ABSTRACT
When cystine is added to Escherichia coli, the bacterium becomes remarkably sensitive to hydrogen peroxide. This effect is due to enlarged intracellular pools of cysteine, which can drive Fenton chemistry. Genetic analysis linked the sensitivity to YdjN, a secondary transporter that along with the FliY-YecSC ABC system is responsible for cystine uptake. FliY-YecSC has a nanomolar Km and is essential for import of trace cystine, whereas YdjN has a micromolar Km and is the predominant importer when cystine is more abundant. Oddly, both systems are strongly induced by the CysB response to sulfur scarcity. The FliY-YecSC system can import a variety of biomolecules, including diaminopimelate; it is therefore vulnerable to competitive inhibition, presumably warranting YdjN induction under low-sulfur conditions. But the consequence is that if micromolar cystine then becomes available, the abundant YdjN massively overimports it, at >30 times the total sulfur demand of the cell. The imported cystine is rapidly reduced to cysteine in a glutathione-dependent process. This action avoids the hazard of disulfide stress, but it precludes feedback inhibition of YdjN by cystine. We conjecture that YdjN possesses no cysteine allosteric site because the isostructural amino acid serine might inappropriately bind in its place. Instead, the cell partially resolves the overaccumulation of cysteine by immediately excreting it, completing a futile import/reduction/export cycle that consumes a large amount of cellular energy. These unique, wasteful, and dangerous features of cystine metabolism are reproduced by other bacteria. We propose to rename ydjN as tcyP and fliY-yecSC as tcyJLN.
IMPORTANCE In general, intracellular metabolite pools are kept at steady, nontoxic levels by a sophisticated combination of transcriptional and allosteric controls. Surprisingly, in E. coli allosteric control is utterly absent from the primary importer of cystine. This flaw allows massive overimport of cystine, which causes acute vulnerability to oxidative stress and is remedied only by wasteful cysteine efflux. The lack of import control may be rationalized by the unusual properties of cysteine itself. This phenomenon justifies the existence of countervailing cysteine export systems, whose purpose is otherwise hard to understand. It also highlights an unexpected link between sulfur metabolism and oxidative damage. Although this investigation focused upon E. coli, experiments confirmed that similar phenomena occur in other species.
INTRODUCTION
Sulfur atoms are essential for life. Imported sulfur species are ultimately channeled into a cytoplasmic pool of cysteine, from which all sulfurous biomolecules are derived, including methionine, iron-sulfur clusters, glutathione, lipoate, biotin, and pantothenate. Escherichia coli can fulfill these requirements by importing and metabolizing a variety of inorganic and organic sulfur compounds: sulfate, sulfite, cystine, cysteine, sulfide, thiosulfate, djenkolate, glutathione (GSH), lanthionine, and others (1).
When environmental sources of sulfur dwindle, bacteria are in trouble. They have minimal pools of stored sulfur, so any interruption of import very quickly causes an interruption of growth. To address this problem, cells respond to sulfur limitation by strongly inducing the synthesis of transporters that import the various sulfur compounds and the enzymes that convert them to cysteine. This response is driven by the CysB regulatory protein. When cysteine pools fall and other sulfur sources are limiting, intracellular N-acetylserine accumulates and binds to CysB, conferring its activity as a positive transcription factor (2). By raising the titers of various sulfur import proteins, CysB enhances the ability of the cell to sustain growth in competitive environments.
Cells depend upon sulfur because it provides chemical activities that enable multiple aspects of metabolism. It is a good metal ligand that is exploited by myriad iron and zinc proteins. Cysteine residues also provide the redox activities by which various proteins sense and degrade oxidants, assimilate sulfate, reduce ribonucleotides, and decontaminate heavy metals. Further, although bacterial cytoplasmic enzymes lack disulfide bonds, periplasmic and secreted enzymes are commonly stabilized by them. These different functions cannot easily be supplied by other amino acids. However, cysteine tends to be avoided as a simple structural residue (3), probably because of the threats of adventitious oxidation and of sulfur deficiency.
Several years ago, our laboratory chanced upon a novel intersection between oxidative stress and sulfur metabolism (4). Bacteria are periodically confronted by hostile organisms that excrete hydrogen peroxide (H2O2) as an antimicrobial agent (5–8). Hydrogen peroxide can penetrate membranes, and a large surge in cytoplasmic H2O2 quickly impairs metabolism by inactivating particular enzyme families (9). However, this stress is rarely lethal, since damaged enzymes are repaired or replaced when H2O2 levels subside; thereafter, growth resumes. Indeed, E. coli can tolerate 15 min of exposure to millimolar concentrations of H2O2 with >70% survival (10). Although some DNA damage occurs through the Fenton reaction (equations 1 and 2), the amount is relatively small, and excision and recombinational DNA repair enzymes quickly repair the lesions and sustain viability.
| (1) |
| (2) |
However, we discovered that when cystine is provided to sulfate-grown cells, the cells become profoundly sensitive to killing by exogenous H2O2, with fewer than 0.1% surviving 1 min of H2O2 exposure (4). Investigation revealed that cysteine can quickly reduce ferric iron formed by the Fenton reaction back to the ferrous form; thus, in the presence of cysteine, iron atoms can redox cycle and catalyze many rounds of hydroxyl radical formation. This phenomenon did not occur if cells had been extensively cultured with cystine prior to the H2O2 exposure. We speculated that when E. coli grows in medium containing only sulfate, which is a relatively poor sulfur source, CysB activates expression of a few sulfur acquisition systems, including one that imports cystine. When cystine is subsequently supplied, it is overimported, thereby driving the cysteine pools to levels that dramatically enhance the Fenton reaction.
In the present study, we report that CysB actually governs two cystine import systems, and we demonstrate the distinctive roles of each. One of these systems can import cystine at a rate that enormously exceeds the cellular demand, and we show that the cell compensates by profusely excreting cysteine. Analysis suggests that the two distinctive features of cysteine—its essentiality for growth and its capacity for redox activity—combine to predispose the cell to import cystine without any effective feedback controls. Because bacteria routinely move from circumstances of sulfur limitation to sulfur sufficiency, this situation will be recapitulated in nature.
MATERIALS AND METHODS
Strains, media, and materials.
Strains, plasmids, and primers are listed in Table S1 in the supplemental material. In physiological experiments, cells were grown in minimal A glucose medium (11) that contained 0.5 mM (each) 18 nonsulfurous amino acids but lacked cystine and methionine unless specified (also known as sulfate medium). Inductively coupled plasma mass spectrometry (ICP-MS) measurements show that this medium contains 2 μM iron, which is pertinent to the Fenton chemistry reported in this paper. When supplied, cystine was added at 0.5 mM (cystine medium), unless otherwise noted. LB medium (10 g Bacto tryptone, 5 g yeast extract, 10 g NaCl per liter, pH 7) was used for strain constructions.
In some experiments, ostensibly sulfur-free medium was prepared, in which the (NH4)2SO4 and MgSO4·7H2O of minimal A glucose medium with 18 amino acids was replaced by 0.405 g NH4Cl and 0.8 mM MgCl2 per liter, respectively. This medium contains some contaminating sulfate. This sulfate was removed by inoculating the medium with wild-type (wt) cells and incubating the culture until growth stopped due to sulfate depletion. Specifically, an overnight culture of wild-type cells that had been grown in standard sulfate medium was washed twice and then suspended to an optical density at 600 nm (OD600) of ∼0.010 in sulfur-free medium. Cells were cultured until growth ceased (after ∼3 h at an OD600 of ca. 0.030). The cells were then removed by centrifugation, and the supernatant was sterilized by filtration and used as a truly sulfur-free medium. Various compounds were then added to test the ability of strains to use these as sole sulfur sources. The ability of FliY-YecSC and YdjN to import 15 μM homocystine as a methionine precursor was tested using metC null mutants (SSK233, SSK234, and SSK235) in standard sulfate medium.
Experiments were invariably performed with cultures that were growing exponentially. In general, strains were inoculated and grown overnight in the specified medium. Cultures were then diluted to an OD600 of 0.005 to 0.010 and grown for at least four generations to an OD600 of 0.1 to 0.2 prior to analysis. Aerobic cultures were grown with vigorous shaking; anoxic cultures were grown in a Coy anaerobic chamber under a gas mixture of 10% H2-5% CO2-85% N2.
The wild-type strain MG1655 was tested for its ability to use cystine as sole carbon or nitrogen source. The base medium contained nitrogen-free salts: per liter, 2 g K2SO4, 13.5 g K2HPO4, 4.7 g KH2PO4, 0.1 g MgSO4·7H2O, 9 g NaCl, and 5 μg thiamine, pH 7. All media also included 0.2 mM isoleucine to compensate for the possibility that excess intracellular cysteine inhibits threonine deaminase (12). Overnight cultures were grown in the base medium supplemented with 5 mM pyruvate and 2 mM proline. Precultures were grown in the same medium for four generations, washed in nitrogen-free salts, and inoculated into the test medium. To test whether cystine can suffice as the sole carbon source, carbon was provided as 5 mM pyruvate or 1 mM cystine or omitted, and 15 mM NH4Cl was included as the nitrogen source. In tests of whether cystine could serve as the sole nitrogen source, nitrogen was provided as 15 mM NH4Cl, 2 mM proline, or 1 mM cystine or omitted, and 5 mM pyruvate was supplied as the carbon source. Growth was monitored by OD at A600.
The expression of the CysB regulon using ydjN′-lacZ as a proxy was tested with a variety of sulfur sources by culturing KCI1230 in sulfur-free Tris medium adapted from the work of Kertesz et al. (13) (150 mM Tris, 0.2% glucose, 20 mM NH4Cl, 2 mM K2HPO4, 0.5 mM MgCl2, 1.3 μM EDTA, and 0.72 μM FeCl2, pH 7.6, supplemented with the 18 amino acids other than Cys and Met). Trace contaminating sulfur had been scavenged from the medium by prior culture with wild-type cells, as described above. KCI1230 was precultured into exponential phase in this medium supplemented with 0.05 mM cystine, and then cells were washed and suspended to an OD600 of 0.007 in the medium devoid of Cys and Met but supplemented with 0.05 mM indicated sulfur compounds. Cells were harvested at an OD600 of 0.2 and then assayed for β-galactosidase (11) and for sulfite reductase by the cytochrome c method (14).
Nonradioactive chemicals were purchased from Sigma-Aldrich, and medium reagents were purchased from Fisher. [14C]cystine was from PerkinElmer or American Radiolabeled Chemicals, Inc. Restriction enzymes were purchased from New England BioLabs. Qiagen kits were used for genomic and plasmid DNA preparation, PCR cleanup, and mRNA and cDNA cleanup, as needed. RNA preparation reagents were from Applied Biosystems/Ambion, Invitrogen, and Molecular Bioproducts. Pico-green dye (from Molecular Probes, now Life Technologies) was used to quantify and standardize the total amount of cDNA that was used for reverse transcription-PCRs (RT-PCRs). SYBR green (Bio-Rad) was used to track amplification of cDNA. Primers were purchased from Fisher/Operon or IDT, PCR reagents were obtained from Invitrogen, and the EZ-Tn5<Kan-2> transposome mutagenesis kit was purchased from Epicentre. DNA sequencing was performed at the University of Illinois CORE-LIMS facility or at ACGT, Inc.
Strain constructions.
Null mutations were made using the lambda red recombinase method to replace the open reading frame (ORF) with a Cam resistance cassette amplified from the pKD3 template (15). P1 transduction was used to introduce mutations into new strains (11). When necessary, Cam or Kan drug resistance markers were excised from strains using the FLP activity of pCP20, followed by loss of the plasmid at nonpermissive temperature. All mutations were verified by PCR and gel analysis.
Complementation plasmids were assembled using the low-copy-number vector pWKS30 (16). The cloned sequences included 200 bases upstream of the complementing ORF so that expression of each gene was, putatively, from its own promoter. Resulting plasmids were verified by sequence analysis.
Single-copy lacZ transcriptional fusions to the fliA, fliY, dcyD, yecS, yecC, and ydjN promoter regions were integrated into the lambda attachment site; unless otherwise noted, the wild-type copy of the gene was maintained at its native site in the chromosome. The putative promoter regions, extending 200 bases upstream from the translational start codon, were amplified by PCR and inserted into the pSJ150 multicloning site, upstream of lacZ, and the sequences were confirmed. The promoter′-lacZ fusions were then integrated into the chromosome at the λ-att site, as described previously (17, 18).
Targeted single-copy transcriptional lac fusions were made in fliY and yecS using lambda red and FLP-mediated site-specific recombination, followed by transformation and integration of pKG137 into the chromosome at the resulting FLP recombination target (FRT) site (19). Correct orientations and chromosomal loci were confirmed by PCR analysis. In the fliY promoter region of both of these transcriptional lacZ fusion mutants, 76 bases directly upstream from the fliY ORF (nucleotides [nt] −3 to −79) were deleted and replaced with the cat cassette (which codes on the opposite strand) by the lambda red recombinase method, in order to study the influence of CysB-regulated fliY expression on downstream expression of yecS.
Tetracycline-controlled expression of ydjN and yecSC was achieved by replacing each of the native promoters with the tetRA cassette amplified by PCR from the chromosome of strain JS519 in a modified lambda red recombinase method (18, 20). In the case of yecSC, this involved deleting the dcyD ORF as well. The native Shine-Dalgarno sequence was retained.
Plasmid p(ydjN) was modified at the single cysteine codon (position 346) by its replacement with either a threonine or serine codon. PCR-generated DNA segments were combined to create a TGC-to-ACC or TGC-to-TCA change in the DNA sequence of ydjN, resulting in p(ydjN C346T) and p(ydjN T346S), respectively.
Hydrogen peroxide toxicity.
Cells were grown aerobically for four generations in aerobic sulfate medium to an OD600 of 0.10. Cultures were diluted in the same prewarmed medium to an OD600 of 0.025. If indicated, cystine was added for 3 min, and cells were then challenged with 2.5 mM H2O2. At intervals, aliquots were diluted into LB medium containing 1,300 U/ml catalase, and cell survival was determined by colony formation after plating in top agar.
Selection of mutants resistant to cystine-H2O2 treatment.
Transposome-mutagenized cells were prepared according to the manufacturer's instructions (EZ-Tn5 <Kan-2>; Epicentre). These mutants contained Tn5 (and an accompanying Kanr cassette) inserted at random locations throughout the chromosome. Eight pools of ∼1,000 mutants were cultured for 4 generations in sulfate medium to an OD600 of 0.1. Each pool was diluted to an OD600 of 0.25 in the same medium supplemented with 0.5 mM cystine, and after 3 min, 2.5 mM H2O2 was added. The exposure was ended at 10 min through the addition of 1,300 U/ml catalase. Cultures were centrifuged, washed to remove catalase, resuspended in warm sulfate medium, and cultured for 1 h. This outgrowth period was necessary to restore H2O2 sensitivity to the surviving cells. The cystine addition and H2O2 exposure were repeated. Survivors were plated on LB plates, and isolated colonies were subsequently tested for sensitivity to the cystine-H2O2 treatment. Only a single isolate, with Kan inserted into ydjN, showed significant resistance.
Cystine import measurements.
Cultures in sulfate medium with or without cystine were grown for four generations to an OD of 0.2. Cells were centrifuged and resuspended in the same prewarmed medium to an OD of 0.2. Chloramphenicol was added to 80 to 100 μg/ml, and cultures were held for 5 min at 37°C. [14C]cystine cocktail in sulfate medium was added to a final concentration of 2 μM and 80 mCi/mmol cystine (for FliY-YecSC-mediated import) or 20 μM and 20 mCi/mmol cystine (YdjN import). At intervals, aliquots were removed and added to washed filters on a vacuum manifold, and these cells were immediately washed three times with 2.5 ml wash buffer (100 mM Tris, pH 8, 0.15 M NaCl, 0.5 mM MgCl2 at room temperature [RT]). Filters were dried and transferred to vials containing 5 ml scintillation fluid. Experiment-specific information is listed with the accompanying figures. Measurements of apparent Km for import were conducted with KCI1137 at an OD600 of 0.1 to 0.2 at 37°C (for the FliY/YesSC transporter) and with KCI1142 at an OD600 of 0.5 at RT (for the YdjN transporter). The lower temperature in the latter reaction was used to decrease the speed with which saturation occurred.
The ability of other biomolecules to inhibit cystine import was tested by mixing 10 μM [14C]cystine and between 10 μM and 1 mM competing compound when performing transport assays.
Analyses of gene expression.
Cultures were grown exponentially for at least four generations in the tested medium to an OD600 of 0.2. The expression of lacZ fusions was evaluated by β-galactosidase assays (11). The responsiveness of fliY::lacZ fusions to diaminopimelate (DAP) restriction was tested by growing dapA mutants in medium containing 1 mM DAP and then monitoring β-galactosidase synthesis for 30 min after DAP was removed by washing. Total protein was measured using Coomassie blue reagent from Thermo-Fisher.
RNA was collected from exponentially growing cultures at OD600s of 0.3 to 0.5. RNA was isolated by hot phenol extraction followed by ethanol extraction, followed by cleanup on Qiagen RNA columns, as needed. cDNA was prepared from DNA-free RNA for reverse transcription-quantitative PCR (qRT-PCR), 5′ rapid amplification of cDNA ends (RACE) (18), and Northern blotting (21) analyses.
Transduction analysis.
Phage P1 cotransduction analysis was used to determine that FliY-YecSC is the sole transporter of diaminopimelic acid (DAP) into E. coli. A P1 lysate from ppk::Tn10∼dapA::cat was used to infect MG1655 (wt) and its ΔfliY and/or ΔydjN derivatives (Table 1) (11). Selection was on LB-12.5 μg/ml tetracycline-1 mM DAP plates. Forty isolates from each transduction were then screened for DAP prototrophy on sulfate medium plates, with or without 1 mM DAP supplements. Our inability to recover any dapA::cat recombinants in the ΔfliY background demonstrated that FliY-YecSC is required for DAP import.
TABLE 1.
The FliY-YecSC system is the sole diaminopimelate (DAP) importer in E. colia
| Recipient genotype | No. of transformants |
% cotransduction | |
|---|---|---|---|
| ppk::Tn10 transductants | dapA::cat cotransduced | ||
| Wild type | 40 | 15 | 38 |
| ΔfliY | 40 | 0 | 0 |
| ΔydjN | 40 | 14 | 35 |
| ΔfliY ΔydjN | 40 | 0 | 0 |
A Tn10 insertion (ppk::Tn10) near the dapA::cat allele of KCI1250 was P1 transduced into strains MG1655, KCI1252, KCI1254, and KCI1262 in DAP-supplemented medium. Successful cotransduction of the dapA null allele indicated that the recipient strain was able to import DAP.
Determination of cellular sulfur requirement.
The sulfur requirement of E. coli was established by measuring the optical density that could be achieved with limited amounts of cystine and methionine. KCI946 (ΔcysDNC ΔcysJIH ΔmetA) cannot assimilate sulfate or synthesize methionine. The strain was grown in cystine- and methionine-supplemented medium to exponential phase. Cells were washed and suspended in the same medium lacking methionine and cystine. To quantify the methionine demand, cystine was added in excess (0.5 mM) and methionine was either omitted or added to 3, 5, 10, 20, 40, or 80 μM final concentration. To quantify the cystine demand exclusive of methionine synthesis, methionine was added in excess (0.5 mM), and cystine was either omitted or added to an 1.5, 3, 5, 7.5, 15, or 20 μM final concentration. Cultures were then incubated until growth stopped.
Final densities were proportionate to the supply of the limiting amino acid. It was found that the strain required 48 μM methionine and 7.8 μM cystine per OD600 of 1.0 of final density. This represents a total sulfur demand of 64 μM sulfur atoms/OD600. Because 1 liter of bacteria of an OD600 of 1.0 contains 0.5 ml total cytoplasm (22), the data indicate a total sulfur concentration in the cytoplasm of 128 mM (incorporated into biomolecules). In this medium, which was also used in the other experiments of this study, the doubling time is 40 min (k = 0.0173 min−1). Thus, the required rate of sulfur influx into the cytoplasm to support this growth rate is 0.0173 min−1 × 128 mM sulfur = 2.2 mM min−1 sulfur atoms.
Measurements of thiol excretion.
Overnight cultures in sulfate medium were diluted to an OD600 of 0.010 and grown to an OD600 of 0.2 in the same aerobic medium. These cultures were then centrifuged and resuspended to an OD600 of 0.10 in the same 37°C fresh medium supplemented with 0.2 mM EDTA, which blocks autoxidation of excreted thiols. After addition of 0.1 mM l-cystine, aliquots were periodically removed using a 5-ml syringe and filtered into 1.5-ml plastic tubes. Filtrate (0.5 ml) was immediately mixed with 0.5 ml of 0.3 mM 5,5′-dithio-bis-(2-nitrobenzoic acid) (DTNB) solution in sulfate medium. The reaction between thiol compounds and DTNB releases 2-nitro-5-thiobenzoate, whose concentration was determined at 412 nm using an extinction coefficient of 13 mM−1 cm−1. In cases where cells were pregrown in cystine-containing medium, 0.1 mg/ml chloramphenicol was added prior to centrifugation and included in the resuspension medium, to avoid induction of ydjN.
Liquid chromatography-mass spectrometry (LC-MS) analysis.
To identify the organic thiol compound that cystine-fed cells excrete, wild-type cells (MG1655) were grown and treated as described above, with resuspension in fresh sulfate medium containing EDTA. Samples were taken immediately before and 10 min after the addition of l-cystine. After filtration, sulfosalicylic acid (0.5% final concentration) was added to filtrates to minimize thiol oxidation. Filtrates were frozen on dry ice and sent for analysis within 1 h in the Joy Carver Metabolomic Center. Calibration standards with cysteine, glutathione, and homocysteine were prepared similarly.
Intracellular cysteine accumulation was measured in log-phase MG1655 (wild type) and KCI1266 (ΔydjN ΔfliY) in sulfate medium. Cells were centrifuged and resuspended in fresh medium at an OD600 of 0.4 (37°C). Cystine (20 μM) was added; this low concentration ensured that little extracellular cystine complicated the analysis. After 1 min, the cell suspension was mixed with an equal volume of 60% methanol-water that had been prechilled to −50°C. An 0.5-ml aliquot of the resulting mixture was filtered through a nitrocellulose membrane (220-nm pore size). Membranes were rinsed twice with 5 ml of prechilled (10 to 12°C) 30% methanol. The membranes were then placed facedown on 0.05 ml of 10% sulfosalicylic acid (4) on an ice-cold plastic petri dish. After 10 min, the liquid was collected by pipette and centrifuged at 12,000 × g for 10 min at 4°C. The resulting supernatant was frozen at −80°C, and organic thiols were quantified by LC-MS at the Metabolomic Center. Intracellular concentrations were calculated using the relation that 1 ml of cell suspension with an OD600 of 1 contains 0.5 μl of total cytoplasmic volume (22).
Reduction of cystine by GSH.
The rate at which glutathione (GSH) directly reduces cystine was determined by coupling this reaction to the subsequent reduction of oxidized glutathione (GSSG) by glutathione reductase. NADPH oxidation was monitored at 340 nm. The 1-ml reaction mixture included 50 mM KPi (pH 7.6), 100 μM EDTA, 200 μM NADPH, 2.3 units of glutathione reductase (Sigma), and 1 mM GSH at 37°C. The reaction was initiated by the addition of 100 μM cystine. Control experiments demonstrated that under these conditions the reduction of cystine is rate limiting for NADPH oxidation. By varying reactant concentration, we determined that the reaction was first order in both cystine and glutathione, and we measured the rate constant to be 1.8 M−1 s−1. The rate of intracellular cystine reduction (in units of molarity per second) can be calculated by multiplying this value by the cytoplasmic concentrations of those compounds.
The rate was also measured in the presence of cell extracts, in order to appraise the ability of cell proteins to catalyze cystine reduction. Extracts were prepared from strains grown to an OD600 of 0.2 in sulfate medium. Cultures (200 ml) were washed and suspended in 2 ml cold KPi buffer, pH 7.6, containing 200 μM EDTA, prior to lysis in a French press. Cell debris was removed by centrifugation for 20 min at top speed in a 4°C microcentrifuge. Protein concentrations were determined by Bradford assay (see above). These extracts were added to the cystine-reduction mixture described above, and NADPH oxidation was monitored. The extracts evinced insignificant NADPH oxidase activity and insignificant NADPH:cystine oxidoreductase activity in the absence of GSH.
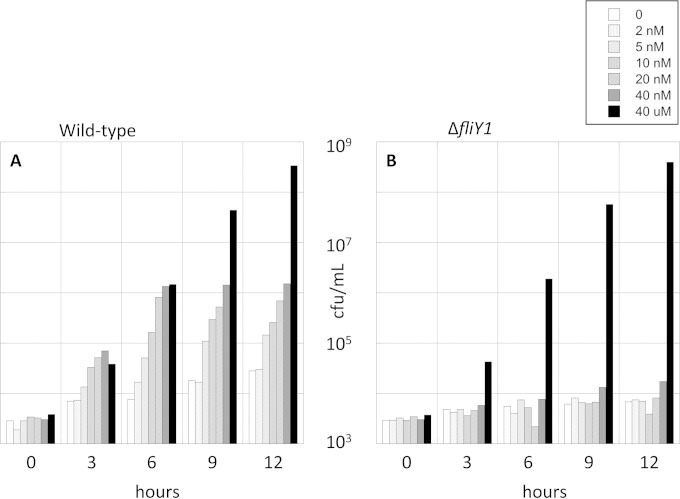
Growth phenotypes with very low cystine.
Wild-type and fliY mutant strains marked with distinctive antibiotic resistance markers were separately precultured to an OD600 of 0.10 in sulfur-free medium that was supplemented with 50 μM cystine. Cells were centrifuged, washed three times with 6 ml sulfur-free medium lacking cystine (see below), and resuspended in the same medium. The two strains were then mixed in equal amounts and diluted to a total calculated OD600 of 0.00001 (∼3 × 103 CFU/ml) in prewarmed sulfur-free medium. Defined amounts of cystine were added, and the growth of each strain was tracked by plating at intervals on selective antibiotic plates.
Because the salt components of the medium contain trace levels of other sulfur compounds, the strains carried ΔcysA ΔcysJIH mutations that prevented the assimilation of inorganic sulfur. Any cystine that contaminated the amino acid mix was removed by preculturing a ΔcysA ΔcysJIH strain in the medium prior to the sterile filtration and inoculation of the competing strains.
Analysis of amino acid content.
The amino acid compositions of proteins, the membership of the CysB regulon, and the Km values for amino acid transporters were obtained either directly from the EcoCyc web site (ecocyc.org) or from articles referenced there. The set of genes whose regulation by CysB has been experimentally demonstrated includes ssuEADCB, cbl, tauABCD, cysK, cysPUWAM, cysDNC, and cysJIH. The amino acid importers (and their substrates) that were considered in evaluating amino acid compositions were ArtIMQP(Arg), ArtJMQP(Arg), GlnQPH(Gln), GltIKJL(Glu, Asp), ArgT/HisQPM(Lys, Arg, His), HisJQPM(His), LivJFGHM(Ile, Leu, Val), LivKFGHM(Leu), MetINQ(Met), ProVWX(Pro), TdcC(Thr, Ser), SdaC(Ser), PutP(Pro), DctA(Asp), GltP(Glu, Asp), SstT(Ser, Thr), MmuP(Met), ArcD(Arg), AroP(Trp, Phe, Tyr), CadB(Lys), GadC(Glu), AsnP(Asn), LysP(Lys), PheP(Phe), ProY(Pro, His), CycA(Ser, Ala, Thr), DcuA(Asp), GltS(Glu), Mtr(Trp), TnaB(Trp), TyrP(Tyr), BrnQ(Ile, Leu, Val), and ProP(Pro, Gly). The amino acid content of the total cellular proteome was obtained from reference 23.
Distribution of ydjN and fliY-yecSC among the bacterial kingdom.
To examine the biotic distribution of these transporters, the protein sequences of YdjN and FliY were employed as queries by the BLAST search software. An identity of 30% was used as a cutoff value. FliY homologues were further filtered according to cysteine content, with the dismissal of those possessing more than two cysteine residues, while YdjN homologues were dismissed if they lacked the critical cysteine residue (C346 in E. coli). These criteria admittedly provide approximate results, since periplasmic binding proteins and secondary transporters may exhibit significant similarity to FliY and YdjN without employing cystine as a substrate. Nevertheless, these searches correctly recovered the YhcL (also known as TcyP) and YtmJKLMN (also known as TcyJKLMN) cystine transporters of Bacillus subtilis (24) as homologues of YdjN and FliY-YecSC, respectively, despite the vast evolutionary distance between these Gram-positive and Gram-negative bacteria. A few phyla lacked recognizable homologues; for example, YdjN homologues were not detected among the Bacteroidetes or Cyanobacteria. However, such an omission may be due to the limited number of genomes sequenced among those phyla. To examine this possibility, the number of positive hits per bacterial phylum was compared to the number of genomes sequenced according to the JGI-IMG database (img.jgi.doe.gov) as of February 2015. This relationship was approximately linear for both transporters, which precluded any conclusion that either transporter is systematically absent from any bacterial phylum.
Impact of futile cycling upon energy consumption.
Calculations based on cell pathways indicate that glucose-grown E. coli growing in unsupplemented medium must expend 18.5 mmol ATP equivalents in order to synthesize the amino acids, nucleotides, and lipids that are incorporated in 1 g cells. (Such cells are used for comparison, since in the present study the cells were supplemented with 18 amino acids. These and other values are obtained from the work of Neidhardt et al. [25].) A similar amount of NADPH is consumed (18.2 mmol). E. coli contains 230 μmol sulfur atoms, primarily as protein methionine and cysteine residues. This much sulfur would be provided by the import of 115 μmol cystine through YdjN at the expense of 115 μmol proton equivalents, or 38 μmol ATP. This number represents 0.2% of the energy that a cell growing in unsupplemented medium will expend for all biosynthesis. A 70-fold excess of cystine import will cost 2.7 mmol ATP, and this will be tripled if the cysteine is exported at a cost of 1 proton per cysteine. Thus, the maximum rate of futile import/export cycles that were observed in this study will require the equivalent of 10 to 30% of the biosynthetic ATP demand of unsupplemented cells. The NADPH that is used in cystine reduction (230 μmol × 70 = 16 mmol) would nearly double the cellular NADPH consumption of such cells.
RESULTS
Identification of the two cystine importers in E. coli.
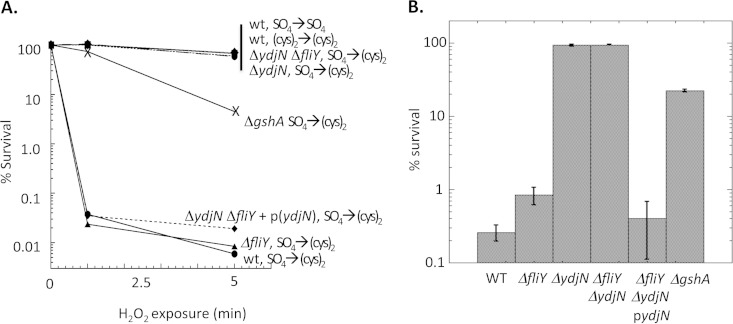
When E. coli is grown with sulfate as the sole sulfur source, the addition of cystine creates a transient hypersensitivity to exogenous H2O2 (Fig. 1). Death is due to DNA damage arising from the Fenton reaction (4). Cysteine can amplify the Fenton reaction by efficiently recycling ferric iron back to the ferrous form after each round of oxidation by H2O2. It was proposed that the period of sulfate growth triggers the induction of a cystine importer, which then brings much cystine into the cell when cystine is added. Reductive processes reduce the cystine to cysteine.
FIG 1.
The addition of cystine immediately sensitizes sulfate-grown cells to H2O2. Exponentially growing E. coli strains were cultured for 5 generations in medium either containing sulfate alone or supplemented with cystine. They were then diluted into the same medium with or without cystine addition for 3 min, and then 2.5 mM H2O2 was added. At the indicated time points, the H2O2 exposure was terminated by the addition of catalase, and cell viability was determined by plating on LB medium (A) or defined medium (B). (A) Representative time courses. (B) Survival at 5-min time point, with standard deviations from three independent experiments. WT, wild type, AB1157; ΔgshA, JTG10; ΔydjN, KCI1137; ΔfliY, KCI1142; ΔydjN ΔfliY, KCI1160; ΔydjN ΔfliY + p(ydjN), KCI1281.
To test this hypothesis, a Tn5 mutant library was generated and screened for isolates that could survive the cystine-H2O2 exposure (see Materials and Methods). Eight strain pools, each containing ∼1,000 independent Tn5 mutants, were cultured in sulfate-based medium and challenged by two rounds of the addition of 0.5 mM cystine and 2.5 mM H2O2. Typical survival frequencies were ∼0.1% per round. Several survivors were screened in follow-up experiments, and one of these isolates exhibited >50% survival after the cystine-H2O2 protocol. Transduction of its Tn5 insertion into a wild-type strain converted it to similar cystine-H2O2 resistance. Sequence analysis revealed that the Tn5 was inserted into the gene ydjN. A precise ydjN insertion-deletion mutant was then constructed independently by the lambda red recombinase method. This mutant exhibited the same resistance phenotype, and complementation by a plasmid copy of ydjN restored sensitivity (Fig. 1). In the absence of cystine, a recA56 ΔydjN double mutant exhibited the same hypersensitivity to H2O2 as did a recA56 single mutant (data not shown), indicating that the mutation specifically affected the impact of cystine upon DNA damage rather than resistance to oxidative DNA damage in general.
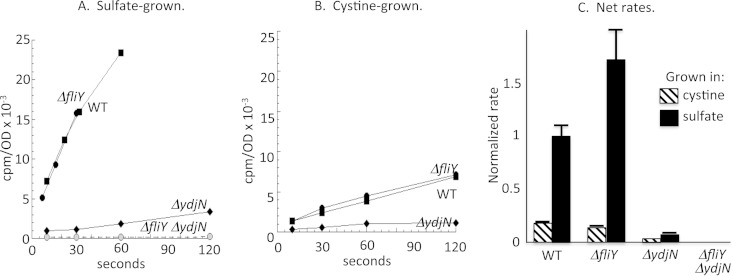
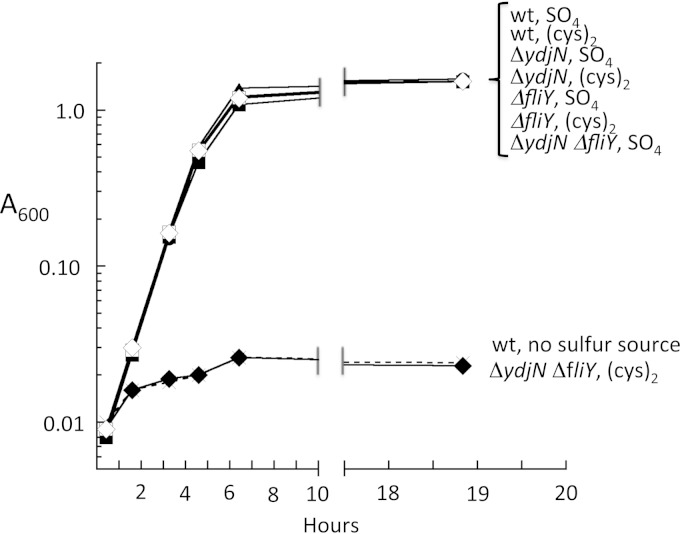
The ydjN ORF resembles members of the family of H+/Na+-dependent symport proteins, such as PutP, PanF, and GltP. These transporters are composed of a single protein, and indeed the ydjN phenotype was not replicated by knockout of ydjM, the nearest gene (KCI1363) (data not shown). The rate of [14C]cystine import into the ΔydjN mutant was reduced by 90% relative to that of wild-type cells, confirming that ydjN is the predominant cystine transporter under these growth conditions (Fig. 2). However, the ΔydjN mutant cells were still able to grow at wild-type rates in medium where cystine was the sole sulfur source. These results suggested that under these conditions E. coli expresses at least one other adequate cystine importer. The FliY periplasmic protein binds cystine and had been proposed to be the periplasmic binding component of an import system (26, 27). Indeed, a ΔfliY ΔydjN double mutant was unable to grow at all using cystine (Fig. 3) and was unable to import detectable [14C]cystine (Fig. 2). The growth phenotype was fully complemented by either ydjN or fliY genes expressed from plasmids (KCI1280 and KCI1310) (data not shown). Although biochemical analysis suggested that the related bacterium Salmonella enterica serovar Typhimurium might contain three cystine-import systems (28), we have found no evidence for a third transporter in E. coli under any growth condition.
FIG 2.
Cystine is imported by the FliY-YecSC and YdjN systems. Cultures were grown in sulfate or cystine medium. Chloramphenicol was added at 37°C for 5 min. [14C]cystine cocktail was then added, and intracellular radioactivity was measured at the indicated time points. The OD600 of cells in the reaction mixtures was 0.2. (A and B) Representative time courses. (C) Import rates (counts per minute per OD unit per second) normalized to the rate for sulfate-grown wild-type cells, with error bars (standard errors of the means) from three independent experiments. WT, wild type, AB1157; ΔydjN, KCI1137; ΔfliY, KCI1142; ΔydjN ΔfliY, KCI1160.
FIG 3.
E. coli requires either YdjN or FliY-YecSC to utilize cystine. Strains were inoculated into sulfur-free medium that was supplemented with sulfate, cystine, or no sulfur compound. Growth was monitored by optical density. The data are representative of more than three independent experiments. Open symbols, sulfate provided; closed symbols, cystine provided; ×, wild-type strain with no sulfur source. Circles, wild-type (wt) strain (AB1157); triangles, ΔfliY strain (KCI1142); squares, ΔydjN strain (KCI1133); diamonds, ΔfliY ΔydjN strain (KCI1160).
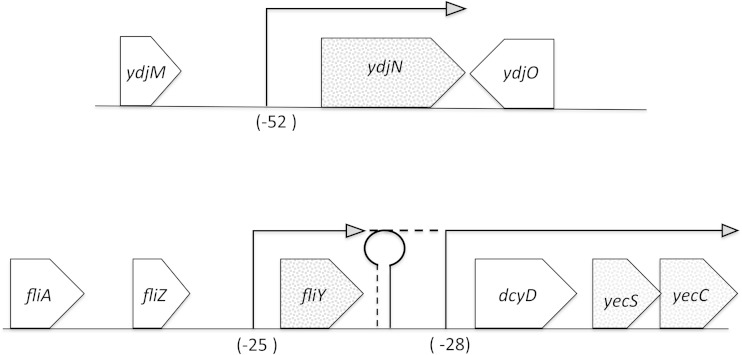
The gene neighborhoods of the ydjN and fliY structural genes are depicted in Fig. 4. The fliY gene owes its name to its position downstream of flagellar structural genes, but inspection suggested that YecS and YecC were likely to be the ATPase and membrane-spanning partners of FliY in an ATP-binding cassette (ABC) transport system. The proximity of fliY to yecS and yecC is broadly conserved among bacteria, whereas proximity to fliA, fliZ, or dcyD is not. Indeed, a ΔydjN ΔdcyD double mutant (KCI1304) grew at wild-type rates in cystine medium, indicating that the FliY system retained function, whereas a ΔydjN ΔyecS mutant (KCI1302) was unable to grow at all (data not shown). We conclude that the YdjN cation symporter and the FliY/YecSC ABC-type transporter comprise the only two cystine import systems in E. coli. As this paper was being prepared, a similar conclusion was reported by Deutch et al. (29), although they reported slow residual cystine import in fliY ydjN mutants. A study in Bacillus subtilis indicated roles for an ABC transporter and a secondary transporter, which show ∼30% homology to E. coli FliY-YecSC and YdjN, respectively (24). That study also identified a second ABC system that was not saturable by cystine and whose synthesis did not respond to sulfur limitation; we infer that its transport activity with cystine was probably adventitious. We conclude that to date only FliY-YecSC and YdjN homologues have been demonstrated to be dedicated cystine importers in bacteria, and in E. coli, these transporters are the only ones capable of using cystine to satisfy its sulfur demand.
FIG 4.
Genomic neighborhoods of genes encoding the cystine importers YdjN and FliY-YecSC. 5′ RACE was used to identify transcriptional start sites for ydjN, fliY, dcyD, and yecS in a wild-type strain (MG1655) grown in sulfate medium. Precise start sites are indicated in Fig. S1 in the supplemental material. Northern analyses indicate that ydjN and fliY transcripts are predominantly monocistronic. No end was found within 500 bases upstream of yecS; hence, transcription most likely is from the dcyD promoter. RT-PCR results suggest that a small amount of read-through occurs from fliY to the dcyD-yecS-yecC operon (see the text).
Regulation of ydjN transcription.
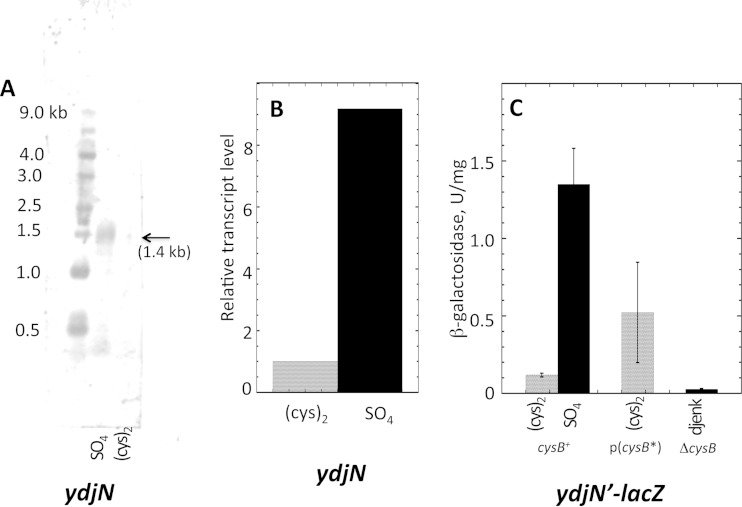
YdjN was responsible for the rapid cystine import and H2O2 sensitivity of E. coli that had been pregrown in sulfate medium (Fig. 1), but the rapid transport and H2O2 sensitivity were absent if cells had been pregrown in cystine medium. That pattern suggested that YdjN might be strongly induced by the CysB regulatory protein (1), which is activated whenever E. coli grows on a relatively poor sulfur source, including sulfate. Northern blot analysis demonstrated that ydjN is expressed as a monocistronic transcript, consistent with the idea that it is a single-protein transporter (Fig. 5A). Transcript analysis by RT-PCR confirmed that ydjN is highly expressed in sulfate medium and repressed in cystine medium (Fig. 5B). A 5′ RACE experiment identified a transcriptional start site 52 nucleotides upstream of the initiating codon (Fig. 4; see also Fig. S1 in the supplemental material). A potential CysB binding site lies 100 bp upstream of a likely promoter. The 200 bp upstream of the translational start site were fused to a lacZ structural sequence, and 11-fold-higher expression of the gene was observed when cells were grown with the poor sulfur sources sulfate and djenkolate than when they were grown with cystine (Fig. 5C). This induction required the presence of CysB protein, and it could be achieved even in cystine medium if the cells expressed a constitutively active form of CysB (also known as CysB*) from a plasmid (Fig. 5C). Thus, YdjN transcription is controlled by CysB. No other growth condition was observed to influence ydjN expression.
FIG 5.
Regulation of ydjN transcription. (A) Northern blot prepared from the RNA of wild-type (MG1655) cells reveals a single 1.4-kb transcript that is visible from sulfate- but not cystine-grown cells. (B) Relative transcript levels in MG1655 analyzed by RT-PCR. (C) Expression of a ydjN′-lacZ transcriptional fusion that was incorporated at the lambda attachment site. The native ydjN allele remained intact. Strains contained either the wild-type cysB+ allele (KCI1230), a constitutively active cysB* allele (32) expressed from a plasmid (KCI1232), or a ΔcysB null allele (KCI1236). Medium contained cystine, sulfate, or djenkolate. (Djenkolate activates the CysB regulon but, unlike sulfate, supports growth of cysB null mutants.)
Regulation of fliY-dcyD-yecS-yecC transcription.
The components of the FliY-YecSC system are transcriptionally regulated in distinct ways. The fliY gene is separated from yecS and yecC by dcyD, which encodes a d-cysteine desulfurase (30). RT-PCR analysis indicated that at least some transcripts bridge the entire region, as positive results were obtained for primer pairs of fliA-fliZ, fliZ-fliY, fliY-dcyD, and dcyD-yecC (data not shown). Experiments in Salmonella suggested that fliA transcription can extend into downstream genes (31). However, 5′ RACE demonstrated that the predominant transcriptional start site for fliY lies immediately upstream of fliY itself (Fig. 4; see also Fig. S1 in the supplemental material), and Northern analysis showed a single monocistronic transcript (Fig. 6A). A transcriptional start was also identified upstream of dcyD (see Fig. S1), and 5′ RACE failed to detect any start site for yecS transcripts within 500 bp of the gene, which suggested that it was cotranscribed with dcyD. Northern analysis did not resolve this point, as neither dcyD nor yecS transcripts were detected. However, this interpretation was supported by the observation that 200-bp DNA sequences 5′ to both fliY (KCI1330) and dcyD (KCI1326) enabled transcription of lacZ fusions inserted into the lambda attachment site, whereas sequences 5′ to either yecS or yecC did not (KCI1322 and KCI1324) (data not shown).
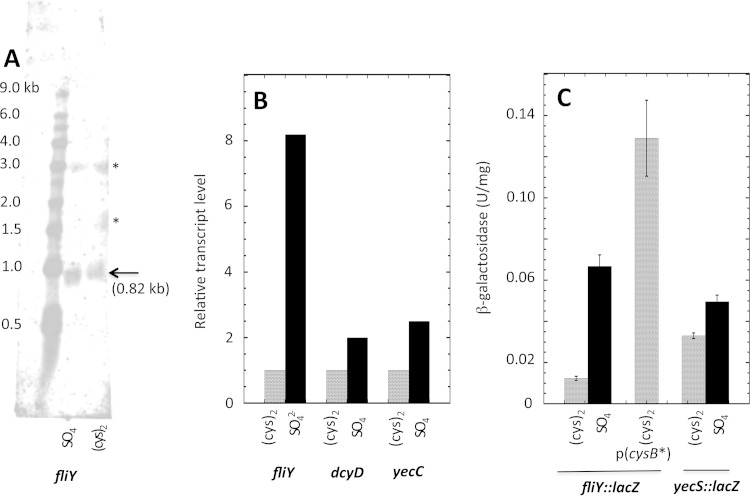
FIG 6.
Regulation of fliY-dcyD-yecSC transcription. (A) Northern blot of fliY with mRNA prepared from wild-type (MG1655) cells reveals a single 0.8-kb transcript. (The asterisks mark nonspecific binding to rRNA.) (B) Relative transcript levels analyzed by RT-PCR. Yields are normalized to the same transcript in cystine medium. (C) Expression of fliY::lacZ and yecS::lacZ transcriptional fusions in their native loci. Strains were KCI1450, KCI1470, and KCI1452.
Northern, qRT-PCR, and lacZ fusion analysis all showed 3- to 10-fold induction of fliY during growth in sulfate medium compared to cystine medium (Fig. 6). This pattern matched that of ydjN and suggested that the transcription of fliY is similarly induced by activated CysB protein. Indeed, it was upregulated even in cystine medium when the strain was transformed with a CysB*-encoding plasmid which constitutively induces the CysB regulon (32) (Fig. 6C). Conversely, the induction of the fliY::lacZ transcriptional fusion was lost when cysB was deleted (see Fig. S2 in the supplemental material).
In contrast, when a dcyD::lacZ transcriptional fusion was inserted into the lambda attachment site—so that the fliY promoter did not lie upstream—its expression was unaffected by sulfur source. (β-Galactosidase activities were 0.19 ± 0.02 unit/mg in both sulfate and cystine media.) Thus, the dcyD promoter, which evidently also drives yecSC transcription, is not regulated by CysB. However, when a yecS::lacZ transcriptional fusion was studied in its native locus downstream of fliY, modest but reproducible induction did occur in sulfate medium (Fig. 6C). We suspected that this result reflected a small amount of read-through from the fliY promoter. To test this, the fliY promoter region was deleted in this fusion strain. There was little overall effect upon yecS::lacZ expression, although what remained seemed insensitive to sulfur source (see Fig. S3 in the supplemental material). These data confirm that little yecSC transcription is due to read-through from fliY, with the majority arising from the dcyD promoter.
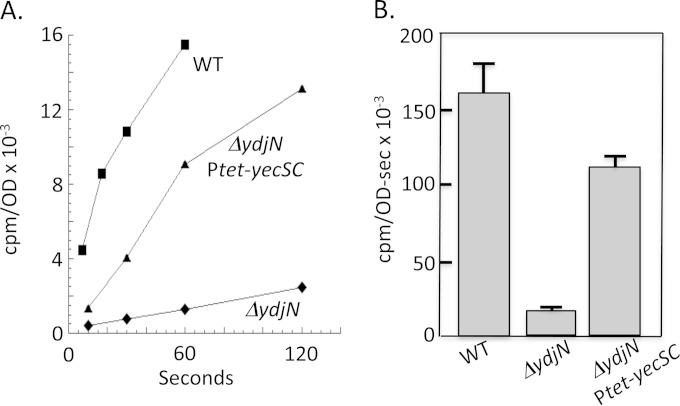
Interestingly, the net effect is that when cells become sulfur deficient, the titers of the FliY periplasmic binding protein will rise substantially, but the titers of the integral membrane components of the transport system will increase very little. This arrangement was compared to the effect of sulfur limitation upon [14C]cystine import in transport assays. Strikingly, FliY-YecSC-mediated cystine import was no faster for cells grown in sulfate than for cells grown in cystine medium, despite induction of the FliY periplasmic protein in the sulfate medium (Fig. 2C). This outcome suggests that when cystine levels are high, as in our assays, FliY is likely to be fully loaded, and the rate-limiting step is passage through the YecSC membrane complex. Since YecSC proteins are not induced in sulfate medium, no increase in transport occurs. This hypothesis was tested by using a Tet-driven promoter to elevate the level of YecSC. Induction of yecSC increased import by ∼5-fold (Fig. 7). Thus, when cystine is provided in saturating levels, the rate-limiting step in transport is movement across the membrane. We note, however, that CysB control is operative when cystine is scarce. Under those conditions, cystine binding by the periplasmic FliY protein is probably the rate-limiting step of the transport process, and so it makes sense that CysB regulates the promoter that drives fliY transcription but not that which drives yecSC transcription.
FIG 7.
The rate-limiting step for import of micromolar cystine by FliY-YecSC is passage through the YecSC complex. Uptake of [14C]cystine was measured for wild-type (WT; AB1157), ΔydjN (KCI1137), and ΔydjN Ptet-yecSC (KCI1392) cells after growth in sulfate medium. Although fliY transcription is induced ∼7-fold when cells are grown in sulfate medium, the rate of cystine import remains as low as in cystine-grown cells unless yecSC transcription is elevated using a Tet-driven promoter (3 μg/ml tetracycline). (A) Representative time course. (B) Import rates (counts per minute per OD unit per second) from three independent experiments, with error bars (standard errors of the means).
A survey of other ABC-type systems in E. coli suggests that it is unique to have the periplasmic binding protein induced independently of the membrane transport proteins when cells are in need of the substrate. Binding proteins are normally encoded in the same operon as their membrane-bound partners. An anonymous reviewer noted that the binding-protein structural gene is often slightly separated on the transcript from genes encoding the integral membrane and ATPase partners, which lie flush with one another; this arrangement results in common transcriptional control but conceivably allows discrete levels of translation. Ribosomal profiles show that binding proteins are typically more abundant than their partners (33).
It is not uncommon for cells to use two amino acid transporters for a single substrate; however, in a typical case one transporter is induced when the substrate amino acid is scarce so that it can be acquired for protein synthesis, while the other transporter is induced when the substrate amino acid is abundant, so that it can be degraded as a carbon source. Lock-step induction of both YdjN and FliY-YecSC during sulfur restriction surprised us, and so we looked for further evidence to confirm that sulfur acquisition is their physiological role.
The cystine transport systems contain virtually no cysteine residues.
The urgency of cysteine acquisition is underscored by the markedly low cysteine content of the proteins that import sulfur compounds and convert them to cysteine (see Fig. S4 in the supplemental material). To date, 22 such enzymes have been determined to be members of the CysB regulon. Cysteine composes only 0.2% of their amino acid residues, compared to 1.7% in the overall E. coli proteome (see Materials and Methods). Further, many of the few exceptional cysteine residues are known to be functionally irreplaceable, such as those that coordinate the iron-sulfur cluster of sulfite reductase and that which channels electrons to the active site of phosphoadenylyl sulfate (PAPS) reductase. The evolutionary selection against cysteine residues in CysB-controlled proteins presumably arises from the requirement that they be efficiently translated when cysteine pools shrink.
Inspection of YdjN and FliY-YecSC shows that they, too, follow this pattern: only one cysteine residue (0.08%) is found among the 1,201 total residues that comprise these four proteins. Twenty cysteine residues would be expected if the proteins conformed to standard cysteine content of the cellular proteome. The exceptional cysteine residue lies on a YdjN strand immediately outside the cytoplasmic membrane (34), and its mutation to either threonine or serine completely ablated activity, indicating that it plays a key functional role (data not shown). We considered the possibility that cysteine residues are scarce in all transport proteins, since their exposure to the oxidizing environment of the periplasm might be problematic. However, analysis of the 33 known importers of other amino acids into E. coli revealed that although their cysteine content was only ∼46% of that of the general proteome, it was still 10-fold higher than that for the YdjN and FliY-YecSC proteins. Furthermore, YhaO—which imports cysteine as a catabolic substrate under anoxic conditions (35)—provides an instructive contrary example; it is induced under cysteine-rich conditions and contains five cysteine residues (1.2% of total). Thus, the low cysteine content of YdjN and FliY-YecSC is consonant with a primary role of replenishing cysteine pools in sulfur-starved cells, rather than supplying cysteine as a carbon or energy source.
This compositional bias is exclusive to cystine importers. Other amino acid importers are not low in their content of the amino acid that they transport: their content of the substrate amino acid averages 4.8%, essentially equivalent to the 5% that would be expected from a random allotment. The greatest underrepresentation among all 33 transporters was for CadB, whose lysine content was only 2.4-fold lower than that for the whole proteome. Thus, the 20-fold underrepresentation of cysteine among the cystine transport proteins is exceptional. The reason is presumably that other amino acids can be synthesized endogenously. Since the element sulfur cannot be synthesized, this option does not apply to cysteine when sulfur compounds are limiting.
E. coli does not effectively catabolize cystine.
Thus, YdjN and FliY-YecSC have amino acid compositions that are congruent with roles in minimal sulfur provision but not cystine catabolism. Indeed, under tested conditions E. coli was unable to grow when cystine was provided as the sole carbon source (see Fig. S5 in the supplemental material). Cells initially exhibited an ability to grow slowly when cystine was provided as a nitrogen source, but that slight advantage faded within two generations, perhaps due to the repression of ydjN transcription and dilution of extant YdjN protein. We infer that E. coli is not configured to use cystine for these purposes. This result conforms with the diagnostic distinction that Salmonella but not E. coli releases copious sulfide when it is incubated with cysteine or cystine (36). Sulfide is a product of catabolic cysteine degradation.
The distinct roles of YdjN and FliY-YecSC: substrate affinities and specificities.
The preceding studies demonstrated that E. coli possesses two independent cystine import systems that are both activated by CysB when intracellular cysteine levels fall. A survey of other bacteria indicates that it is not unusual for microbes to maintain both transporters; this arrangement prevails among Enterobacteriaceae and is also found among Firmicutes, including Bacillus subtilis. We infer that these two transport systems must each provide a particular benefit.
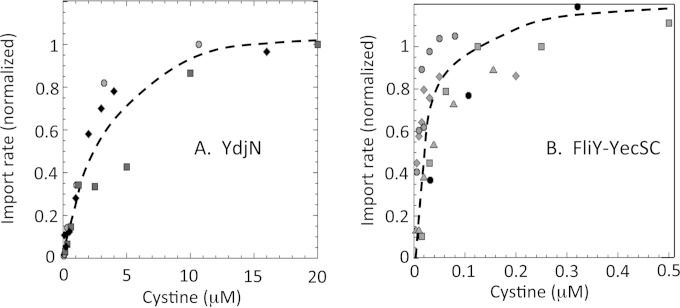
Apparent Km values for each transporter were determined with whole-cell transport assays. (A significant caveat is that simultaneous cysteine efflux from the cell, discussed below, diminished radioactivity accumulation; these Km values might differ from those of purified enzyme.) The half-maximal rate of import by YdjN occurred at 2 μM cystine (Fig. 8A). In contrast, a half-maximal value of ∼30 nM was observed for FliY-YecSC—100 times lower than that of YdjN (Fig. 8B). ABC-type transporters commonly have higher binding affinities than do H+/Na+-driven substrate symporters; our survey of the known E. coli ABC amino acid importers (see Materials and Methods) revealed that they almost invariably exhibit apparent Km values of <1 μM, whereas ion-driven secondary transporters range up to 10 μM. The saturation curves suggested that YdjN might be the primary transporter at higher concentrations of cystine. Consistent with this idea, the deletion of ydjN triggered induction of the CysB regulon even in cystine-containing (0.5 mM) medium, indicating that in these ΔydjN mutants the cystine import was aberrantly slow; under the same condition, the CysB regulon was not activated in either fliY mutants or wild-type cells (see Fig. S6 in the supplemental material). Thus, YdjN is indeed the primary transporter when substantial levels of cystine are provided.
FIG 8.
Transport kinetics of the FliY-YecSC and YdjN systems. (A) Transport by YdjN. KCI1142 (ΔfliY) was suspended to an OD600 of 0.5 in fresh sulfate medium and incubated with chloramphenicol for 5 min at RT. [14C]cystine was added, and at certain time points, samples were removed for counting. Data points represent initial linear rates; distinct symbols represent data from three independent experiments. Half-maximum transport occurred at ∼2 μM cystine. (B) Transport by FliY-YecSC. KCI1137 (ΔydjN) was suspended to an OD600 of 0.2 in fresh sulfate medium and incubated with chloramphenicol for 5 min at 37°C. Prewarmed [14C]cystine was added, and at certain time points samples were removed for counting. Data points represent initial linear rates; distinct symbols represent data from four independent experiments. Half-maximum transport occurred at ∼30 nM cystine.
Conversely, the transport data predict that FliY-YecSC may provide a growth advantage when cystine is scarce. To test this idea, it was necessary to create medium in which cystine could be provided at very low concentrations, yet without its being quickly exhausted in the course of the experiment. These conditions were met by studying the competition between a 50:50 mixture of fliY+ ydjN+ and ΔfliY ydjN+ strains during outgrowth at very low cell densities (∼103/ml). Additional steps were taken to avoid any interference by contaminating sulfur compounds (see Materials and Methods). The mixed culture was then supplemented with low-nanomolar cystine concentrations, and cell growth was tracked by plating the competing strains on discriminatory selective medium. The outcome was that the FliY-YecSC system provided a strong growth advantage at these low cystine concentrations (Fig. 9). The strains competed equally when cystine was provided at a concentration (40 μM) that saturated both transporters. These results fit very well with the measured apparent Km values, and they comprise a clear demonstration of the benefit of a very-high-affinity transporter.
FIG 9.
The FliY-YecSC system is essential for competitive growth at nanomolar cystine concentrations. Wild-type (KCI826) and ΔfliY (KCI1726) strains were mixed at equivalent titers and inoculated at very low cell density into sulfur-free medium supplemented with 0 to 40 nM or 40 μM cystine. Cell outgrowth was monitored by colony formation. The wild-type strain grew well (A), but the fliY mutant was able to grow only when 40 μM cystine was provided (B). The data are representative of three independent experiments.
The sulfur-free compound diaminopimelate (DAP) is a structural analogue of cystine that has been suggested to be an additional substrate of cystine-import systems. DAP is an essential component of the cell wall, and dap biosynthetic mutants require supplementation. We reproduced other workers' observation that DAP-supplemented ΔdapA mutants grow well in sulfate medium but struggle to grow when cystine is added, supporting the idea that cystine and DAP compete for transport (26). Transductional analysis demonstrated that ΔdapA ΔfliY double mutants are inviable even when DAP is provided (Table 1), confirming that DAP uptake into E. coli is mediated exclusively by the FliY-YecSC system.
We considered the possibility that the FliY-YecSC system is optimized for DAP rather than cystine import. However, transport measurements revealed that a 10-fold excess of DAP is needed to inhibit by 50% the import of cystine by the FliY-YecSC transporter, which suggests that the transporter binds cystine much more effectively than DAP. Further, the FliY-YecSC system does not respond to DAP shortages: the fliY::lacZ fusion was not induced when DAP was withdrawn from dapA mutants (KCI1540) (data not shown). DAP import by the FliY-YecSC system appears to be adventitious rather than an evolutionarily honed phenomenon.
The fact that FliY-YecSC can transport DAP while YdjN cannot also suggests fundamental differences in the way that these two transporters recognize cystine. Even a 100-fold excess of DAP did not have any effect on the rate of cystine import by YdjN (data not shown). Other cystine analogues were also examined (Table 2; see structures in Fig. S7 in the supplemental material). The FliY-YecSC system enabled cells to grow using djenkolate, lanthionine, and d-cystine as sulfur sources and homocystine as a methionine precursor. In contrast, YdjN was active only with homocystine and d-cystine (Table 2), and it was not inhibited by the other compounds. Selenocystine competitively blocked cystine import by YdjN. This pattern suggests the possibility that YdjN operates only with compounds containing disulfide bonds that can be reductively cleaved (below).
TABLE 2.
Growth of the wild type and cystine import mutants on various sulfur sourcesa
| Sole sulfur source | Growth type in strain |
|||||||
|---|---|---|---|---|---|---|---|---|
| Aerobic growth |
Anaerobic growth |
|||||||
| Wild type | ΔfliY mutant | ΔydjN mutant | ΔfliY ΔydjN mutant | Wild type | ΔfliY mutant | ΔydjN mutant | ΔfliY ΔydjN mutant | |
| Sulfate | + | + | + | + | + | + | + | + |
| d-Cystine | + | + | + | − | ++ | ++ | ++ | − |
| l-Cystine | ++ | ++ | ++ | − | ++ | ++ | ++ | − |
| d-Cysteine | + | + | + | − | + | + | + | + |
| l-Cysteine | + | + | + | − | ++ | ++ | ++ | ++ |
| Djenkolate | + | − | + | − | ND | ND | ND | ND |
| Lanthionine | + | − | + | − | ND | ND | ND | ND |
| Glutathione, reduced | + | + | + | + | + | + | + | + |
| Glutathione, oxidized | − | − | − | − | − | − | − | − |
| l-Homocystine | − | − | − | − | − | − | − | − |
| l-Methionine | − | − | − | − | − | − | − | − |
| Spent medium | − | − | − | − | − | − | − | − |
Growth was tested in sulfur-free medium under aerobic and anaerobic conditions. ++, doubling time of <50 min; +, doubling time of >50 min; −, no growth in 1 day; ND, not determined. The wild type and ΔfliY and ΔydjN single mutants could employ 15 mM l-homocystine as a methionine precursor, but ΔfliY ΔydjN double mutants could not.
Extreme overimport of cystine by YdjN.
When sulfate-grown cells encounter cystine, the rate of cystine import by YdjN far exceeds the rate of import by FliY-YecSC, even though the latter is sufficient to support wild-type growth rates (Fig. 2A and 3). From measuring the yield of biomass under conditions of limiting cystine, we calculate that E. coli uses its internal cysteine pool at a rate of 2.2 mM/min (see Materials and Methods).
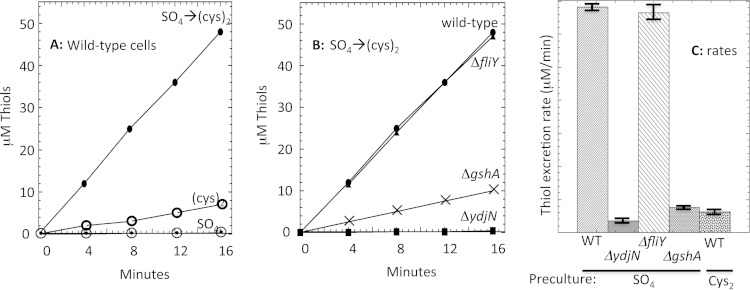
In striking contrast, when cystine was abruptly added to sulfate-grown cells, the rate of cystine import was substantially higher. This excessive import reflected the prior induction of YdjN and persisted for long periods, gradually subsiding only as YdjN titers declined via dilution as cells grew and divided (see Fig. S8 in the supplemental material). Storage of such immense levels of cystine inside the cell was implausible, so we sought to learn its fate. Measurements demonstrated that these cells continuously excreted large quantities of a thiol compound (Fig. 10A); mass spectrometry identified the molecule as cysteine. The rate of cysteine efflux, normalized to cytoplasmic volume, was 70 mM/min. Thus, in this circumstance the rate of cystine import by YdjN exceeded the cellular sulfur demand by 30-fold.
FIG 10.
Cystine import through YdjN drives rapid thiol excretion. (A) Thiol release was measured from sulfate-grown wild-type cells (dotted circles), cystine-grown cells (open circles), and sulfate-grown cells immediately after the addition of 0.1 mM cystine (filled circles). (B) Thiol release was measured upon the addition of 0.1 mM cystine to sulfate-grown cells. Strains were wild type (MG1655; circles), ΔfliY mutant (KCI1252; triangles), ΔydjN mutant (KCI1248), and ΔgshA mutant (KCI1220). (C) Mean thiol excretion rates after cystine addition, from three independent experiments with standard errors of the means. WT, wild type.
When wild-type cells were cultured continuously in cystine medium, so that YdjN was expressed only at basal levels, cysteine efflux occurred at a lower rate (Fig. 10A). Quantitation indicated that these cells still imported cysteine at 9 times the rate of utilization. Cysteine export did not occur from ΔydjN mutants, indicating that FliY-YecSC does not overimport cystine (Fig. 10B). The futile cycle of cystine import and cysteine release is a remarkable phenomenon, and its basis is considered in Discussion.
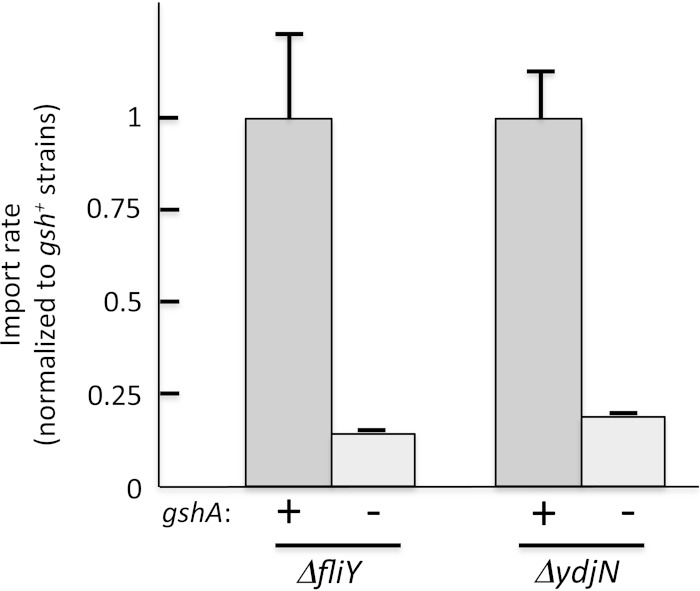
Reduction of imported cystine to cysteine.
Imported cystine must be reduced to cysteine before it can be incorporated into proteins or used as a sulfur donor in biosynthetic pathways. Mutants that lack glutathione (gshA) imported cystine at a much-diminished rate through both transporters (Fig. 11). The overimport of cystine by YdjN was slowed in gshA mutants: cells did not become sensitive to H2O2 (Fig. 1), and they excreted far less cysteine (Fig. 10). This phenotype is largely recapitulated in Δgor mutants, which lack glutathione reductase (4). Not all cystine import is lost, since even in gshA backgrounds both YdjN and FliY-YecSC systems transport enough cystine to sustain wild-type growth rates. However, routine YdjN function is diminished—like ydjN mutants, gshA mutants exhibit partial induction of the CysB regulon even in cystine medium (see Fig. S6 in the supplemental material).
FIG 11.
Glutathione is necessary for rapid cystine import. ΔfliY (KCI1142), ΔfliY ΔgshA (KCI1210), ΔydjN (KCI1254), and ΔydjN ΔgshA (KCI1516) strains grown in sulfate medium were suspended to an OD600 of 0.2 in the same medium. The cells were incubated for 5 min with chloramphenicol at 37°C, and then [14C]cystine cocktail was added. Mean rates normalized to gshA+ strains, with error bars (standard errors of the means) from three independent experiments.
We infer that GSH is likely to be, directly or indirectly, the electron donor that reduces imported cystine to cysteine. This process is probably important not only for the provision of cysteine but also for the avoidance of sulfur exchange reactions that would transfer disulfide bonds from cystine to proteins. The most straightforward mechanism of GSH-dependent cystine reduction would be by analogous sulfur exchange reactions, with oxidized glutathione (GSSG) subsequently being recycled by glutathione reductase:
| (3) |
| (4) |
| (5) |
To appraise this possibility, we measured the rate constant for reaction 3 (1.8 M−1 s−1) and then quantified GSH and cystine pools inside cells. Metabolite extraction indicated that when cystine is added to sulfate-grown cells, intracellular levels of cysteine become high (ca. 2 mM), while cystine levels are below the detection limit (<50 μM). GSH levels are ∼5 mM. Using 50 μM as an upper limit for cystine, we calculate (see Materials and Methods) that chemical reduction processes (reactions 3 and 4) would generate at most 30 μM cysteine per minute. This is 70-fold lower than the cellular rate of cysteine utilization and >2,000-fold lower than the observed rate of cysteine excretion by overimporting cells. Thus, the GSH-dependent reduction of imported cystine must be an enzyme-catalyzed process.
The fact that YdjN maintains a single cysteine residue, whereas most cysteine acquisition gene products do not, raised the possibility that this residue is important for its function and is involved in cystine reduction. Indeed, its mutation to serine inactivated the transporter, as a plasmid encoding the mutant protein was unable to restore cystine-dependent growth to fliY ydjN mutants (KCI1286) (data not shown). Unlike FliY-YecSC, YdjN cannot transport nonreducible cystine analogues like DAP and djenkolate, and so we consider it possible that formation of a mixed disulfide between the catalytic cysteine residue and the cystine substrate is an integral part of the transport process. More work will be necessary to test this idea.
In contrast, the FliY-YecSC system imports nonreducible substrates such as DAP, and so it presumably releases cystine as a disulfide compound into the cytoplasm. Again, a gshA mutation slows transport (Fig. 11). In vitro assays determined that wild-type extracts catalyzed GSH-dependent cystine reduction at a rate (0.5 mM mg−1 min−1) that is sufficient to allow the observed rate of cystine reduction in wild-type cells (70 mM/min, with cytoplasmic protein at 300 mg/ml). Glutaredoxins II and III (encoded by grxB and grxC, respectively) were the primary catalysts, as ΔgrxB and ΔgrxC mutants each exhibited only about 50% of the wild-type activity and a ΔgrxB ΔgrxC double mutant had ∼10% the activity (see Fig. S9A in the supplemental material). However, mutants lacking all glutaredoxins continued to import cystine at wild-type rates through the FliY-YecSC transporter (KCI1514; see Fig. S9B). These mutations also failed to phenocopy the H2O2 resistance phenotype of ΔgshA mutations during cystine import through YdjN (KCI1260), as all individual and combined grx mutants exhibited <0.25% survival upon cystine/H2O2 challenge, compared to 20% for the gshA mutant. The same was true of individual mutations in candidate glutathione S-transferase genes (yghU, yibF, yciW, yliJ, gst, yncG, yfcG, sspA, and yfcF). Therefore, redundancies have prevented us from identifying a single predominant route of cystine reduction.
DISCUSSION
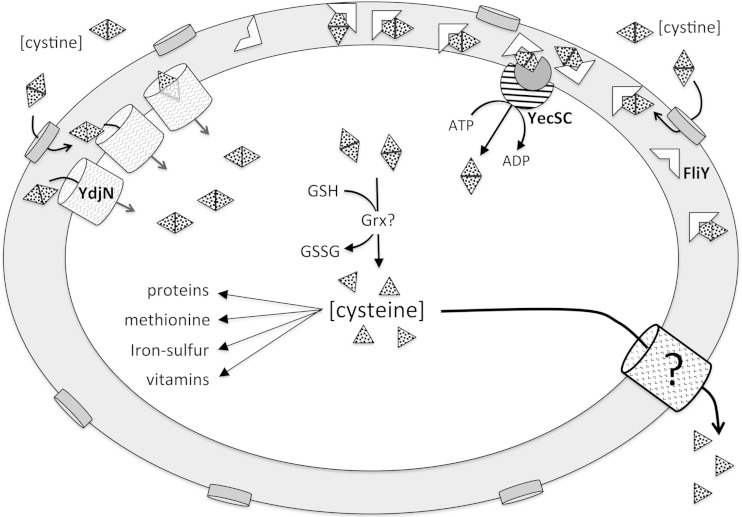
The two special features of sulfur compounds—their essentiality for growth and their reactivity in vivo—lead to cystine transport phenomena that are very unusual. The desperation of cells for sulfur-containing compounds can predispose them to import far more cystine than is usable, and the redox behavior of excess cysteine can put the cell at risk of oxidative damage. E. coli responds by excreting cysteine, thereby establishing a futile import/export cycle that is wasteful but, apparently, the lesser of two evils (Fig. 12).
FIG 12.
Cystine metabolism in Escherichia coli. Extracellular cystine enters the periplasm through porins and then is imported into the cytoplasm by either the YdjN or FliY-YecSC system. Cystine reduction to cysteine is glutathione dependent and probably occurs either during YdjN transport or subsequently in a glutaredoxin-dependent reaction. The intracellular pool of cysteine is ∼0.2 mM in sulfate-grown cells (4) but surges to millimolar levels when cystine becomes available. Excess cysteine is excreted through unidentified exporters. Ribosomal profiling studies suggest that the relative ratio of importer-protein synthesis in sulfate-grown cells is ∼8 YdjN per ∼24:1:1 FliY-YecSC (33). The larger flux is through YdjN when cystine is ample but through FliY-YecSC when it is scarce.
Sulfur is not stored in E. coli: the 5 mM concentration of intracellular glutathione, the most plausible sulfur storage species, can provide enough sulfur atoms for only 2 min of good growth. Thus, the cell must continuously harvest sulfur compounds from its environment. Cystine and cysteine are the sulfur sources of choice, as they are the only sulfur compounds that provide intracellular cysteine to E. coli at a rate sufficient to deactivate the CysB transcription factor (see Fig. S10 in the supplemental material). Cystine is more likely than cysteine to be encountered in oxic habitats, since in standard medium cysteine autoxidizes within a few minutes. Therefore, it appears that cystine import is often the preferred source of sulfur.
Why does E. coli maintain two import systems?
At first blush, it might seem that E. coli uses two cystine transporters in order to exploit their distinct energetics. When extracellular cystine levels are substantial, import through YdjN is more economical. As an ion-driven importer, YdjN expends only about one-third the energy of an ATP-driven importer like FliY; this reduces by ∼1% the total ATP demand of the cell. However, when sulfur substrates drop to submicromolar levels, the relatively high Km of YdjN is inadequate. Transporters of other amino acids also slow when their substrates are scarce, but in those cases, the cell can forge on by synthesizing such amino acids de novo. Sulfur atoms, however, cannot be synthesized. Thus, when sulfur is scant and trace cystine is available, the cell relies upon FliY to acquire it: being half-saturated by 30 nM, the FliY system fully satisfies sulfur demand at exceedingly low cystine concentrations. An inescapable consequence of tight binding by the periplasmic binding protein is a reliance upon ATP to push the transport process forward. A typical binding-protein KD (equilibrium dissociation constant) of ∼10−7 M (37) represents a ΔG°′ of +10 kcal, so that highly exergonic ATP hydrolysis is ultimately required to pry substrate off the binding protein. Thus, YdjN is more economical at high cystine concentrations, whereas only FliY remains effective at very low concentrations.
The puzzle is that YdjN is induced alongside FliY when sulfur is scarce. Why induce YdjN if FliY-YecSC will be fully adequate? We think the answer may lie in the fact that FliY can be competitively inhibited by other biomolecules, including DAP, a structural molecule that seems likely to be found at significant levels in some of the habitats native to E. coli. When cystine is scarce, even moderate amounts of such competing molecules may impair FliY turnover. In contrast, YdjN, which seems to be specific for disulfide compounds, is unaffected by those molecules and will continue to bind and import cystine. If C346 does indeed form a transient disulfide bond with transported substrates, YdjN may retain this residue as a device to exclude nonsulfur compounds and thereby avoid the inhibition to which FliY is vulnerable. Basal levels of YdjN are not especially effective at nanomolar concentrations of cystine—but its strong induction can substantially compensate. For example, despite the high effective Km of YdjN (2 μM), the fact that its Vmax is 30 times the maximum cell demand allows it to import enough cystine to avoid growth problems even when the external cystine concentration is as little as 70 nM.
Several other amino acids have more than one importer, and in most cases, a similar justification can be made. Tryptophan, for example, is imported into E. coli through both a general aromatic transporter (AroP) and a tryptophan-specific system (Mtr). Tryptophan import through AroP can be competitively blocked by tyrosine and phenylalanine—and, accordingly, the Mtr system is induced by phenylalanine and tyrosine complexes of the TyrR transcription factor, thereby restoring the capacity for tryptophan uptake.
The pairing of YdjN and FliY transporters is not uncommon. Among the Enterobacteriaceae, 22 of 41 genera use both systems. Another 13 employ YdjN alone, while four have neither (presumably relying upon other sulfur sources). Two genera are comprised of a mixture of species, some containing both transporters and others containing only YdjN. Looking more broadly, apparent homologues of both systems can be found throughout the bacterial kingdom (see Materials and Methods). Most of the homologues are found among Actinobacteria, Firmicutes, and Proteobacteria, which is roughly in proportion to the overrepresentation of these phyla among sequenced strains in the database. While the substrate specificity of ABC-type transporters cannot be predicted with full confidence from protein sequence, the presence of true FliY and YdjN homologues has been confirmed by genetic analysis of Bacillus subtilis (24). In that bacterium, both transporters are induced during sulfur limitation, just as in E. coli. We conclude that the fact that sulfur is indispensable has prompted many microbes to take an extra step and rely upon a combination of transporters. This arrangement contrasts with other amino acid acquisition strategies.
Although YdjN and FliY-YecSC import an oxidized substrate, they are also found among committed anaerobes such as clostridial species. Even anaerobes periodically encounter oxygen, as can be inferred from their possession of enzymes that scavenge oxygen species (38), so in that light their ability to import cystine may not be surprising. Consistent with this view, we did not observe any impact upon fliY::lacZ or ydjN::lacZ expression when E. coli was cultured anaerobically rather than aerobically (KCI1230 and KCI1330) (data not shown), indicating that it, too, is prepared to recover cystine in anoxic habitats. Many bacteria do lack apparent homologues of these transporters, but we were unable to correlate their distribution with any particular lifestyle or habitat.
Why is cystine import uncontrolled?
Although YdjN induction may help cells cope with sulfur scarcity, the possession of so many transport molecules is risky because a subsequent encounter with micromolar cystine causes cystine to be imported at rates far beyond the cellular need. Cysteine pools swell. This situation potentially accelerates Fenton chemistry, both because cysteine autoxidation generates H2O2 and because subsequent Fenton chemistry is facilitated when the cysteine repeatedly reduces Fenton-active iron (4). To minimize cysteine accumulation, the cell exports cysteine. This response carries a startlingly heavy price: our calculations (see Materials and Methods) imply that the resultant futile cycle of cystine import, reduction, and excretion consumes the equivalent of at least 10% of the biosynthetic ATP budget and doubles the NADPH demand. The outcome is not a quirk of E. coli K-12 strains, as we observed the same behavior with Salmonella (see Fig. S11 in the supplemental material).
Such a situation is, to our knowledge, unprecedented. It would seem that both the hazards of Fenton chemistry and those of energy waste would be avoided if YdjN would stop importing cystine once cytoplasmic cysteine levels were high. Why does it not do so? In principle, three mechanisms might achieve feedback control: arrival at thermodynamic equilibrium, product inhibition, and allosteric inhibition. YdjN imports cystine at the expense of a proton equivalent, which means that even for a moderate proton motive force (−0.17 V), equilibrium would not be achieved until intracellular cystine levels were >500-fold higher than their external concentration. Thus, influx of 2 μM external cystine would continue until internal cystine rose to 1 mM—an unacceptable situation, given the likelihood that disulfide exchange reactions between cystine and protein sulfhydryls would derivatize catalytic residues and inactivate enzymes. Indeed, we saw that E. coli avoids cystine accumulation by rapidly reducing it to cysteine. This reaction is exergonic (E0′ of cystine = −0.250 V [39]; E0′ of NADPH, the ultimate electron donor = −0.320 V). The upshot is that cystine import through YdjN can never approach equilibrium.
Product inhibition occurs when accumulated products bind in the active sites of the producing enzymes, slowing them down even before thermodynamic equilibrium has been reached. This may have happened in our gshA mutants, when cystine reduction was disrupted. But because cystine is quickly converted to cysteine upon import into wild-type cells, product inhibition does not normally happen.
Allostery is a final possibility. Methionine and histidine importers, for example, are inhibited once the pertinent amino acid accumulates inside E. coli (40, 41). Cytoplasmic methionine binds to an allosteric site within the ATPase domain of the MetNIQ importer, blocking turnover (42). However, this option may not be available for cysteine, for the unique reason that it has an isostructural twin inside the cell: serine. It may be difficult to evolve an allosteric site that distinguishes between the two; the risk would be that high levels of serine would occupy a putative cysteine allosteric site, conveying a misimpression of cysteine sufficiency.
An exception that supports this interpretation is that both biosynthetic and transcriptional control of cysteine synthesis rest upon the ability of cysteine to specifically inhibit CysE, serine O-acetyltransferase (1). In this one case of cysteine-mediated inhibition, cysteine exerts its effect by binding exactly in the position of the serine substrate (43, 44). The enzyme distinguishes the two molecules only through the distinct chemical behaviors of their oxygen and sulfur atoms. Thus, an allosteric site—which must rely upon shape rather than reactivity to detect cysteine—might not be an option for YdjN. An alternative feedback mechanism that is available in some organisms would be the use of methionine as a proxy for cysteine sufficiency. However, in E. coli methionine and cysteine pools do not equilibrate, since methionine cannot be converted to cysteine.
Interestingly, the absence of YdjN control is reminiscent of MntH, the manganese importer (45). MntH also can massively overimport its substrate due to the lack of any effective feedback device. When it overshoots, the cell induces an efflux system to pump the excess manganese back out (46). In this case, allosteric control may be impossible because ferrous iron easily mispopulates manganese binding sites, as the metals are virtually identical in size, charge, and preferred binding geometry. This is a striking analogy to potential cysteine/serine competition and the nonregulation of YdjN.
This logic implies that FliY-YecSC should also be uncontrolled. Indeed, this system can also overshoot, as we particularly observed when YecSC was overproduced. It does not naturally do so in E. coli only because the low YecS-YecC content establishes a limit on the total flux. However, a homologous transporter does appear to overimport cystine into the Gram-positive bacterium Lactobacillus fermentum. This bacterium exclusively employs a FliY-YecSC-type system for cystine uptake (47). In Gram-positive bacteria, extracytoplasmic binding proteins are tethered to the cell surface; consequently, a single YecSC-type channel cannot service a multitude of binding proteins. Thus, the relative number of channels must be high during sulfur limitation. The upshot is that upon cystine upshift, the flux capacity is high. Indeed, experimenters noted that when cystine was added to erstwhile sulfur-poor cells, large amounts of an unidentified thiol compound accumulated in the medium. Given our results with E. coli, we presume that the excreted compound was cysteine, and this example suggests that the problem of cystine overimport—and compensation by export—may be general among microbes. Ongoing work in the lab is focused upon the identification and control of the key cysteine efflux systems.
Synergistic action of cysteine and H2O2.
We note that the stunning sensitivity of cysteine-rich cells to H2O2 seems like a vulnerability that might be exploited by inhospitable hosts. The only other situation in which H2O2 is nearly so toxic is when nitric oxide blocks the respiratory cytochrome oxidases, causing NADH to accumulate and indirectly promote iron reduction (48). Macrophages generate both NO and H2O2, raising the prospect that these species synergize in poisoning captured bacteria (49). We wonder whether host organisms might analogously use the cysteine-H2O2 combination as an antimicrobial weapon, or whether further work might develop it as an antibiotic therapy.
Renaming YdjN and FliY-YecSC.
Finally, we propose to rename E. coli YdjN as TcyP and FliY-YecS-YecC as TcyJ-TcyL-TcyN, in order to conform with the names given to functional and structural homologues that have these names in Bacillus subtilis (24). B. subtilis TcyP exhibits 45% identity to E. coli YdjN, and both proteins are half-saturated by ∼1 μM cystine. The relationship is less clear for the ABC transporters, as B. subtilis has two that can import cystine. A three-component system (TcyABC) shows slightly greater homology to the E. coli FliY-YecSC, but tcyABC is not regulated in response to cystine, and it imports cystine effectively only when it is provided at nonphysiological concentrations (1 mM). Instead, the ABC system that is functionally homologous to FliY-YecSC is comprised of TcyJKLMN. TcyJ and TcyK are substrate binding proteins affixed to the outer aspect of the Gram-positive membrane; they both exhibit 28% identity to FliY. TcyL and TcyM are paired integral membrane proteins and are 35% and 32% identical to YecS, respectively. TcyN is the ATP-binding component and is 50% identical to YecC. Like FliY-YecSC, TcyJKLMN can transport nonreducible cystine analogues such as djenkolate. The reported Km of TcyJKLMN (2.5 μM) greatly exceeds that of the E. coli system, but this disparity may arise from the difference in dynamics between fixed and free substrate binding proteins. Thus, we feel the literature will be clarified by the renaming of the E. coli genes.
Supplementary Material
ACKNOWLEDGMENT
This study was funded by grant GM101012 from the National Institutes of Health.
Footnotes
Supplemental material for this article may be found at http://dx.doi.org/10.1128/JB.00277-15.
REFERENCES
- 1.Kredich NM. 1996. Biosynthesis of cysteine, p 514–527. In Neidhardt FC, Curtiss R III, Ingraham JL, Lin ECC, Low KB, Magasanik B, Reznikoff WS, Riley M, Schaechter M, Umbarger HE (ed), Escherichia coli and Salmonella: cellular and molecular biology, 2nd ed, vol 1 ASM Press, Washington, DC. [Google Scholar]
- 2.Kredich NM. 1992. The molecular basis for positive regulation of cys promoters in Salmonella typhimurium and Escherichia coli. Mol Microbiol 6:2747–2753. doi: 10.1111/j.1365-2958.1992.tb01453.x. [DOI] [PubMed] [Google Scholar]
- 3.Marino SM, Gladyshev VN. 2012. Analysis and functional prediction of reactive cysteine residues. J Biol Chem 287:4419–4425. doi: 10.1074/jbc.R111.275578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Park S, Imlay JA. 2003. High levels of intracellular cysteine promote oxidative DNA damage by driving the Fenton reaction. J Bacteriol 185:1942–1950. doi: 10.1128/JB.185.6.1942-1950.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Glass GA, DeLisle DM, DeTogni P, Gabig TG, Magee BH, Markert M, Babior BM. 1986. The respiratory burst oxidase of human neutrophils. Further studies of the purified enzyme. J Biol Chem 261:13247–13251. [PubMed] [Google Scholar]
- 6.Mehdy MC. 1994. Active oxygen species in plant defense against pathogens. Plant Physiol 105:467–472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Seki M, Iida K, Saito M, Nakayama H, Yoshida S. 2004. Hydrogen peroxide production in Streptococcus pyogenes: involvement of lactase oxidase and coupling with aerobic utilization of lactate. J Bacteriol 186:2046–2051. doi: 10.1128/JB.186.7.2046-2051.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bedard K, Lardy B, Krause K-H. 2007. NOX family NADPH oxidases: not just in mammals. Biochemie 89:1107–1112. doi: 10.1016/j.biochi.2007.01.012. [DOI] [PubMed] [Google Scholar]
- 9.Imlay JA. 2013. The molecular mechanisms and physiological consequences of oxidative stress: lessons from a model bacterium. Nat Rev Microbiol 11:443–454. doi: 10.1038/nrmicro3032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Imlay JA, Linn S. 1986. Bimodal pattern of killing of DNA-repair-defective or anoxically grown Escherichia coli by hydrogen peroxide. J Bacteriol 166:519–527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Miller JH. 1972. Experiments in molecular genetics. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY. [Google Scholar]
- 12.Harris CL. 1981. Cysteine and growth inhibition of Escherichia coli: threonine deaminase as the target enzyme. J Bacteriol 145:1031–1035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kertesz MA, Leisinger T, Cook AM. 1993. Proteins induced by sulfate limitation in Escherichia coli, Pseudomonas putida, or Staphylococcus aureus. J Bacteriol 175:1187–1190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Messner KR, Imlay JA. 1999. The identification of primary sites of superoxide and hydrogen peroxide formation in the aerobic respiratory chain and sulfite reductase complex of Escherichia coli. J Biol Chem 274:10119–10128. doi: 10.1074/jbc.274.15.10119. [DOI] [PubMed] [Google Scholar]
- 15.Datsenko KA, Wanner BL. 2000. One-step inactivation of chromosomal genes in Escherichia coli K-12 using PCR products. Proc Natl Acad Sci U S A 97:6640–6645. doi: 10.1073/pnas.120163297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wang RF, Kushner SR. 1991. Construction of versatile low-copy-number vectors for cloning, sequencing and gene expression in Escherichia coli. Gene 100:195–199. doi: 10.1016/0378-1119(91)90366-J. [DOI] [PubMed] [Google Scholar]
- 17.Haldimann A, Wanner BL. 2001. Conditional-replication, integration, excision, and retrieval plasmid-host systems for gene structure-function studies of bacteria. J Bacteriol 183:6384–6393. doi: 10.1128/JB.183.21.6384-6393.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Martin JE, Imlay JA. 2011. The alternative aerobic ribonucleotide reductase of Escherichia coli, NrdEF, is a manganese-dependent enzyme that enables cell replication during periods of iron starvation. Mol Microbiol 80:319–334. doi: 10.1111/j.1365-2958.2011.07593.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ellermeier CD, Janakiraman A, Slauch JM. 2002. Construction of targeted single copy lac fusions using lambda Red and FLP-mediated site-specific recombination in bacteria. Gene 290:153–161. doi: 10.1016/S0378-1119(02)00551-6. [DOI] [PubMed] [Google Scholar]
- 20.Merighi M, Ellermeier CD, Slauch JM, Gunn JS. 2005. Resolvase-in vivo expression technology analysis of the Salmonella enterica serovar Typhimurium PhoP and PmrA regulons in BALB/c mice. J Bacteriol 187:7407–7416. doi: 10.1128/JB.187.21.7407-7416.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Masse E, Gottesman S. 2002. A small RNA regulates the expression of genes involved in iron metabolism in Escherichia coli. Proc Natl Acad Sci U S A 99:4620–4625. doi: 10.1073/pnas.032066599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Imlay JA, Fridovich I. 1991. Assay of metabolic superoxide production in Escherichia coli. J Biol Chem 266:6957–6965. [PubMed] [Google Scholar]
- 23.Neidhardt FC, Umbarger HE. 1996. Chemical composition of Escherichia coli, p 13–16. In Neidhardt FC, Curtiss R III, Ingraham JL, Lin ECC, Low KB, Magasanik B, Reznikoff WS, Riley M, Schaechter M, Umbarger HE (ed), Escherichia coli and Salmonella: cellular and molecular biology, 2nd ed, vol 1 ASM Press, Washington, DC. [Google Scholar]
- 24.Burguiere P, Auger S, Hullo M-F, Danchin A, Martin-Verstraete I. 2004. Three different systems participate in l-cystine uptake in Bacillus subtilis. J Bacteriol 186:4875–4884. doi: 10.1128/JB.186.15.4875-4884.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Neidhardt FC, Ingraham JL, Schaechter M. 1990. Physiology of the bacterial cell: a molecular approach. Sinauer Associates, Inc, Sunderland, MA. [Google Scholar]
- 26.Berger EA, Heppel LA. 1972. A binding protein involved in the transport of cystine and diaminopimelic acid in Escherichia coli. J Biol Chem 247:7684–7694. [PubMed] [Google Scholar]
- 27.Butler JD, Levin SW, Facchiano A, Miele L, Mukherjee AB. 1993. Amino acid composition and N-terminal sequence of purified cystine binding protein of Escherichia coli. Life Sci 52:1209–1215. doi: 10.1016/0024-3205(93)90103-A. [DOI] [PubMed] [Google Scholar]
- 28.Baptist EW, Kredich NM. 1977. Regulation of l-cystine transport in Salmonella typhimurium. J Bacteriol 131:111–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Deutch CE, Spahija I, Wagner CE. 2014. Susceptibility of Escherichia coli to the toxic l-proline analogue l-selenaproline is dependent on two l-cystine transport systems. J Appl Microbiol 117:1487–1499. doi: 10.1111/jam.12623. [DOI] [PubMed] [Google Scholar]
- 30.Soutourina J, Blanquet S, Plateau P. 2001. Role of d-cysteine desulfhydrase in the adaptation of Escherichia coli to d-cysteine. J Biol Chem 276:40864–40872. doi: 10.1074/jbc.M102375200. [DOI] [PubMed] [Google Scholar]
- 31.Ikebe T, Iyoda S, Kutsukake K. 1999. Structure and expression of the fliA operon of Salmonella typhimurium. Microbiology 145:1389–1396. doi: 10.1099/13500872-145-6-1389. [DOI] [PubMed] [Google Scholar]
- 32.Colyer TE, Kredich NM. 1994. Residue threonine-149 of the Salmonella typhimurium CysB transcription activator: mutations causing constitutive expression of positively regulated genes of the cysteine regulon. Mol Microbiol 13:797–805. doi: 10.1111/j.1365-2958.1994.tb00472.x. [DOI] [PubMed] [Google Scholar]
- 33.Li G-W, Burkhardt D, Gross C, Weissman JS. 2014. Quantifying absolute protein synthesis rates reveals principles underlying allocation of cellular resources. Cell 157:624–635. doi: 10.1016/j.cell.2014.02.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Daley DO, Rapp M, Granseth E, Melen K, Drew D, von Heijne G. 2005. Global topology analysis of the Escherichia coli inner membrane proteome. Science 308:1321–1323. doi: 10.1126/science.1109730. [DOI] [PubMed] [Google Scholar]
- 35.Mendez J, Reimundo P, Perez-Pascual D, Navais R, Gomez E, Guijarro JA. 2011. A novel cdsAB operon is involved in the uptake of l-cysteine and participates in the pathogenesis of Yersinia ruckeri. J Bacteriol 193:944–951. doi: 10.1128/JB.01058-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Oguri T, Schneider B, Reitzer L. 2012. Cysteine catabolism and cysteine desulfhydrase (CdsH/STM0458) in Salmonella enterica serovar Typhimurium. J Bacteriol 194:4366–4376. doi: 10.1128/JB.00729-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Prossnitz E, Gee A, Ames GF. 1989. Reconstitution of the histidine periplasmic transport system in membrane vesicles. Energy coupling and interaction between the binding protein and the membrane complex. J Biol Chem 264:5006–5014. [PubMed] [Google Scholar]
- 38.Mishra S, Imlay JA. 2013. An anaerobic bacterium, Bacteroides thetaiotaomicron, uses a consortium of enzymes to scavenge hydrogen peroxide. Mol Microbiol 90:1356–1371. doi: 10.1111/mmi.12438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rodrigues SD, Batista GB, Ingberman M, Pecoits-Filho R, Nakao LS. 2012. Plasma cysteine/cystine reduction potential correlates with plasma creatine levels in chronic kidney disease. Blood Purif 34:231–237. doi: 10.1159/000342627. [DOI] [PubMed] [Google Scholar]
- 40.Kadner RJ. 1975. Regulation of methionine transport activity in Escherichia coli. J Bacteriol 122:110–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Liu CE, Ames GF. 1997. Characterization of transport through the periplasmic histidine permease using proteoliposomes reconstituted by dialysis. J Biol Chem 272:859–866. doi: 10.1074/jbc.272.2.859. [DOI] [PubMed] [Google Scholar]
- 42.Kadaba NS, Kaiser JT, Johnson E, Lee A, Rees DC. 2008. The high-affinity E. coli methionine ABC transporter: structure and allosteric regulation. Science 321:250–253. doi: 10.1126/science.1157987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hindson VJ. 2003. Serine acetyltransferase of Escherichia coli: substrate specificity and feedback control by cysteine. Biochem J 375:745–752. doi: 10.1042/bj20030429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pye VE, Tingey AP, Robson RL, Moody PCE. 2004. The structure and mechanism of serine acetyltransferase from Escherichia coli. J Biol Chem 279:40729–40736. doi: 10.1074/jbc.M403751200. [DOI] [PubMed] [Google Scholar]
- 45.Martin JE, Waters LS, Storz G, Imlay JA. 2015. The Escherichia coli small protein MntS and exporter MntP optimize the intracellular concentration of manganese. PLoS Genet 11:e1004977. doi: 10.1371/journal.pgen.1004977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Waters LS, Sandoval M, Storz G. 2011. The Escherichia coli MntR miniregulon includes genes encoding a small protein and an efflux pump required for manganese homeostasis. J Bacteriol 193:5887–5897. doi: 10.1128/JB.05872-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Turner MS, Woodberry T, Hafner LM, Giffard PM. 1999. The bspA locus of Lactobacillus fermentum BR11 encodes an l-cystine uptake system. J Bacteriol 181:2192–2198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Woodmansee AN, Imlay JA. 2003. A mechanism by which nitric oxide accelerates the rate of oxidative DNA damage in Escherichia coli. Mol Microbiol 49:11–22. doi: 10.1046/j.1365-2958.2003.03530.x. [DOI] [PubMed] [Google Scholar]
- 49.Vazquez-Torres A, Jones-Carson J, Mastroeni P, Ischiropoulos H, Fang FC. 2000. Antimicrobial actions of the NADPH phagocyte oxidase and inducible nitric oxide synthase in experimental salmonellosis. I. Effects on microbial killing by activated peritoneal macrophages in vitro. J Exp Med 192:227–236. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.