Abstract
Lung cancer is the most common cause of cancer related mortality in the United States. Surgical resection with a lobectomy is the standard treatment for Stage I non-small cell lung cancer (NSCLC). With an aging population however, there are a significant number of patients who have other co-morbidities that preclude surgical resection. Image-guided radiofrequency ablation is a new emerging modality of treatment which may be applicable in this high-risk group of patients. In this article, we review the principles of radiofrequency ablation, the common devices in use, the results of ablate and resect studies, future directions, and the results of treatment for Stage I non-small cell lung neoplasm.
Keywords: radiofrequency ablation, early stage lung cancer, minimally invasive surgery, high-risk patients, new technology, Stage I non-small cell lung cancer, elderly patients
Introduction
Surgical resection with a lobectomy is the standard treatment for Stage I non-small cell lung cancer (NSCLC) (1–3). In patients who are medically inoperable due to significant co-morbidities precluding surgical resection, conventional external beam radiation is typically used, although the results of this treatment have been suboptimal (4–6). In this high-risk group of patients, Radiofrequency ablation (RFA), a newer modality of treatment and may be applicable (7–9). In this article, we review the principles of RFA, the results of ablate and resect studies, future directions, and the results of treatment for Stage I non-small cell lung neoplasm (7,10,11).
Principles of Radiofrequency Ablation
The use of interstitial hyperthermia to treat lung neoplasm was initially reported by Lilly and colleagues in 1983 (8). RFA is performed utilizing a thermal energy delivery system. An alternating current is applied by a radiofrequency energy generator and this, in turn is delivered through a needle electrode (9). Image-guidance, most commonly computed tomography (CT) is utilized for placement of the needle electrode and the tines are deployed within the tumor. The alternating current generates ionic agitation, which results in heat and thermal ablation. The temperature can reach 90 degrees centigrade leading to coagulative necrosis and tissue destruction in the area of the probe.
Technique and Devices for Radiofrequency Ablation
RFA is generally performed percutaneously under CT guidance. It can also be performed with a thoracotomy as a parenchymal-sparing adjunct to lung resection, particularly in patients with limited pulmonary metastases (11). Currently there are 3 United States FDA-approved RFA devices available in the United States for ablation of soft tissue lesions. Boston Scientific (Boston, MA, USA) manufactures one RFA system which consists of a radiofrequency generator and LeVeen needle electrodes (LeVeen Needle Electrode, RadioTherapeutics Corporation, Sunnyvale, CA). The second system comprises a RF generator and the RITA Starburst XL Electrosurgical Device (Angiodynamics, NY). The third FDA-approved system is the Valley Lab RFA device (Covidien, Boulder, CO). The Valley Lab electrode has a proximal insulated portion and a distal, uninsulated active tip. The electrode is irrigated with a continuous infusion of ice-water, and for this reason, is sometimes referred to as a “cool-tip” electrode. Different algorithms are currently used by the different devices to determine the length of time that the alternating current is delivered and RFA performed (7,10).
Ablate and Resect Studies
There have been few investigations where the completeness of ablation after RFA has been evaluated by “Ablate and Resect” studies. In an initial study, Yang and colleagues presented the results of a multicenter ablate and resect study in 13 patients (12), and in this series, seven patients (55%) had 100% ablation. They also demonstrated a median tumor ablation of 70% as well as a learning curve which exists in achieving a 100% ablation.
In another interesting study, Nguyen and colleagues reported the results of a prospective “ablate and resect” study in 8 patients with Stage I or II NSCLC patients (13). RFA of the tumor was performed after a standard thoracotomy, and subsequently a resection was performed. Tumor cell viability was determined by routine histology as well as supravital dye staining. Three of eight patients (38%) had complete ablation of tumors, and in seven of the eight patients (87.5%) there was more than 80% non-viability of tumor. Ambrogi and colleagues, in a prospective study evaluated the efficacy of ablation in patients who underwent RFA either by the CT guided approach or by thoracotomy, followed by resection (14). These authors were able to demonstrate complete ablation in six out of nine patients (67%). In summary, these studies show that complete ablation is possible, and the rate of effective 100% ablation varied from 38–67%. Further refinements in techniques and advances in technology may allow for more effective ablation.
Evaluation of Response after Radiofrequency Ablation
Clinically, the assessment of response after RFA or other ablative therapies such as stereotactic radiosurgery is difficult because, unlike surgical resection, there is a scar which persists after therapy. There is considerable variation in how response after treatment and progression during follow-up are defined and evaluated. Chest CT scans, changes in contrast enhancement and positron emission tomography (PET) scans have all been used to assess the response to treatment. Consequently the response rates reported in the literature vary considerably (7,10).
RFA also results in inflammation, and therefore the treated lesion is actually larger initially. This area of inflammation slowly decreases over time (15). Hence, using size alone as a criterion to determine response early after RFA may not accurately determine the initial response rate. Investigators from our group at the University of Pittsburgh have described a modified Response Evaluation Criteria in Solid Tumors (RECIST) incorporating not only the size of the lesion on CT scanning, but also the density of the lesion and metabolic activity on PET scanning (16,17,18). This method combines the standard RECIST criteria with evaluation of lesion quality on CT scanning and PET scanning and appears to be a comprehensive method to determine response (Table 1).
Table 1.
Modified RECIST Criteria
| RESPONSE | CT MASS SIZE | CT MASS QUALITY | PET * |
|---|---|---|---|
| COMPLETE (Two of the following) | Lesion disappearance (scar) or less than 25% original size |
Cyst cavity formation Low density |
SUV<2.5 |
| PARTIAL (One of the following) | More than 30% decrease in the sum LD of target lesions | Mass central necrosis or central cavity with liquid density | Decreased SUV or area of FDG uptake |
| STABLE LESION (One of the following) | Less than 30% decrease in the sum LD of target lesions | Mass solid appearance, no central necrosis or cavity | Unchanged SUV or area of FDG uptake |
| PROGRESSION (Two of the following) | Increase of more than 20% in sum LD of target lesions | Solid mass, invasion adjacent structures | Higher SUV or larger area of FDG uptake |
PET done selectively; SUV: Standardized uptake value of fluorodeoxy glucose F18
FDG: Fluorodeoxy glucose F18
LD: Lesion diameter
(Reprinted from Fernando et al. ref 16 with permission)
Clinical Studies of Radiofrequency Ablation for Stage I NSCLC
There are few reports in the literature with an emphasis on Stage I non-small cell lung cancer. We have summarized these results in Table 2. When reviewing the literature, interpretation of results after RFA should be done after review not only of the stage of the disease, the patient population being treated, the protocol used for follow-up, the duration of follow up, and the criteria used to evaluate progressive disease. All these are very important since the methods utilized for the determination of recurrence or progression varies in the literature, and should be taken into consideration while evaluating the results.
Table 2.
Summary of selected clinical studies with radiofrequency ablation for the treatment of Stage I non-small cell lung cancer
| Study Author, Year (ref) | No. of Patients | Pathology | Duration of Follow-up | Outcome Results |
|---|---|---|---|---|
| Simon et al., 2008 (23) | 75 | Stage I NSCLC | Median: 20.5 months* | Estimated overall survival 1, 2 and 5 years was 78%, 57% and 27% Median survival: 29 months. |
| Lencioni et al, 2008 (22) | 13 | Stage I NSCLC | Mean: 15 months* | Overall Survival at 2 year –75% (1 year not provided) |
| Fernando et al, 2005 (16) | 18 | NSCLC (Stage I, 9 patients) | Median: 14 months* | Local Progression – 38% of lesions Median PFI – 17.6 months |
| Ambrogi et al, 2006 (19) | 54 | NSCLC & Metastases | Mean: 23.7 months* | Median Local PFI – 24.1 months |
| Hiraki et al, 2007 (21) | 20 | Stage I NSCLC | Median: 21 months | Local Progression: 35% Estimated overall 1, 2 and 3 year survival: 90%, 84% and 74% respectively |
| Pennathur et al, 2007 (18) | 19 | Stage I NSCLC | Mean:29 months | Overall Survival at 1 year – 95%, 2 years: 68%; Median Survival – NR Local Progression:42% Median time to Local Progression – 27 months |
| Beland et al 2010 (24) | 79 | NSCLC (Stage I, 67 patients) | Mean: 16 months | Overall recurrence in 43% Median disease free survival 23 months |
| Lanuti et al 2009 (25) | 31 | Stage I NSCLC | Median: 17.3 Months | 2 year survival: 78% median survival: 30 months Local Progression: 31.5% |
PFI: Progression Free Interval
NR: Not reached
Follow-up not provided specifically for Stage I and includes all patients (primary and metastatic lung cancer) in this report
Ambrogi and colleagues reported the results of RFA in 54 patients with 64 lung lesions (40 NSCLC, 24 metastases) (19). However, staging information was not provided for the 40 patients with NSCLC. The complete response rate was 62%. The follow-up in this series is one of the longest in the literature with a mean follow-up of 23.7 months (6–50 months). The median overall survival in this cohort of patients was 28.9 months and median local progression free interval was 24.1 months. Lee and colleagues reported their experience with RFA in 10 patients with Stage I NSCLC, of which four patients were considered high-risk patients (20). A total of 80% were alive at a mean follow-up of 14.8 months.
In a more recent study, Hiraki and colleagues reported the results of 20 patients (14 who were medically inoperable and 6 who had refused surgery) with Stage I NSCLC who were treated with RFA (21). The most common complication was pneumothorax which occurred in 57% of patients. At a median follow-up of 21 months, local progression occurred in 7 patients (35%). The median time to local progression was 9 months. The estimated overall survival was 90%, 84% and 74% at one, two and three years respectively. In another interesting multicenter international study, Lencioni and colleagues reported the results of RFA for the treatment of 106 patients, of which 33 patients had NSCLC (22). Among the 33 patients with NSCLC, 13 patients had Stage I NSCLC. The primary endpoints in this study were technical success, safety and assessment of response rates and the secondary endpoints were overall survival, cancer-specific survival and quality of life. Technical success in performing the radiofrequency ablation was achieved in 99% of the patients. The mean follow-up in this series was 15 months. Among patients with NSCLC who were evaluated for response 12.5% had an incomplete response or progression of disease. The overall survival of all patients with NSCLC was 70% and 48% at one and two years respectively. The estimated two year overall survival in patients with Stage I NSCLC was 75%. In both the Hiraki study and the Lencioni study, PET scans were not routinely utilized for the assessment of response. In addition, another limitation is the relatively short follow-up in these studies.
In one of the largest studies reported in the literature, Simon and colleagues presented the results of RFA in 75 patients with NSCLC (23). The reported overall survival at 1, 2 and 5 years was 78%, 57% and 27% respectively. The median survival was 29 months. These authors also reported a 30 day perioperative mortality rate of 3.9%, with a 2.6% procedure specific mortality rate. The estimated five-year local progression-free survival of patients with tumors less than 3 cm was better than those with tumors greater than 3 cm. Overall, this is an interesting study; however, there was significant perioperative mortality associated with RFA in these compromised patients.
In a recent article, Beland and colleagues reported the results of 79 patients with NSCLC who were treated with radiofrequency ablation (24). The overall mean follow-up was 16 months (range 1–72). Patients were followed with CT scans and selectively with PET scans. These investigators utilized either size or enhancement in CT scans and/ or PET scan data to determined progression. There were a total of 54 patients with Stage 1A NSCLC and another 13 patients with Stage 1B NSCLC. Recurrence was seen in a total of 34 patients (43%) of patients. In patients with recurrent disease, the progression was local in 38%, intrapulmonary (same lobe) in 18%, distal in 21%, and mixed (local and nodal) in 6%. At 2 years the estimated local progression was 28%.
Lanuti and colleagues reported the results of RFA for the treatment of Stage I NSCLC in 31 patients over a four and half year period (25). The majority of patients were staged 1A, and the mean size of the tumor was 2 cm. The predominant histology was adenocarcinoma. In this series, 11 patients (35%) with co-morbidities refused surgery. RFA was performed with a cool tip or cluster probe. There was no peri-operative mortality. The median follow-up for alive patients was 17.3 months. During follow-up, Local recurrence or progression occurred in 31.5% of patients. The overall median survival was 30 months and median disease-free survival was 25.5 months. The overall 2 and 3 year survival was 78% and 47% respectively. The estimated disease-free survival at 2 and 3 years was 57% and 39% respectively. These investigators also evaluated pulmonary function at baseline and 3–6 months after the procedure. There were no significant differences, indicating preservation of pulmonary function during early follow-up.
University of Pittsburgh Experience
We initially reported the results of RFA in 18 patients with NSCLC, of which 9 patients were Stage I patients (16). The median follow-up was 14 months and local progression occurred in 38% of nodules. The principal findings of our early reports from the University of Pittsburgh were that RFA was more effective for smaller (≤5 cm) tumors, with better early survival and response to treatment. In addition, we described a modification of the RECIST criteria (Table 1) that was used to assess treatment response and progression at the ablated sites.
In an updated study from the University of Pittsburgh, we reported the results in medically inoperable high-risk patients with Stage I NSCLC treated with radiofrequency ablation under CT guidance (18). One of the primary strengths of this study was the methodical follow-up and this study has one of the longest follow-up period reported in the literature. We evaluated response with CT and PET scan as described before. A total of nineteen patients underwent RFA over a three-year period. There were 8 men and 11 women with a median age of 78 years (range 68–88). The mean follow-up was 29 months (median, 28 months; range, 9–52). An initial complete response was observed in 2 patients (10.5%), partial response in 10 (53%), and stable disease in 5 (26%). Early progression occurred in 2 patients (10.5%).
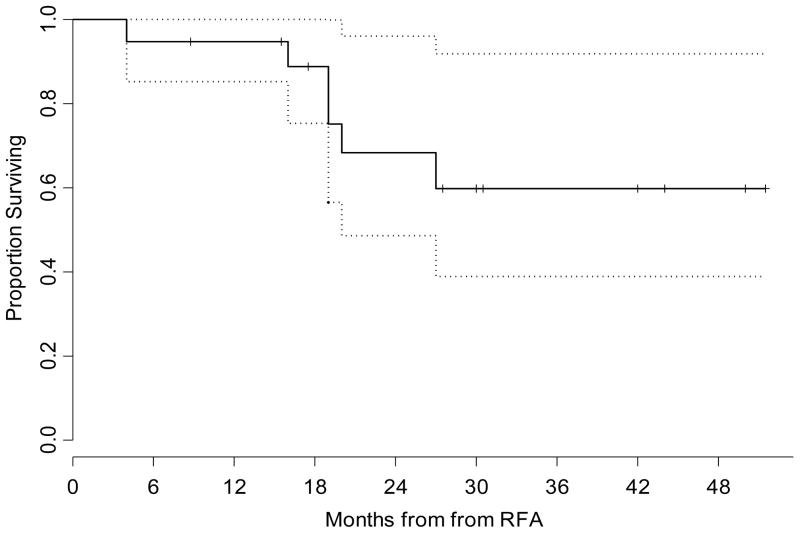
During follow-up, local progression occurred in 8 nodules (42%) and the median time to progression was 27 months. The procedure-related mortality was zero. The probability of survival at 1 year was estimated to be 95% (95% confidence interval: 85%–100%) and probability of survival at 2 years was estimated to be 68% (95% confidence interval 49%–96%), respectively (Figure 1). The median survival was not reached. Our experience indicates RFA is safe in high-risk, Stage I NSCLC patients with reasonable results in patients who are not fit for surgery.
Figure 1.
Kaplan-Meier Plot illustrating the overall survival for the entire group with confidence limits. The time shown is in x axis is in months from RFA. The dotted lines are 95% confidence bands for the probability of overall survival. Reprinted with permission (18).
Complications
In general, RFA appears to be a safe procedure with minimal morbidity and mortality (26). The most common complication appears to be pneumothorax, however, prolonged air leak is rare (18). In our early experience with RFA, there was one mortality in a patient with a central lesion, who developed massive hemoptysis about 3 weeks after the procedure (17). This patient was also treated with brachytherapy. We, therefore, do not recommend treatment with RFA in patients with central lung lesions. Steinke and colleagues reported the results of a world-wide survey in 493 patients from seven centers (27). There were two deaths. In addition, pneumothorax occurred in 30% of patients and pleural effusion requiring aspiration occurred in less than 10% of patients. Death due to hemorrhage and pneumonia/ respiratory distress syndrome has been reported, but is rare (26). However, Simon and colleagues reported a 30-day perioperative mortality rate of 3.9%, with a 2.6% procedure-specific mortality rate in their study (23).
It is important to note that patients undergoing RFA for lung lesions typically have significant comorbidities. Proper patient selection is critical and it is important to follow these patients closely and manage complications effectively. Thoracic surgeons are, therefore, ideally positioned not only to perform RFA but also to provide peri-operative care and long term follow-up to these patients (28).
Improving Outcomes after Radiofrequency Ablation
Although initial local control is good after treatment with radiofrequency ablation, there is a significant incidence of local progression of lung neoplasm during follow-up (18, 21, 28). There are several factors that may influence local recurrence or progression of disease, including progression are technical issues such as the degree of ablation, and whether complete ablation is achieved during the procedure. Another important consideration is the adequacy of the margins of ablation around the tumor. Our data on margins and sublobar resection demonstrated that local recurrence rates were decreased when the margins were greater than 1 cm (29). In general, we strive to attain a 0.5- to 1.0-cm margin around the tumor. A further consideration is the size of the lesion treated. In several studies, the progression rate was improved during follow-up, after treatment of smaller lesions with RFA (11, 23). In our current protocol, we limit RFA to lesions less than 5 cm.
Another approach to improve the ablation zone and the margins of ablation is to increase the conductivity of the tissue with saline infusion (30). Thus, in the future, further advances in technology as described in the section below or adjuvant therapy may be useful in decreasing progression after RFA and, perhaps, in improving survival.
Future Directions
The technology for ablation is continuously evolving in several facets. For example, newer ablative modalities such as Microwave ablation are currently being investigated. The mechanism of action of MWA is dielectric heating with fractional heating of water molecules, resulting in changes in the polarity of these molecules and heating, leading to cell death in the area of the ablation (31). While there are some potential advantages of this technology, clinical experience is very limited at this time.
In addition, advances to facilitate transthoracic placement of the probe with navigation are being investigated (32). These technologies have the potential to decrease instrument adjustments, which are currently needed, and aid in probe placement. Further technological improvements with more precise placement of the probe may aid in more accurate ablation and lead to better local control of tumors. More recently, the feasibility of CT-guided bronchoscopic radiofrequency ablation for lung cancer has been reported (33). It is clear that this technology is rapidly evolving, is increasingly being investigated and future advances and refinements are anticipated.
Conclusions
In summary, the early results of RFA for the treatment of medically inoperable patients with early non-small cell lung cancer appear encouraging. Surgery remains the best treatment for resectable lung cancer (1–3); however, emerging technologies, such as RFA or stereotactic radiosurgery, may have a role in patients who are medically inoperable. There are, however, several factors which merit further investigation including optimal patient selection for RFA and measures to improve local control of the tumor. Prospective studies are underway in our institution and others to evaluate the role of RFA in the treatment of lung neoplasm. We currently have an ongoing IRB approved protocol which continues to accrue at the University of Pittsburgh. There are emerging technological advances in the field of image-guided ablation in the treatment of cancer and thoracic surgeons should continue to investigate this new image-guided modality, which may offer an alternative option to medically inoperable patients (28). Thoracic surgeons should continue to evaluate new technologies and add these to their armamentarium in the treatment of lung neoplasm.
Acknowledgments
This research was funded in part by the National Institutes of Health (NIH) Specialized Program of Research Excellence in Lung Cancer (P50 CA090440), and in part by research grants from Angiodynamics (RITA Medical) Inc.
The authors wish to thank Dr. Shannon Wyszomierski for her excellent assistance in editing this manuscript
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Arjun Pennathur, Email: apennathur@aol.com, Assistant Professor of Surgery, The Heart, Lung, and Esophageal Surgery Institute, University Of Pittsburgh Medical Center, 200 Lothrop Street, C-800, Pittsburgh, PA 15213, Tel: 412-647-7556 Fax: 412-647-7550.
Ghulam Abbas, Assistant Professor of Surgery, The Heart, Lung, and Esophageal Surgery Institute, University Of Pittsburgh Medical Center, 200 Lothrop Street, C-800, Pittsburgh, PA 15213.
James D. Luketich, Email: luketichjd@upmc.edu, Henry T. Bahnson Professor of Cardiothoracic Surgery, Director and Chief, The Heart, Lung, and Esophageal Surgery Institute, University of Pittsburgh Medical Center, 200 Lothrop Street; C-800, Pittsburgh PA 15213, Tel: (412) 647-2911; Fax (412) 647-0050.
References
- 1.Ginsberg RJ, Martini N. Thoracic Surgery. 2. Philadelphia, PA: Churchill Livingstone; 2002. Non – Small Cell Lung Cancer/Surgical Management; pp. 837–59. [Google Scholar]
- 2.Ginsberg RJ, Rubinstein LV Lung Cancer Study Group. Randomized trial of lobectomy versus limited resection for T1 N0 non-small cell lung cancer. Ann Thorac Surg. 1995;60:615–623. doi: 10.1016/0003-4975(95)00537-u. [DOI] [PubMed] [Google Scholar]
- 3.Landreneau RJ, Sugarbaker DJ, Mack MJ, et al. Wedge resection versus lobectomy for stage I (T1 N0 M0) non-small-cell lung cancer. J Thorac Cardiovasc Surg. 1997;113:691–700. doi: 10.1016/S0022-5223(97)70226-5. [DOI] [PubMed] [Google Scholar]
- 4.Jeremic B, Classen J, Bamberg M. Radiotherapy alone in technically inoperable, medically inoperable, early stage (I/II) non-small cell lung cancer. Int J Radiat Oncol Biol Phys. 2002;54:119–30. doi: 10.1016/s0360-3016(02)02917-6. [DOI] [PubMed] [Google Scholar]
- 5.Sibley G, Jamieson T, Marks L, et al. Radiotherapy alone for medically inoperable stage I non–small-cell lung cancer: The Duke experience. Int J Radiat Oncol Biol Phys. 1998;40:149–54. doi: 10.1016/s0360-3016(97)00589-0. [DOI] [PubMed] [Google Scholar]
- 6.Kaskowitz L, Graham MV, Emami B, et al. Radiation therapy alone for stage I non-small cell lung cancer. Int J Radiat Oncol Biol Phys. 1993;27:517–23. doi: 10.1016/0360-3016(93)90374-5. [DOI] [PubMed] [Google Scholar]
- 7.Pennathur A, Abbas G, Schuchert M, et al. Radiofrequency Ablation for the treatment of lung neoplasm. Expert Rev Med Devices. 2008;5:613–621. doi: 10.1586/17434440.5.5.613. [DOI] [PubMed] [Google Scholar]
- 8.Lilly MB, Brezovich IA, Atkinson W, et al. Hyperthermia with implanted electrodes: in vitro and in vivo correlations. Int J Radiat Oncol Biol Phys. 1983;9:373–82. doi: 10.1016/0360-3016(83)90299-7. [DOI] [PubMed] [Google Scholar]
- 9.Thomsen S. Advanced Therapy in Thoracic Surgery. 2. Hamilton, Ontario: BC Decker Inc; 2005. Radio Frequency Ablation of Thoracic malignancies; pp. 75–90. [Google Scholar]
- 10.Pennathur A, Abbas G, Landreneau RJ, Luketich JD. Radiofrequency ablation for the treatment of stage I non-small cell lung neoplasm. Semin Thorac Cardiovasc Surg. 2008;20(4):279–84. doi: 10.1053/j.semtcvs.2008.11.003. [DOI] [PubMed] [Google Scholar]
- 11.Pennathur A, Abbas G, Qureshi I, et al. Radiofrequency ablation for the treatment of pulmonary metastases. Ann Thorac Surg. 2009 Apr;87(4):1030–1039. doi: 10.1016/j.athoracsur.2008.12.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yang S, Whyte R, Askin F, et al. Radiofrequency ablation of primary and metastatic lung tumors. Analysis of an ablate and resection study. Presented at the AATS, 82nd Annual meeting; Washington DC. 2002. p. 212. [Google Scholar]
- 13.Nguyen CL, Scott WJ, Young NA, Rader T, Giles LR, Goldberg M. Radiofrequency ablation of primary lung cancer: results from an ablate and resect pilot study. Chest. 2005;128(5):3507–11. doi: 10.1378/chest.128.5.3507. [DOI] [PubMed] [Google Scholar]
- 14.Ambrogi M, Fontanini G, Cioni R, et al. Biologic effects of radiofrequency thermal ablation on non-small cell lung cancer: Results of a pilot study. J Thorac Cardiovasc Surg. 2006;131:1002–1006. doi: 10.1016/j.jtcvs.2005.12.040. [DOI] [PubMed] [Google Scholar]
- 15.Steinke K, Glenn D, King J, et al. Percutaneous imaging-guided radiofrequency ablation in patients with colorectal pulmonary metastases 1-year follow-up. Ann Surg Oncol. 2004;11:207–212. doi: 10.1245/aso.2004.04.008. [DOI] [PubMed] [Google Scholar]
- 16.Fernando HC, De Hoyos A, Landreneau RJ, et al. Radiofrequency ablation for the treatment of non-small cell lung cancer in marginal surgical candidates. J Thorac Cardiovasc Surg. 2005;129:639–44. doi: 10.1016/j.jtcvs.2004.10.019. [DOI] [PubMed] [Google Scholar]
- 17.Herrera LJ, Fernando HC, Perry Y, et al. Radiofrequency ablation of pulmonary malignant tumors in nonsurgical candidates. J Thorac Cardiovasc Surg. 2003;125:929–37. doi: 10.1067/mtc.2003.18. [DOI] [PubMed] [Google Scholar]
- 18.Pennathur A, Luketich JD, Abbas G, et al. Radiofrequency Ablation for Stage I non-small cell lung neoplasm. J Thorac Cardiovasc Surg. 2007;134:857–64. doi: 10.1016/j.jtcvs.2007.04.060. [DOI] [PubMed] [Google Scholar]
- 19.Ambrogi MC, Lucchi M, Dini P, et al. Percutaneous radiofrequency ablation of lung tumours: results in the mid-term. Eur J Cardiothorac Surg. 2006;30:177–83. doi: 10.1016/j.ejcts.2006.03.067. [DOI] [PubMed] [Google Scholar]
- 20.Lee JM, Jin GY, Goldberg SN, et al. Percutaneous radiofrequency ablation for inoperable non-small cell lung cancer and metastasis: preliminary results. Radiology. 2004;230:125–134. doi: 10.1148/radiol.2301020934. [DOI] [PubMed] [Google Scholar]
- 21.Hiraki T, Gobara H, Iishi T, et al. Percutaneous radiofrequency ablation for clinical stage I non-small cell lung cancer: results in 20 nonsurgical candidates. J Thorac Cardiovasc Surg. 2007;134:1306–12. doi: 10.1016/j.jtcvs.2007.07.013. [DOI] [PubMed] [Google Scholar]
- 22.Lencioni R, Crocetti L, Cioni R, et al. Response to radiofrequency ablation of pulmonary tumours: a prospective, intention-to-treat, multicentre clinical trial (the RAPTURE study) Lancet Oncol. 2008;9:621–8. doi: 10.1016/S1470-2045(08)70155-4. [DOI] [PubMed] [Google Scholar]
- 23.Simon CJ, Dupuy DE, DiPetrillo TA, et al. Pulmonary radiofrequency ablation: long-term safety and efficacy in 153 patients. Radiology. 2007;243:268–75. doi: 10.1148/radiol.2431060088. [DOI] [PubMed] [Google Scholar]
- 24.Beland MD, Wasser EJ, Mayo-Smith WW, Dupuy DE. Primary non-small cell lung cancer: review of frequency, location, and time of recurrence after radiofrequency ablation. Radiology. 2010 Jan;254(1):301–7. doi: 10.1148/radiol.00000090174. [DOI] [PubMed] [Google Scholar]
- 25.Lanuti M, Sharma A, Digumarthy SR, Wright CD, Donahue DM, Wain JC, Mathisen DJ, Shepard JA. Radiofrequency ablation for treatment of medically inoperable stage I non-small cell lung cancer. J Thorac Cardiovasc Surg. 2009 Jan;137(1):160–6. doi: 10.1016/j.jtcvs.2008.08.034. [DOI] [PubMed] [Google Scholar]
- 26.Nguyen CL, Scott WJ, Goldberg M. Radiofrequency Ablation of Lung Malignancies. Ann Thorac Surg. 2006;82:365–371. doi: 10.1016/j.athoracsur.2005.11.069. [DOI] [PubMed] [Google Scholar]
- 27.Steinke K, Sewell PE, Dupuy D, et al. Pulmonary radiofrequency ablation – an international study survey. Anticancer Res. 2004;24:339–343. [PubMed] [Google Scholar]
- 28.Pennathur A, Abbas G, Gooding WE, et al. Image-guided Radiofrequency Ablation of Lung Neoplasm in 100 Consecutive Patients by a Thoracic Surgical Service. Ann Thorac Surg. 2009 Nov;88(5):1601–8. doi: 10.1016/j.athoracsur.2009.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.El-Sherif A, Fernando HC, Santos R, et al. Margin and local recurrence after sublobar resection of non-small cell lung cancer. Ann Surg Oncol. 2007;14:2400–5. doi: 10.1245/s10434-007-9421-9. [DOI] [PubMed] [Google Scholar]
- 30.Lee JM, Youk JH, Kim YK, et al. Radio-frequency thermal ablation with hypertonic saline solution injection of the lung: ex vivo and in vivo feasibility studies. Eur Radiol. 2003;13:2540–7. doi: 10.1007/s00330-003-1876-x. [DOI] [PubMed] [Google Scholar]
- 31.Simon CS, Dupuy DE, Mayo-Smith WW. Microwave ablation: Principles and applications. Radiographics. 2005;25:S69–S83. doi: 10.1148/rg.25si055501. [DOI] [PubMed] [Google Scholar]
- 32.Santos RS, Gupta A, Ebright MI, et al. Electromagnetic navigation to aid radiofrequency ablation and biopsy of lung tumors. Ann Thorac Surg. 2010 Jan;89(1):265–8. doi: 10.1016/j.athoracsur.2009.06.006. [DOI] [PubMed] [Google Scholar]
- 33.Tanabe T, Koizumi T, Tsushima K, et al. Comparative study of three different catheters for CT-Bronchoscopy-guided radiofrequency ablation as a potential and novel intervention therapy for lung cancer. Chest. doi: 10.1378/chest.09-1065. Prepublished online October 26, 2009. [DOI] [PubMed] [Google Scholar]