Abstract
In this issue of the Journal of Bacteriology, Chonoles Imlay et al. (K. R. Chonoles Imlay, S. Korshunov, and J. A. Imlay, J Bacteriol 197:3629–3644, 2015, http://dx.doi.org/10.1128/JB.00277-15) show that oxidative stress kills sulfur-restricted Escherichia coli grown with sublethal H2O2 when challenged with cystine. Killing requires rapid and seemingly unregulated cystine transport and equally rapid cystine reduction to cysteine. Cysteine export completes an energy-depleting futile cycle. Each reaction of the cycle could be beneficial. Together, a cystine-mediated vulnerability emerges during the transition from a sulfur-restricted to a sulfur-replete environment, perhaps because of complexities of sulfur metabolism.
TEXT
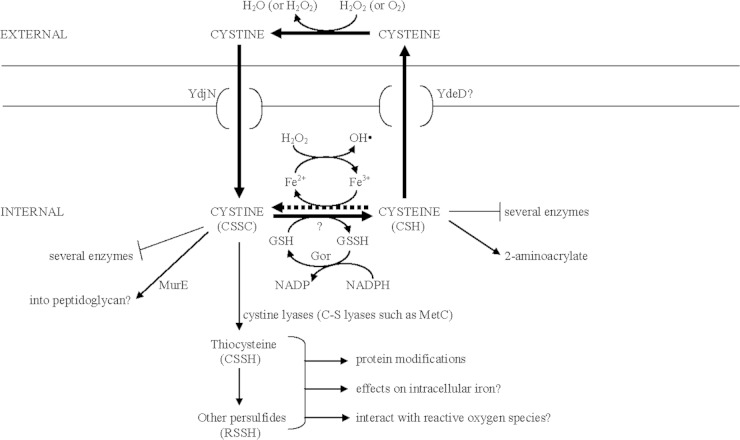
Sulfur is a component of the amino acids cysteine and methionine; the antioxidant glutathione; the cofactors coenzyme A (CoA), thiamine, and lipoamide; and other essential molecules. Sulfur-containing compounds in bacteria are usually reduced intracellularly (e.g., sulfide, cysteine, and proteins with free sulfhydryls) but oxidized extracellularly (e.g., sulfate, cystine, and proteins with either oxidized thiols or disulfide bonds). Sulfur-containing molecules undoubtedly reflect and contribute to intracellular redox balance. Scattered reports have described effects of cysteine/cystine on oxidative stress and antibiotic resistance, and some might be the result of altered redox balance (1–3). Park and Imlay have observed that 0.5 mM cystine killed sulfur-restricted Escherichia coli exposed to 2.5 mM H2O2 (2). They proposed that intracellular cystine is reduced to cysteine, which in turn reduces intracellular ferric to ferrous iron, providing the limiting factor for the Fenton reaction and generating hydroxyl radicals (2). This chain of events will be called cystine-mediated hypersensitivity (CMH). In a study reported in this issue of the Journal of Bacteriology, Chonoles Imlay et al. isolated mutants resistant to CMH and found that death results from excessive and seemingly unregulated cystine transport and rapid glutathione-dependent reduction of cystine to cysteine (Fig. 1) (4). Cysteine export and subsequent extracellular oxidation complete a futile cycle that was calculated to consume 10% of the ATP requirement for biosynthesis and double the total NADPH demand. Each component of this futile cycle is probably beneficial, which suggests that CMH may be an emergent property resulting from high intracellular levels of sulfur-containing compounds. Figure 1 summarizes the reactions and compounds considered here.
FIG 1.
The cystine/cysteine futile cycle, CMH, and possible problems associated with sulfur metabolism. The thick solid arrows represent the cystine/cysteine futile cycle. The thick dotted arrow shows the reaction that is proposed to kill cells during CMH.
The cystine transporters, their regulation, and prolonged H2O2 sensitivity.
Chonoles Imlay et al. selected survivors of CMH and found that the mutants lost YdjN, which is an H+/Na+ cystine symporter (4). A previously described cystine transporter did not contribute to CMH (4). The rate of cystine transport is 30 times the cellular sulfur requirement, and CMH persists for 1 to 2 h after cystine challenge (2, 4). This persistence is the basis for an unexpected cellular vulnerability, which led to an analysis of cystine transport regulation. A relatively poor sulfur source (sulfate) was required to induce YdjN via CysB, but addition of cystine, which is a good sulfur source, should repress YdjN (4). Therefore, prolonged sensitivity suggests either that YdjN synthesis persists after cystine addition or that growth slowly dilutes YdjN even if ydjN transcription ceases. Another factor contributing to prolonged sensitivity may be cysteine-dependent induction of a cysteine/cystine transport system (5; M. Loddeke and L. Reitzer, unpublished observation), which may not occur in a ydjN mutant. Prolonged sensitivity also suggests the absence of kinetic inhibition of YdjN, because either such an inhibitor does not exist or the inhibitor is rapidly degraded. Evidence for the latter possibility is that loss of cystine reduction to cysteine impairs cystine transport and intracellular cystine is undetectable after cystine challenge (4).
Why is cystine rapidly reduced to cysteine?
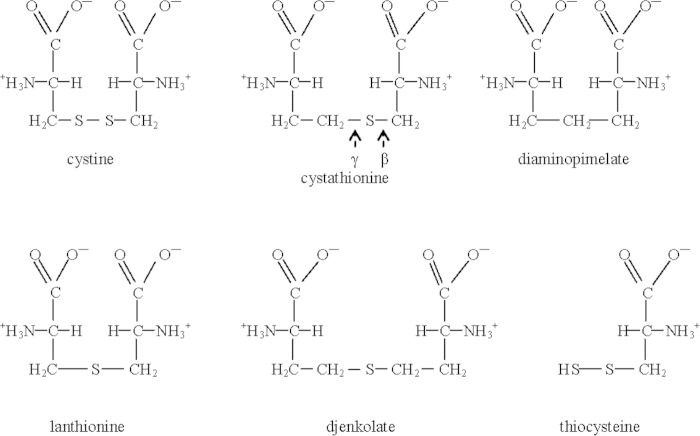
Cystine-challenged cells had undetectable intracellular cystine and higher intracellular cysteine, which indicates that cystine reduction is as rapid as cystine transport (4). Glutathione was required for both cystine reduction and CMH, but the genes and proteins for cystine reduction are not known. One rationale for rapid reduction is cystine toxicity. Several proteins fail to distinguish between cystine and its analogs, which implies that cystine could interfere with their function (Fig. 2). These proteins include FliY, MetC, MurE, some transaminases, and NifS-like proteins (5–9). MetC is a cystathionine β-lyase (a C-S lyase) that cleaves cystathionine in methionine synthesis and also cleaves cystine, lanthionine, and djenkolate with Kms less than 1 mM (6). MurE ligates a peptidoglycan precursor to diaminopimelate, cystathionine, or lanthionine in vitro and in vivo (8). Cystine is probably also a substrate for MurE, although it has not been explicitly tested. If it is, then cystine may be incorporated into peptidoglycan.
FIG 2.
Structures of cystine analogs and thiocysteine. The structures are drawn to emphasize the similarities and differences. The cystathionine β- and γ-lyases cleave at the C-S bonds shown above, which generates homocysteine and cysteine, respectively. The figure was adapted from reference 4 with permission.
A second rationale for rapid cystine reduction is to avoid some toxic products of cystine metabolism. One possible toxic product is thiocysteine, which is also called cysteine persulfide (Fig. 2). Thiocysteine is produced from cystine lyase activity, which is usually a secondary reaction of several enzymes described above, but especially the cystathionine β- and γ-lyases (6, 10). Thiocysteine is an important and abundant effector of the cellular thiol landscape, at least in eukaryotes (10, 11). Thiocysteine and other persulfides (RSSH) can modify or inactivate proteins (10, 12). Persulfide intermediates are part of Fe-S cluster assembly (13), and the interaction of low-molecular-weight persulfides with iron-containing proteins may affect both protein activity and intracellular iron. In addition to indirect effects via iron, thiocysteine and persulfides directly interact with reactive oxygen species (10, 12).
Rapid cysteine export and function of extracellular thiols.
Rapid cysteine export has several possible benefits. First, export can lower intracellular cysteine, which would prevent generation of ferrous iron and impair the Fenton reaction (2). The product of this one-electron reduction could be a thiyl radical, which could exacerbate oxidative stress. Second, cysteine export can also prevent inhibition of several enzymes, such as homoserine dehydrogenase I (ThrA) (14). Third, C-S lyase degradation of cysteine produces 2-aminoacrylate and 2-iminopropionate, which are toxic since the loss of the enzyme that degrades these intermediates, RidA, impairs growth in medium with cysteine (15, 16). E. coli contains several C-S lyases, but cysteine degradation is not their primary function (17). Fourth, cysteine and thiol secretion can protect against oxidative stress by removing H2O2 (18–21). The protection for E. coli is modest (21), and CMH presumably exceeds the capacity of this protection. Finally, cysteine export could generate an extracellular signal. An example of such signaling is regulation of the immune response. Dendritic cells import cystine when they encounter lipopolysaccharides, presumably from bacteria. The cystine is reduced to cysteine, which is exported. The extracellular cysteine is imported into T cells, which lack a cystine transporter, and T cells proliferate (22).
CMH as an emergent property.
Each reaction in the possible cysteine futile cycle is potentially beneficial. Rapid transport provides a competitive advantage in a sulfur-limited environment, rapid cystine reduction removes a potentially dangerous compound, and rapid cysteine export may have several different functions. CMH emerges because of seemingly unregulated cystine transport and high intracellular concentrations of cysteine and perhaps other reactive sulfur species (Fig. 1). This emergent property is apparent when sulfur-restricted cells encounter cystine. The resulting high-intracellular-cysteine or other sulfur-containing compounds are not lethal, although cells may be stressed because of higher levels of intracellular iron and reactive oxygen species. Exogenous H2O2 is lethal, perhaps because the capacity to handle the additional oxidative stress is exceeded.
Chonoles Imlay et al. speculate that the lethal combination of H2O2 and iron-reducing cysteine contributes to host defense mechanisms (4). This defense mechanism may also involve toxic products of cystine metabolism that generate reactive sulfur species, such as persulfides, and killing may involve a synergism between reactive oxygen and sulfur species. Cysteine/cystine in E. coli and other organisms can either diminish or amplify oxidative stress (2, 4, 19, 21), which suggests that controlling the effects on sulfur metabolism could be difficult. The contribution of sulfur-containing compounds to physiology is complex, difficult to study and control, poorly understood, and underappreciated. The genetics-based analysis of CMH-resistant mutants by Chonoles Imlay et al. has provided an interesting perspective on the metabolism of sulfur-containing compounds (4). Prokaryotes and eukaryotes probably encounter similar problems with the basic chemistry of sulfur-containing compounds, although studies of sulfur metabolism in the former have generally lagged behind those in the latter (e.g., references 10 and 23). More studies using bacterial genetics should provide new information about this basic biochemistry.
The views expressed in this Commentary do not necessarily reflect the views of the journal or of ASM.
REFERENCES
- 1.Berglin EH, Edlund MB, Nyberg GK, Carlsson J. 1982. Potentiation by l-cysteine of the bactericidal effect of hydrogen peroxide in Escherichia coli. J Bacteriol 152:81–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Park S, Imlay JA. 2003. High levels of intracellular cysteine promote oxidative DNA damage by driving the Fenton reaction. J Bacteriol 185:1942–1950. doi: 10.1128/JB.185.6.1942-1950.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Turnbull AL, Surette MG. 2010. Cysteine biosynthesis, oxidative stress and antibiotic resistance in Salmonella typhimurium. Res Microbiol 161:643–650. doi: 10.1016/j.resmic.2010.06.004. [DOI] [PubMed] [Google Scholar]
- 4.Chonoles Imlay KR, Korshunov S, Imlay JA. 2015. Physiological roles and adverse effects of the two cystine importers of Escherichia coli. J Bacteriol 197:3629–3644. doi: 10.1128/JB.00277-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Berger EA, Heppel LA. 1972. A binding protein involved in the transport of cystine and diaminopimelic acid in Escherichia coli. J Biol Chem 247:7684–7694. [PubMed] [Google Scholar]
- 6.Dwivedi CM, Ragin RC, Uren JR. 1982. Cloning, purification, and characterization of β-cystathionase from Escherichia coli. Biochemistry 21:3064–3069. doi: 10.1021/bi00256a005. [DOI] [PubMed] [Google Scholar]
- 7.Kessler D. 2004. Slr0077 of Synechocystis has cysteine desulfurase as well as cystine lyase activity. Biochem Biophys Res Commun 320:571–577. doi: 10.1016/j.bbrc.2004.06.007. [DOI] [PubMed] [Google Scholar]
- 8.Mengin-Lecreulx D, Blanot D, van Heijenoort J. 1994. Replacement of diaminopimelic acid by cystathionine or lanthionine in the peptidoglycan of Escherichia coli. J Bacteriol 176:4321–4327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Munt O, Prufer D, Schulze Gronover C. 2013. A novel C-S lyase from the latex-producing plant Taraxacum brevicorniculatum displays alanine aminotransferase and l-cystine lyase activity. J Plant Physiol 170:33–40. doi: 10.1016/j.jplph.2012.08.018. [DOI] [PubMed] [Google Scholar]
- 10.Ida T, Sawa T, Ihara H, Tsuchiya Y, Watanabe Y, Kumagai Y, Suematsu M, Motohashi H, Fujii S, Matsunaga T, Yamamoto M, Ono K, Devarie-Baez NO, Xian M, Fukuto JM, Akaike T. 2014. Reactive cysteine persulfides and S-polythiolation regulate oxidative stress and redox signaling. Proc Natl Acad Sci U S A 111:7606–7611. doi: 10.1073/pnas.1321232111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Miranda KM, Wink DA. 2014. Persulfides and the cellular thiol landscape. Proc Natl Acad Sci U S A 111:7505–7506. doi: 10.1073/pnas.1405665111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mishanina TV, Libiad M, Banerjee R. 2015. Biogenesis of reactive sulfur species for signaling by hydrogen sulfide oxidation pathways. Nat Chem Biol 11:457–464. doi: 10.1038/nchembio.1834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kessler D. 2006. Enzymatic activation of sulfur for incorporation into biomolecules in prokaryotes. FEMS Microbiol Rev 30:825–840. doi: 10.1111/j.1574-6976.2006.00036.x. [DOI] [PubMed] [Google Scholar]
- 14.Reitzer L. 2005. Catabolism of amino acids and related compounds. EcoSal Plus. doi: 10.1128/ecosalplus.3.4.7. [DOI] [PubMed] [Google Scholar]
- 15.Ernst DC, Lambrecht JA, Schomer RA, Downs DM. 2014. Endogenous synthesis of 2-aminoacrylate contributes to cysteine sensitivity in Salmonella enterica. J Bacteriol 196:3335–3342. doi: 10.1128/JB.01960-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Muller A, Langklotz S, Lupilova N, Kuhlmann K, Bandow JE, Leichert LI. 2014. Activation of RidA chaperone function by N-chlorination. Nat Commun 5:5804. doi: 10.1038/ncomms6804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Awano N, Wada M, Mori H, Nakamori S, Takagi H. 2005. Identification and functional analysis of Escherichia coli cysteine desulfhydrases. Appl Environ Microbiol 71:4149–4152. doi: 10.1128/AEM.71.7.4149-4152.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hung J, Cooper D, Turner MS, Walsh T, Giffard PM. 2003. Cystine uptake prevents production of hydrogen peroxide by Lactobacillus fermentum BR11. FEMS Microbiol Lett 227:93–99. doi: 10.1016/S0378-1097(03)00653-0. [DOI] [PubMed] [Google Scholar]
- 19.Lo R, Turner MS, Barry DG, Sreekumar R, Walsh TP, Giffard PM. 2009. Cystathionine γ-lyase is a component of cystine-mediated oxidative defense in Lactobacillus reuteri BR11. J Bacteriol 191:1827–1837. doi: 10.1128/JB.01553-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ohtsu I, Wiriyathanawudhiwong N, Morigasaki S, Nakatani T, Kadokura H, Takagi H. 2010. The l-cysteine/l-cystine shuttle system provides reducing equivalents to the periplasm in Escherichia coli. J Biol Chem 285:17479–17487. doi: 10.1074/jbc.M109.081356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ohtsu I, Kawano Y, Suzuki M, Morigasaki S, Saiki K, Yamazaki S, Nonaka G, Takagi H. 2015. Uptake of l-cystine via an ABC transporter contributes defense of oxidative stress in the l-cystine export-dependent manner in Escherichia coli. PLoS One 10:e0120619. doi: 10.1371/journal.pone.0120619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Angelini G, Gardella S, Ardy M, Ciriolo MR, Filomeni G, Di Trapani G, Clarke F, Sitia R, Rubartelli A. 2002. Antigen-presenting dendritic cells provide the reducing extracellular microenvironment required for T lymphocyte activation. Proc Natl Acad Sci U S A 99:1491–1496. doi: 10.1073/pnas.022630299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Go YM, Chandler JD, Jones DP. 2015. The cysteine proteome. Free Radic Biol Med 84:227–245. doi: 10.1016/j.freeradbiomed.2015.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]