Abstract
Brain size is widely used as a measure of behavioural complexity and sensory-locomotive capacity in avians but has largely relied upon laborious dissections, endoneurocranial tissue displacement, and physical measurement to derive comparative volumes. As an alternative, we present a new precise calculation method based upon coupled magnetic resonance (MR) imaging and computed tomography (CT). Our approach utilizes a novel interactive Fakir probe cross-referenced with an automated CT protocol to efficiently generate total volumes and surface areas of the brain tissue and endoneurocranial space, as well as the discrete cephalic compartments. We also complemented our procedures by using sodium polytungstate (SPT) as a contrast agent. This greatly enhanced CT applications but did not degrade MR quality and is therefore practical for virtual brain tissue reconstructions employing multiple imaging modalities. To demonstrate our technique, we visualized sex-based brain size differentiation in a sample set of Ring-necked pheasants (Phasianus colchicus). This revealed no significant variance in relative volume or surface areas of the primary brain regions. Rather, a trend towards isometric enlargement of the total brain and endoneurocranial space was evidenced in males versus females, thus advocating a non-differential sexually dimorphic pattern of brain size increase amongst these facultatively flying birds.
Cognitive, behavioural, ecological and sensory-motor mechanisms of the central nervous system (CNS) in vertebrates have been inferred from total size and dimensional compartmentalization of the brain and endoneurocranial space; however, the manual procedures for measuring these structures are known to be imprecise1. This has critical implications for correlative neuroanatomy as well as the reconstruction of fossils in which the neural tissues do not preserve. Moreover, detailed mapping of intra/interspecific variability2 and developmental trajectories3 is vital because these can influence the interpretation of evolutionary modifications in the CNS through time. Advanced digital imaging technologies offer an optimal tool for accurate quantitative investigations of complex neural structures. Yet, the technical manipulation of virtual tomographic data for specialized evolutionary studies is still a nascent field4,5,6,7. This contribution therefore presents a new benchmark protocol for 3D visualization and rigorous volumetric measurement of the brain and endoneurocranial space in vertebrates. The utility of our technique is demonstrated via a case study of differential sex-related size modularization of the main cerebral divisions in an extant representative of Phasianidae (Galliformes). This particular problem is of interest because overall brain enlargement is thought to result from relative dimensional increases in major components according to their importance8. Nevertheless correlation between behavioural complexity and size of the brain is not entirely clear1. Modular changes are also potentially independent within each neurological region9, but have not yet been tomographically compared against sexual dimorphism, which appears to select for larger total brain volumes in the males of some species10,11,12,13. Furthermore, our experimental model of the Ring-necked pheasant (Phasianus colchicus), a mid-sized (<one meter maximum snout-tail length and up to ~3 kg) facultative terrestrial bird that is capable of short-distance flight, represents a viable proxy for the lifestyle and locomotive capabilities of early stem avians14,15,16. These likewise trended towards gross brain enlargement via differential development of key cerebral components17,18,19,20.
Our analysis methods employed a combination of Magnetic resonance (MR) imaging, a gold standard for intact brain visualization, and Computed tomography (CT) as a superior modality for digitally recreating the endoneurocranial space. These were augmented by innovative interactive software coupled with automated segmentation procedures to enable fast and efficient compilation of accurate cross-referenced volumetric measurements. Experimentation with different soft tissue fixation parameters and the inclusion of sodium polytungstate (SPT) as a contrast agent21 also illustrates an ideal sample treatment technique that can be used for discriminating detailed surface geometry and spatial relations between the brain tissue and endoneurocranium, as well as internal segregation of major brain divisions (prosencephalon, mesencephalon and rhombencephalon) in an avian paradigm of other vertebrates.
Results and discussion
Our MR and μCT data acquisitions obtained sufficient contrast and resolution, as well as a high signal-to-noise ratio necessary for quantitative analysis. Image artefacts and bulk motion from scanner vibration were completely negated. The addition of SPT substantially enhanced CT visualization (Fig. 1a,b; Supplementary Figure 1). Moreover, it did not degrade MR image quality; on the contrary, some brain structures possessed better contrast (Fig. 2a–c) after prolonged fixation, and were measurable through a marked increase in the signal-to-noise ratio after three months (83.5+/−0.8) versus six days (51.5+/−0.9) of immersion. Screening for MR contrast deterioration during prolonged scanning (Supplementary Figure 2) also revealed <1% standard deviation between normalized images (T2 relaxation time = 64.6+/−2.0 ms at commencement as opposed to 63.8+/−3.2 ms after 16 hours). Relaxometry, on the other hand, returned only marginal improvement: T1 = 2170.5+/−3.5 ms (4% increases), T2 = 1606.8+/−0.6 (10% decreases). These results compliment recent findings by Kotrotsou et al.22, who demonstrated that post-mortem subject volume variation was significantly reduced across time points relative to inter-subject volume variation over one week/six months. Kotrotsou et al.22 further concluded that ex vivo and in vivo brain volume metrics are linearly correlated, thus ex vivo MR volumetry can accurately capture ante-mortem endoneurocranial anatomy. The most important outcome of this study, however, was that our combined technique of incorporating an interactive seven-fold Fakir probe for MR with an automated Watershed application for CT (both detailed in the materials and methods section) produced complementary information about brain versus interstitium/endoneurocranial volume and surface area (Table 1). Our endoneurocranial values differed by 8.0 ± 1.3%, probably reflecting manual segmentation error from poorer MR imaging of bone23. In contrast, MR measurements of brain soft tissues were found to deviate by only 0.4+/−1.9% (Table 1, Supplementary Figure 3).
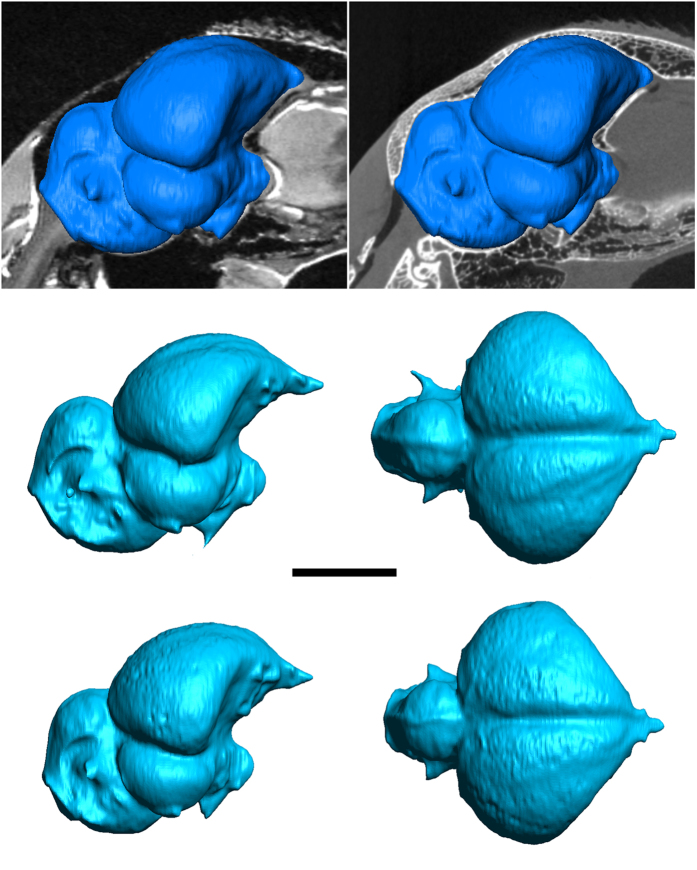
Figure 1. Automated surface renderings of segmented avian endoneurocrania (Phasianus colchicus).
Mid-sagittal section of (a) 3D RARE MR versus Watershed method CT (b) generated image with inset endoneurocranial surface (adult male specimen). Comparative proportions of male (c,d) and female (e,f) endoneurocrania in lateral (c,e) and dorsal (d,f) views.
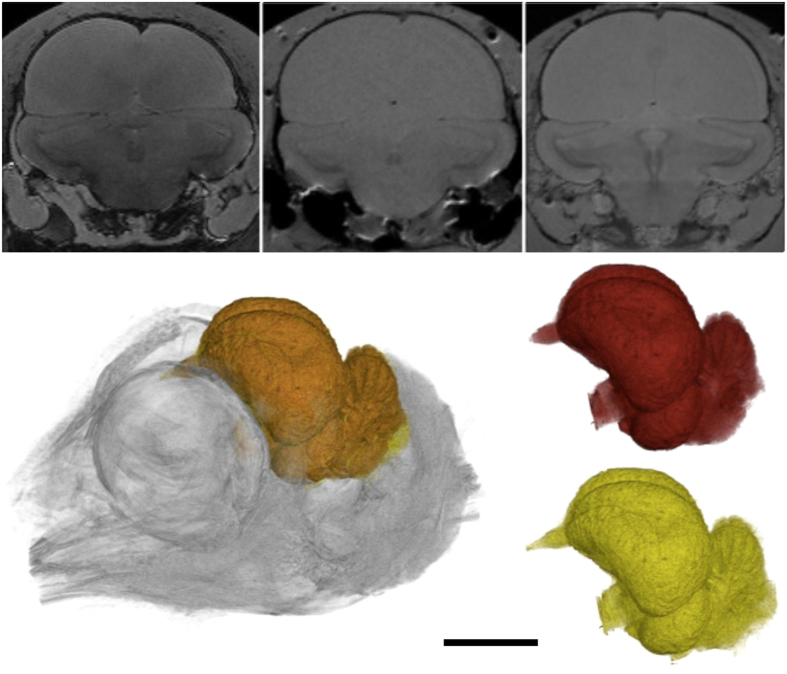
Figure 2. Effects of SPT fixation on avian brain tissue (Phasianus colchicus).
MR visualization of juvenile specimen after: (a) three days with no SPT; (b) six days with SPT + water; (c) three months with SPT + formalin. Surface rendering of 3D RARE MR data (adult male specimen) illustrating: (d) the in situ endoneurocranial space; (e) isolated brain tissues; (f) and isolated endoneurocranial space.
Table 1. Volumetric and surface area calculations for total brain tissue versus endocranial space of both adult female and male Phasianus colchicus.
| Segmentation | Total Brain Tissue |
Total Endocranium |
||
|---|---|---|---|---|
| Female | Male | Female | Male | |
| MR manual volume (mm3) | 3335+/−84 | 3752+/−154 | 4003+/−103 | 4344+/−160 |
| Volume (mm3) | •3383+/−131 | •3729+/−197 | ••3642+/−52 | ••4036+/−172 |
| Surface area (mm2) | •1394+/−39 | •1538+/−80 | ••1500+/−37 | ••1572+/−91 |
•MR and Fakir.
••CT and Watershed methods.
The benefits of using MR to visualize brain structures are well known6,24, and have been illustrated elsewhere in extant avians23,25,26. However, indistinct discrimination of the osseous interstitial border is usually overcome by CT co-registration of both modalities with automated segmentation24. Our new procedure of separate computation for each major brain compartment (Supplementary Figure 4) augments this approach by contributing robustly correlated volume fractions and surface areas (Table 2 and 3; Pearson’s correlation coefficient of volume versus surfaced weighted mean distance = 0.997). Such accuracy demonstrates that reliable data can be derived from our rapid processing procedure, and that a combination of MR and CT offers a very precise means of generating cross-referenced volumetric calculations of not only proportional values but also total brain space parameters (including in (including in vivo22) across the entire endoneurocranium.
Table 2. Brain compartment volumes (mm3) and surface areas (mm2).
| Specimen | Prosencephalon |
Mesencephalon |
Rhombencephalon |
Total | Total | |||
|---|---|---|---|---|---|---|---|---|
| Volume | Area | Volume | Area | Volume | Area | Volume | Area | |
| Male 1 | 2363 | 922 | 652 | 277 | 726 | 301 | 3741 | 1500 |
| Male 2 | 2229 | 882 | 563 | 296 | 734 | 306 | 3526 | 1485 |
| Male 3 | 2375 | 890 | 661 | 370 | 883 | 370 | 3919 | 1630 |
| Female 1 | 2343 | 893 | 511 | 248 | 638 | 267 | 3493 | 1408 |
| Female 2 | 2204 | 882 | 570 | 262 | 643 | 280 | 3418 | 1424 |
| Female 3 | 2026 | 808 | 553 | 256 | 660 | 285 | 3238 | 1350 |
| Juvenile male 1 | 1784 | 758 | 443 | 222 | 558 | 293 | 2785 | 1273 |
| Juvenile male 2 | 1964 | 734 | 492 | 235 | 612 | 309 | 3068 | 1278 |
| Juvenile female 1 | 1913 | 829 | 437 | 222 | 569 | 312 | 2919 | 1363 |
| Juvenile female 2 | 1731 | 692 | 425 | 262 | 579 | 351 | 2735 | 1305 |
Calculated using the enhanced Fakir probe on MR data.
Table 3. Brain compartments volumes (mm3) and surface areas (mm2) relative to equivalent total brain measurements.
| Specimen | Prosencephalon |
Mesencephalon |
Rhombencephalon |
|||
|---|---|---|---|---|---|---|
| Volume | Area | Volume | Area | Volume | Area | |
| Male 1 | 0.63 | 0.61 | 0.17 | 0.18 | 0.19 | 0.20 |
| Male 2 | 0.63 | 0.59 | 0.16 | 0.20 | 0.21 | 0.21 |
| Male 3 | 0.61 | 0.55 | 0.17 | 0.23 | 0.23 | 0.23 |
| Female 1 | 0.67 | 0.63 | 0.15 | 0.18 | 0.18 | 0.19 |
| Female 2 | 0.65 | 0.62 | 0.17 | 0.18 | 0.19 | 0.20 |
| Female 3 | 0.63 | 0.60 | 0.17 | 0.19 | 0.20 | 0.21 |
| Mean (adult) | 0.64 ± 0.02 | 0.60 ± 0.03 | 0.16 ± 0.01 | 0.19 ± 0.02 | 0.20 ± 0.02 | 0.21 ± 0.01 |
| Juvenile male 1 | 0,64 | 0,60 | 0,16 | 0,17 | 0,20 | 0,23 |
| Juvenile male 2 | 0,64 | 0,57 | 0,16 | 0,18 | 0,20 | 0,24 |
| Juvenile female 1 | 0,66 | 0,61 | 0,15 | 0,16 | 0,19 | 0,23 |
| Juvenile female 2 | 0,63 | 0,53 | 0,16 | 0,20 | 0,21 | 0,27 |
| Mean (juvenile) | 0.64 ± 0.01 | 0.58 ± 0.04 | 0.16 ± 0.01 | 0.18 ± 0.02 | 0.20 ± 0.01 | 0.24 ± 0.02 |
Calculated using the enhanced Fakir probe on MR data.
Critical to our particular test case was the discovery that no marked differentiation existed in compartmental measurements between the sexes of our adult-stage Phasianus colchicus sample (Table 2 and 3). Conversely, distinct variation (p < 0.05) occurred in absolute volume/surface area with males being demonstrably larger than 10% (Fig. 1c–f; Table 1). Although it is clear that fundamental changes in the separate brain parts do alter overall brain size, our precise calculations detected an isometric, perhaps hormonally driven1 increase that accords with other documented examples of avian sexual dimorphism10,11,12,13; a phenomenon similarly influencing gross brain size in mammals27,28. This effect is presumably coupled with other intraspecific proportional changes including ontogenetic variability3 and ecomorphology (e.g. lifestyle specific and seasonal fluctuations1,2) to overlay the intrinsic mosaic evolution of avian9 and mammalian29 brain development. As an extension, dimorphic segregation might also be discernible amongst the restored brains of fossil avians, and could be used to discriminate disputed sexual morphs accepting the constraints of adequate diagenetic retro-deformation and sample size30,31,32,33. More importantly, exact quantitative measure of different brain regions, especially the proportional enlargement of the forebrain, which has been qualitatively contested as an indicator of enhanced somatosensory integration and the capacity for flight19,20, requires further investigation. Likewise, relative surface areas of key endoneurocranial compartments particularly those housing the avian eminentia sagittalis34 (=exterior expression of the upper hyperstriatum or Wulst35) would greatly assist with reconstructing comparative visual evolution and palaeoecology relative to extant analogues36. Given this substantial potential, the broader application of optimizing the efficiency of existing MR and CT analyses offers a significant advancement for both comparative neurology and virtual tissue visualization in biological research.
Methods
Sample preparation and scanning
All animal experimentation protocols were approved by the Ethics Committee of the Institute for Clinical and Experimental Medicine and the Ministry of Health of Czech Republic in accordance with the European Communities Council Directive 86/609/EEC. The severed heads of six adult (six months old; three male, three female), and four early stage juvenile (seven weeks old; two male, two female) Phasianus colchicus were fixed in formalin for four weeks. These specimens were each sealed in airtight plastic bags to prevent drying and shrinkage1. They were then scanned at high resolution (voxel volume = 0.002775 mm3) using a 4.7T MR spectrometer (Bruker BioSpec) equipped with a commercially available resonator coil (Bruker), and 3D Rapid Acquisition incorporating a Relaxation Enhancement (RARE) multi-spin echo sequence (basic parameters in Table 4). Prolonged MR scan time (>13 hours) generated images with sufficient contrast-to-noise ratio for quantitative analysis (Supplementary Figure 3c, 4, 5). We also processed one adult control sample of a Hoatzin (Opisthocomus hoazin) using identical procedures to examine possible changes in MR contrast using 2D RARE multi-spin echo sequence (Table 4). Hampering of image contrast during long term MR scanning was assessed via relaxometry undertaken at 25 °C with a standard Car-Purcel-Meiboom-Gill (CPMG) multi-spin echo sequence. Two T2 maps (Table 1) were calculated with an evolution delay of 17 hours between maps. Each sample was retained within the MR scanner for the full duration of the study (>20 hours). CT data was acquired on an Albira (Bruker BioSpin) μCT/PET system scanner and reconstructed using the Feldkamp, Davis, and Kress (FDK) algorithm in Plastimatch37 (resolution = 125 × 125 × 125 μm3).
Table 4. Parameters for RARE and relaxometry sequences.
| Parameters | 3D RARE | 2D RARE | Relaxometry |
|---|---|---|---|
| Repetition time (TR ms) | 500 | 3000 | 5000 |
| Effective echo time (TE ms) | 36 | 36 | 7.2 |
| Turbo factor | 8 | 8 | − |
| Number of acquisitions | 8 | 2 | 8 |
| Field of view (FOV cm3) | 5.0 × 6.2 × 6.0 | 4.6 × 4.6 | 4.6 × 4.6 |
| Spatial resolution (μm2) | 98 × 121 | 180 × 180 | 359 × 359 |
| Effective spectral bandwidth (Hz) | 69444 | 34722 | 101010 |
| Scan time (hrs) | 18.12 | 2.24 | 1.25 |
| Slice thickness (mm) | 0.234 | 2 | 2 |
| Echo number | 8 | 8 | 256 |
To directly compare our MR and μCT results, each sample was immersed in a saline solution mixed with neutral formalin and SPT to enhance CT contrast. The effects of SPT were also examined relative to early-stage ex vivo P. colchicus ontogenetic proxies scanned under varying conditions (contrasting tissue preparation and media): (1) saline solution + neutral formalin but without SPT after 3 days of fixation; (2) an SPT water solution (50g/l) after 6 days of fixation; and (3) a saline + neutral formalin + SPT solution after 3 months of fixation. Because relaxation times determine contrast of MR images, we additionally measured T1 (saturation recovery sequence) and T2 (CPMG sequence) of fixative medium with and without SPT on a 0.5T relaxometer (Bruker Minispec) at 25 °C (Supplementary Table 1). Our MR images differed according to the dominant influence (weighting) of proton density during T1 or T2 (T2*) relaxation times. This was due to chemical fixation of the tissue by formalin (identified via comparisons with in vivo samples), which encourages protein cross-linking and immobilization of water molecules, and thus a decrease in T2 relaxation times38,39. Fixation effects are dependent on agent concentrations, scan time, and positioning within the sample39,40,41,42. Dawe et al. showed that T2 values near the surface of human brain hemispheres are constant during six-month fixation periods, and therefore longer fixation times (>three months) are necessary to stabilize T2 relaxation times throughout the whole tissue body42. To accommodate, we used concentrated media and proportionately small avian brains to achieve complete fixation in about four weeks; evidenced by stable T2 values and MR contrast.
Volumetric calculations
Brain tissue and endoneurocranial volumes/surface areas were calculated from manual delineation in VGStudio MAX (http://www.volumegraphics.com), and employed as a comparison for Fakir method and Watershed segmentation protocols. We also developed a new software package based on the Fakir method for interactive manipulation of our MR data. The Fakir method estimates the volume and surface area of visualized objects from their intersections with a random 3D grid of lines (Supplementary Figure 5). Cruz-Orive43 originally developed the Fakir probe with a single set of parallel lines, but this suffered from estimation variance because the line intersection lengths differed directionally, thus impinging measurement of anisotropic objects. Sandau44 included perpendicular fixation of the grid with an orthogonal triplet of parallel lines, yet again this required isotropic orientation of surface elements for non-biased surface area estimation. The optimized Fakir method45 has solved these problems by employing multiple grids with a random orientation. These benefit from an antithetic effect (negative covariance of partial estimates from multidirectional lines46) to decrease variance. As an example, the maximum coefficient of variation (CV) achieved for planar objects is 0.58 for a single probe, but can be significantly improved via a triple probe (CV = 0.10). To elaborate this further, we initiated a seven-fold probe47, which achieved an orientation CV < 0.04. Grid geometry was further enhanced by mutually adjusting the sets of parallel lines to halve the number of intersects across the given surface area required for achieving precision. More information is available from: http://www2.biomed.cas.cz/~janacek/fakir/3dtools.htm.
To increase speed and efficiency, we also used an automated 3D image analysis for μCT. This utilized a Watershed method, which is a powerful tool based on basin-like contour forms defined by closed peaks and troughs48,49. Such a technique offers marked advantage for image segmentation, and is augmented by fast computation times in comparison to other approaches. We implemented our Watershed algorithm in purpose-developed modules using Amira (FEI).
Additional Information
How to cite this article: Jirak, D., Janacek, J. & Kear, B. P. A combined MR and CT study for precise quantitative analysis of the avian brain. Sci. Rep. 5, 16002; doi: 10.1038/srep16002 (2015).
Supplementary Material
Acknowledgments
D.J. and J.J. were funded by the Czech Science Foundation (P302/12/1207). B.P.K. was funded by the Swedish Research Council. We thank Martin Kundrát for valuable comments and Luis Fabio Silveira for hoatzin sample collection.
Footnotes
Author Contributions D.J. and J.J. designed research. B.P.K., D.J. and J.J. wrote the manuscript. All authors discussed the results and reviewed the manuscript.
References
- Healy S. D. & Rowe C. A critique of comparative studies of brain size. Proc. R. Soc. B 274, 453–464 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwaniuk A. N. & Nelson J. E. Can endocranial volume be used as an estimate of brain size in birds? Can. J. Zool. 80, 16–23 (2002). [Google Scholar]
- Iwaniuk A. N. & Nelson J. E. Developmental differences are correlated with relative brain size in birds: a comparative analysis. Can. J. Zool. 81, 1913–1928 (2003). [Google Scholar]
- Marino L., Sol D., Toren K. & Lefebvre L. Does diving limit brain size in cetaceans? Mar. Mammal. Sci. 22, 413–425 (2006). [Google Scholar]
- Sutton M. D. Tomographic techniques for the study of exceptionally preserved fossils. Proc. R. Soc. B 275, 1587–1593 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corfield J. R., Wild J. M., Cowan B. R., Parsons S. & Kubke M. F. MRI of postmortem specimens of endangered species for comparative brain anatomy. Nature Protoc. 3, 597–605 (2008). [DOI] [PubMed] [Google Scholar]
- Zelenitsky D. K., Therrien F., Ridgely R. C., McGee A. R. & Witmer L. M. Evolution of olfaction in non-avian theropod dinosaurs and birds. Proc. R. Soc. B 278, 3625–3634 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mace G. M., Harvey P. H. & Clutton-Brock T. H. Brain size and ecology in small mammals. J. Zool. 193, 333–354 (1981). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwaniuk A. N., Dean K. M. & Nelson J. E. A mosaic pattern characterizes the evolution of the avian brain. Proc. R. Soc. B 271, S148–S151 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sahin B. et al. Brain volumes of the lamb, rat and bird do not show hemispheric asymmetry: a stereological study. Image Anal. Stereol. 20, 9–13 (2001). [Google Scholar]
- Garamszegi L. Z., Eens M., Erritzoe J. & Møller A. P. Sperm competition and sexually size dimorphic brains in birds. Proc. R. Soc. B 272, 159–166 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garamszegi L. Z., Eens M., Erritzoe J. & Møller A. P. Sexually size dimorphic brains and song complexity in passerine birds. Behav. Ecol. 16, 335–345. [Google Scholar]
- Møller A. P., Erritzoe J. & Garamszegi L. Z. Covariation between brain size and immunity in birds: implications for brain size evolution. J. Evol. Biol. 18, 223–237 (2005). [DOI] [PubMed] [Google Scholar]
- Glen C. L. & Bennett M. B. Foraging modes of Mesozoic birds and non-avian theropods. Curr. Biol. 17, R911–R912 (2007). [DOI] [PubMed] [Google Scholar]
- Dial K. P., Jackson B. E. & Segre P. A fundamental avian wing-stroke provides a new perspective on the evolution of flight. Nature 451, 985–989 (2008). [DOI] [PubMed] [Google Scholar]
- Wang X., McGowan A. J. & Dyke G. J. Avian wing proportions and flight styles: first step towards predicting the flight modes of Mesozoic birds. PloS ONE 6(12), e28672 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopson J. A. Relative brain size and behavior in archosaurian reptiles. Ann. Rev. Ecol. Syst. 8, 429–448 (1977). [Google Scholar]
- Larsson H. C., Sereno P. C. & Wilson J. A. Forebrain enlargement among non-avian theropod dinosaurs. J. Vertebr. Paleont. 20, 615–618 (2000). [Google Scholar]
- Dominguez P. A., Milner A. C., Ketcham R. A., Cookson M. J. & Rowe T. B. The avian nature of the brain and inner ear of Archaeopteryx. Nature 430, 666–669 (2004). [DOI] [PubMed] [Google Scholar]
- Kurochkin E. N., Dyke G. J., Saveliev S. V., Pervushov E. M. & Popov E. V. A fossil brain from the Cretaceous of European Russia and avian sensory evolution. Biol. Lett. 3, 309–313 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metscher B. D. MicroCT for comparative morphology: simple staining methods allow high-contrast 3D imaging of diverse non-mineralized animal tissues. BMC Physiology 9, 11 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kotrotsou A. et al. Ex vivo MR volumetry of human brain hemispheres. Magn. Reson. Med. 71, 364–374 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vellema M., Verschueren J., Van Meir V. & Van der Linden A. A customizable 3-dimensional digital atlas of the canary brain in multiple modalities. NeuroImage 57, 352–361 (2011). [DOI] [PubMed] [Google Scholar]
- Ebel K.-D. & Benz-Bohm G. CT and MRI features of brain neoplasms. In Ebel K.-D., Blickman H., Willich E. & Richter E. Eds. Differential Diagnosis in Pediatric Radiology. Stuttgart, Thieme, 538–547 (1999). [Google Scholar]
- Güntürkün O., Verhoye M., De Groof G. & Van der Linden A. A 3-dimensional digital atlas of the ascending sensory and the descending motor systems in the pigeon brain. Brain Struct Funct 218, 269–281 (2013). [DOI] [PubMed] [Google Scholar]
- De Groof G. et al. A three-dimensional digital atlas of the starling brain. Brain Struct Funct (2015) 10.1007/s00429-015-1011-1. [DOI] [PubMed] [Google Scholar]
- Cosgrove K. P., Mazure C. M. & Staley J. K. Evolving knowledge of sex differences in brain structure, function, and chemistry. Biol. Psychiat. 62, 847–55 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoenemann P. T. Brain size scaling and body composition in mammals. Brain Behav. Evol. 63, 47–60 (2004). [DOI] [PubMed] [Google Scholar]
- Barton R. A. & Harvey P. H. Mosaic evolution of brain structure in mammals. Nature 405, 1055–1058 (2000). [DOI] [PubMed] [Google Scholar]
- Chiappe L. M., Marugán-Lobón J. & Zhou Z. Life history of a basal bird: morphometrics of the Early Cretaceous Confuciusornis. Biol. Lett. 4, 719–723 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters W. S. & Peters D. S. Life history, sexual dimorphism and ‘ornamental’ feathers in the Mesozoic bird Confuciusornis sanctus. Biol. Lett. 5, 817–820 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiappe L. M., Marugán-Lobón J. & Chinsamy A. Palaeobiology of the Cretaceous bird Confuciusornis: a comment on Peters & Peters (2009). Biol. Lett. 6, 529–530 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters W. S. & Peters D. S. Sexual size dimorphism is the most consistent explanation for the body size spectrum of Confuciusornis sanctus. Biol. Lett. 6, 531–532 (2010). [Google Scholar]
- (2010).
- Milner A. C. & Walsh S. A. Avian brain evolution: new data from Palaeogene birds (Lower Eocene) from England. Zool. J. Linn. Soc. 155, 198–219 (2009). [Google Scholar]
- Jarvis E. D. et al. Avian brains and a new understanding of vertebrate brain evolution. Nature Rev. 6, 151–159 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garamszegi L. Z., Møller A. P. & Erritzoe J. Coevolving avian eye size and brain size in relation to prey capture and nocturnality. Proc. R. Soc. B 269, 961–967 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharp G., Kandasamy N., Singh H. & Folkert M. GPU-based streaming architectures for fast cone-beam CT image reconstruction and demons deformable registration. Phys. Med. Biol. 52, 5771–5783 (2007). [DOI] [PubMed] [Google Scholar]
- Kennan R. P., Richardson K. A., Zhong J., Maryanski M. J. & Gore J. C. The effects of cross-link density and chemical exchange on magnetization transfer in polyacrylamide gels. J. Magn. Reson. B 110, 267–277 (1996). [DOI] [PubMed] [Google Scholar]
- Fishbein K. W. et al. Effects of formalin fixation and collagen cross-linking on T2 and magnetization transfer in bovine nasal cartilage. Magn. Reson. Med. 57, 1000–1011 (2007). [DOI] [PubMed] [Google Scholar]
- Medawar P. B. The rate of penetration of fixatives. J. Roy. Microsc. Soc. 61, 46–57 (1941). [Google Scholar]
- Dempster W. T. Rates of penetration of fixing fluids. Am. J. Anat. 107(1), 59–72 (1960). [DOI] [PubMed] [Google Scholar]
- Dawe R. J., Bennett D. A., Schneider J. A., Vasireddi S. K. & Arfanakis K. Postmortem MRI of human brain hemispheres: T2 relaxation times during formaldehyde fixation. Magn. Reson. Med. 61, 810–818 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cruz-Orive L. M. Stereology of single objects. J. Microsc. 186, 93–107 (1997). [Google Scholar]
- Sandau K. How to estimate the area of a surface using the spatial grid. Acta Stereol. 6, 31 (1987). [Google Scholar]
- Kubínová L. & Janáček J. Estimating surface area by the isotropic Fakir method from thick slices cut in an arbitrary direction. J. Microsc. 191, 201–211 (1998). [DOI] [PubMed] [Google Scholar]
- Mattfeldt T., Mobius H. J. & Mall G. Orthogonal triplet probes: an efficient method for unbiased estimation of length and surface of objects with unknown orientation in space. J. Microsc. 139, 279–289 (1985). [DOI] [PubMed] [Google Scholar]
- Janáček J. & Kubínová L. Variances of length and surface area estimates by spatial grids: preliminary study. Image Anal. Stereol. 29, 45–52 (2011). [Google Scholar]
- Beucher S. & Lantuéjoul C. Use of watersheds in contour detection. In Proceedings International Workshop on Image Processing, Real-Time Edge and Motion Detection/Estimation, Rennes, France. September (1979).
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.