Abstract
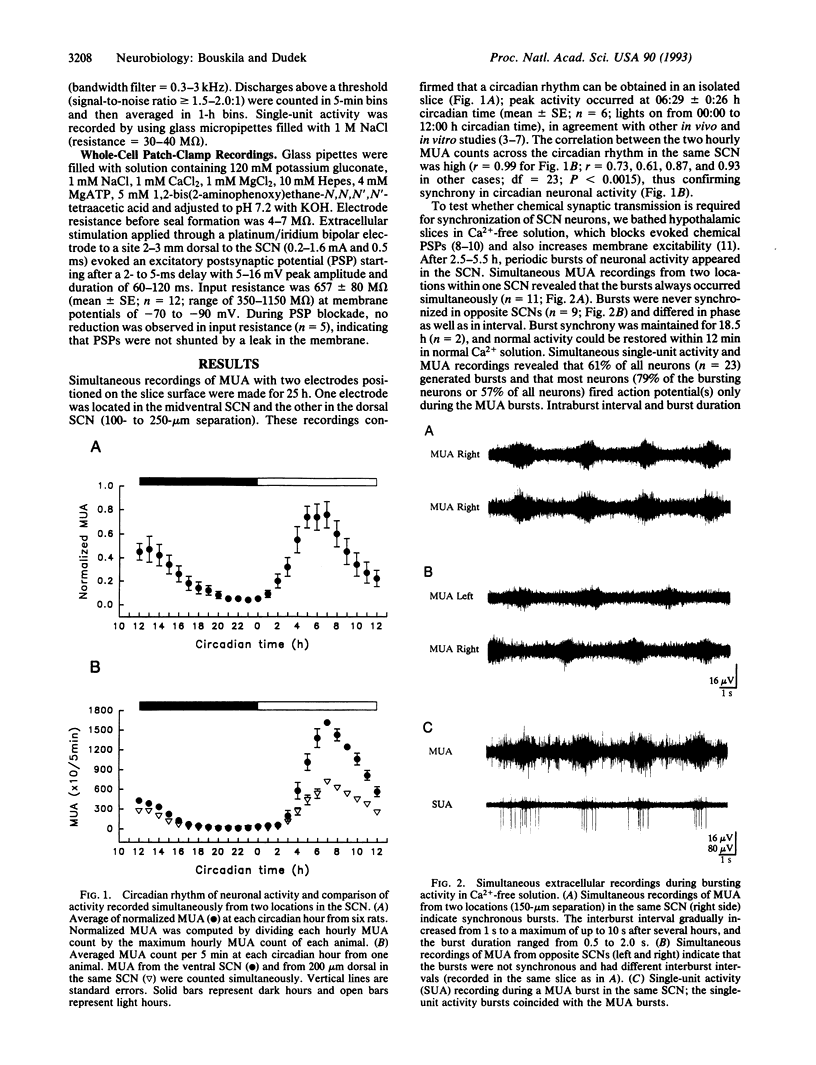
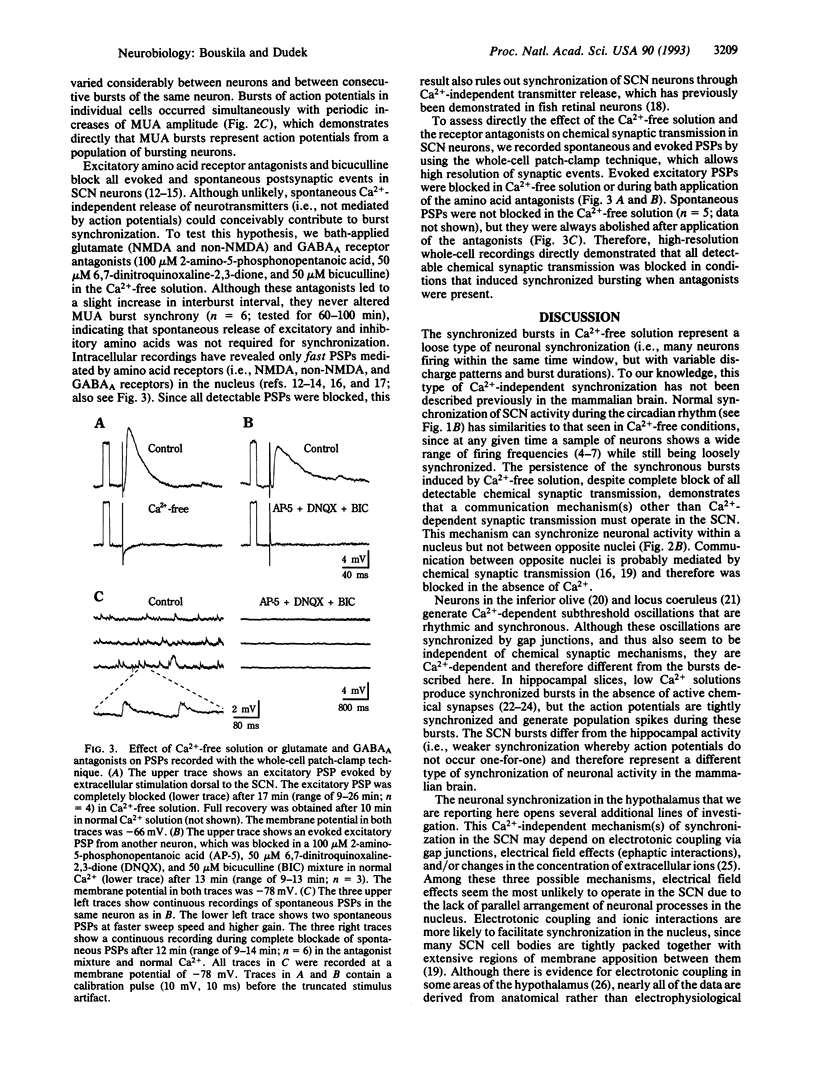
A critical question in understanding the mammalian brain is how populations of neurons become synchronized. This is particularly important for the neurons and neuroendocrine cells of the hypothalamus, which are activated synchronously to control endocrine glands and the autonomic nervous system. It is widely accepted that communication between neurons of the adult mammalian brain is mediated primarily by Ca(2+)-dependent synaptic transmission. Here we report that synchronous neuronal activity can occur in the hypothalamic suprachiasmatic nucleus without active Ca(2+)-dependent synaptic transmission. Simultaneous extracellular recordings of neuronal activity in the suprachiasmatic nucleus, which contains the mammalian biological clock, confirmed a circadian rhythm of synchronized activity in hypothalamic slices. Ca(2+)-free medium, which blocks chemical synaptic transmission and increases membrane excitability, produced periodic and synchronized bursts of action potentials in a large population of suprachiasmatic nucleus neurons with diverse firing patterns. N-Methyl-D-aspartic acid, non-N-methyl-D-aspartic acid, and gamma-aminobutyric acid type A receptor antagonists had no effect on burst synchrony. Whole-cell patch-clamp recordings confirmed that the Ca(2+)-free solution blocked evoked postsynaptic potentials and that the mixture of antagonists blocked the remaining spontaneous postsynaptic potentials. Therefore, mechanisms other than Ca(2+)-dependent synaptic transmission can synchronize neurons in the mammalian hypothalamus and may be important wherever neuronal networks are synchronized.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Buzsáki G., Horváth Z., Urioste R., Hetke J., Wise K. High-frequency network oscillation in the hippocampus. Science. 1992 May 15;256(5059):1025–1027. doi: 10.1126/science.1589772. [DOI] [PubMed] [Google Scholar]
- Cahill G. M., Menaker M. Effects of excitatory amino acid receptor antagonists and agonists on suprachiasmatic nucleus responses to retinohypothalamic tract volleys. Brain Res. 1989 Feb 6;479(1):76–82. doi: 10.1016/0006-8993(89)91337-1. [DOI] [PubMed] [Google Scholar]
- Christie M. J., Williams J. T., North R. A. Electrical coupling synchronizes subthreshold activity in locus coeruleus neurons in vitro from neonatal rats. J Neurosci. 1989 Oct;9(10):3584–3589. doi: 10.1523/JNEUROSCI.09-10-03584.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DEL CASTILLO J., STARK L. The effect of calcium ions on the motor end-plate potentials. J Physiol. 1952 Apr;116(4):507–515. doi: 10.1113/jphysiol.1952.sp004720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Douglas W. W. Stimulus-secretion coupling: variations on the theme of calcium-activated exocytosis involving cellular and extracellular sources of calcium. Ciba Found Symp. 1978;(54):61–90. doi: 10.1002/9780470720356.ch4. [DOI] [PubMed] [Google Scholar]
- Dudek F. E., Snow R. W., Taylor C. P. Role of electrical interactions in synchronization of epileptiform bursts. Adv Neurol. 1986;44:593–617. [PubMed] [Google Scholar]
- FRANKENHAEUSER B., HODGKIN A. L. The action of calcium on the electrical properties of squid axons. J Physiol. 1957 Jul 11;137(2):218–244. doi: 10.1113/jphysiol.1957.sp005808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green D. J., Gillette R. Circadian rhythm of firing rate recorded from single cells in the rat suprachiasmatic brain slice. Brain Res. 1982 Aug 5;245(1):198–200. doi: 10.1016/0006-8993(82)90361-4. [DOI] [PubMed] [Google Scholar]
- Groos G., Hendriks J. Circadian rhythms in electrical discharge of rat suprachiasmatic neurones recorded in vitro. Neurosci Lett. 1982 Dec 31;34(3):283–288. doi: 10.1016/0304-3940(82)90189-6. [DOI] [PubMed] [Google Scholar]
- Hatton G. I. Emerging concepts of structure-function dynamics in adult brain: the hypothalamo-neurohypophysial system. Prog Neurobiol. 1990;34(6):437–504. doi: 10.1016/0301-0082(90)90017-b. [DOI] [PubMed] [Google Scholar]
- Inouye S. T., Kawamura H. Persistence of circadian rhythmicity in a mammalian hypothalamic "island" containing the suprachiasmatic nucleus. Proc Natl Acad Sci U S A. 1979 Nov;76(11):5962–5966. doi: 10.1073/pnas.76.11.5962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jefferys J. G., Haas H. L. Synchronized bursting of CA1 hippocampal pyramidal cells in the absence of synaptic transmission. Nature. 1982 Dec 2;300(5891):448–450. doi: 10.1038/300448a0. [DOI] [PubMed] [Google Scholar]
- Kim Y. I., Dudek F. E. Intracellular electrophysiological study of suprachiasmatic nucleus neurons in rodents: excitatory synaptic mechanisms. J Physiol. 1991 Dec;444:269–287. doi: 10.1113/jphysiol.1991.sp018877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim Y. I., Dudek F. E. Intracellular electrophysiological study of suprachiasmatic nucleus neurons in rodents: inhibitory synaptic mechanisms. J Physiol. 1992 Dec;458:247–260. doi: 10.1113/jphysiol.1992.sp019416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konnerth A., Heinemann U., Yaari Y. Slow transmission of neural activity in hippocampal area CA1 in absence of active chemical synapses. Nature. 1984 Jan 5;307(5946):69–71. doi: 10.1038/307069a0. [DOI] [PubMed] [Google Scholar]
- Llinás R., Yarom Y. Oscillatory properties of guinea-pig inferior olivary neurones and their pharmacological modulation: an in vitro study. J Physiol. 1986 Jul;376:163–182. doi: 10.1113/jphysiol.1986.sp016147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meijer J. H., Rietveld W. J. Neurophysiology of the suprachiasmatic circadian pacemaker in rodents. Physiol Rev. 1989 Jul;69(3):671–707. doi: 10.1152/physrev.1989.69.3.671. [DOI] [PubMed] [Google Scholar]
- Prosser R. A., Gillette M. U. The mammalian circadian clock in the suprachiasmatic nuclei is reset in vitro by cAMP. J Neurosci. 1989 Mar;9(3):1073–1081. doi: 10.1523/JNEUROSCI.09-03-01073.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ralph M. R., Foster R. G., Davis F. C., Menaker M. Transplanted suprachiasmatic nucleus determines circadian period. Science. 1990 Feb 23;247(4945):975–978. doi: 10.1126/science.2305266. [DOI] [PubMed] [Google Scholar]
- Schwartz E. A. Depolarization without calcium can release gamma-aminobutyric acid from a retinal neuron. Science. 1987 Oct 16;238(4825):350–355. doi: 10.1126/science.2443977. [DOI] [PubMed] [Google Scholar]
- Schwartz W. J., Gross R. A., Morton M. T. The suprachiasmatic nuclei contain a tetrodotoxin-resistant circadian pacemaker. Proc Natl Acad Sci U S A. 1987 Mar;84(6):1694–1698. doi: 10.1073/pnas.84.6.1694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shibata S., Oomura Y., Kita H., Hattori K. Circadian rhythmic changes of neuronal activity in the suprachiasmatic nucleus of the rat hypothalamic slice. Brain Res. 1982 Sep 9;247(1):154–158. doi: 10.1016/0006-8993(82)91041-1. [DOI] [PubMed] [Google Scholar]
- Taylor C. P., Dudek F. E. Synchronous neural afterdischarges in rat hippocampal slices without active chemical synapses. Science. 1982 Nov 19;218(4574):810–812. doi: 10.1126/science.7134978. [DOI] [PubMed] [Google Scholar]
- Thomson A. M., West D. C. Factors affecting slow regular firing in the suprachiasmatic nucleus in vitro. J Biol Rhythms. 1990 Spring;5(1):59–75. doi: 10.1177/074873049000500106. [DOI] [PubMed] [Google Scholar]
- Van den Pol A. N. The hypothalamic suprachiasmatic nucleus of rat: intrinsic anatomy. J Comp Neurol. 1980 Jun 15;191(4):661–702. doi: 10.1002/cne.901910410. [DOI] [PubMed] [Google Scholar]
- Wheal H. V., Thomson A. M. The electrical properties of neurones of the rat suprachiasmatic nucleus recorded intracellularly in vitro. Neuroscience. 1984 Sep;13(1):97–104. doi: 10.1016/0306-4522(84)90262-8. [DOI] [PubMed] [Google Scholar]


