Nanomaterials: Self-Structural Modifications. Chem. Rev. 114, 9890–9918 (2014). [DOI] [PubMed] [Google Scholar]
- Chen X., Liu L. & Huang F. Black titanium dioxide (TiO2) nanomaterials. Chem. Soc. Rev. 44, 1861–1885 (2015). [DOI] [PubMed] [Google Scholar]
- Srivastava S. et al. Size-Selected TiO2 Nanocluster Catalysts for Efficient Photoelectrochemical Water Splitting. ACS Nano 8, 11891–11898 (2014). [DOI] [PubMed] [Google Scholar]
- Lang S. M. & Bernhardt T. M. Gas Phase Metal Cluster Model Systems for Heterogeneous Catalysis. Phys. Chem. Chem.Phys. 14, 9255–9269 (2012). [DOI] [PubMed] [Google Scholar]
- Jun Y. W. et al. Surfactant-assisted elimination of a high energy facet as a means of controlling the shapes of TiO2 nanocrystals. J. Am. Chem. Soc. 125,15981–15985 (2003). [DOI] [PubMed] [Google Scholar]
- Qiu B. et al. Facile Synthesis of the Ti3+ Self-doped TiO2-graphene Nanosheet Composites with Enhanced Photocatalysis. Sci. Rep. 5, 8591–8596 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su J. et al. Porous Titania with Heavily Self-Doped Ti3+ for Specific Sensing of CO at Room Temperature. Inorg. Chem. 52, 5924–5930 (2013). [DOI] [PubMed] [Google Scholar]
- Myung S. T. et al. Black Anatase Titania Enabling Ultra High Cycling Rates for Rechargeable Lithium Batteries. Energy Environ. Sci. 6, 2609–2614 (2013). [Google Scholar]
- Chen J. S. et al. Constructing Hierarchical Spheres from Large Ultrathin Anatase TiO2Nanosheets with Nearly 100% Exposed (001) Facets for Fast Reversible Lithium Storage. J. Am. Chem. Soc. 132, 6124–6130 (2010). [DOI] [PubMed] [Google Scholar]
- Randorn C. & Irvine J. T. S. Synthesis and Visible Light Photoactivity of a High Temperature Stable Yellow TiO2 Photocatalyst. J. Mater. Chem. 20, 8700–8704 (2010). [Google Scholar]
- Naldoni A. et al. Pt and Au/TiO2 Photocatalysts for Methanol Reforming: Role of Metal Nanoparticles in Tuning Charge Trapping Properties and Photoefficiency. Appl. Catal. B 130, 239–248 (2013). [Google Scholar]
- Hoang S., Berglund S. P., Hahn N. T., Bard A. J. & Mullins C. B. Enhancing Visible Light Photo-oxidation of Water with TiO2 Nanowire Arrays via Cotreatment with H2 and NH3: Synergistic Effects between Ti3+ and N. J. Am. Chem. Soc. 134, 3659–3662 (2012). [DOI] [PubMed] [Google Scholar]
- Grabstanowicz L. R. et al. Facile Oxidative Conversion of TiH2 to High-Concentration Ti3+-Self-Doped Rutile TiO2 with Visible-Light Photoactivity. Inorg. Chem. 52, 3884–3890 (2013). [DOI] [PubMed] [Google Scholar]
- Dong J. et al. Defective Black TiO2 Synthesized via Anodization for Visible-Light Photocatalysis. ACS Appl. Mater. Interfaces 6, 1385–1388 (2014). [DOI] [PubMed] [Google Scholar]
- Ren R. et al. Controllable Synthesis and Tunable Photocatalytic Properties of Ti3+-doped TiO2. Sci. Rep. 5, 10714–10724 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hosono E., Fujihara S., Kakiuchi K. & Imai H. Growth of Submicrometer-Scale Rectangular Parallelepiped Rutile TiO2 Films in Aqueous TiCl3 Solutions under Hydrothermal Conditions. J. Am. Chem. Soc. 126, 7790–7791 (2004). [DOI] [PubMed] [Google Scholar]
- Kavan L., O’Regan B., Kay A. & Gratzel M. Preparation of TiO2 (anatase) Films on Electrodes by Anodic Oxidative Hydrolysis of TiCl3. J. Electroanal. Chem. 346, 291–307 (1993). [Google Scholar]
- Prakasam H. E., Shankar K., Paulose M., Varghese O. K. & Grimes C. A. A New Benchmark for TiO2 Nanotube Array Growth by Anodization. J. Phys. Chem.C 111, 7235–7241 (2007). [Google Scholar]
- Paulose M. et al. Anodic Growth of Highly Ordered TiO2 Nanotube Arrays to 134 μm in Length. J. Phys. Chem. B 110, 16179–16184 (2006). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.