Abstract
The production of “natural” autoantibodies or antibodies, i.e., Ig that bind a variety of self- and/or exogenous Ag and arise independently of known immunization, is thought to be a feature of CD5+ B lymphocytes. To determine whether other lymphocyte subsets exist that might be committed to the production of natural antibodies, human peripheral blood B cells were sorted on the basis of surface CD5 expression and differential expression of surface CD45RA (CD5+CD45RAintermediate(int), CD5− CD45RAlow(lo), and CD5−CD45RAhigh(hi)), and analyzed for the type of Ig produced after EBV infection and culture. Like their CD5+ counterparts, most CD5−CD45RAlo B lymphocytes were precursors of cells producing IgM, a major proportion of which displayed the Ag-binding features of natural antibodies. In contrast, CD5−CD45RAhi B cells comprised a high frequency of IgG-producing cell precursors, possibly including memory B lymphocytes. Six of seven IgM mAb generated from sorted CD5−CD45RAlo B cells and three of four IgM mAb from sorted CD5+ B cells were polyreactive, binding with different affinities (Kd, 10−5 to 10−8 M) to two or more Ag; the remaining mAb from CD5−CD45RAlo and the mAb from CD5+ B cells each bound to a single Ag (Kd, 10−7 to 10−8 M), β-galactosidase and ssDNA, respectively. CD5−CD45RAlo B cells account for 4.1 ± 1.2% (mean ± SD in 11 healthy subjects; CD5+ B cells, 23.3 ± 6.9%) of total B lymphocytes and display the features of quiescent cells. In a given individual, the number of CD5−CD45RAlo B cells remains constant over time. CD5−CD45RAlo and CD5+ B cells bear surface CD11b and CD14, at densities and/or frequencies apparently higher than those of CD5−CD45RAhi B lymphocytes. Despite their surface CD5− phenotype, CD45RAlo B cells express CD5 mRNA at levels comparable with those of CD5+ B lymphocytes, whereas CD5−CD45RAhi B cells express only trace amounts of CD5 mRNA. The commitment to natural antibody production and the degree of CD5 mRNA expression suggest that the newly defined CD5−CD45RAlo B cell subset is related to CD5+ B lymphocytes, and may constitute the human homologue of the mouse Ly-1− “sister” B cell population.
Murine Ly-1+ B cells are thought to constitute a lineage different from that of “conventional” (Ly-1−) B cells (1). Ly-1 is unrelated to the surface receptor for Ag and was previously thought to be expressed uniquely on T lymphocytes. The Ly-1+ and Ly-1− B lymphocyte populations are distinct in expression of surface molecules, organ location, ontogeny, and development (reviewed in References 2 to 4). The human homologues of murine Ly-1+ B cells, CD5+ B lymphocytes (reviewed in References 2 to 7), account for the vast majority of B cells in the late developmental stages of the fetus and in the newborn (8). Early in life, “conventional” CD5− B cells progressively increase in number, effectively reducing the CD5+ to CD5− B cell ratio. In the adult, CD5+ B lymphocytes still constitute a significant proportion of the B cell repertoire, accounting for 10 to 30% of circulating, tonsillar, and splenic B lymphocytes (9–11).
In normal humans and mice, a major proportion of CD5+ and Ly-1+ B cells, respectively, are committed to the production of antibodies, mainly IgM, but also IgG and IgA, binding a variety of self-Ag, e.g., IgG Fc fragment, ssDNA, insulin, thyroglobulin, and actin, as well as exogenous Ag, e.g., viruses or bacterial components and products (9, 10, 12, 13). Ig with such reactivities are referred to as “natural” autoantibodies or antibodies (5, 7, 9, 12–21). A major proportion of these antibodies is polyreactive, binding with different affinities to two or more Ag that may differ widely in nature (e.g., proteins, polynucleotides, polysaccharides, or phospholipids) (5, 7, 9, 12, 13, 15–25). Some natural antibodies are mono-reactive and can display affinities for self-Ag comparable with those of autoantibodies isolated from autoimmune patients (20, 26). Supporting an autoantibody-producing role for CD5+ B cells not only in the normal, but also in the autoimmune B cell repertoire, is the demonstration that Ly-1+ B cells are greatly increased in number and secrete autoantibodies in autoimmune NZB mice (2, 3, 14). Moreover, CD5+ B cells are greatly increased in number and/or produce autoantibodies in certain human autoimmune conditions, such as rheumatoid arthritis, SLE, Sjogren syndrome, and, possibly, primary antiphospholipid syndrome (18, 27–33). Finally, in patients with chronic lymphocytic leukemia, the occurrence of neoplastic CD5+ B cells is associated with that of autoantibodies and autoimmune traits (4, 34).
Evidence, however, has accumulated suggesting that natural antibodies may also be produced by some CD5− B lymphocytes (9, 10, 12, 35–37). Accordingly, a major proportion of anti-DNA IgG-producing cell precursors has been consistently detected among CD5− B lymphocytes in SLE patients (38). To segregate natural antibody-producing CD5− B cells as a discrete subset, we sorted normal human CD5− B lymphocytes into two subpopulations displaying low and high surface levels of the high m.w. (220 and 200 kDa) isoforms (CD45RA) of the leukocyte-common Ag (L-CA, CD45): CD5− CD45RAlo and CD5−CD45RAhi B cells, respectively. CD5+ B cells were sorted for comparison. The rationale for our approach was derived from experiments in the mouse suggesting that B cells possibly expressing the high m.w. isoforms of the murine L-CA at low density produce Ig with the features of natural antibodies (39–41). Analysis of the Ig produced by our sorted lymphocytes showed that, like CD5+ B cells, the CD5−CD45RAlo, but not the CD5−CD45RAhi, B cell subset is highly enriched in natural antibody-producing cell precursors. This and the results of detailed phenotypic studies suggest that the surface CD5−CD45RAlo B cell subset is related to its CD5+ counterpart.
MATERIALS AND METHODS
PBMC and purified B lymphocytes
Healthy blood donors (New York Blood Center, New York, NY) were used as source of PBMC. These were separated using lymphocyte separation medium (Organon Teknika-Cappel, Malvern, PA). To prepare purified B lymphocytes, PBMC were depleted of T cells and monocytes by SRBC (Colorado Serum Co., Denver, CO) rosetting and treatment with l-leucine methyl ester, respectively (12, 18, 26, 42). Purified T cells were recovered from the SRBC-rosette fraction by lysing SRBC with H2O.
mAb to PBMC surface markers and flow cytometry studies
The following mouse mAb to human Ag were used: biotinylated, FITC-or PE4-labeled mAb to CD20 (clone H299, IgG2aκ; B1, Coulter, Hialeah, FL); biotin-, FITC-, or PE-labeled mAb to CD5 (clone SFC124T6G12, IgG2aκ; T1, Coulter); FITC-labeled mAb to CD45RA (220 and 200 kDa isoforms of the L-CA) (clone 2H4LDH11LDB9, IgG1κ; 2H4; Coulter); FITC-labeled mAb to CD45RO (180 kDa isoform of the L-CA) (clone UCHL1, IgG2aκ; DAKO-UCHL1, Dakopatts, Glostrup, Denmark); mAb to CD11b (clone 94, IgMκ; Mol, Coulter); biotinylated mAb to CD11b (clone LM2/1.6.11, IgG1κ; American Type Culture Collection, Bethesda, MD, HB 204); and biotinylated mAb to CD14 (clone 322A-1, IgG2bκ; My4, Coulter). In addition, mouse IgM (clone R4A3–22-12, IgM/κ; Coulter), IgG1 (clone SK7; IgG1; Becton Dickinson, Mountain View, CA) or mouse IgG2b (Coulter) mAb with irrelevant specificities were used as isotype controls as appropriate in the experiments. B lymphocytes were incubated with the indicated FITC-, PE- or biotin-conjugated mAb in ice-cold sterile PBS, pH 7.4, containing 1% BSA and 1% human AB serum (GIBCO-BRL, Life Technologies, Gaithersburg, MD). Cell-bound biotinylated mAb were detected using streptavidin labeled with FITC, PE, or RED613 (GIBCO). For fluorescence flow cytometric analysis, cells were fixed using PBS, pH 7.4, containing 1% paraformaldehyde, and applied to a FACScan equipped with an argon ion laser (Becton Dickinson). For sorting, lymphocytes were resuspended in ice-cold PBS and applied to an Ortho Cytofluorograf equipped with an argon ion laser (Ortho Instruments, Westwood, MA). Sorted cells were collected into frozen RPMI 1640 containing 2 mM glutamine, 1% ampicillin, and 20% FCS (GIBCO-BRL, Life Technologies).
To analyze surface CD11b expression, purified B cells that had been sorted by tagging with the various PE- and FITC-labeled mAb were washed three times and reacted with the biotinylated mouse IgGl mAb to human CD11b, followed by RED613-labeled streptavidin. Sorted B lymphocytes were similarly analyzed for surface CD14, using biotinylated IgG2a mAb to CD14. In some cases, CD11b expression was analyzed by reacting the cells with a mouse IgM mAb to CD11b, followed by a biotinylated affinity-purified goat F(ab′)2 fragment to mouse IgM (Organon Teknika-Cappel) and RED613-labeled streptavidin. In unfractionated PBMC, surface CDllb and CD14 expression were analyzed using three-color fluorescence flow cytometry involving PE-labeled mAb to CD5, FITC-labeled mAb to CD20, the CD11b- or CD14-specific reagents, and RED613-labeled streptavidin. The final RED613 (a conjugate of PE and Texas Red) light emission peak (613 nm) is well separated from the emission peaks of FITC and PE (515 and 575 nm, respectively).
Culture of B cells and detection of antibody-producing cell precursors
Concentrated EBV was prepared and aliquoted as described (42). B cells were resuspended in a freshly thawed EBV aliquot and incubated at 37°C for 2 h. After addition of fresh RPMI 1640 containing 2 mM glutamine, 1% ampicillin, and 10% FCS (FCS-RPMI), cells were distributed at 2000, 1000, 500, 250, 125, 60, 30, 10, 5, 2, and 1/well, in the presence of 105 irradiated (1800 rad) PBMC as feeders, in microculture plates (12, 18, 26). Forty-eight wells, containing a total volume of 200 µ1, were seeded for each cell density. After a 3-wk culture at 37°C in a humidified 5% CO2 atmosphere, 180 µl of culture fluid were harvested, and divided into six 30-µl aliquots. The aliquots were diluted 1/2 with PBS-Tween, and analyzed for total IgM, IgG, IgA, κ and λ L chains using specific ELISA, and peroxidase-labeled anti-Ig H and L chain antibody probes, as previously detailed (9, 12, 18, 26, 28, 36, 38). In each experiment, appropriate titration reference curves were constructed. Calculations of the frequencies of IgM-, IgG-, and IgA-producing cell precursors were performed based on Poisson distribution analysis of the data derived from plots of the fraction of negative microcultures (wells) for antibody production vs input cell dose of the limiting dilution culture experiments, as described (9, 18, 26, 42–44).
Analysis of the segregation of the precursors of cells producing antibodies with various Ag-binding activity were performed using fluids from microcultures seeded with 250, 125, 60, and 30 sorted and EBV-infected CD5+, CD5−CD45RAlo, and CD5−CD45RAhi B cells/ well in 96-well plates, and specific ELISA. The following Ag, diluted to the indicated concentration in 0.1 M carbonate/bicarbonate buffer, pH 9.6, were used to coat ELISA plates: polyclonal human IgG Fc fragment (m.w., 25,000, Organon Teknika-Cappel) (5 µg/ml); calf thymus ssDNA (average m.w., 500,000, Sigma Chemical Co., St. Louis, MO) (10 µg/ml); recombinant human insulin (m.w., 6000, a gift from Eli Lilly Corp., Indianapolis, IN) (2.5 µg/ml); actin from porcine heart (m.w., 43,000, Sigma) (5 µg/ml); PC (m.w., 258, Sigma) (20 µg/ml); tetanus toxoid (TT) (m.w., 110,000, Massachusetts Public Health Biological Laboratories, Jamaica Plain, MA) (2 µg/ml); and β-galactosidase from Escherichia coli (monomer m.w., 135,000, Sigma) (5 µg/ml).
Generation of human monoclonal EBV-transformed cell lines, construction of somatic cell hybrids, and analysis of mAb Ag-binding activities
Cell lines producing mAb of selected Ag-binding activity were established from EBV-transformed B cells by three sequential subculturing steps under limiting dilution conditions. EBV-transformed cell lines were stabilized by fusion with F3B6 cells, an Ig-nonsecretor, HAT-sensitive, and ouabain-resistant human-mouse hybrid, as previously described (12, 26, 44, 45). Cell hybrids were recloned and mAb were prepared as described (44, 45).
The Ag-binding activities were analyzed by dose-dependent binding, homologous Ag inhibition, and cross-competitive Ag inhibition studies as detailed elsewhere (12, 18, 26). In competitive inhibition assays, increasing amounts (0.025 to 50 µg) of soluble ssDNA (5.0 × 10−10 to 1.0 × 10−6 M), actin (5.7 × 10−9 to 1.1 × 10−5 M), PC (7.6 × 10−7 to 1.5 × 10−3 M), TT (2.2 × 10−9 to 4.5 × 10−6 M), or β-galactosidase (1.8 × 10−9 to 3.7 × 10−6 M) were reacted for 24 h with the indicated mAb (present in at least 10-fold lower molar amounts) in PBS (100 µl) containing 0.05% Tween 20 (PBS-Tween) and 1% BSA. After an additional 18 h incubation at room temperature, the mixtures were transferred to ELISA plates precoated with either the same Ag used in soluble form in the preincubation step (homologous competition) or a different Ag (heterologous or cross-competition). After a 2-h incubation and subsequent washing with PBS-Tween, the amount of mAb bound to the solid phase Ag was measured using a peroxidase-conjugated affinity-purified goat antibody to human IgM. Binding activity of a given mAb observed in the presence of soluble ligand was expressed as percentage of binding activity measured after incubation of the same mAb under identical conditions but in the absence of any soluble ligand. The data derived from the homologous competitive inhibition experiments were used to calculate the Kd of each mAb for different Ag (12, 18, 26).
Analysis of CD5-specific mRNA expression in the different B cell subsets
A PCR method was developed to analyze CD5 mRNA expressed by B and T cells. Three sets of B cell cultures were studied. Each set consisted of three discrete B lymphocyte (107) cultures generated by EBV transformation of freshly sorted CD5+, CD5−CD45RAlo and CD5−CD45RAhi B cells from a single subject. Poly(A)+ RNA from B or T cells was purified by affinity absorption to oligo(dT)-cellulose. mRNA (2 or 0.4 µg) from each B cell subset, and mRNA (2.0 to 0.004 µg) from T cells was divided into two aliquots, which were distributed in two microtubes eventually used throughout the β-actin and CD5 cDNA amplification procedures. mRNA was transcribed into cDNA using M-MLV reverse transcriptase (Super-Script RNase H- Reverse Transcriptase. GIBCO-BRL, Life Technologies) in conjunction with a poly(dT)12−18 primer. After cDNA synthesis, RNA was degraded by RNase H. cDNA was then amplified using the appropriate oligonucleotide primers (β-actin- or CD5-specific) and Taq DNA polymerase (Perkin-Elmer Cetus, Emeryville, CA). The oligonucleotide primers were designed on the basis of the human genomic β-actin DNA (46) and CD5 cDNA (47) sequences. Each of them yielded DNA products different in size when amplifying genomic DNA and cDNA. The β-actin-specific 5′-(5′ GTAC-CACTGGCATCGTGATGGACT 3′) and 3′-(5′ ATCCACACGGAG-TACTTGCGCTCA 3′) primers encompassed sequences corresponding to residues 1013–1036 and 1779–1802, respectively, of the published β-actin DNA sequence (46). These two exonic sequences flank two intervening introns and one intervening exon, and yielded ~0.6 and ~0.9 kbp products when amplifying cDNA and genomic DNA, respectively. The CD5-specific 5′-(5′ AGGACGGATGGCA-CATGGTTT 3′) and 3′-(5′ TTGTCCTGGGCCTCATAGCT 3′) primers encompassed sequences corresponding to residues 230–250 and 628–647, respectively, of the pT1-1 CD5 cDNA (47). These two primers yielded ~0.45 and ~ 1.1 kbp products when amplifying cDNA and genomic DNA, respectively. Each PCR reaction was carried out in a 50-µl volume. Denaturing, annealing, and extension temperatures were 94° (1 min), 52° (2 min), and 72°C (2 min), respectively. To amplify β-actin and CD5 cDNA, 18 and 25 cycles, respectively, were performed. The amplified β-actin (5 and 20 µl) and CD5 DNA, respectively, were applied to 1.2% agarose gels containing 1 µg/ml ethidium bromide. Fractionated DNA was transferred onto nylon membranes (GenScreen, Du Pont Co., NEN Research Products, Boston, MA) for hybridization with the 32P-labeled CD5-specific oligonucleotide (5′ CCAGAAGACAACACCTCCAA 3′) probe, encompassing residues 477–496 of the CD5 cDNA sequence (47). Autoradiograpy was carried out using Kodak XAR-5 film (Eastman Kodak Co., Rochester, NY).
RESULTS
Surface expression of CD45RA and CD5 by human B lymphocytes, and resolution and sorting of three B cell subsets
Mouse B lymphocytes display a differential expression of surface high m.w. isoforms of the L-CA (48). Treatment of murine B lymphocytes with mAb to these cell-surface molecules results in modulation of selected antibody responses (39–41). We reasoned that differential expression of CD45 by human B cells, in concert with that of CD5, might serve in segregating discrete B cell subsets, possibly committed to the production of different populations of antibodies. We analyzed the surface expression of both the high (220 and 200 kDa) and low (180 kDa) m.w. isoforms of CD45, i.e., CD45RA and CD45RO, respectively, by B cells, and compared it with that of autologous T lymphocytes. Fluorescence flow cytometric analyses using FITC-labeled mAb to CD45RA (2H4) or to CD45RO (UCHL1), and PE-labeled mAb to human CD20 (Bl) revealed that all human peripheral B cells consistently express, although at variable degrees, surface CD45RA but not CD45RO (Fig. 1, A and G), and suggested that the B cells with the lowest degree of CD45RA expression could be consistently resolved as a relatively small shoulder (Fig. 1, A and B). The exclusive CD45RA expression by B cells contrasts with the expression of CD45RA and/or CD45RO by T lymphocyte sub- populations (Fig. 1, H and I, respectively).
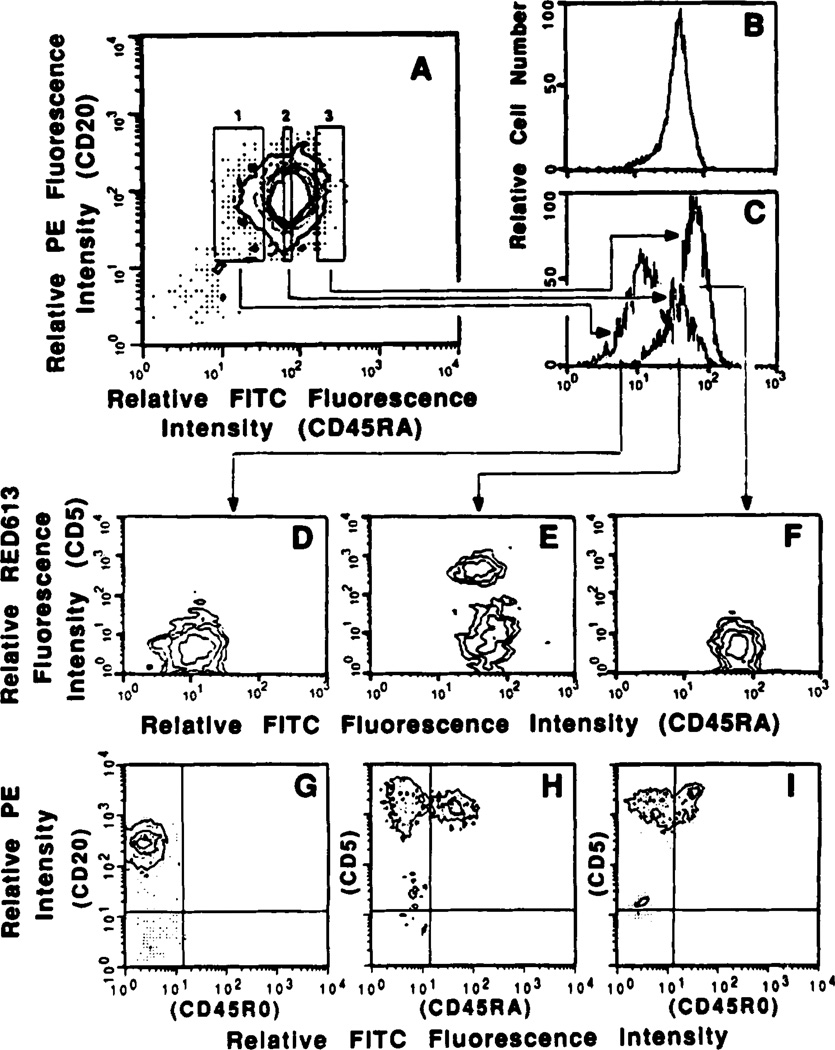
Figure 1.
Expression of surface CD45RA and CD5 by human B lymphocytes. Peripheral blood B cells (more than 98% CD20+) from a healthy subject were reacted with PE-labeled mAb to CD20 and FITC-labeled mAb to CD45RA and applied to the FACS. Lymphocytes within rectangles 1, 2, and 3 (A) were sorted as CD45RAlo, CD45RAint, and CD45RAhi B cells, respectively. (Each sorted fraction contained more than 99% CD20+ cells). Profiles of sorted cells analyzed for FITC fluorescence intensity (C) were compared with those obtained from similar analysis of the unfractionated cells (B). The mean FITC fluorescence intensities of the sorted B lymphocytes as determined by reanalysis were: CD5−CD45RAlo B cells, 11: CD5+ B cells, 45; and CD5−CD45RAhi B cells, 64. To determine which cell fraction contained CD5+ B lymphocytes, sorted CD45RAlo, CD45RAint, and CD45RAhi cells were reacted with biotinylated mAb to CD5 followed by RED613-labeled streptavidin, and analyzed by two-color fluorescence flow cytometry (D, E, and F, respectively). G depicts the contourgrams derived from the analysis of the unfractionated B cells after reaction with PE-labeled mAb to CD20 in conjunction with FITC-labeled mAb to CD45RO. H and I depicts the contourgrams derived from the analysis of autologous T lymphocytes after reaction with PE-labeled mAb to CD5 in conjunction with FITC-labeled mAb to CD45RA and FITC-labeled mAb to CD45RO, respectively. In B to F, only the first three logs of the four-log range of green fluorescence intensity are depicted.
To segregate B cells on the basis of their different levels of surface CD45RA and to analyze their surface CD5 expression, we conventionally defined the boundaries of two (CD45RAlo and CD45RAhi) B cell subsets (Fig. 1A, rectangles 1 and 3, respectively). In addition, we arbitrarily delimited the boundaries of a third B cell fraction expressing CD45RA at an intermediate degree (CD45RAint) (Fig. 1A, rectangle 2). CD45RAlo, CD45RAhi, and CD45RAint B lymphocytes were sorted as discrete fractions, as indicated by the reanalysis of some of the sorted cells (Fig. 1, cf. profiles in C and B). To determine the levels of cell-surface CD5, sorted CD45RAlo, CD45RAhi and CD45RAint B lymphocytes were reacted with biotinylated mAb to CD5 and RED613-streptavidin and analyzed by FACS. Neither CD45RAlo nor CD45RAhi lymphocytes contained detectable CD5+ B cells (Fig. 1, D and F). Rather, CD5+ B cells were found within the CD45RAint fraction (Fig. 1E); those CD5+ displayed a relatively lower density of CD45RA than did the CD5− cells of that subset. Similar results were derived from the analyses of B cells from two additional subjects (not shown).
For further phenotypic analysis of the lymphocytes and characterization of the antibodies they produce, we purified the three B cell subsets in a single step procedure by simultaneously reacting 5 × 107 B lymphocytes with PE-labeled mAb to CD5 and FITC-labeled mAb to CD45RA and applying them to the fluorescence-activated cell sorter. CD5−CD45RAlo, CD5−CD45RAhi and CD5+ B cells were segregated as three homogeneous fractions, as exemplified by reanalysis of some of the sorted lymphocytes (Fig. 2, A, D, E, and F). Similar sortings using B lymphocytes from 10 additional subjects (Fig. 2, B and C show representative data) consistently yielded comparable results. Based on these experiments, we calculated that CD5−CD45RAlo B cells account for 4.1 ± 1.2% (mean ± SD) of total peripheral blood B lymphocytes, i.e., less than one-fifth the proportion of CD5+ B cells in the same individuals (23.3 ± 6.9%, mean ± SD). Sequential samplings of PBMC from four healthy individuals revealed that, like CD5+ B lymphocytes, the proportion of CD5−CD45RAlo B lymphocytes remained remarkably constant over a 7-week period (Table I).
Figure 2.
Sorting of CD5−CD45RAlo, CD5−CD45RAhi, and CD5+ B cells and analysis of their light scattering properties. Purified B lymphocytes from three subjects (A to C) were simultaneously reacted with PE-labeled mAb to CD5 and FITC-labeled mAb to CD45RA. Cells within rectangles 1, 2, and 3 of A were sorted as CD5−CD45RAlo, CD5+, and CD5−CD45RAhi B lymphocytes, respectively. D to F show contourgrams derived from the reanalyses of some of the cells from each sorted fraction. The percentages of sorted lymphocytes falling back within the original sorting windows, as determined by reanalysis, was 80% for CD5+ (D), 93% for CD5− CD45RAlo(E), and 90% for CD5−D45RAhi (F). Contourgrams in insets depict the forward and side light scattering properties of the unsorted cells (A), and of the three sorted cell fractions (D to F). Single parameter profiles in insets of B and C depict the forward light scattering properties of CD5−CD45RAlo (.....), CD5−CD45RAhi (-.-), and CD5+ B cells (——). The forward light-scattering profiles of CD5−CD45RAlo and CD5+ B cells overlap.
TABLE I.
Proportions of B cell subsets in the peripheral blood of healthy subjects over timea
| Subject | CD5−CD45RAlo B Cells |
CD5+ B Cells |
||||||
|---|---|---|---|---|---|---|---|---|
| Day 0 | Day 10 | Day 30 | Day 50 | Day 0 | Day 10 | Day 30 | Day 50 | |
| 1 | 3.7 | 4.0 | 3.9 | 4.1 | 33.1 | 26.9 | 23.6 | 21.8 |
| 2 | 2.5 | 3.1 | 2.2 | 2.0 | 23.2 | 25.1 | 25.6 | N.D. |
| 3 | 4.0 | 4.1 | 4.4 | 4.5 | 26.2 | 26.8 | 24.0 | 24.8 |
| 4 | 5.4 | 5.7 | 5.3 | 5.5 | 28.4 | 26.5 | 23.5 | 24.2 |
PBMC from four healthy individuals were analyzed at the indicated time intervals. Lymphocytes were tagged with PE-labeled mAb to CD5, FITC-labeled mAb to CD45RA, and biotinylated mAb to CD20, followed by RED613-labeled streptavidin. Values are the percentages of total B lymphocytes (CD20+) that were CD5+ or CD5−CD45RAlo as determined by three-color fluorescence flow cytometry.
CD5−CD45RAlo and CD5+ B lymphocytes were small, resting cells. Similar to their CD5−CD45RAhi counterparts, they consistently displayed low degrees of forward and right-angle light scattering, as exemplified by the contourgrams (insets of A, D, E, and F) and the single parameter profiles (insets of B and C) of the B cells from the three subjects analyzed in Figure 2. These light scattering values are two- to four-fold lower than those we measured in previous experiments for in vivo activated B cells (18, 28, 38). Over the same 7-week period of the experiments reported above (Table 1), there was no variation in the light scattering properties of any of the three B cell subsets in any of the four subjects studied (not shown).
Surface CD11b and CD14 expression by CD5−CD45RAlo, CD5− CD45RAhi, and CD5+ B lymphocytes
Although the CD11b and CD14 molecules are characteristically expressed on the surface of cells of the myelomonocytic lineage, they have also been detected on CLL as well as “normal” CD5+ B lymphocytes (4). To analyze surface CD11b and CD14 expression by total B lymphocytes, we reacted PBMC from a healthy subject with PE-labeled mAb to CD5, FITC-labeled mAb to CD20, and mAb to CD11b or CD14, followed by the appropriate second reagents. Three-color FACS analysis revealed that B lymphocytes (CD20+ cells) expressed surface CD11b and/or CD14, but at levels 50 to 100 times lower than those detected on the surface of autologous monocytes and similar to those expressed by a major proportion of T cells (Fig. 3, A and B, respectively). Comparable results were obtained using PBMC from six additional individuals (not shown).
Figure 3.
Surface CD11b and CD14 in PBMC and purified B lymphocyte subsets. PBMC were reacted with PE-labeled mAb to CD5, FITC-labeled mAb to CD20, and i) mAb to CD11b (IgMκ), followed by biotinylated goat F(ab′)2 fragments to mouse IgM, and then RED613-labeled streptavidin (A), or ii) biotinylated mAb to CD14 (IgG2aκ), followed by RED613-labeled streptavidin (B). Three-color fluorescence analysis was performed. T cells were gated according to their high level of expression of CD5, B cells according to their expression of CD20, and monocytes according to their high forward and right angle light-scattering properties. The RED613 fluorescence intensity of each cell fraction was then determined. To analyze CD11b and CD14 surface expression in the different B cell subsets, CD5+, CD5−CD45RAlo, and CD5−CD45RAhi B lymphocytes sorted from a healthy subject were reacted with: i) biotinylated mouse IgG1κ of irrelevant specificity (open profiles) or biotinylated mouse mAb to human CD11b (IgG1κ) (solid profiles), followed by RED613-labeled streptavidin (C, E, and G); or ii) biotinylated mouse IgG2aκ of irrelevant specificity (open profiles) or biotinylated mouse mAb to human CD14 (IgG2aκ) (solid profiles), followed by RED613-labeled streptavidin (D, F, and H). Single parameter analyses of RED613 fluorescence were performed.
CD5+, CD5−CD45RAlo, and CD5−CD45RAhi B lymphocytes sorted using PBMC from two of these subjects were reacted with the biotinylated specific CD11b or CD14 reagents, followed by RED613-labeled streptavidin, and applied to the FACS. Single-color analyses of the cells from one of the two individuals showed that, similar to CD5+, a large proportion of CD5−CD45RAlo B cells expressed surface CD11b and/or CD14 (Fig. 3, C, D, E, and F), whereas fewer CD5−CD45RAhi B cells express either marker (Fig. 3, G and H). In the second individual, CD11b+ cells accounted for 20, 52, and 19% of CD5+, CD5−CD45RAlo, and CD5−CD45RAhi B lymphocytes, respectively. CD14+ cells accounted for 14, 30, and 2% of CD5+, CD5−CD45RAlo, and CD45RAhi B lymphocytes, respectively. These experiments show that peripheral blood human B cells bear surface CD11b and CD14, albeit at low density, and suggest that greater proportions of CD5+ and of CD5−CD45RAlo B lymphocytes express CD11b and CD 14 as compared with CD5−CD45RAhi cells.
Analysis of the classes and Ag-binding activities of the antibodies produced by CD5−CD45RAlo, CD5−CD45RAhi, and CD5+ B lymphocytes
We previously showed that EBV is equally efficient at activating B cells committed to the production of IgM, IgG, or IgA, as well as CD5+ and CD5” B cells (5,18, 26). To determine the frequencies of IgM-, IgG- and IgA-producing cell precursors among CD5+, CD5−CD45RAlo, and CD5−CD45RAhi B lymphocytes, these cells were sorted from PBMC from different subjects, infected with EBV, and distributed in limiting dilution conditions. After a 3- wk culture, microculture fluids were tested for Ig content and the frequency of precursors committed to the production of each Ig class was determined by Poisson distribution analysis of the limiting dilution data. Whereas the vast majority of CD5−CD45RAlo and CD5+ B cells produced IgM, less than 40% of CD5−CD45RAhi B lymphocytes produced this Ig class (Table II). Conversely, only a minority of CD5−CD45RAlo or CD5+ B cells were committed to the production of IgG, but more than 50% of CD5−CD45RAhi B lymphocytes produced this Ig class. Most of the remaining B cells in each subset were committed to the production of IgA. The ratio of κ to λ L chain- producing cells was the highest among CD5−CD45RAlo and the lowest among CD5−CD45RAhi B lymphocytes (Table II).
TABLE II.
Frequency of circulating IgM-, IgG-, or IgA-producing cell precursors in CD5−CD45RAloCD5−CD45RAhi, CD5+and unfractionated B lymphocytes
| B Cellsa |
% of Total B Cellsb |
Cells Committed to Production of Igc |
Ratio of κ to λ Ig-Producing Cellsd |
||
|---|---|---|---|---|---|
| IgM | IgG | IgA | |||
| CD5+ | 23.3 ± 6.9 | 91.9 ± 8.6 | 4.4 ± 0.2 | 3.6 ± 1.9 | 1.1 |
| CD5−CD45RAlo | 4.1 ± 1.2 | 75.1 ± 12.1 | 11.5 ± 6.4 | 13.4 ± 0.3 | 1.3 |
| CD5−CD45RAhi | 2.5 ± 0.5 | 38.1 ± 4.7 | 53.4 ± 11.5 | 8.6 ± 1.5 | 0.8 |
| Unfractionated | 100 | 82.4 ± 8.1 | 8.2 ± 0.7 | 9.6 ± 1.4 | 1.4 |
CD5−CD45RAlo, CD5−CD45RAhi. and CD5+ B lymphocytes were purified by fluorescence-activated cell sorting. Unfractionated B lymphocytes consisted of total B cells passed through the flow cytometer.
The magnitude of each B cell subset was determined as the proportion of lymphocytes (percentage of total B cells) arbitrarily gated within the appropriate windows and sorted. Numbers are means ± SD of the values measured in five healthy individuals.
B cells were infected with EBV and cultured under limiting dilution conditions to produce Ig. For each subject, 48 microcultures were analyzed at each cell concentration. The frequencies of lymphocytes producing Ig of different classes and L chain isotypes were calculated by statistical analysis according to Poisson distribution. Numbers represent the frequencies of B cells producing IgM, IgG, and IgA as percentages of the total Ig-producing cells (mean ± SD of values measured using B lymphocytes from five healthy subjects). For each sorted cell type, the proportion of B cells induced by EBV to secrete Ig was approximately 20% of the actually plated cells.
Ratios between the frequencies of κ and λ L chain-producing cell precursors as determined by limiting dilution analysis of B cells from the five healthy subjects.
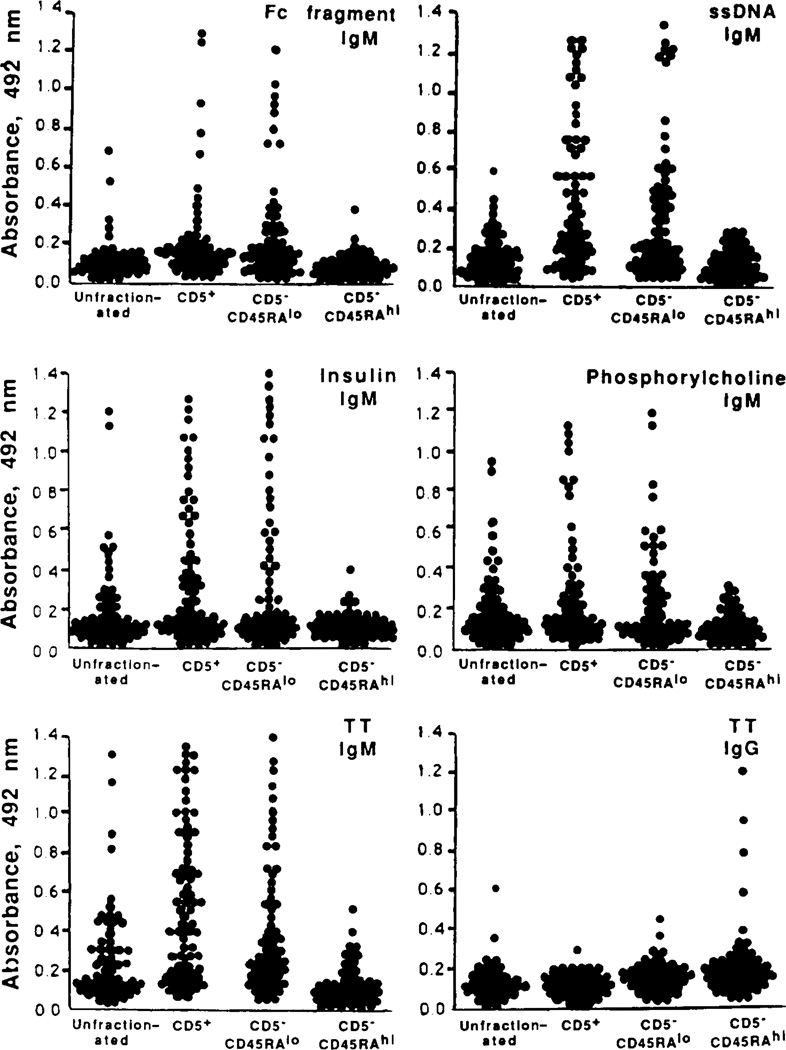
The distribution of the precursors of B cells producing antibodies with various Ag-binding activities was analyzed using microcultures that had been seeded with 250 EBV-infected CD5+, CD5−CD45RAlo and CD5−CD45RAhi B cells/well. The fluids harvested from individual micro-cultures were analyzed for content of antibodies to four self Ag: IgG Fc fragment, ssDNA, insulin and PC, and a prototypic exogenous Ag, TT. As expected, cells committed to the production of IgM binding to self- and exogenous Ag were readily detected among CD5+ B lymphocytes (Fig. 4). Precursors of cells producing antibodies with similar Ag-binding activities were also detected among CD5−CD45RAlo but not CD5−CD45RAhi B lymphocytes (Fig. 4). Instead, the CD5−CD45RAhi B cell subset contained the majority of the lymphocytes committed to the production of IgG to TT in these subjects, who had been previously vaccinated but not recently boosted with TT (Fig. 4). A similar clear-cut segregation of the natural antibody-producing cell precursors with CD5+ and CD5−CD45RAlo, but not CD5−CD45RAhi, B cells was observed when microculture wells seeded with 125, 60, and 30 cells were analyzed (data not shown). Thus, like CD5+ B cells, CD5−CD45RAlo B lymphocytes are enriched in precursors of cells producing IgM, a major proportion of which display the Ag-binding activities generally associated with natural antibodies. In contrast, CD5−CD45RAhi B lymphocytes are highly enriched in precursors of cells producing IgG, including those to an exogenous Ag, TT, known to induce memory B cells.
Figure 4.
Ag-binding activities of the antibodies produced by unfractionated, CD5+, CD5−CD45RAlo, CD5−CD45RAhi B lymphocytes. Sorted B cells were infected with EBV for 1 h and then seeded in culture at 250/well in the presence of irradiated feeders. After 3 wk, culture fluids were tested for the presence of antibodies to five Ag using specific ELISA. Each dot represents the Ag-binding activity (expressed as absorbance at 492 nm) of IgM or IgG in the fluid of a single microculture. Eighty microcultures established using B cells from three different subjects were assayed in each column. Fc fragment is the Fc fragment of human IgG. The amount of IgM produced over a 3-wk period by 250 EBV-transformed B cells ranged from about 1500 ng (CD5−CD45RAhi B cells) to 3000 ng (CD5−CD45RAlo and CD5+ B cells).
Generation of mAb-producing B cell lines using CD5−CD45RAlo and CD5+ B lymphocytes and analysis of Ag-binding activities
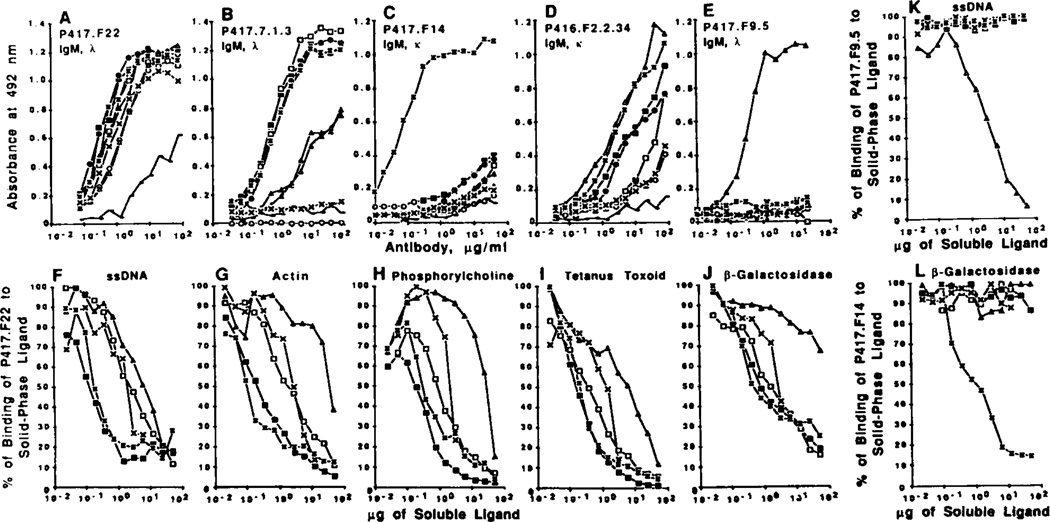
To analyze at the clonal level the Ag-binding activities of the mAb produced by CD5−CD45RAlo B cells and to compare them with those of the Ig produced by CD5+ B cells, we generated seven mAb-producing cell lines from purified CD5−CD45RAlo B cells and four from CD5+ B lymphocytes of three subjects using EBV-transformation and somatic cell hybridization techniques. These cell lines were selected for the production of IgM binding to ssDNA, TT, or β-galactosidase. Each mAb was tested for binding to the Ag used for its selection and to eight other Ag. Six mAb from CD5−CD45RAlo and three from CD5+ B cells were poly-reactive. Two of the CD5−CD45RAlo-derived mAb, P417.F22 (Fig. 5A) and P417.F2.2.8.8 (binding curve not shown) bound in a dose-dependent fashion and with different efficiencies to all nine Ag used in this study. The remaining seven mAb efficiently bound in a dose-dependent fashion to two or more Ag, as exemplified by the binding curves of mAb P417.7.1.3 (Fig. 5B) from CD5−CD45RAlo B cells, and those of mAb P416.F2.2.34 (Fig. 5D) from CD5+ B cells. The polyreactivity of some of the mAb produced by CD5+ CD45RAlo and CD5+ B cells was confirmed in cross-competition studies in which the binding of one mAb to a solid-phase Ag was inhibited not only by the soluble homologous ligand but also by soluble heterologous ligands, as exemplified by the analysis of mAb P417.F22 (Fig. 5, F to J). Finally, one mAb generated from CD5−CD45RAlo B cells (mAb P417.F14) (Fig. 5C), and one from CD5+ B cells (mAb P417.F9.5) (Fig. 5E) were monoreactive, binding only to the Ag used for their selection, β-galactosidase and ssDNA, respectively. Cross-competitive inhibition experiments using these antibodies showed that the binding of mAb P417.F9.5 to ssDNA was inhibited only by ssDNA (Fig. 5K) and that of mAb P417.F14 to β-galactosidase was inhibited only by β-galactosidase (Fig. 5L). All 11 mAb displayed different (low, moderate, or high) affinities for different Ag, as indicated by the calculated Kd values for binding to ssDNA, actin, PC, TT, and β-galactosidase (Table III). In addition to those generated from sorted CD5+ and CD5−CD45RAlo B lymphocytes, four mAb were generated from the sorted CD5−CD45RAhi B cells of subject P417. Two mAb were IgM (P417.F28, IgM, κ, and P417.F29, IgM, λ) and two were IgG (P417.F27, IgG, κ, and P417.F30, IgG, λ). None of these mAb displayed any detectable binding activity to any of the Ag used in our experiments, suggesting that they are monoreactive to yet to be identified Ag. These experiments show that, similar to CD5+ B cells, a major proportion of CD5−CD45RAlo B cells are committed to the production natural antibodies, most of which are polyreactive. They also show that monoreactive antibodies to self or exogenous antigens can be readily isolated from both the CD5−CD45RAlo and CD5+ B cell subsets.
Figure 5.
A to E, dose-dependent binding of IgM mAb generated using CD5−CD45RAlo (A to C) and CD5+ B cells (D and E) to solid phase ligands. Ag-binding activity of each mAb is expressed as optical absorbance at 492 nm. F to J, dose-dependent inhibition of the binding of mAb P417.F22 to solid phase ligands by soluble homologous or heterologous ligands. Samples of mAb P417.F22 (0.4 µg) were incubated with increasing amounts of soluble ligand. After 18 h, mixtures were transferred to ELISA plates precoated with ssDNA (F), actin (G), phosphorylcholine (H), tetanus toxoid (I) or β-galactosidase (J), K and L, dose-dependent inhibition of the binding of mAb P417.F9.5 (K) and mAb P417.F14 (L) to solid phase ssDNA and β-galactosidase, respectively. In the competitive inhibition experiments, the amount of mAb bound to the solid phase Ag is expressed as a percentage of the binding activity measured in the absence of any soluble ligand (100% of binding activity). The following Ag were used: IgG Fc fragment (○), ssDNA (Δ), insulin (●), thyroglobulin (▲), actin (□), phosphorylcholine (×), tetanus toxoid (▪), β-galactosidase (*), and LPS (——).
TABLE III.
Dissociation constant (Kd. M) of mAb generated/mm CD5−CD45RAlo and CD5+ B lymphocytes for different Aga
| mAb | B Cell Subset | Chain |
Selecting Ag |
Ag |
|||||
|---|---|---|---|---|---|---|---|---|---|
| H | L | ssDNA | Actin | Phosphoryleholine | TT | β-Galactosldase | |||
| P417.F22 | CD5−CD45RAlo | µ | λ | ssDNA | 1.5 × 10−7 | 7.2 × 10−7 | 2.9 × 10−5 | 3.2 × 10−8 | 4.4 × 10−7 |
| P417.7.1.3 | CD5−CD45RAlo | µ | λ | TT | nilb | 9.9 × 10−6 | nil | 5.8 × 10−7 | 1.4 × 10−6 |
| P417.F21.5 | CD5−CD45RAlo | µ | κ | TT | nil | nil | nil | 4.3 × 10−7 | 2.6 × 10−7 |
| P417.F2.2.8.8 | CD5−CD45RAlo | µ | λ | ssDNA | 8.0 × 10−7 | 4.5 × 10−6 | 5.4 × 10−4 | 2.0 × 10−8 | 1.0 × 10−8 |
| P418.F20.1 | CD5−CD45RAlo | µ | λ | TT | nil | 8.4 × 10−6 | nil | 3.7 × 10−7 | 1.6 × 10−6 |
| P418.F63.35 | CD5−CD45RAlo | µ | λ | TT | 3.6 × 10−7 | 2.8 × 10−8 | nil | 1.9 × 10−6 | nil |
| P417.F14 | CD5−CD45RAlo | µ | κ | β-Galacto- sidase |
nil | nil | nil | nil | 4.6 × 10−7 |
| P416.13.3.1.1 | CD5+ | µ | λ | ssDNA | 5.6 × 10−4 | nil | nil | nil | 3.5 × 10−8 |
| P417.14.2.2 | CD5+ | µ | κ | TT | 8.5 × 10−7 | 3.0 × 10−5 | nil | 3.0 × 10−6 | 3.9 × 10−7 |
| P416.F2.2.34 | CD5+ | µ | λ | ssDNA | 1.9 × 10−7 | 2.8 × 10−6 | nil | 1.2 × 10−8 | N.D. |
| P417.F9.5 | CD5+ | µ | λ | ssDNA | 4.6 × 10−8 | nil | nil | nil | nil |
Eleven mAb were generated using B cells from three different healthy subjects (P416, P417, and P418). mAb P417.7.13, P416.13.3.1.1, and P417.14.2.2 were produced by cloned EBV-transformed B cells. All other mAb were produced by EBV-transformed B cell hybrids. Kd values were calculated from the results of competitive inhibition experiments involving pairs of homologous ligands. The m.w. of each Ag used for the calculation of the Kd is given in Materials and Methods.
Kd greater than 1 × 10−3 M.
Expression of CD5 mRNA by CD5−CD45RAlo, CD5−CD5RAhi, and CD5+ B cells
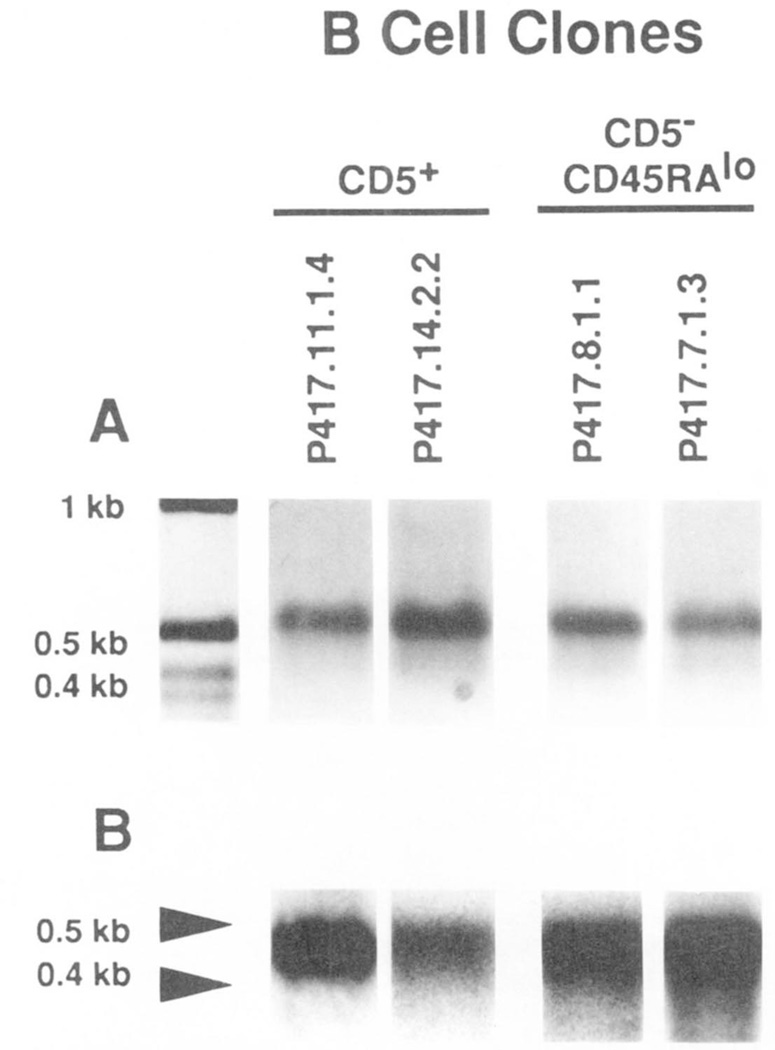
CD5 mRNA has been detected in human surface CD5− B cell lines (49, 50). We analyzed the level of CD5 mRNA in CD5−CD45RAlo B cells and compared it with that in autologous CD5+ and CD5−CD45RAhi B cells. To avoid any interference by T lymphocytes potentially contaminating freshly sorted B cells and because of the difficulty in obtaining sufficient amounts of mRNA for cDNA synthesis from the small numbers of sorted B cells, we amplified sorted lymphocytes in culture using EBV. Three sets of B cells, each from a different subject (P416, P417, and P418) and consisting of three 4-wk-old bulk lymphoblast cultures established by EBV transformation of CD5+, CD5−CD45RAlo, and CD5−CD45RAhi B cells were analyzed. In addition, we analyzed four EBV-transformed B cell clones established by sequential subculturing over a 3-mo period: two were generated using CD5−CD45RAlo B cells, P417.8.1.1 (monoclonal parental line of the P417.F22 cell hybrid) (Table III; Fig. 5A) and P417.7.1.3 (Table III; Fig. 5B), and two using CD5+ B cells, P417.11.1.4 (monoclonal parental line of the P417.F9.5 cell hybrid) (Table III; Fig. 5E) and P417.14.2.2 (Table III), from donor P417. None of the bulk or monoclonal B cell cultures contained T cells in detectable numbers, as assessed by FACS analysis (not shown). In each bulk cell culture and clone, the level of CD5 mRNA expression was compared with that of cell surface CD5 expression.
Cells from bulk cultures established using sorted surface CD5+ B cells expressed moderate, but consistent, levels of surface CD5, as exemplified by analysis of lymphocytes from donor P417 (Fig. 6B, continuous line). In contrast, cells from bulk cultures established using autologous surface CD5− B cells, whether CD45RAlo or CD45RAhi, were consistently negative for surface CD5 (Fig. 6B, dotted and broken lines, respectively). Accordingly, the two monoclonal cell lines established using CD5+ B cells from experiment P417 (P417.11.1.4 and P417.14.2.2) consistently expressed higher levels of surface CD5 than their counterparts established using autologous CD5−CD45RAlo B cells (P417.8.1.1 and P417.7.1.3) (Fig. 6, C and D).
Figure 6.
Expression of cell surface CD5 in the different B cell subsets. Sorted CD5−CD45RAlo (·····), CD5−CD45RAhi (-·-), and CD5+ (——) B cells (donor P417) were transformed with EBV, grown in bulk culture for 3 wk, and reacted with: biotinylated mouse IgG2aκ, of irrelevant specificity, followed by PE-labeled streptavidin (A, three profiles virtually overlapping) or PE-labeled mAb to CD5 (IgG2aκ) (B, two profiles virtually overlapping). The monoclonal EBV-transformed cell lines generated by sequential cloning from sorted CD5−CD45RAlo or CD5+ B cells were reacted with PE-labeled mAb to CD5 and subjected to FACS analysis. The fluorescence profiles of the CD5−CD45RAlo B cell-derived lines P417.8.1.1 and P417.7.1.3 were compared one to one with those of the CD5+ B cell-derived lines P417.11.1.4 and P417.14.2.2 (C and D).
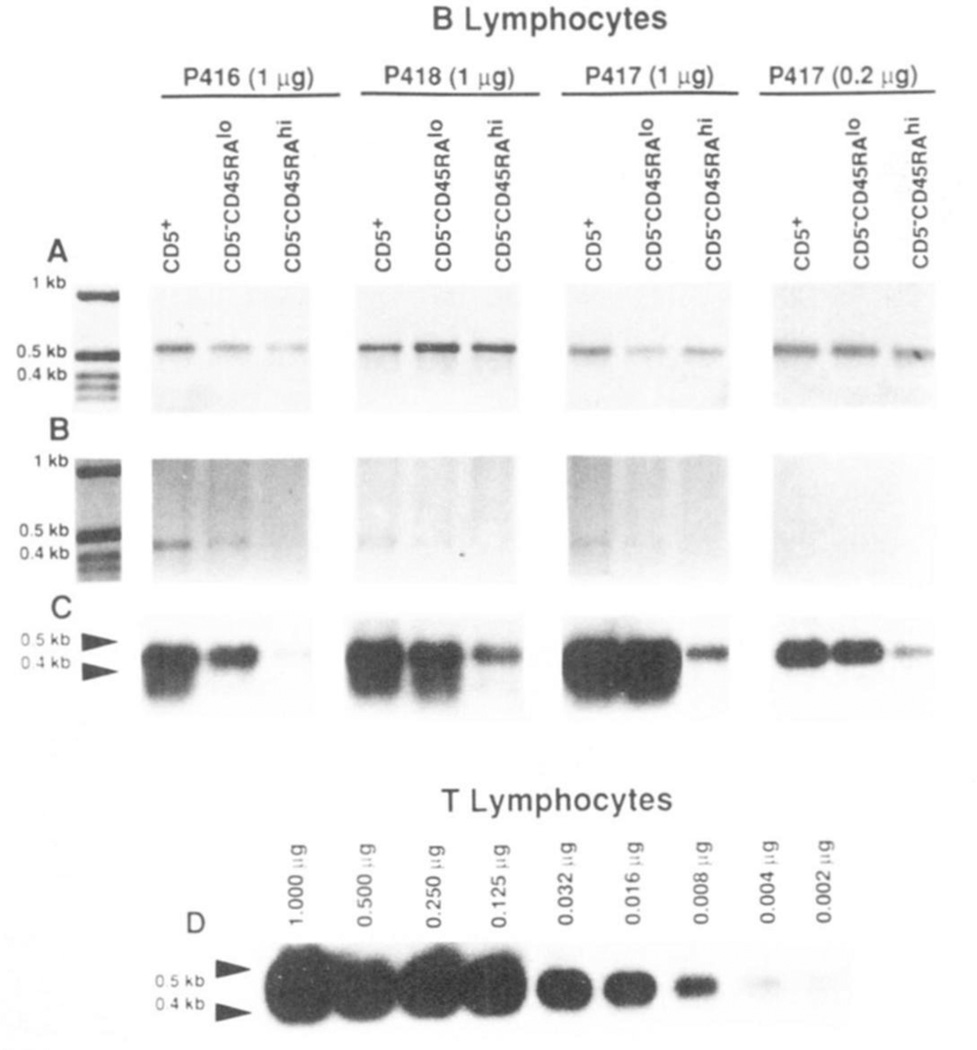
The CD5-specific PCR amplification technique detailed in Materials and Methods was applied to the analysis of the cDNA prepared using mRNA from the EBV-transformed lymphocytes that had been established with CD5+, CD5−CD45RAlo and CD5−CD45RAhi B cells of three subjects (P416, P417. and P418). In each subject, using the CD5-specific primers, we amplified comparable amounts of ~0.45 kbp DNA in CD5+ and CD5−CD45RAlo B cells, but significantly lower amounts of DNA of similar size in CD5−CD45RAhi B cells (Fig.7B). In each case, as suggested by its size and strong hybridization with the 32P-labeled CD5-specific (477–496 bp) oligonucleotide probe, the DNA was amplified from the 230–647 stretch of the CD5 cDNA sequence (Fig. 7, B and C). Using β-actin-specific primers and the same reverse transcribed cDNA samples as templates, we amplified comparable amounts of −0.6 kbp DNA products in B cells from all subsets (Fig. 7A). This suggested that the different degrees of CD5 cDNA amplification achieved in the B cell subsets were not caused by differences in the amounts of cDNA templates (resulting from variable efficiency in reverse transcribing the mRNA samples). The amounts of B cell mRNA (0.2 and 1.0 µg) used in these experiments were within the range over which the PCR technique amplified CD5-specific cDNA at degrees proportional to the amounts of mRNA that had been used in each case to reverse transcribe the sscDNA used as PCR template. Such a proportionality is indicated by the PCR analysis of the decreasing amounts of T cell mRNA depicted in Figure 7D. Slot-blot experiments showed that CD5+ B cells expressed 10- to 30-fold lower amounts of CD5 mRNA than did autologous T lymphocytes (not shown). This supported the results of the PCR amplification experiments. For instance, the level of CD5 mRNA found in 0.2 and 1.0 µg of mRNA from EBV-transformed CD5+ B cells was comparable with that found in 0.008 and 0.125 µg. respectively, of mRNA from T cells (Fig. 7, C and D: P417). The expression of CD5 mRNA by CD5−CD45RAlo B cells was confirmed at the clonal level, as exemplified by analysis of mRNA from the monoclonal B cell lines P417.8.1.1 and P417.7.1.3, and comparison with mRNA from the surface CD5+ monoclonal B cell lines P417.11.1.4 and P417.14.2.2 (Fig. 8).
Figure 7.
Expression of CD5 mRNA by CDS+, CD5−CD45RAlo, and CD5−CD45RAhi B cells from three healthy subjects (P416, P417, and P418). mRNA from each B cell subset (1 µg or 0.2 µg) or T lymphocytes (1.0 to 0.002 µg) was reverse transcribed. cDNA was amplified by PCR using β-actin-specific or CD5-specific primers, and analyzed on a 1.2% agarose gel (see Materials and Methods for details). A and B show ethidium bromide stained gels with amplified β-actin (~0.6 kb) and CD5 (~0.45 kb) cDNA. respectively, prepared from the three B cell subsets. C depicts the hybridization of the 32P-labeled CD5-specific oligonucleotide probe to fractionated amplified CD5 cDNA of the material in B. D depicts the hybridization of the 32P-labeled CD5-specific oligonucleotide probe to fractionated, amplified cDNA reverse transcribed from different amounts (1.0 to 0.002 µg) of purified T cell mRNA.
Figure 8.
Expression of CD5 mRNA by EBV-transformed CD5+ and CD5−CD45RAlo B cell lines. mRNA (1 µg) was extracted from two EBV-transformed monoclonal B cell lines derived from the sorted CD5+ B cells (P417.11.1.4 and P417.14.2.2) and two derived from the sorted CD5−D45RAlo B cells (P417.8.1.1 and P417.7.1.3) of donor P417. mRNA was divided Into two aliquots, and then reverse transcribed. cDNA was amplified by PCR using the β-acttn (A, ethidium bromide staining) or CD5 (B, Southern hybridization)-specific primers and then fractionated on a 1.2% agarose gel. Southern hybridization was performed using the 32P-labeled CD5-specific oligonucleotide probe.
These experiments suggest that CD5 mRNA is expressed not only by surface CD5+ B cells, but also, and in comparable amounts, by autologous CD5−CD45RAlo B cells. In contrast, CD5 mRNA is expressed only in trace amounts by autologous surface CD5−CD45RAhi B lymphocytes.
DISCUSSION
These studies show that two polar fractions, CD45RAlo and CD45RAhi B cells, can be consistently segregated from human CD5− B lymphocytes on the basis of their differential expression of surface CD45RA. These CD5− B cell subsets differ in clonal composition and in the Ag-binding properties of the antibodies they produce. Like CD5+ B cells (which were found to express surface CD45RA at low to intermediate density), most CD5−CD45RAlo B cells are committed to the production of IgM, a significant proportion of which display the Ag-binding features of natural antibodies. In contrast, more than 50% of CD5−CD45RAhi B cells consist of IgG-producing cell precursors, some of which are probably memory cells. The analysis of the clonal composition of the surface CD5−CD45RAint B cells was beyond the scope of the present investigation. Preliminary data, however. suggest that these lymphocytes contain a relatively higher proportion of IgG-producing cell precursors than do CD5−CD45RAlo B lymphocytes, and few or virtually no natural antibody-producing cell precursors (M. T. Kasaian and P. Casali, unpublished observations).
The fact that CD5−CD45RAlo B lymphocytes account for a very low percentage of B cells (about 4% of total peripheral blood B cells or 6% of CD5− B cells) provides an explanation for the infrequent detection of natural antibodies in cultures established using unfractionated CD5− B lymphocytes (9, 10, 18, 35, 36). The present binding and competitive inhibition experiments show that many of the natural mAb generated from CD5−D45RAlo B cells display a wide range of binding affinities (Kd, from 10−4 to 10−8 M) for different Ag and are indistinguishable from the well characterized poly-reactive antibodies produced by CD5+ B cells (present data) (References 5, 12, 18, 26, 36, and 38). The present analyses also show that certain natural antibodies, from both the CD5−D45RAlo and CD5+ B cell subsets, are monoreactive, binding to a self (e.g., mAb P417.F9.5 to ssDNA) or an exogenous (e.g., mAb P417.F14 to β-galactosidase) Ag. Preliminary experiments suggest that such monoreactive antibodies can account for up to 30% of the natural antibodies produced by CD5−CD45RAlo and CD5+ B lymphocytes in healthy subjects (M. T. Kasaian and P. Casali. unpublished observations). In addition, they show that, like their polyreactive counterparts (29, 51, 52), monoreactive high affinity human natural antibodies, e.g., an IgM mAb to thyroglobulin (26, 52) and an IgM mAb to β-galactosidase (N. Harindranath, H. Ikematsu, A. L. Notkins, and P. Casali, manuscript in preparation) isolated from healthy subjects, can be encoded in germ-line V genes. The germ-line configuration of the vast majority of the natural antibodies produced by CD5+ and CD5−D45RAlo B cells would contrast with the somatically hypermutated configuration of the V genes of the antibodies generated through a process of affinity maturation, possibly produced by “memory” CD5−CD45RAhi B cells. Further gene sequencing will determine whether this is a general rule, or whether some CD5+ B cells can express hypermutated V genes, as a result of an Ag-driven process, as recently suggested by us (29, 53) and others (54). Such experiments may also elucidate the relationship between natural antibodies and anti-self reactivity and/or natural mechanisms of defense against infections.
Several lines of evidence support the hypothesis that human CD5−D45RAlo B lymphocytes constitute a discrete cell subset rather than a result of phenotypic alterations associated with an activation state of CD5+ B cells: i) CD5−D45RAlo B cells are small size, likely resting cells, as indicated by their very low degrees of forward and right angle light scattering; ii) in different individuals, these lymphocytes account for comparable proportions (2 to 6%) of the total peripheral blood B lymphocytes: and iii) in a given individual, the proportion of CD5−D45RAlo B cells remains remarkably constant over time, suggesting that, as shown for CD5+ B lymphocytes (11), the number of these cells may be under genetic control.
Our findings indicate that despite the lack of surface CD5. CD5−D45RAlo B cells can express CD5 mRNA at levels comparable with those of their surface CD5+ counterparts. Expression of high levels of CD5 mRNA by CD5−D45RAlo B cells is unlikely a result of EBV infection, because EBV infection of autologous surface CD5−CD45RAhi B cells under similar conditions is not associated with induction of high levels of CD5 mRNA (Fig. 7). It is also unlikely that surface CD5−CD45RAlo/ CD5 mRNA+ B cells result from EBV-induced phenotypic changes in surface CD5+ B cells potentially contaminating the sorted CD5−CD45RAlo lymphocyte fractions, because: 1) reanalysis of freshly sorted CD5−CD45RAlo B lymphocytes never showed any significant number of CD5+ B cells, and ii) these experiments and those by others (49, 50, 54) suggest that EBV transformation of surface CD5+ B cells is associated with either preservation of high levels, or some down-regulation, but rarely complete loss, of surface CD5. The present data also demonstrate that CD5 mRNA is expressed, although at very low levels, by (possibly “conventional”) CD5−CD45RAhi B cells. The previously reported inability to detect CD5 mRNA in all but a minority of surface CD5− B cells by other authors (49, 50) might rest in the relative insensitivity of the Northern analysis used as compared to the PCR cDNA amplification technique applied here. As for the detection of CD5 mRNA in a minority of surface CD5− EBV-transformed B cell lines by the same authors (49, 50), it seems possible that such surface CD5−/CD5 mRNA+ B cell lines originated from CD5−CD45RAlo B lymphocytes because unfractionated lymphocytes were used in those experiments. Such a possibility is particularly supported by the findings of Paavonen et al. (49), showing that of a panel of six surface CD5− B cell lines the two expressing CD5 mRNA at high levels produced natural antibodies. Finally, although our experiments fall short of elucidating the post-transcriptional events underlying the failure of a major proportion of lymphocytes to express surface CD5 in the normal human B cell repertoire, they may offer an explanation for the ability of EL4 thymoma (35) or tetradecanoyl phorbol acetate (55) to induce surface CD5 expression in CD5− B lymphocytes. Similar to human surface CD5− B cells that express CD5 mRNA, murine surface Ly-1− B cells may express Ly-1 mRNA. This could be at the basis of the ability of these Ly-1− B cells to express surface Ly-1 after activation by antibodies to µH chains, as recently shown by Ying-zi et al. (56).
The high frequency of IgM-producing cell precursors, the prominent commitment to the production of natural antibodies, and the relatively high levels of CD5 mRNA expression of the surface CD5−CD45RAlo B cells suggest that these lymphocytes are related to CD5+ B cells. This relationship may be based upon common origin, ontogeny, and/or development. CD5−CD45RAlo B cells may represent the human equivalent of the mouse Ly-1− “sister” B cell population, a B subset that lacks cell-surface Ly-1 but shares many of the phenotypic and functional features of Ly-1+ B cells (2, 3, 57–59). Like Ly-1+ B lymphocytes, their Ly-1− “sister” counterparts: i) are preferentially represented in the peritoneal cavity (2, 3); ii) display the surface IgMhi/IgDlo, Mac-1+ (the equivalent of human CD11b), B220lo phenotype characteristic of Ly-1 + B cells (2, 3, 57, 58); iii) comprise a high number of natural antibody-producing cell precursors (57); and iv) may constitute a lineage distinct from that of “conventional” (Ly-1−) B cells (2, 3, 59, 60, 61). In accordance with mouse Ly-1+ and Ly-1− “sister” B cells, human CD5+ and CD5−CD45RAlo B lymphocytes express surface CD45RA at lower density as compared with the (possibly “conventional”) CD5−CD45RAhi B cells. Moreover, they appear to express surface CD11b and CD14 at higher frequency than do CD5−CD45RAhi B lymphocytes. However, in contrast to the mouse, human CD5+ and CD5−CD45RAlo B lymphocytes are not readily distinguishable from “conventional” B lymphocytes in terms of surface IgM/IgD expression (4, 18) (M. T. Kasaian and P. Casali, unpublished observations). A major distinguishing feature of human CD5−CD45RAlo and CD5+ B lymphocytes is their commitment to the production of natural antibodies. In this context, we show here that the Ig made by significant proportions of both of these B cell subsets bind to PC (Fig. 4), a reactivity that underlies the prominent natural antibody response of murine peritoneal Ly-1+ and Ly-1− “sister” B cells to bromelain-treated mouse RBC (57, 62, 63). The finding that CD5+ and CD5−CD45RAlo B cells share this reactivity further supports the relatedness between these two human natural antibody-producing B cell subsets and emphasizes their similarities to the mouse Ly-1 B cell lineage.
The function of CD45RA on the B cell begins to be elucidated. The cytoplasmic domain of CD45, which is shared by all isoforms, is a protein tyrosine phosphatase (64). CD45 likely regulates signal transduction by modulating the phosphorylation state of the Ag receptor sub-units (65, 66). The amount of CD45 present on mature B cells apparently exceeds that needed for the function of the receptor for Ag (66). In the mouse, treatment of B cells with mAb to the high m.w. isoforms of the L-CA (Ly-5.1, Ly-5.2) inhibits the antibody response to T-dependent Ag, e.g., SRBC, but not to T-independent Ag, particularly type 1, such as TNP-Brucella abortus (39–41). This may be consistent with the speculation that murine surface Ly-5lo B cells (i.e., Ly-1+ and Ly-1− “sister” B lymphocytes) are the cellular elements recruited by some T cell-independent Ag to produce Ig, mainly IgM, possibly including natural antibodies (57). A similar role may be envisaged for human CD5−CD45RAlo and CD5+ B cells because CD5+ B lymphocytes are recruited by T-independent stimuli to proliferate and secrete natural antibodies (67).
The identification and functional characterization of human CD5+ B lymphocytes, and their murine homologues, Ly-1+ B cells, has revealed that discrete B lymphocyte subsets are endowed with different functions, a notion that lies at the basis of a “layered” immune system, as postulated by Herzenberg and Herzenberg (68). Our findings of a dichotomy in the putatively homogeneous human CD5− B cell population introduce an additional layer of complexity in our understanding of the human B cell repertoire. CD5−CD45RAlo and CD5−CD45RAhi B cells are clearly distinct in terms of the class, reactivity, and functional role of the antibodies they produce. Current studies are focused on the role of CD5−CD45RAlo B lymphocytes in those autoimmune diseases, particularly SLE, in which high levels of monoreactive IgG and IgM autoantibodies to DNA are produced mainly by lymphocytes within the CD5− B cell compartment (38). Preliminary observations indicate that in the peripheral blood of SLE patients CD5−CD45RAlo B cells account for a large proportion of CD5− B lymphocytes and the CD5−CD45RAhi subset is dramatically reduced in size (M. T. Kasaian, H. Ikematsu, J. E. Balow, and P. Casali, unpublished observations). It is conceivable that such a dramatic reduction of the CD5−CD45RAhi B cell subset results from the cytolytic activity of antibodies to the high m.w. isoforms of CD45, including CD45RA, which have been detected in the circulation of these patients (69, 70). Experiments are in progress to determine whether CD5−CD45RAlo and CD5+ B cells in SLE patients secrete such anti-CD45RA autoantibodies. This, along with the analysis of CD5−CD45RAlo B cells in newborns and healthy adults, should help to elucidate the roles of this novel lymphocyte subset in the human B cell repertoire.
Acknowledgments
We are grateful to Dr. J. Hirst (Cell Sorting Unit, Kaplan Cancer Center, Dept. of Pathology, N.Y.U. School of Medicine, New York, NY) for his help with cell sorting; Dr. L. Mantovani (Dept. of Pathology, N.Y.U. School of Medicine) for kindly preparing biotinylated mouse mAb to CDllb; Dr. T. F. Davies (Dept. of Medicine, The Mount Sinai Hospital Medical School, New York, NY) for his gift of purified human thyroglobulin; Dr. G. Inghirami (Dept. of Pathology, Coll. of Physicians & Surgeons, Columbia University, New York, NY) for his gift of oligonucleotides; and Dr. W. R. Fields (Eli Lilly Research Laboratories, Indianapolis, IN) for his gift of recombinant human insulin. We acknowledge the technical assistance of Mr. H. Liu.
Footnotes
This work was supported by United States Public Health Service Grants AR-40908. CFAR AI-27741, and CA-09161; and by the BRSG S07 RR05399-30 awarded by the National Center for Research Resources, National Institutes of Health, Bethesda, MD. The contribution of the Lila Motley Cancer Fund is gratefully acknowledged.
This is Publication 8 from the “The Jeanette Greenspan Laboratory for Cancer Research”. P. C. is a Kaplan Cancer Scholar. Some of the data contained in this paper have been presented at the New York Academy of Sciences conference on “CD5 B Cells in Development and Disease” held in Palm Beach, FL, on June 3–6, 1991. During this conference it was unanimously decided by the convenants that both Ly-1+ and CD5+ B lymphocytes be renamed B-1a cells. Ly-1 “sister” B lymphocytes were renamed B-1b cells. Accordingly, we propose here that CD5−CD45RAlo B lymphocytes be renamed human B-lb cells.
Abbreviations used in this paper: PE, phycoerythrin; PCR, polymerase chain reaction; PC. phosphorylcholine clorlde; TT, tetanus toxoid; CD45RAlo(low); CD45RAint(intermediate); CD45RAhi(high); PBS-Tween, PBS containing 0.05% Tween-20.
REFERENCES
- 1.Hayakawa K, Hardy RR, Herzenberg LA. Progenitors for Ly-1 B cells are distinct from progenitors for other B cells. J. Exp. Med. 1985;161:1554. doi: 10.1084/jem.161.6.1554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Herzenberg LA, Stall AM, Lalor PA, Sidman C, Moore WA, Parks DR, Herzenberg LA. The LY-1 B cell lineage. Immunol. Rev. 1986;93:81. doi: 10.1111/j.1600-065x.1986.tb01503.x. [DOI] [PubMed] [Google Scholar]
- 3.Hayakawa K, Hardy RR. Normal, autoimmune, and malignant CD5+ B cells: the Ly-1 B lineage? Annu. Rev. Immunol. 1988;6:197. doi: 10.1146/annurev.iy.06.040188.001213. [DOI] [PubMed] [Google Scholar]
- 4.Kipps TJ. The CD5 B cell. Adv. Immunol. 1989;47:117. doi: 10.1016/s0065-2776(08)60663-x. [DOI] [PubMed] [Google Scholar]
- 5.Casali P, Notkins AL. Probing the human B cell repertoire with EBV: polyreactive antibodies and CD5+ B lymphocytes. Annu. Rev. Immunol. 1989;7:513. doi: 10.1146/annurev.iy.07.040189.002501. [DOI] [PubMed] [Google Scholar]
- 6.Raveche ES. Possible immunoregulatory role for CD5+ B cells. Clin. Immunol. Immunopathol. 1990;56:135. doi: 10.1016/0090-1229(90)90136-e. [DOI] [PubMed] [Google Scholar]
- 7.Kasaian MT, Ikematsu H, Casali P. CD5+ B lymphocytes. Proc. Soc. Exp. Biol. Med. 1991;197:226. doi: 10.3181/00379727-197-43250. [DOI] [PubMed] [Google Scholar]
- 8.Antin JH, Emerson SG, Martin P, Gadol N, Ault KA. LEU-1+ (CD5+) B cells: a major lymphoid subpopulation in human fetal spleen: phenotypic and functional studies. J. Immunol. 1986;136:505. [PubMed] [Google Scholar]
- 9.Casali P, Burastero SE, Nakamura M, Inghirami G, Notkins AL. Human lymphocytes making rheumatoid factor and antibody to ssDNA belong to the Leu-1+ B-cell subset. Science. 1987;236:77. doi: 10.1126/science.3105056. [DOI] [PubMed] [Google Scholar]
- 10.Hardy RR, Hayakawa K, Shimizu M, Yamasaki K, Kishimoto T. Rheumatoid factor secretion from human Leu-1+ B cells. Science. 1987;236:81. doi: 10.1126/science.3105057. [DOI] [PubMed] [Google Scholar]
- 11.Kipps TJ, Vaughan JH. Genetic influence on the levels of circulating CD5 B lymphocytes. J. Immunol. 1987;139:1060. [PubMed] [Google Scholar]
- 12.Nakamura M, Burastero SE, Notkins AL, Casali P. Human monoclonal rheumatoid factor-like antibodies from CD5 (Leu-l)* B cells are polyreactive. J. Immunol. 1988;140:4180. [PubMed] [Google Scholar]
- 13.Hayakawa K, Carmack CE, Hyman R, Hardy RR. Natural autoantibodies to thymocytes: origin. VH genes, fine specificities, and the role of Thy-1 glycoprotein. J. Exp. Med. 1990;172:869. doi: 10.1084/jem.172.3.869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hayakawa K, Honda RR, Hardy M, Herzenberg LA, Steinberg AD, Herzenberg LA. Ly-1 B cells: functionally distinct lymphocytes that secrete IgM autoantibodies. Proc. Natl. Acad. Sci USA. 1984;81:2494. doi: 10.1073/pnas.81.8.2494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dighiero G, Lymberi P, Guilbert B, Ternyck T, Avrameas S. Natural autoantibodies constitute a substantial part of normal circulating immunoglobulins. Ann. N.Y. Acad. Sci. 1986;475:135. doi: 10.1111/j.1749-6632.1986.tb20863.x. [DOI] [PubMed] [Google Scholar]
- 16.Monastier M, Manheimer-Lory A, Bellon B, Painter C, Dang H, Talal N, Zanetti M, Schwartz RS, Pisetesky D, Kuppers R, Rose N, Brochler J, Klareskog L, Holmdahl R, Erlanger B, Alt F, Bona CA. Shared idiotypes and restricted immunoglobulin variable region heavy chain genes characterize murine autoantibodies of various specificities. J. Clin. Invest. 1986;78:753. doi: 10.1172/JCI112637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bona CA. V genes encoding autoantibodies: molecular and phenotypic characteristics. Annu. Rev. Immunol. 1988;6:327. doi: 10.1146/annurev.iy.06.040188.001551. [DOI] [PubMed] [Google Scholar]
- 18.Burastero S, Casali P. Characterization of human CD5 (Leu-1, OKT1)+ B lymphocytes and the antibodies they produce. Contrib. Microbiol. Immunol. 1989;11:231. [PubMed] [Google Scholar]
- 19.Avrameas S. Natural autoantibodies: from “horror autotoxicus” to “gnothi seauton”. Immunol. Today. 1991;12:154. doi: 10.1016/S0167-5699(05)80045-3. [DOI] [PubMed] [Google Scholar]
- 20.Riboldi P, Kasaian MT, Mantovani L, Ikematsu H, Casali P. Natural antibodies. In: Bona CA, Siminovitch K, Zanetti M, Theofilopoulos AN, editors. The Molecular Pathology of Autoimmunity. New York: Harwood Academic Publishers; 1992. In press. [Google Scholar]
- 21.Casali P. Immunoglobulin M. In: Roitt IM, Delves PJ, editors. In Encyclopaedia of Immunology. London: Academic Press. Harcourt Brace Jovanovlch; 1992. pp. 743–747. [Google Scholar]
- 22.Shoenfield Y, Rauch J, Massicotte H, Datta SK, Andre-Schwartz J, Stollar BD, Schwartz RS. Polyspeclficity of monoclonal autoantibodies produced by human-human hybridomas. JV. Engl. J. Med. 1983;308:414. doi: 10.1056/NEJM198302243080802. [DOI] [PubMed] [Google Scholar]
- 23.Theofilopoulos AN, Dixon FJ. Murine models of systemic lupus erythematosus. Adv. Immunol. 1985;37:269. doi: 10.1016/s0065-2776(08)60342-9. [DOI] [PubMed] [Google Scholar]
- 24.Carroll P, Stafford D, Schwartz RS, Stollar BD. Murine monoclonal anti-DNA autoantibodies bind to endogenous bacteria. J. Immunol. 1985;135:1086. [PubMed] [Google Scholar]
- 25.Zouali M, Stollar BD, Schwartz RS. Origin and diversification of anti-DNA antibodies. Immunol. Rev. 1988;105:137. doi: 10.1111/j.1600-065x.1988.tb00770.x. [DOI] [PubMed] [Google Scholar]
- 26.Nakamura M, Burastero SE, Ueki Y, Larrick JW, Notkins AL, Casali P. Probing the normal and autoimmune B cell repertoire with Epstein-Barr virus: Frequency of B cells producing monoreactive high affinity autoantibodies in patients with Hashimoto’s disease and systemic lupus erythematosus. J. Immunol. 1988;141:4165. [PubMed] [Google Scholar]
- 27.Bona CA. CD5-Lyl B cells. In: Bona CA, Siminovitch M, Zanetti K, Theoficopoulos AN, editors. In the molecular pathology of autoimmunity. New York: Harwood Academic Publishers; 1992. In press. [Google Scholar]
- 28.Burastero SE, Casali P, Wilder RL, Notkins AL. Monoreactive high affinity and polyreactive low affinity rheumatoid factors are produced by CD5+ B cells from patients with rheumatoid arthritis. J. Exp. Med. 1988;168:1979. doi: 10.1084/jem.168.6.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Harindranath N, Goldfarb IS, Ikematsu H, Burastero SE, Wilder RL, Notkins AL, Casali P. Complete sequence of the genes encoding the VH and VL regions of low and high-affinity monoclonal IgM and IgA1 rheumatoid factors produced by CD5+ B cells from a rheumatoid arthritis patient. Int. Immunol. 1991;3:865. doi: 10.1093/intimm/3.9.865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dauphinee M, Tovar Z, Talal N. B cells expressing CD5 are increased in Sjogren’s syndrome. Arthritis Rheum. 1988;31:642. doi: 10.1002/art.1780310509. [DOI] [PubMed] [Google Scholar]
- 31.Suzuki N, Sakane T, Engleman EG. Anti-DNA antibody production by CD5+ and CD5− by cells of patients with systemic lupus erythematosus. J. Clin. Invest. 1990;85:238. doi: 10.1172/JCI114418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Valasquillo MC, Alcocer-Varela J, Alarcon-Segovia D, Cabiedes J, Sanchez-Guerrero J. Some patients with primary antiphospholipid syndrome have increased circulating CD5+ B cells that correlate with levels of IgM antiphospholipid. Clin. Exp. Rheum. 1991;9:1. [PubMed] [Google Scholar]
- 33.Kasaian MT, Casali P. Natural autoantibody-producing and autoimmunity-prone B lymphocyte subsets. Autoimmunity. 1992 In press. [Google Scholar]
- 34.Sthoeger ZM, Wakai M, Tse DB, Vinciguerra VP, Allen SL, Budman DR, Lichtman SM, Schulman P, Weiselberg LR, Chiorazzi N. Production of autoantibodies by CD5-expressing B lymphocytes from patients with chronic lymphocytic leukemia. J. Exp. Med. 1989;169:255. doi: 10.1084/jem.169.1.255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Werner-Favre C, Vischer TL, Wohlwend D, Zubler RH. Cell surface CD5 is a marker for activated human B cells. Eur. J. Immunol. 1989;19:1209. doi: 10.1002/eji.1830190709. [DOI] [PubMed] [Google Scholar]
- 36.Ueki Y, Goldfarb IS, Harindranath N, Gore M, Koprowski H, Notkins AL, Casali P. Clonal analysis of a human antibody response. Quantitation of precursors of antibody-producing cells and generation and characterization of monoclonal IgM, IgG, and IgA to rabies virus. J. Exp. Med. 1990;171:19. doi: 10.1084/jem.171.1.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mackenzie LE, Youinou PY, Hicks R, Yuksel B, Mageed RA, Lydyard PM. Auto- and polyreactivity of IgM from CD5+ and CD5− cord blood B cells. Scand. J. Immunol. 1991;33:329. doi: 10.1111/j.1365-3083.1991.tb01778.x. [DOI] [PubMed] [Google Scholar]
- 38.Casali P, Burastero SE, Balow JE, Notkins AL. High affinity antibodies to ssDNA are produced by CD5− B cells in SLE patients. J. Immunol. 1989;143:3476. [PubMed] [Google Scholar]
- 39.Yakura H, Shen F-W, Bourcet E, Boyse EA. On the function of Ly-5 in the generation of antigen-driven B cell differentiation: comparison and contrast with Lyb-2. J. Exp. Med. 1983;157:1077. doi: 10.1084/jem.157.4.1077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yakura H, Kawabata I, Shen F-W, Katagiri M. Selective inhibition of lipopolysaccharide-induced polyclonal IgG response by monoclonal Ly-5 antibody. J. Immunol. 1986;136:2729. [PubMed] [Google Scholar]
- 41.Yakura H, Kawabata I, Ashida T, Katagiri M. Differential regulation by Ly-5 and Lyb-2 of IgG production induced by lipopolysaccharide and B cell stimulatory factor-1 (IL-4) J. Immunol. 1988;141:875. [PubMed] [Google Scholar]
- 42.Inghirami G, Nakamura M, Balow JE, Notkins AL, Casali P. Model for studying virus attachment: identification and quantitation of Epstein-Barr virus-binding cells by using biotinylated virus in flow cytometry. J. Virol. 1988;62:2453. doi: 10.1128/jvi.62.7.2453-2463.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Casali P, Nakamura M, Ginsberg-Fellner F, Notkins AL. Frequency of B cells committed to the production of antibodies to insulin in newly diagnosed patients with insulin-dependent diabetes mellltus and generation of high affinity human monoclonal IgG to insulin. J. Immunol. 1990;144:3741. [PubMed] [Google Scholar]
- 44.Ikematsu H, Goldfarb IS, Harindranath N, Kasaian MT, Casali P. Generation of human monoclonal antibody-producing cell lines by Epstein-Barr virus (EBV)-transformation of B lymphocytes and a somatic cell hybridization technique. J. Tiss. Cult. Meth. 1992;14:9. [Google Scholar]
- 45.Larrick JW, Chiang YL, Sheng-Dong R, Shenyk G, Casali P. Generation of specific human monoclonal antibodies by in vitro expansion of human B cells: a novel recombinant DNA approach. In: Borrebaeck CAK, editor. In Vitro Immunization in Hybridoma Technology. Amsterdam: Elsevier Science Publishers; 1988. pp. 231–246. [Google Scholar]
- 46.Nakajima-Iijima S, Reddy H, Hamada P, Kakunaga T. Molecular structure of the human cytoplasmic β-actin gene: interspecies homology of sequences in the introns. Proc. Natl. Acad. Sci. USA. 1985;82:6133. doi: 10.1073/pnas.82.18.6133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Jones NH, Clabby ML, Dialynas DP, Huang H-JS, Herzenberg LA, Strominger JL. Isolation of complementary DNA clones encoding the human lymphocyte glycoprotein T1 /Leu-1. Nature. 1986;323:346. doi: 10.1038/323346a0. [DOI] [PubMed] [Google Scholar]
- 48.Thomas ML. The leukocyte common antigen family. Annu. Rev. Immunol. 1989;7:339. doi: 10.1146/annurev.iy.07.040189.002011. [DOI] [PubMed] [Google Scholar]
- 49.Paavonen T, Quartey-Papafio R, Delves PJ, Mackenzie L, Lund T, Youinou P, Lydyard PM. CDS mRNA expression and auto-antibody production in early human B cells immortalized by EBV. Scand. J. Immunol. 1990;31:269. doi: 10.1111/j.1365-3083.1990.tb02768.x. [DOI] [PubMed] [Google Scholar]
- 50.Mayer R, Logtenberg T, Strauchen J, Dimitriu-Bona A, Mayer L, Mechanic S, Chiorazzi N, Borche L, Dighiero G, Mannheimer-Lory A, Diamond B, Alt F, Bona CA. CD5 and immunoglobulin V gene expression in B-cell lymphomas and chronic lymphocytic leukemia. Blood. 1990;75:1518. [PubMed] [Google Scholar]
- 51.Sanz I, Capra JD. The genetic origin of human autoantibodies. J. Immunol. 1988;140:3283. [PubMed] [Google Scholar]
- 52.Sanz I, Casali P, Thomas JW, Notkins AL, Capra JD. Nucleotide sequences of eight human natural autoantibody VH regions reveals apparent restricted use of VH families. J. Immunol. 1989;142:4054. [PubMed] [Google Scholar]
- 53.Ikematsu H, Harindranath N, Casali P. Somatic mutations in the VH genes of high affinity antibodies to self and foreign antigens produced by human CD5+ and CD5− B cells. Ann. N.Y. Acad. Sci. 1992 doi: 10.1111/j.1749-6632.1992.tb24631.x. In press. [DOI] [PubMed] [Google Scholar]
- 54.Van Der Heijden RW, Buncshoten H, Hoek A, Van Es J, Punter M, Osterhaus ADME, Uytdehaag FGCM. A human CD5+ B cell clone that secretes an anti-idiotype-specific high affinity IgM monoclonal antibody. J. Immunol. 1991;146:1503. [PubMed] [Google Scholar]
- 55.Freedman AS, Freeman G, Whitman J, Segil J, Daley J, Levine H, Nadler LM. Expression and regulation of CD5 on in vitro activated human B cells. Eur. J. Immunol. 1989;19:849. doi: 10.1002/eji.1830190511. [DOI] [PubMed] [Google Scholar]
- 56.Ying-zi C, Rabin E, Wortis HH. Treatment of murine CD5− B cells with anti-lg, but not LPS. induces surface CD5: two B-cell activation pathways. Int. Immunol. 1991;3:467. doi: 10.1093/intimm/3.5.467. [DOI] [PubMed] [Google Scholar]
- 57.Klinman DM, Holmes KL. Differences in the repertoire expressed by peritoneal and splenic Ly-1 (CD5)+ B cells. J. Immunol. 1990;144:4520. [PubMed] [Google Scholar]
- 58.Mclntyre TM, Holmes KL, Steinberg AD, Kastner DL. CD5+ peritoneal B cells express high levels of membrane, but not secretory, Cµ mRNA. J. Immunol. 1991;146:3639. [PubMed] [Google Scholar]
- 59.Kantor AB. The development and repertoire of B-1 cells (CD5 B cells) Immunol. Today. 1991;12:389. doi: 10.1016/0167-5699(91)90136-H. [DOI] [PubMed] [Google Scholar]
- 60.Lalor PA, Stall AM, Adams S, Herzenberg LA. Permanent alteration of the murine Ly-1 B repertoire due to selective depletion of Ly-1 B cells in neonatal animals. Eur. J. Immunol. 1989;19:501. doi: 10.1002/eji.1830190314. [DOI] [PubMed] [Google Scholar]
- 61.Solvason N, Lehuen A, Kearney JF. An embryonic source of Ly-1 but not conventional B cells. Int. Immunol. 1991;3:543. doi: 10.1093/intimm/3.6.543. [DOI] [PubMed] [Google Scholar]
- 62.Mercolino TJ, Arnold LW, Hawkins LA, Haughton G. Normal mouse peritoneum contains a large population of Ly-1+ (CD5) B cells that recognize phosphatidyl choline: relationship to cells that secrete hemolytic antibody specific for autologous erythrocytes. J. Exp. Med. 1988;168:687. doi: 10.1084/jem.168.2.687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Poncet P, Kocher HP, Pages J, Jaton J-C, Bussard AE. Monoclonal autoantibodies against mouse red blood cells: a family of structurally restricted molecules. Mol. Immunol. 1985;22:541. doi: 10.1016/0161-5890(85)90177-4. [DOI] [PubMed] [Google Scholar]
- 64.Clark EA, Ledbetter JA. Leukocyte cell surface enzymology: CD45 (LCA, T200) is a protein tyrosine phosphatase. Immunol. Today. 1989;10:225. doi: 10.1016/0167-5699(89)90257-0. [DOI] [PubMed] [Google Scholar]
- 65.Ledbetter JA, Tonks NK, Fischer EH, Clark EA. CD45 regulates signal transduction and lymphocyte activation by specific association with receptor molecules on T or B cells. Proc. Natl. Acad. Sci. USA. 1988;85:8628. doi: 10.1073/pnas.85.22.8628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Justement LB, Campbell KS, Chien NC, Cambier JC. Regulation of cell receptor signal transduction and phosphorylation by CD45. Science. 1991;252:1839. doi: 10.1126/science.1648262. [DOI] [PubMed] [Google Scholar]
- 67.Zupo S, Dono M, Azzoni L, Chiorazzi N, Ferrarini M. Evidence for differential responsiveness of human CD5+ and CD5− B cell subsets to T cell-independent mitogens. Eur. J. Immunol. 1991;21:351. doi: 10.1002/eji.1830210216. [DOI] [PubMed] [Google Scholar]
- 68.Herzenberg LA, Herzenberg LA. Toward a layered immune system. Cell. 1989;59:953. doi: 10.1016/0092-8674(89)90748-4. [DOI] [PubMed] [Google Scholar]
- 69.Tanaka S, Matsuyama T, Steinberg AD, Schlossman SF, Morimoto C. Anti-lymphocyte antibodies against CD4+2H4 cell populations in patients with systemic lupus erythematosus. Arthritis Rheum. 1989;32:398. doi: 10.1002/anr.1780320408. [DOI] [PubMed] [Google Scholar]
- 70.Mimura T, Fernsten P, Jarjour W, Winfield JB. Autoantibodies specific for different isoforms of CD45 in systemic lupus erythematosus. J. Exp. Med. 1990;172:653. doi: 10.1084/jem.172.2.653. [DOI] [PMC free article] [PubMed] [Google Scholar]