Abstract
Rationale: Malignant airway obstruction is commonly found in patients with lung cancer and is associated with significant morbidity and mortality. Relieving malignant obstruction may improve symptoms, quality of life, and life expectancy.
Objectives: The objective of this study was to analyze our experience with bronchoscopic endobronchial intratumoral injection of cisplatin for malignant airway obstruction.
Methods: We conducted a retrospective analysis of patients with malignant airway obstruction treated with bronchoscopic intratumoral injection of cisplatin. Patient characteristics, histology, degree of airway obstruction, procedural methods, treatment cycles, performance status, and therapeutic outcomes were evaluated. Tumor response was analyzed based on bronchoscopic measurements performed on completion the of final treatment session. Adverse events and overall survival were abstracted.
Measurements and Main Results: Between January 2009 and September 2014, 22 patients (10 men, 12 women; mean age ± SD, 64.4 ± 9.5 yr) were treated with one to four injections of 40 mg of cisplatin mixed in 40 ml of 0.9% NaCl. Treatments were completed 1 week apart. The primary etiologies of airway obstruction included squamous cell carcinoma (n = 11), adenocarcinoma (n = 6), small cell carcinoma (n = 2), large cell undifferentiated carcinoma (n = 1), and metastatic endobronchial cancer (n = 2). Twenty-one of 22 patients were evaluable for response. The majority of patients (15/21, 71.4%) responded to therapy, defined as greater than 50% relative reduction in obstruction from baseline. Treatment response was obtained regardless of tumor histology, concurrent systemic therapy, number of treatment cycles administered, performance status, or use of additional ablative interventions. Responders had significantly improved overall survival as compared with nonresponders, although the difference was small. Severe treatment-related side effects or complications were not observed.
Conclusions: Subject to the limitations of a single-center retrospective study and a subjective primary outcome measure, we have demonstrated the feasibility of improving the patency of central airways that are largely or completely occluded by endobronchial malignant tumor using intraluminal injection of cisplatin. Additional longer-term, larger-scale safety and comparative effectiveness studies of this palliative treatment modality are warranted.
Keywords: endobronchial injection of chemotherapy, malignant airway obstruction, cisplatin
In the United States, approximately 220,000 patients are diagnosed with lung cancer annually (1). Mortality attributable to lung cancer outnumbers the next four leading causes of cancer-related death combined. Approximately one-third of lung cancers are complicated by malignant airway obstruction with associated symptoms (2). Other primary malignancies that may metastasize to the airway include melanoma and cancers of the thyroid, kidney, colon, esophagus, and breast. Sequelae resulting from malignant airway obstruction include cough, dyspnea, hemoptysis, postobstructive pneumonia, and respiratory failure. Malignant airway obstruction may occur in patients with known cancer or may be the presenting symptom in new diagnoses. Malignant airway obstruction is responsible for significant morbidity and, if untreated, death from suffocation is not uncommon. In cases with life-threatening obstruction, urgent recanalization of the airways is imperative (3). The goals of treatment are to reestablish and/or maintain airway patency, achieve stability of endobronchial lesions, and allow for the administration of additional cancer treatments that may improve symptoms, lung function, functional status, and quality of life.
Conventional treatment using systemic chemotherapy and/or radiotherapy has proven unsatisfactory in yielding rapid restoration of airway patency in patients with malignant airway obstruction (4–6). Various palliative modalities have been pursued in an attempt to effectively manage airway complications (3, 7, 8). Current local treatment options include mechanical debulking via rigid bronchoscopy, neodymium-doped yttrium aluminium garnet laser photoresection, cryotherapy, electrocautery, argon plasma coagulation (APC), brachytherapy, stents, and photodynamic therapy. No modality has proven superior. The choice of endobronchial therapy is generally dictated by the patient’s stability, nature of the underlying problem, overall prognosis, quality of life, physician’s personal experience, and technology available at a particular institution. The most successful strategies use systemic treatment with combined local interventional modalities (8, 9).
Effective recanalization of obstructed airways reduces postobstructive infections, respiratory insufficiencies, and duration of hospitalization, while improving quality of life (10–12). Local therapies also enhance the effect of subsequent systemic chemotherapy and/or external radiotherapy and have a favorable effect on survival outcomes (8). In contrast, failure to treat malignant airway obstruction, even if followed by radiotherapy, has been associated in several series with a poor prognosis (11, 12).
Although debulking of malignant airway obstruction with rigid bronchoscopy, neodymium-doped yttrium aluminium garnet laser photoresection, electrocautery, and APC is effective, improvements are usually short-lived. Photodynamic therapy and brachytherapy have emerged as options for debulking in obstructed airways and peribronchial disease, with slower response but longer-lasting effect (13, 14). However, their use is generally limited to superficial tumors with limited mass and extension. Massive hemoptysis also remains a concern, particularly with brachytherapy (15). Celikoglu and colleagues have observed and reported that direct injection of chemotherapeutic agents via needle-catheter into endobronchial tumors is effective at achieving airway patency and has long-lasting effect (16–18).
We at the University of Florida have used bronchoscopic intratumoral injection of cisplatin to palliate endobronchial obstruction, with and without concurrent destructive/ablative techniques, and/or stenting. The aim of the present work was to assess the feasibility of initial endobronchial intratumoral chemotherapy (EITC) with and without concurrent airway interventions based on our experience to date. Additionally, we analyzed our data to determine if EITC has any impact on overall survival and to ascertain the optimal number of EITC treatment cycles.
Methods
We retrospectively reviewed the medical records of all patients treated with EITC for malignant airway obstruction at the University of Florida Health Shands Hospital between January 2009 and September 2014. All research and analysis was approved by the University of Florida’s Institutional Review Board (#IRB201400823). Based on previously published literature (18, 19) we considered EITC an additional therapeutic modality for patients whom we anticipated would not have significant benefit from standard ablative therapy. Patients were treated with EITC in addition to other therapies as indicated. All patients signed informed clinical consent for intratumoral cisplatin before the procedure.
Procedural Considerations, Cisplatin Dosing, and Schedule
The procedure was performed under moderate sedation on spontaneously breathing patients. The target lesion was localized and degree of endobronchial obstruction was visually estimated. Endobronchial lesions with large tumor bulk, extensive involvement of the mucosa, rapid recurrence after initial ablative therapy, or those where recanalization of distal airways was thought to be difficult with standard ablative therapy (argon plasma coagulation at our institution) on radiographic evaluation or bronchoscopic airway examination were considered for intratumoral cisplatin therapy. Based on the bronchoscopist’s discretion, the lesions were initially debulked with flexible forceps and/or argon plasma coagulation before intratumoral cisplatin injection. This was especially true for, but not limited to, lesions causing respiratory compromise. Cisplatin was then administered to endoscopically visible tumor tissue via a flexible 19-gauge Wang needle inserted directly into the tumor. Injections were made in a fan pattern to disperse the drug solution throughout the tumor. Larger tumors required wider distribution of injections.
After cisplatin injection, the bronchoscope was passed distal to the tumor and excess cisplatin was suctioned from the distal airways to prevent the potential for alveolar toxicity secondary to residual drug. At the end of EITC, the decision to place a stent in the affected airway was made by the bronchoscopist based on anatomic characteristics and perceived benefit. During subsequent cycles of cisplatin injection, no additional airway interventions were performed. After each bronchoscopy, patients were allowed to recover from sedation according to standard hospital practice. Vital signs were closely monitored.
We chose to use cisplatin for our EITC study as it has been demonstrated to be one of the most active single agents against lung cancer and is frequently used in systemic combination (20, 21). A maximal dose of 40 mg per session was administered, based on previous published literature (19). Patients were treated with aqueous cisplatin solution (concentration of 1 mg/ml) injected directly into the tumor. The lyophilized (freeze-dried) cisplatin powder was reconstituted in 0.9% NaCl solution just before use. A maximum cisplatin dose of 40 mg, divided into four 10-ml syringes, was administered during each EITC treatment session. Each site was treated with one to four injections per session depending on the size and location of the lesion. Patients were treated weekly for a total of one to four sessions.
Data Abstraction and Analysis
The following data were retrospectively abstracted from patients’ medical records: demographic information, malignancy site of origin, histologic subtype of cancer, site of malignant airway obstruction, degree of airway obstruction, systemic cancer therapies received before EITC, concurrent systemic treatment during EITC, Eastern Cooperative Group (ECOG) performance status, additional maneuvers performed to restore airway patency (i.e., APC, debulking, and/or airway stenting), and radiographic improvement. Detailed chart review was performed for all patients to evaluate for toxicity and overall survival after final EITC session.
We classified the degree of airway obstruction at initial bronchoscopy, before any form of intervention, into three distinct categories: less than 50% obstruction, 50 to 75% obstruction, and greater than 75% obstruction, based on visual estimation by the bronchoscopist. All measurements for visual estimation of the degree of obstruction were made in real time during the bronchoscopy procedure by a single interventional bronchoscopist. The residual airway obstruction was estimated by visual assessment after the final intratumoral injection procedure by the same proceduralist. Residual airway obstruction was classified in the same manner as for initial degree of obstruction.
The primary end point, response to treatment, was calculated as the percent reduction in airway obstruction [(original obstruction − residual obstruction)/original obstruction × 100%]. Percentage reduction in airway obstruction was then divided into three categories: (1) good response: greater than 50% relative reduction in obstruction; (2) moderate response: 25 to 50% relative reduction in obstruction; and (3) small response: less than 25% relative reduction in obstruction. Response was evaluated at the completion of the final EITC procedure. Because intratumoral injection of cytotoxic medications is most often followed by an immediate endoscopically visible reduction in tumor bulk (19), we believe that our assessment of reduction in airway obstruction immediately after therapy is representative of response to EITC. For purposes of statistical analysis, we divided the patients into two distinct groups. One group, classified as “responders,” included patients who had good response. The other group was classified as “nonresponders,” which primarily included patients with a small to moderate response to EITC.
We sought to analyze any association between response and performance status, additional airway interventions, concurrent systemic therapies, tumor histology, and the number of EITC cycles delivered. We report these here as secondary outcomes. We also evaluated safety outcomes and feasibility of EITC. Finally, we report overall survival based on response. A separate analysis was conducted to determine whether good responders had better overall survival than moderate and small responders.
Statistical Methods
The majority of analyses conducted were descriptive in nature. Kaplan-Meier curves were used to estimate survival probabilities over time, overall, and stratified by response (good [>50% reduction] vs. poor [<50% reduction]). We compared survival for good response versus poor response by the logrank test, but no causality can be inferred. Associations between response and other variables were conducted by Fisher exact test. Cox regression analysis was not used due to the small size of study.
Results
Patient Characteristics
We treated 22 patients, 10 men and 12 women, aged between 49 and 80 years (mean age, 64.4 ± 9.5 yr), who presented with symptomatic malignant airway obstruction of the trachea or a major bronchus, secondary to inoperable histologically confirmed lung cancer (small cell and non-small cell lung cancer) or metastatic endobronchial cancer. Twenty-one of 22 patients had profound airway obstruction (>75%) based on bronchoscopic visual estimation, and one patient had moderate obstruction (50–75%).
Sites treated with EITC included right main stem bronchus (n = 6), left main stem bronchus (n = 6), right upper lobe bronchus (n = 3), bronchus intermedius (n = 1), left upper lobe bronchus (n = 2), left lower lobe bronchus (n = 3), and trachea (n = 1). Nearly half of the patients had ECOG scores of 2, as shown in Table 1. Ten patients (5 with right main obstruction, 4 with left main obstruction, and 1 with tracheal obstruction) had significant respiratory compromise, defined as requiring increasing supplemental O2 from their baseline and close monitoring in an intermediate care or intensive care unit. Sixteen patients had postobstructive atelectasis on chest radiograph performed preintervention.
Table 1.
Response to endobronchial injection of chemotherapy with cisplatin based on tumor type, concurrent systemic therapy, number of cycles, and additional airway maneuvers
| Responders (n = 15) | Nonresponders (n = 6) | P Value | |
|---|---|---|---|
| Histology (n) | 0.68 | ||
| Adenocarcinoma (6) | 5 (83) | 1 (17) | |
| Squamous (11) | 6 (55) | 5 (45) | |
| Small cell (1) | 1 (100) | 0 | |
| Large cell (1) | 1 (100) | 0 | |
| Metastatic (2) | 2 (100) | 0 | |
| Concurrent Rx (n) | 0.78 | ||
| Chemotherapy (3) | 2 (67) | 1 (33) | |
| Radiation (2) | 1 (50) | 1 (50) | |
| Chemotherapy/radiation (13) | 9 (69) | 4 (31) | |
| None (3) | 3 (100) | 0 | |
| No. of EITC treatments (n) | 0.12 | ||
| One (3) | 1 (33) | 2 (67) | |
| Two (1) | 0 | 1 (100) | |
| Three (2) | 2 (100) | 0 | |
| Four (15) | 12 (75) | 3 (25) | |
| Additional airway Rx (n) | 0.25 | ||
| None (6) | 5 (83) | 1 (17) | |
| Debulk (1) | 0 | 1 (100) | |
| APC/debulk (7) | 4 (57) | 3 (43) | |
| APC/debulk/stent (7) | 6 (86) | 1 (14) | |
| ECOG performance status (n) | 0.61 | ||
| ECOG 0 (1) | 1 (100) | 0 | |
| ECOG 1 (6) | 5 (83) | 1 (17) | |
| ECOG 2 (12) | 9 (75) | 3 (25) | |
| ECOG 3 (2) | 0 | 2 (100) |
Definition of abbreviations: APC = argon plasma coagulation; ECOG = Eastern Cooperative Group; EITC = endobronchial injection of chemotherapy; Rx = treatment.
Data presented as n (%).
One-half of all patients had squamous cell cancer, and six had adenocarcinoma (Table 1). A majority of patients (n = 14, 63.6%) did not undergo concurrent therapy. Three received concurrent systemic chemotherapy, two had external beam radiation therapy, and three had both chemotherapy and external beam radiation therapy. A majority (n = 15) received four cycles of EITC. Six patients (27%) received EITC with cisplatin as monotherapy (Table 1). All patients (n = 10) with significant airway compromise as defined above received additional airway maneuvers with the first bronchoscopy session along with intratumoral cisplatin to provide immediate symptomatic relief.
Treatment Effect
Twenty-one of 22 patients with airway obstruction were evaluable for local treatment response. One patient with moderate airway obstruction at the outset could not be evaluated for response due to lack of appropriate documentation. All 22 patients were evaluated for overall survival. Fifteen of 21 patients (71.5%) achieved good response with reduction in airway obstruction greater than 50% from baseline. One patient (5%) had moderate response, with a 25 to 50% reduction in airway obstruction, and the remaining 5 patients (24.5%) had a small response, with reduction in airway obstruction of less than 25% from baseline.
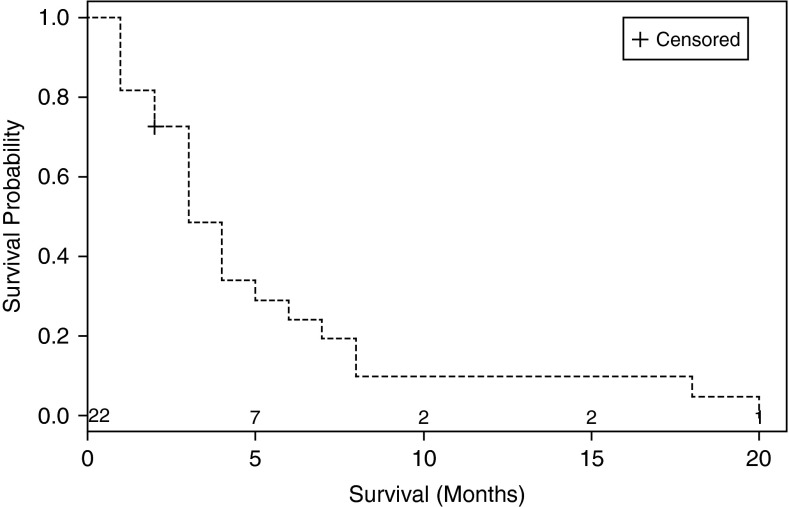
Table 1 displays response based on histologic subtype of cancer, concurrent therapy, additional airway intervention, number of cycles, ECOG performance status, and statistical significance for each of those variables. Overall median survival for the group was 3 months (95% confidence interval [CI] of 1–5 mo). Figure 1 shows the Kaplan-Meier curve for overall survival. In the subgroup analysis, median survival for responders was 4 months (95% CI of 3–8 mo), and for nonresponders it was 2.5 months (95% CI of 1–3 mo; P value, 0.026), suggesting improvement in survival for those patients who responded to EITC with cisplatin. Figure 2 displays the Kaplan-Meier curve for overall survival in responders and nonresponders.
Figure 1.
Kaplan-Meier curve showing overall survival for all patients.
Figure 2.
Kaplan-Meier curve showing overall survival by response, with responders having statistically improved overall survival as compared with nonresponders
Eleven of sixteen patients (69%) with postobstructive atelectasis on preintervention chest radiograph showed improvement in lung aeration on postintervention imaging performed between 5 and 7 days after the final bronchoscopy session. A few patients had transient nausea post-procedure, which improved significantly after a single dose of intravenous ondansetron. No local adverse effects such as fistula formation, hemoptysis, or any significant damage to normal airway mucosa were noted on follow-up bronchoscopies. Although we did not assess patients for long-term systemic side effects, no intraprocedural adverse events were noted.
Discussion
Our results indicate that EITC with cisplatin can improve the patency of airways largely or entirely occluded by endobronchial malignant tumor. The majority of patients included in our study achieved good response regarding reduction in the degree of airway obstruction. No major adverse events were recorded.
A statistically significant difference was not observed when analyzing the degree of response to EITC with cisplatin based on histologic subtype of tumor, concurrent systemic therapy, performance status, or additional airway maneuvers. Although not statistically significant and limited by low numbers, a higher proportion of patients who had three or four cycles had a response than those patients who only had one or two cycles.
Median survival in our cohort was only 3 months, which is similar to previously reported literature describing bronchoscopic treatment of patients with malignant airway obstruction (22), indicating that our patients presented with advanced-stage lung cancer and an overall poor prognosis. Although our study population was small and the overall survival too short to demonstrate a clinically important survival benefit with EITC, we observed that patients who responded to EITC and associated therapies had slightly better survival than nonresponders.
Approximately 30% of patients with lung cancer have tumor obstruction of the central airways manifested by symptoms of respiratory distress, bleeding, or infection (10, 23). Therapeutic bronchoscopy procedures, such as endobronchial laser and insertion of airway stents, are commonly used with a goal to relieve respiratory distress, improve quality of life, and potentially prolong survival. Several studies have demonstrated that in patients with unresectable obstructive lung cancer, a preliminary efficient debulking of the airway by laser photoresection or cryotherapy, before irradiation, lessens morbidity by reducing the number of local complications such as postobstructive infection, respiratory insufficiency, and hemoptysis (11, 12, 24–27). Chhajed and colleagues showed that patients with advanced lung cancer and malignant airway obstruction who underwent interventional bronchoscopy and systemic chemotherapy had survival similar to those without malignant airway obstruction who received systemic treatment (28).
The present work confirms and extends previously reported favorable experience with EITC (18, 19). Considered together with the earlier studies, our observations demonstrate the feasability of restoring the patency of airways occluded by malignant airway obstruction using EITC as part of a multimodality treatment approach. Based on the limited number of experiences recorded to date, EITC appears to be tolerably safe for the palliative treatment of patients who have a short life expecancy.
This preliminary, nonrandomized, retrospective observational study was not designed to adequately assess whether the benefit of intratumoral chemotherapy alone or in combination with argon plasma coagulation, stent placement, and/or debulking is uniquely due to the effect of debulking or to a neoadjuvant therapeutic and immunotherapeutic action of locoregionally administered cisplatin. No causal relationship can be inferred from this small observational study. Other unmeasured confounders could contribute to differences, or lack thereof, seen in this study. It is also not possible to determine from our study whether intratumoral chemotherapy combined with or without additional airway maneuvers is superior to existing modalities for management of malignant airway obstruction such as rigid bronchoscopic debulking, laser, argon plasma coagulation, electrocautery, cryotherapy, etc.
Our study is further limited by lack of standardization in airway obstruction measurement technique (29). Another limitation of our study is the absence of a standardized protocol to guide therapeutic recommendations for our study population.
Limitations notwithstanding, this case series does contribute additional information to support the feasability of EITC with cisplatin for management of malignant airway obstruction. We showed that EITC can be used as a part of multimodality therapy. Response appears to be similar regardless of tumor histology. A significant response can be obtained in patients with concurrent systemic therapy. On average our responders experienced modest mortality benefit. This study thus serves as a basis to study bronchoscopic intratumoral cisplatin in a controlled, randomized, and prospective fashion.
Bronchoscopic intratumoral cisplatin appears to offer several advantages compared with other methods for palliative debulking of malignant endobronchial tumors. EITC is relatively easy to perform for experienced interventionists and appears to be well-tolerated. EITC does not require expensive additional instrumentation aside from ordinary bronchoscopy equipment. There is no immediate sloughing of tumor, which is commonly seen with other thermal modalities. Mechanical debulking and thermal modalities often have difficulty achieving results in segmental airways, especially when the tumor is distal; one may postulate that EITC could provide an advantage over traditional modalities in these cases. None of the patients in our study developed major side effects from EITC with cisplatin. This safety and efficacy was observed when intratumoral chemotherapy was used as monotherapy and when used in conjunction with additional bronchoscopic therapeutic interventions.
Further studies are warranted to determine whether intratumoral chemotherapy is superior to other interventional methods, especially regarding duration of response, as well as to standardize the treatment protocol and determine the effects of EITC on patient symptoms and quality of life. Intratumoral chemotherapy may possibly be able to be used to treat tumors that are not visible centrally, potentially in combination with stereotactic radiation. This application should also be studied in future trials.
Acknowledgments
Acknowledgment
The authors thank Dr. Ingram Olkin, Stanford University, for his helpful comments on an early draft of this paper and insight on the clinical circumstances in which this intervention may be most appropriate. They also thank Dr. Eugene Goldberg from the University of Florida for his pioneering work in the field of intratumoral chemotherapy injections and for providing information on this technique.
Footnotes
Partially supported by National Institutes of Health grant 1UL1TR000064 from the National Center for Advancing Translational Sciences (J.J.S.).
Author Contributions: H.J.M. contributed to the study design, data collection, and manuscript writing. A.B. contributed to the study design, data collection, and manuscript writing. A.M.P. contributed to the study design, data collection, and manuscript writing. J.W. contributed to manuscript writing. S.F.-B. contributed to data collection and manuscript writing. J.C. contributed to data collection and manuscript writing. P.M. contributed to the study design and manuscript writing. J.J.S. contributed to data analysis, statistics, and manuscript writing. M.A.J. contributed to the study design, data collection, and manuscript writing.
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2013. CA Cancer J Clin. 2013;63:11–30. doi: 10.3322/caac.21166. [DOI] [PubMed] [Google Scholar]
- 2.Minna JD, Pass H, Glatstein E, Ilhde DC.Cancer of the lung. In: DeVita VT Jr., Hellman S, Rosenberg SA, editors. Cancer principles and practice of Oncology. Philadelphia: J. B. Lippincott; 1989. pp. 591-705. [Google Scholar]
- 3.Freitag L. Interventional endoscopic treatment. Lung Cancer. 2004;45:S235–S238. doi: 10.1016/j.lungcan.2004.07.970. [DOI] [PubMed] [Google Scholar]
- 4.Chetty KG, Moran EM, Sassoon CS, Viravathana T, Light RW. Effect of radiation therapy on bronchial obstruction due to bronchogenic carcinoma. Chest. 1989;95:582–584. doi: 10.1378/chest.95.3.582. [DOI] [PubMed] [Google Scholar]
- 5.Hazuka MB, Bunn PA., Jr Controversies in the nonsurgical treatment of stage III non-small cell lung cancer. Am Rev Respir Dis. 1992;145:967–977. doi: 10.1164/ajrccm/145.4_Pt_1.967. [DOI] [PubMed] [Google Scholar]
- 6.Roswit B, Patno ME, Rapp R, Veinbergs A, Feder B, Stuhlbarg J, Reid CB. The survival of patients with inoperable lung cancer: a large-scale randomized study of radiation therapy versus placebo. Radiology. 1968;90:688–697. doi: 10.1148/90.4.688. [DOI] [PubMed] [Google Scholar]
- 7.Cortese DA, Edell ES. Role of phototherapy, laser therapy, brachytherapy, and prosthetic stents in the management of lung cancer. Clin Chest Med. 1993;14:149–159. [PubMed] [Google Scholar]
- 8.Ernst A, Feller-Kopman D, Becker HD, Mehta AC. Central airway obstruction. Am J Respir Crit Care Med. 2004;169:1278–1297. doi: 10.1164/rccm.200210-1181SO. [DOI] [PubMed] [Google Scholar]
- 9.Freitag L, Ernst A, Thomas M, Prenzel R, Wahlers B, Macha HN. Sequential photodynamic therapy (PDT) and high dose brachytherapy for endobronchial tumour control in patients with limited bronchogenic carcinoma. Thorax. 2004;59:790–793. doi: 10.1136/thx.2003.013599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cavaliere S, Venuta F, Foccoli P, Toninelli C, La Face B.Endoscopic treatment of malignant airway obstructions in 2,008 patients Chest 19961101536–1542.[Published erratum appears in Chest 111:1476.] [DOI] [PubMed] [Google Scholar]
- 11.Eichenhorn MS, Kvale PA, Miks VM, Seydel HG, Horowitz B, Radke JR. Initial combination therapy with YAG laser photoresection and irradiation for inoperable non-small cell carcinoma of the lung: a preliminary report. Chest. 1986;89:782–785. doi: 10.1378/chest.89.6.782. [DOI] [PubMed] [Google Scholar]
- 12.Vergnon JM, Schmitt T, Alamartine E, Barthelemy JC, Fournel P, Emonot A. Initial combined cryotherapy and irradiation for unresectable non-small cell lung cancer: preliminary results. Chest. 1992;102:1436–1440. doi: 10.1378/chest.102.5.1436. [DOI] [PubMed] [Google Scholar]
- 13.Vincent RG, Dougherty TJ, Rao U, Boyle DG, Potter WR. Photoradiation therapy in advanced carcinoma of the trachea and bronchus. Chest. 1984;85:29–33. doi: 10.1378/chest.85.1.29. [DOI] [PubMed] [Google Scholar]
- 14.Macha HN, Freitag L. The role of brachytherapy in the treatment and control of central bronchial carcinoma. Monaldi Arch Chest Dis. 1996;51:325–328. [PubMed] [Google Scholar]
- 15.Furuse K, Fukuoka M, Kato H, Horai T, Kubota K, Kodama N, Kusunoki Y, Takifuji N, Okunaka T, Konaka C, et al. The Japan Lung Cancer Photodynamic Therapy Study Group. A prospective phase II study on photodynamic therapy with photofrin II for centrally located early-stage lung cancer. J Clin Oncol. 1993;11:1852–1857. doi: 10.1200/JCO.1993.11.10.1852. [DOI] [PubMed] [Google Scholar]
- 16.Celikoğlu SI, Karayel T, Demirci S, Celikoğlu F, Cağatay T. Direct injection of anti-cancer drugs into endobronchial tumours for palliation of major airway obstruction. Postgrad Med J. 1997;73:159–162. doi: 10.1136/pgmj.73.857.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Celikoğlu F, Celikoğlu SI. Intratumoural chemotherapy with 5-fluorouracil for palliation of bronchial cancer in patients with severe airway obstruction. J Pharm Pharmacol. 2003;55:1441–1448. doi: 10.1211/0022357021936. [DOI] [PubMed] [Google Scholar]
- 18.Celikoglu SI, Celikoglu F, Goldberg EP. Endobronchial intratumoral chemotherapy (EITC) followed by surgery in early non-small cell lung cancer with polypoid growth causing erroneous impression of advanced disease. Lung Cancer. 2006;54:339–346. doi: 10.1016/j.lungcan.2006.09.004. [DOI] [PubMed] [Google Scholar]
- 19.Celikoglu F, Celikoglu SI, York AM, Goldberg EP. Intratumoral administration of cisplatin through a bronchoscope followed by irradiation for treatment of inoperable non-small cell obstructive lung cancer. Lung Cancer. 2006;51:225–236. doi: 10.1016/j.lungcan.2005.10.012. [DOI] [PubMed] [Google Scholar]
- 20.Sandler AB, Nemunaitis J, Denham C, von Pawel J, Cormier Y, Gatzemeier U, Mattson K, Manegold C, Palmer MC, Gregor A, et al. Phase III trial of gemcitabine plus cisplatin versus cisplatin alone in patients with locally advanced or metastatic non-small-cell lung cancer. J Clin Oncol. 2000;18:122–130. doi: 10.1200/JCO.2000.18.1.122. [DOI] [PubMed] [Google Scholar]
- 21.Ciotti R, Ucci G, Belotti G, Facchi E, Cremonesi M, Gatti C, Baccheta G. Prospective evaluation of anthracycline-related early cardiac damage: how do we monitor it? J Clin Oncol. 2001;19:4269–4270. doi: 10.1200/JCO.2001.19.22.4269. [DOI] [PubMed] [Google Scholar]
- 22.Neyman K, Sundset A, Espinoza A, Kongerud J, Fosse E. Survival and complications after interventional bronchoscopy in malignant central airway obstruction: a single-center experience. J Bronchology Interv Pulmonol. 2011;18:233–238. doi: 10.1097/LBR.0b013e318222a7da. [DOI] [PubMed] [Google Scholar]
- 23.Santos RS, Raftopoulos Y, Keenan RJ, Halal A, Maley RH, Landreneau RJ. Bronchoscopic palliation of primary lung cancer: single or multimodality therapy? Surg Endosc. 2004;18:931–936. doi: 10.1007/s00464-003-9202-x. [DOI] [PubMed] [Google Scholar]
- 24.Dumon JF, Shapshay S, Bourcereau J, Cavaliere S, Meric B, Garbi N, Beamis J. Principles for safety in application of neodymium-YAG laser in bronchology. Chest. 1984;86:163–168. doi: 10.1378/chest.86.2.163. [DOI] [PubMed] [Google Scholar]
- 25.Dumon JF, Reboud E, Garbe L, Aucomte F, Meric B. Treatment of tracheobronchial lesions by laser photoresection. Chest. 1982;81:278–284. doi: 10.1378/chest.81.3.278. [DOI] [PubMed] [Google Scholar]
- 26.Gelb AF, Epstein JD. Laser in treatment of lung cancer. Chest. 1984;86:662–666. doi: 10.1378/chest.86.5.662. [DOI] [PubMed] [Google Scholar]
- 27.Kvale PA, Eichenhorn MS, Radke JR, Miks V. YAG laser photoresection of lesions obstructing the central airways. Chest. 1985;87:283–288. doi: 10.1378/chest.87.3.283. [DOI] [PubMed] [Google Scholar]
- 28.Chhajed PN, Baty F, Pless M, Somandin S, Tamm M, Brutsche MH. Outcome of treated advanced non-small cell lung cancer with and without central airway obstruction. Chest. 2006;130:1803–1807. doi: 10.1378/chest.130.6.1803. [DOI] [PubMed] [Google Scholar]
- 29.Begnaud A, Connett JE, Harwood EM, Jantz MA, Mehta HJ. Measuring central airway obstruction: what do bronchoscopists do? Ann Am Thorac Soc. 2015;12:85–90. doi: 10.1513/AnnalsATS.201406-268OC. [DOI] [PMC free article] [PubMed] [Google Scholar]