Abstract
The search continues for optimal markers that can be utilized to improve bladder cancer detection and to predict disease recurrence. Although no single marker has yet replaced the need to perform cystoscopy and urine cytology, many tests have been evaluated and are being developed. In the future, these promising markers may be incorporated into standard practice to address the challenge of screening in addition to long-term surveillance of patients who have or are at risk for developing bladder cancer.
Keywords: Biomarkers, bladder cancer, transitional cell carcinoma, urothelial carcinoma
INTRODUCTION
Urothelial carcinoma of the bladder (UCB) is the 7th most common cancer worldwide in men and the 17th most common cancer worldwide in women. Approximately 75% of newly diagnosed UBCs are non-muscle invasive (carcinoma in situ, Ta and T1). Smoking is the most common risk factor and accounts for approximately half of all UBCs. Occupational exposure to aromatic amines and polycyclic aromatic hydrocarbons are other important risk factors for the development of UBC.[1] Bladder cancer has the highest cost of any malignancy when categorized on a per patient basis. The direct economic cost of non-muscle invasive bladder cancer (NMIBC) is primarily related to the need for lifelong cystoscopic examination as non-muscle invasive tumors are characterized by a high recurrence rate (50–70% within 5 years).[2] Stringent surveillance protocols are followed because of the high likelihood of recurrence and poor prognosis of patients who may subsequently develop muscle-invasive disease.
Development of ideal biomarkers that will enable diagnosis at an earlier stage of disease and accurately monitor for recurrence remains challenging. Urinary markers that could be used in place of or as an adjunct to current screening and surveillance techniques, or markers that could be used to risk-stratify patients and predict progression of disease, would be beneficial to clinicians for determining surveillance regimens and potential therapeutic response. This review examines the clinically available tests and emerging biomarkers in the context of potential application for bladder cancer diagnosis, prognostication and surveillance.
An ideal biomarker for bladder cancer would provide sufficient negative predictive value to allow patients to avoid invasive tests such as cystoscopy or to risk-stratify patients with indolent versus aggressive disease. The Food and Drug Administration (FDA)-approved tests include Bladder Tumor Antigen (BTA) stat®, BTA TRAK®, nuclear matrix protein (NMP22)/BladderChek® and UroVysion™ for diagnosis and surveillance, while ImmunoCyt™/uCyt™ is approved for surveillance [Table 1].
Table 1.
Summary of clinically available bladder cancer urinary biomarkers
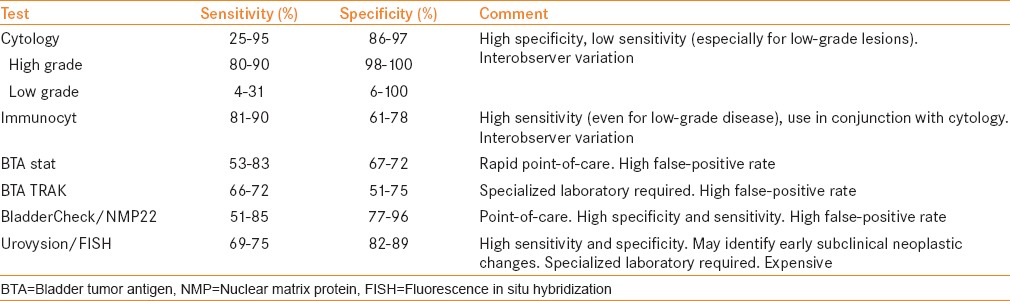
URINE CYTOLOGY
Voided urine cytology remains the gold standard for non-invasive testing for bladder cancer, and is the most commonly used urinary marker in clinical practice. The overall sensitivity of voided urine cytology ranges from 25% to 95%.[3,4,5,6,7,8,9] Cytology has proven to be very useful for the detection of high-grade and high-stage disease. High-grade lesions and carcinoma in situ (CIS) can be detected by voided cytology with a sensitivity of 80–90% and a specificity of 98–100%.[4,10] Morphologic changes associated with high-grade malignant cells include increased size, increased nuclear-to-cytoplasmic ratio, nuclear pleomorphism, coarse and irregular chromatin and frequent mitotic figures. These characteristics are associated with a higher risk for bladder cancer, even in the presence of a negative cystoscopic examination.[11] Despite its effectiveness in detecting high-grade lesions, cytology has the propensity to miss low-grade disease. In a review by Renshaw and colleagues, the sensitivity for detecting low-grade lesions ranged from 0% to 100% and the specificity ranged from 6% to 100%.[12] Low-grade malignant cells may only appear slightly different from dysplastic or normal cells and can pose a challenge for cytopathologic interpretation. Conditions that can cause inflammatory changes in the bladder, such as recent intravesical therapy, radiation treatment and infection, may result in false-positive readings up to 12% of the time.[13] Moreover, the definition of a positive cytology reading can be highly variable.[7,8]
Cytology is also relatively expensive and time-consuming, costing approximately $100 per test and taking over 24 h for the results to become available.[13] The positive predictive value of atypical, suspicious and malignant reports has been reported to be 12%, 39% and 67%, respectively.[14] Urine cytology is highly specific but has intermediate sensitivity, indicating that it has a role in adjunct diagnosis but not in screening for primary bladder cancer. High tumor grade is associated with significantly higher sensitivity compared with low and intermediate grades combined.[14]
ImmunoCyt/uCyt+ assay
In 1997, Fradet and Lockhart developed the ImmunoCyt test to augment urine cytology by using an immunocytofluorescent technique that consisted of antibodies (M344 and LDQ10) labeled with fluorescein, which have been shown to react with a mucin glycoprotein, and another antibody (19A211) that reacted with a glycosylated form of carcinoembryonic antigen. These antigens are expressed by tumor cells found in the majority of bladder cancer patients and occasionally on tumor cells of some patients with prostate cancer. The antigens can be detected in tumor cells exfoliated in the urine and are not expressed in the normal genitourinary tissues, with the exception of a few umbrella cells in a small percentage of patients.[15,16]
ImmunoCyt/uCyt+ is performed under microscopy by a trained cytopathologist. A relatively large number of exfoliated cells are necessary to perform an accurate test. A cytology slide must contain a minimum of 500 cells for a negative score to be valid, while the presence of one fluorescent cell is considered positive. Sensitivity of urinary cytology could be increased from 50% to 90% (range, 81–90%) when incorporating the ImmunoCyt/uCyt+ test, but the specificities of the combined assays were less than that achieved by cytology alone (range, 61–78%).[17,20,22,24] Studies also suggest that ImmunoCyt/uCyt+ has a superior sensitivity to cytology for early pathological stage (Ta–T1) and low-grade tumors, and can significantly improve the detection of CIS.[18,19,21] Comploj et al. evaluated 7422 cytology and ImmunoCyt/uCyt+ tests. The overall sensitivity was 35% for cytology, 68% for ImmunoCyt and 73% for the two tests combined. The overall specificity was 98% for cytology, 72% for ImmunoCyt/uCyt+ and 72% for the two tests combined. Cytology and ImmunoCyt/uCyt+ together had an overall sensitivity of 73%, with 59% for grade 1, 77% for grade 2 and 90% for grade 3 tumors (1973 World Health Organization grading classification).[23]
ImmunoCyt/uCyt+ is less affected by hematuria and inflammatory conditions because it is a cellular assay, but the test is subjective and depends on specimen stability and handling as well as interobserver variation.[17] These limitations restrict the ImmunoCyt/uCyt+ test to being recommended as an adjunct to cytology, and it is only approved for the surveillance of patients with a history of bladder cancer.
BTA assays
Two forms of the BTA test (Polymedco Inc., Cortlandt Manor, NY, USA) capable of detecting human complement factor H-related protein (cFH) are available and FDA approved for diagnosis and follow-up of bladder cancer. The BTA stat test is a qualitative dipstick, point-of-care immunoassay[25] while the BTA TRAK is a quantitative enzyme-linked immunosorbent assay (ELISA).[27] The reported sensitivity is 53–83% for BTA stat and 66–72% for BTA TRAK. The specificity is 67–72% and 51–75% for BTA stat and BTA TRAK, respectively.[26,27,28,29,33]
While sensitivities can be better for BTA compared with cytology, the disadvantage of these assays is that false-positive results can occur with hematuria, highly concentrated urine, cystitis and previous treatment with BCG. A number of reports have noted that there is often a high correlation between BTA data and hematuria levels, and the presence of hematuria in subjects without malignant disease can result in false-positive BTA assay tests.[30,31] Rather than detecting a bladder tumor antigen, urinary BTA assays may be measuring serum cFH introduced by bleeding,[32] a common presenting factor in bladder cancer patients.[25] BTA stat and BTA TRAK tests cannot replace cystoscopy and have limited utility as an adjunct for surveillance in patients with NMIBC.[34]
NMP22
NMP22 (BladderCheck) is an immunoassay for the detection of nuclear matrix protein 22 in urine. It is a marker of urothelial cell death and is elevated in the urine of patients with bladder cancer. The recommended NMP22 cut-off for diagnosis of urothelial carcinoma by the manufacturer is 10 u/mL; however, different cut-offs (3.6–12 u/mL) have been used. A level of 6.4 units/mL demonstrated sensitivity for urothelial carcinoma (UC) as much as twice that of cytology.[35] NMP22 is a point-of-care assay, making the test an attractive adjunct for cystoscopy. Rather than detecting a specific tumor antigen, urinary NMP22 assays measure the cellularity or amount of cell turnover that may be introduced into the urine by a variety of conditions, including surface shedding from bladder tumors. Thus, false-positive results may be encountered in hematuria and benign inflammatory conditions.[36,41]
NMP22 demonstrated a sensitivity of 51–85% and a specificity of 77–96% in detecting bladder cancer.[37,39,40] Patients with positive NMP22 and negative cystoscopy have a 9.57-times greater risk of recurrence during 1 year of follow-up compared with patients with a negative test result.[39] Hwang et al. reported a lower overall sensitivity for NMP22 of 32% compared with 38% for cytology. The sensitivity of NMP22 for low-grade tumors was higher than that of cytology, and when NMP22 was combined with cytology, the sensitivity for detecting bladder cancer increased.[38]
Fluorescence in situ hybridization (FISH)
The first report of a novel FISH probe set for bladder cancer detection was published in 2000.[42] This assay is referred to as UroVysion (Abbott Molecular Inc., Des Plaines, IL, USA). It is a molecular genetic technique used for detecting aneuploidy of chromosomes 3, 7 and 17 and loss of the 9p21 locus in exfoliated urothelial cells. Suggested criteria for a positive assay include finding five or more urinary cells with gains of two or more chromosomes, ≥10 cells with gain of a single chromosome (e.g. trisomy 7) or homozygous deletion of 9p21 in >20% of epithelial cells.
An overall sensitivity of 84.2% and specificity of 91.8% in detecting urothelial carcinoma was initially reported. A meta-analysis showed a pooled sensitivity and specificity of 72% (69–75%) and 83% (82–85%), respectively.[43] In a large study by Dimashkieh et al., the overall sensitivity, specificity, positive predictive value and negative predictive value in detecting UC were 61.9%, 89.7%, 53.9% and 92.4%, respectively. The performance was better in high-grade UC than in low-grade UC, with sensitivities of 75.5% and 40.8%, respectively.[44] The accumulation of copy number variations (CNVs, represented as DNA loss or gain) during the development of bladder cancer can begin 3 years before diagnosis, but the extent required for a positive UroVysion™ test is usually achieved no earlier than 1 year before diagnosis.[45] FISH-based molecular grading has been shown to increase the accuracy of prognostic models to predict both recurrence and progression.[46]
It may be challenging to distinguish inflammatory and reactive changes from recurrent tumor with cystoscopy and urine cytology in patients who have recently been treated with BCG. A positive UroVysion test following BCG treatment is associated with failure of therapy, and patients with superficial bladder cancer who had positive UroVysion at the end of BCG treatment were at a higher risk for progression to muscle-invasive disease. Patients with a positive FISH test should be closely monitored even if cytology and cystoscopy are negative because of the risk of disease recurrence.[47,48,49,56]
UroVysion has been evaluated as a reflex test when equivocal or atypical cytology has been reported.[50] A negative FISH test result under these situations likely correlates with benign cytological changes. For patients with atypical cytology and negative cystoscopy, 3-year recurrence free survival with negative and positive FISH results were 67% and 34%, respectively.[51,52,53] The test appears to have a high specificity among patients who have a variety of benign genitourinary conditions, including microhematuria, BPH, infection and inflammation.[54] In addition to UC, adenocarcinoma, squamous carcinoma, small cell carcinoma of the bladder and renal cell carcinoma have been found to be associated with positive FISH results in urinary specimens.[55]
In summary, UroVysion™ seems to have a high specificity for the detection of bladder cancer and the ability to detect bladder tumor recurrence prior to clinical symptoms. Thus, it may be used as a confirmatory test for either cytology or uCyt+™ testing. One major limitation of the FISH assay is the lack of consensus regarding criteria used to evaluate abnormal cells. Additionally, the test has relatively low sensitivity in the detection of low-grade bladder tumors and may not improve sensitivity as an adjunct to cytomorphologic analysis.
Other protein markers
Many proteins are expressed in the urine of patients with bladder cancer and have potential application as diagnostic or prognostic tumor markers. These include blood group antigens, tumor-associated antigens, proliferating antigens, oncogenes, peptide growth factors and their receptors, cell adhesion molecules, tumor angiogenesis and angiogenesis inhibitors and cell cycle regulatory proteins.[57]
Cytokeratins: UBC tests and Cyfra 21.1
Cytokeratins are intracellular proteins in the intracytoplasmic cytoskeleton of epithelial cells. These proteins are released in urine following cell death. UBC-Rapid and UBC-ELISA tests are immunological assays (IDL Biotech, Borlange, Sweden) that detect cytokeratin 8 and 18 fragments in urine. The UBC-Rapid test is a qualitative point-of-care assay, UBC-ELISA is a quantitative assay requiring trained personnel to perform the ELISA and UBC rapid quantitative is a new point-of-care assay.[58,59] In a recent study, performance of UBC rapid determined visually and quantitatively and UBC-ELISA showed sensitivities of 53%, 61% and 50%, respectively, and specificities of 82%, 69% and 69%, respectively.[59] Prior studies revealed a high variability of sensitivities (36–78%) and specificities (63–97%) for the UBC rapid test (evaluated visually).[33,60,61] The UBC ELISA demonstrated a sensitivity and specificity of 40–70% and 63–75%, respectively.[61,62] High false-positive rates and the inability to detect low-grade tumors have limited the utility of these tests for bladder tumor surveillance.[63]
Cyfra 21.1 is an ELISA-based assay that detects fragments of CK19 in urine with monoclonal antibodies. Inflammatory bladder conditions can cause false-positive results.[64] The reported sensitivity and specificity of Cyfra 21.1 was 61–85% and 75–91%, respectively.[65,66]
BLCA-1 and BLCA-4
BLCA-1 and BLCA-4 are nuclear transcription factors expressed early in the development of urothelial carcinoma. These proteins are associated with tumor cell proliferation, survival and angiogenesis. BLCA-4 is expressed in tumor and adjacent benign areas of the bladder, but not in bladders without malignancy. Its expression does not appear to be affected by tumor grade or by various benign urologic disorders such as urinary tract infection, catheterization or cystitis, but may be elevated in patients with spinal cord injuries.[67] It may also be a biomarker of field changes.[68] The ELISA assay for detection of BLCA-4 in urine has a sensitivity of 89–96% and specificity of 90–100%.[69,70,71,72,73] In a study by Myers-Irvin et al., BLCA-1 demonstrated 80% sensitivity and 87% specificity.[69] These markers may help to identify individuals with earlier stages of bladder cancer, but still need further refinement and validation.[67]
INVESTIGATIONAL BIOMARKERS
Advances in proteomic technologies and urinary proteome profiling studies for bladder cancer have identified many biomarker candidates for UC, including alfa-defensin, apolipoprotein A-1 (APOA1) and alfa 1-antitrypsin (A1AT).[74,75,76] Alfa-defensin was able to detect bladder cancer with better sensitivity and specificity than commercial tests.[75] APOA1 was significantly elevated in urine samples from bladder cancer patients and has a high diagnostic potential. A1AT achieved a sensitivity of 74% and a specificity of 80% for bladder cancer detection.[77,78] A panel consisting of an eight-protein biomarker combination achieved 92% sensitivity and 97% specificity for the detection of bladder cancer.[79] These multiplex biomarker panels are undergoing further validation.
DNA biomarkers
Understanding bladder cancer genomics may provide opportunities for discovery of new biomarkers for diagnosis as well as possible therapeutic targets. Genomic changes in bladder cancer are complex and vary with different histological types.[80] Low-grade, papillary, non-invasive tumors are generally characterized by constitutive activation of the receptor tyrosine kinase-Ras pathway, such as activating mutations in the HRAS and fibroblast growth factor receptor 3 (FGFR3) genes. In contrast, high-grade invasive tumors are characterized by alterations in the tumor suppressor protein p53 (TP53) and retinoblastoma 1 (RB1) pathways. Recent genomic platforms have revealed that urothelial cancer is much more complex and heterogeneous, and many new significantly mutated genes have been reported.
Amplified focal regions include genes such as E2F3, CCND1, MDM2, ERBB2, CCNE1, MYC and FGFR3, and deleted regions contain genes such as CDKN2A, RB1 and CREBBP.[81,82] The most common focal deletion contained CDKN2A and was observed in approximately 50% of bladder cancer samples. Three clusters have been identified based on somatic mutations and focal copy number alterations (CNAs): Cluster A was enriched in focal somatic CNAs in several genes and mutations in MLL2. Cluster B was characterized by deletion of CDKN2A and mutations in FGFR3 and papillary morphology. Cluster C showed TP53 mutations as well as enrichment with RB1 mutations and amplifications of E2F3 and CCNE1.[82] Kompier et al. monitored multiple mutations in five genes including FGFR3 in bladder tumors.[83] Mutations in individual genes were not overly prevalent (11–63%), but mutations in one or more target genes were found in 88% of primary tumors and 88% of recurrent tumors. Mutational analyses have been applied successfully to voided urine sediments, and tumor-specific mutational screening assays may have utility for diagnosis and surveillance of bladder cancer patients.[84,85]
DNA methylation
Epigenetic alterations, such as DNA methylation, are frequently observed in tumors.[86] DNA methylation occurs at cytosines located at CpG dinucleotides. CpG islands are enriched for these base pairs and typically overlap with gene regulatory regions. DNA methylation is associated with downregulation of tumor suppressor genes and may contribute to the development of bladder cancer.[87] In the past decade, several methylation markers have been identified that could aid in the detection of bladder tumors based on urinary assays. Chung et al. identified a panel of eight genes (A2BP1, NPTX2, SOX11, PENK, NKX6-2, DBC1, MYO3A and CA10) that were highly methylated in bladder cancer, with methylation frequencies ranging from 62% to 92%.[88] Reinert et al. found that POU4F2 and HOXA9 genes had a high frequency of methylation (92%) in bladder cancer tissues and were unmethylated in the normal urothelium.[89]
There is also potential utility of using gene methylation as a prognostic marker. Methylation of CDH1, FHIT, LAMC2, RASSF1A, TIMP3, SFRP1, SOX9, PMF1 and RUNX3 genes is associated with poor survival in muscle-invasive bladder cancer.[90,93] Methylation of RASSF1A and HOXB2 were associated with bladder tumor stage, grade and tumor progression.[91,92] Several studies have examined methylation markers in the urine of patients with bladder cancer. Methylation of VAX1, KCNV1, TAL1, PPOX1 and CFTR in 212 urine samples from patients (157 with primary tumors and 55 with recurrent tumors) and 190 samples from healthy controls was found to detect both primary and recurrent disease, with a sensitivity of 89% and a specificity of 88%.[94] Zuiverloon et al. studied the use of APC, TERT and EDNRB methylation in a urinary test for the detection of recurrent bladder cancer. A sensitivity of 63% was reported with a specificity of 58%.[95] In conclusion, methylation markers for bladder cancer diagnosis are still at an early stage compared with other FDA-approved markers and need further validation. Most methylation markers show higher sensitivity compared with cytology, but potentially at the cost of a lower specificity.[96]
RNA markers
Non-invasive detection of RNA tumor markers in urine samples represents another attractive diagnostic option. RNA isolation procedures, stability of polymerase chain reaction (PCR) amplicons as well as the amount and particularly the quality of RNA are factors that may influence the outcome of gene expression studies. Development of one general standard operating protocol is important in order to compare gene expression data across studies.[97]
Survivin
Survivin is an anti-apoptotic protein. The urinary levels of survivin are elevated in bladder cancer, and this protein has shown promise as a biomarker for UC.[98] A reverse transcription (RT)-PCR assay called BioDot has been used to detect survivin. Urinary levels of survivin have been shown to have high sensitivity (64–83%) and specificity (88–93%) for the detection of UC.[99,100,101] In a large, prospective study that examined survivin performance for bladder cancer screening, the marker demonstrated good negative predictive value (99%) and specificity (98%), but a low positive predictive value and sensitivity. Survivin was not influenced by confounders such as inflammation and hematuria, and the test was associated with a relatively low number of false-positive results.[102]
Telomerase
Telomerase is a RT responsible for adding tandem repeat sequences (TTAGGG) to the ends of chromosomes. Telomerase activity is increased in many cancers, and urinary telomerase activity has been shown to be an accurate marker for the detection of bladder tumors, with a specificity in the range of 87–100%.[103] Different assays for detecting telomerase activity have variable sensitivities because of the low stability of telomerase in urine. In a prospective, case–control series of 218 men, the sensitivity of telomerase with the telomeric repeat amplification protocol assay was 90% and the specificity was 88%.[63] The clinical application of this biomarker has been limited due to lack of standardization.
Other potential markers
Measurement of urinary hTERT, SENP1, PPP1CA and MCM5 mRNAs has been used to identify bladder cancer recurrence. All of these mRNA markers have been shown to be more sensitive than cytology. The combination of each marker with cytology resulted in increased detection rates.[104] Nicotinamide N-methyltransferase (NNMT) has been reported to be highly expressed in bladder cancer. Urinary NNMT expression levels have been suggested as a tool for early diagnosis.[105] Holyoake et al. developed a quantitative RT-PCR urine-based assay for bladder cancer detection. Overexpressed genes identified in bladder cancer tissues (CDC2, MDK, IGFBP5 and HOXA13) were selected for this assay. Measurement of the combination of mRNA markers detected UC at a sensitivity of 85% and a specificity of 80% across all stages, with the best performance obtained with stage T1 disease.[106] In a cohort of 485 patients presenting with hematuria, an assay derived from this biomarker panel, the uRNA test, achieved a higher sensitivity (62%) compared with NMP22 and cytology, with a specificity of 85%.[107] Further validation studies are needed before these markers can be incorporated into routine clinical practice.
MicroRNA (miRNA)
miRNAs are endogenous, non-coding RNA molecules approximately 22 nucleotides in length that regulate gene expression by inhibition of translation or by degradation of mRNA.[108,115] miRNAs play an important role in normal development, cell growth, differentiation and apoptosis.[109] Dysregulated miRNAs can ultimately lead to aberrant expression of genes that may predispose normal cells to malignant transformation.[119]
Downregulation of miRNAs, including those that target the FGFR3 pathway, such as miR-145, miR-101, miR-100 and miR-99a, may be associated with increased expression of FGFR3 in the absence of FGFR3 mutation and has been observed in low-grade NMIBC.[110,111] In contrast, increased expression of miRNAs is observed in high-grade muscle-invasive bladder cancer compared with adjacent normal bladder urothelium, including miRNAs predicted to target p53, such as miR-21 and miR373.[111]
Ratert et al. screened 723 miRNAs for potential diagnostic and prognostic value in bladder cancer and found seven upregulated miRNAs (miR-20a, miR-106b, miR-130b, miR-141, miR-200a, miR-200a and miR-205) and eight downregulated miRNAs (miR-100, miR-125b, miR-130a, miR-139-5p, miR-145, miR-199a-3p, miR-214 and miR-222).[112] Analyses demonstrated a robust ability for miRNAs to distinguish between normal tissue and bladder cancer tissue, highlighting the prognostic potential for miR-141 in muscle-invasive bladder cancer. The combination of four miRNAs (miR-130b, miR-141, miR-199-3p and miR-205) resulted in correct classification of 100% of tissue samples. Rosenberg et al. found that miR-29c was significantly decreased in NMIBC that progressed and showed potential utility for risk stratification of patients with T1 disease into those with high vs. low risk of progression.[113] Only two of 36 cases with high expression of miR-29c progressed, only one of which was in the first 5 years; while 50% of patients with low expression progressed with a median progression-free survival of 35 months.
The RNA ratio of miR-126 to miR-182 in urine samples was found to detect bladder cancer with a sensitivity of 72% and specificity of 82%.[120] The sensitivity and specificity of miR-145 levels in urine to distinguish bladder cancer patients from non-cancer controls was 80% and 60%, respectively. Furthermore, miR-200a has been shown to be an independent predictor of NMIBC recurrence, with lower levels associated with a higher risk of recurrence.[117] Mengual et al. used a 6-miRNA diagnosis model to accurately diagnose UC with a sensitivity and specificity of 83% and 87%, respectively.[114] Another panel of miRNAs including miR-135b, miR-15b and miR-1224-3p could detect bladder cancer with 94% sensitivity and 51% specificity.[121] A diagnostic test based on three miRNAs (miR-200c, miR-141 and miR-30b) demonstrated sensitivity of 100% and specificity of 96% for the identification of invasive cancers and was able to identify invasive bladder tumors misclassified in pathologic evaluation of bladder biopsy specimens.[118] Although urinary miRNAs show promise as potential biomarkers for bladder cancer, a major challenge is that most miRNAs are down-regulated, making it difficult to use reduced miRNA levels as a diagnostic tool.[116] The clinical value and application of miRNA signatures requires external validation in larger trials as well as comparison against current standards of care.
CONCLUSIONS
The search for the ideal bladder cancer marker(s) remains challenging. A multitude of tests for bladder cancer detection and surveillance have been evaluated, but most assays are currently not routinely used in clinical practice. Identification of novel genomic alterations and advances in molecular techniques may result in the development of a new generation of molecules that could be used in clinical practice. The use of any single biomarker may prove to be inadequate for bladder cancer testing. Instead, a combination of biomarkers or panels that include the use of exfoliated cells, DNAs, RNAs, proteins and/or metabolites may yield the best approach for bladder cancer detection, surveillance and prognostication.[122,123,124]
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Burger M, Catto JW, Dalbagni G, Grossman HB, Herr H, Karakiewicz P, et al. Epidemiology and risk factors of urothelial bladder cancer. Eur Urol. 2013;63:234–41. doi: 10.1016/j.eururo.2012.07.033. [DOI] [PubMed] [Google Scholar]
- 2.Mossanen M, Gore JL. The burden of bladder cancer care: Direct and indirect costs. Curr Opin Urol. 2014;24:487–91. doi: 10.1097/MOU.0000000000000078. [DOI] [PubMed] [Google Scholar]
- 3.Giannopoulos A, Manousakas T, Mitropoulos D, Botsoli-Stergiou E, Constantinides C, Giannopoulou M, et al. Comparative evaluation of the BTAstat test, NMP22, and voided urine cytology in the detection of primary and recurrent bladder tumors. Urology. 2000;55:871–5. doi: 10.1016/s0090-4295(00)00489-1. [DOI] [PubMed] [Google Scholar]
- 4.Grégoire M, Fradet Y, Meyer F, Têtu B, Bois R, Bédard G, et al. Diagnostic accuracy of urinary cytology, and deoxyribonucleic acid flow cytometry and cytology on bladder washings during follow-up for bladder cancer. J Urol. 1997;157:1660–4. [PubMed] [Google Scholar]
- 5.Grossman HB. New methods for detection of bladder cancer. Semin Urol Oncol. 1998;16:17–22. [PubMed] [Google Scholar]
- 6.Loening S, Fallon B, Narayana A, Penick GD, Hawtrey C, Culp DA, et al. Urinary cytology and bladder biopsy in patients with bladder cancer. Urology. 1978;11:591–5. doi: 10.1016/0090-4295(78)90009-2. [DOI] [PubMed] [Google Scholar]
- 7.Pode D, Shapiro A, Wald M, Nativ O, Laufer M, Kaver I. Noninvasive detection of the bladder cancer with the BTA stat test. J Urol. 1999;161:443–6. [PubMed] [Google Scholar]
- 8.Ramakumar S, Bhuiyan J, Besse JA, Roberts SG, Wollan PC, Blute ML, et al. Comparison of screening methods in the detection of bladder cancer. J Urol. 1999;161:388–94. [PubMed] [Google Scholar]
- 9.Zippe C, Pandragni L, Agarwal A. NMP22 is a sensitive, cost-effective test in patients at risk for bladder cancer. J Urol. 1999;161:62–5. [PubMed] [Google Scholar]
- 10.Brown FM. Urine cytology: Is it still the gold standard for screening? Urol Clin North Am. 2000;27:25–37. doi: 10.1016/s0094-0143(05)70231-7. [DOI] [PubMed] [Google Scholar]
- 11.Murphy WM, Soloway MS, Jukkola AF, Crabtree WN, Ford KS. Urinary cytology and bladder cancer. The cellular features of transitional cell neoplasms. Cancer. 1984;53:1555–65. doi: 10.1002/1097-0142(19840401)53:7<1555::aid-cncr2820530723>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- 12.Renshaw AA, Nappi D, Weinberg DS. Cytology of grade 1 papillary transitional cell carcinoma: A comparison of cytologic, architectural and morphometric criteria in cystoscopically obtained urine. Acta Cytol. 1996;40:676–82. doi: 10.1159/000333938. [DOI] [PubMed] [Google Scholar]
- 13.Burchardt M, Burchardt T, Shabsigh A, De La Taille A, Benson MC, Sawczuk I. Current concepts in biomarker technology for bladder cancers. Clin Chem. 2000;46:595–605. [PubMed] [Google Scholar]
- 14.Turco P, Houssami N, Bulgaresi P, Troni GM, Galanti L, Cariaggi MP, et al. Is conventional urinary cytology still reliable for diagnosis of primary bladder carcinoma? Accuracy based on data linkage of a consecutive clinical series and cancer registry. Acta Cytol. 2011;55:193–6. doi: 10.1159/000320861. [DOI] [PubMed] [Google Scholar]
- 15.Greene KL, Berry A, Konety BR. Diagnostic utility of the ImmunoCyt/uCyt+ test in bladder cancer. Rev Urol. 2006;8:190–7. [PMC free article] [PubMed] [Google Scholar]
- 16.Li HX, Li M, Li CL, Ma JH, Wang MR, Rao J, et al. ImmunoCyt and cytokeratin 20 immunocytochemistry as adjunct markers for urine cytologic detection of bladder cancer: A prospective study. Anal Quant Cytol Histol. 2010;32:45–52. [PubMed] [Google Scholar]
- 17.Hautmann S, Toma M, Lorenzo Gomez MF, Friedrich MG, Jaekel T, Michl U, et al. Immunocyt and the HA-HAase urine tests for the detection of bladder cancer: A side-by-side comparison. Eur Urol. 2004;46:466–71. doi: 10.1016/j.eururo.2004.06.006. [DOI] [PubMed] [Google Scholar]
- 18.Lodde M, Mian C, Comploj E, Palermo S, Longhi E, Marberger M, et al. uCyt+test: Alternative to cystoscopy for less-invasive follow-up of patients with low risk of urothelial carcinoma. Urology. 2006;67:950–4. doi: 10.1016/j.urology.2005.11.057. [DOI] [PubMed] [Google Scholar]
- 19.Messing EM, Teot L, Korman H, Underhill E, Barker E, Stork B, et al. Performance of urine test in patients monitored for recurrence of bladder cancer: A multicenter study in the United States. J Urol. 2005;174:1238–41. doi: 10.1097/01.ju.0000173918.84006.4d. [DOI] [PubMed] [Google Scholar]
- 20.Mian C, Maier K, Comploj E, Lodde M, Berner L, Lusuardi L, et al. uCyt+/ImmunoCyt in the detection of recurrent urothelial carcinoma: An update on 1991 analyses. Cancer. 2006;108:60–5. doi: 10.1002/cncr.21712. [DOI] [PubMed] [Google Scholar]
- 21.Têtu B, Tiguert R, Harel F, Fradet Y. ImmunoCyt/uCyt+ improves the sensitivity of urine cytology in patients followed for urothelial carcinoma. Mod Pathol. 2005;18:83–9. doi: 10.1038/modpathol.3800262. [DOI] [PubMed] [Google Scholar]
- 22.Toma MI, Friedrich MG, Hautmann SH, Jakel KT, Erbersdobler A, Hellstern A, et al. Comparison of the ImmunoCyt test and urinary cytology with other urine tests in the detection and surveillance of bladder cancer. World J Urol. 2004;22:145–9. doi: 10.1007/s00345-003-0390-8. [DOI] [PubMed] [Google Scholar]
- 23.Comploj E, Mian C, Ambrosini-Spaltro A, Dechet C, Palermo S, Trenti E, et al. uCyt+/ImmunoCyt and cytology in the detection of urothelial carcinoma: An update on 7422 analyses. Cancer Cytopathol. 2013;121:392–7. doi: 10.1002/cncy.21287. [DOI] [PubMed] [Google Scholar]
- 24.Pfister C, Chautard D, Devonec M, Perrin P, Chopin D, Rischmann P, et al. Immunocyt test improves the diagnostic accuracy of urinary cytology: Results of a French multicenter study. J Urol. 2003;169:921–4. doi: 10.1097/01.ju.0000048983.83079.4c. [DOI] [PubMed] [Google Scholar]
- 25.Gutiérrez Baños JL, del Henar Rebollo Rodrigo M, Antolín Juárez FM, García BM. Usefulness of the BTA STAT test for the diagnosis of bladder cancer. Urology. 2001;57:685–9. doi: 10.1016/s0090-4295(00)01090-6. [DOI] [PubMed] [Google Scholar]
- 26.van Rhijn BW, van der Poel HG, van der Kwast TH. Urine markers for bladder cancer surveillance: A systematic review. Eur Urol. 2005;47:736–48. doi: 10.1016/j.eururo.2005.03.014. [DOI] [PubMed] [Google Scholar]
- 27.Thomas L, Leyh H, Marberger M, Bombardieri E, Bassi P, Pagano F, et al. Multicenter trial of the quantitative BTA TRAK assay in the detection of bladder cancer. Clin Chem. 1999;45:472–7. [PubMed] [Google Scholar]
- 28.Mahnert B, Tauber S, Kriegmair M, Nagel D, Holdenrieder S, Hofmann K, et al. Measurements of complement factor H-related protein (BTA-TRAK assay) and nuclear matrix protein (NMP22 assay)—useful diagnostic tools in the diagnosis of urinary bladder cancer? Clin Chem Lab Med. 2003;41:104–10. doi: 10.1515/CCLM.2003.018. [DOI] [PubMed] [Google Scholar]
- 29.Ellis WJ, Blumenstein BA, Ishak LM, Enfield DL. Clinical evaluation of the BTA TRAK assay and comparison to voided urine cytology and the Bard BTA test in patients with recurrent bladder tumors. The Multi-Center Study Group. Urology. 1997;50:882–7. doi: 10.1016/s0090-4295(97)00508-6. [DOI] [PubMed] [Google Scholar]
- 30.Oge O, Kozaci D, Gemalmaz H. The BTA stat test is nonspecific for hematuria: An experimental hematuria model. J Urol. 2002;167:1318–20. doi: 10.1016/s0022-5347(05)65290-1. [DOI] [PubMed] [Google Scholar]
- 31.Hennenlotter J, Huber S, Todenhöfer T, Kuehs U, Schilling D, Aufderklamm S, et al. Point-of-care tests for bladder cancer: The influencing role of hematuria. Adv Urol 2011. 2011 doi: 10.1155/2011/937561. 937561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Miyake M, Goodison S, Rizwani W, Ross S, Bart Grossman H, Rosser CJ. Urinary BTA: Indicator of bladder cancer or of hematuria. World J Urol. 2012;30:869–73. doi: 10.1007/s00345-012-0935-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Schroeder GL, Lorenzo-Gomez MF, Hautmann SH, Friedrich MG, Ekici S, Huland H, et al. A side by side comparison of cytology and biomarkers for bladder cancer detection. J Urol. 2004;172:1123–6. doi: 10.1097/01.ju.0000134347.14643.ab. [DOI] [PubMed] [Google Scholar]
- 34.Serretta V, Pomara G, Rizzo I, Esposito E. Urinary BTA-stat, BTA-trak and NMP22 in surveillance after TUR of recurrent superficial transitional cell carcinoma of the bladder. Eur Urol. 2000;38:419–25. doi: 10.1159/000020318. [DOI] [PubMed] [Google Scholar]
- 35.Stampher DS, Carpinito GA, Villanueva JR. Evaluation of NMP 22 in the detection of transitional cell carcinoma of bladder. J Urol. 1998;159:394–8. doi: 10.1016/s0022-5347(01)63930-2. [DOI] [PubMed] [Google Scholar]
- 36.Miyake M, Goodison S, Giacoia EG, Rizwani W, Ross S, Rosser CJ. Influencing factors on the NMP-22 urine assay: An experimental model. BMC Urol. 2012;12:23. doi: 10.1186/1471-2490-12-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Schlake A, Crispen PL, Cap AP, Atkinson T, Davenport D, Preston DM. NMP-22, urinary cytology, and cystoscopy: A 1 year comparison study. Can J Urol. 2012;19:6345–50. [PubMed] [Google Scholar]
- 38.Hwang EC, Choi HS, Jung SI, Kwon DD, Park K, Ryu SB. Use of the NMP22 BladderChek test in the diagnosis and follow-up of urothelial cancer: A cross-sectional study. Urology. 2011;77:154–9. doi: 10.1016/j.urology.2010.04.059. [DOI] [PubMed] [Google Scholar]
- 39.Gupta NP, Sharma N, Kumar R. Nuclear matrix protein 22 as adjunct to urine cytology and cystoscopy in follow-up of superficial TCC of urinary bladder. Urology. 2009;73:592–7. doi: 10.1016/j.urology.2008.04.051. [DOI] [PubMed] [Google Scholar]
- 40.Choi HS, Lee SI, Kim DJ, Jeong TY. Usefulness of the NMP22BladderChek test for screening and follow-up of bladder cancer. Korean J Urol. 2010;51:88–93. doi: 10.4111/kju.2010.51.2.88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Shariat SF, Marberger MJ, Lotan Y, Sanchez-Carbayo M, Zippe C, Lüdecke G, et al. Variability in the performance of nuclear matrix protein 22 for the detection of bladder cancer. J Urol. 2006;176:919–26. doi: 10.1016/j.juro.2006.04.017. [DOI] [PubMed] [Google Scholar]
- 42.Sokolova IA, Halling KC, Jenkins RB, Burkhardt HM, Meyer RG, Seelig SA, et al. The development of a multitarget, multicolor fluorescence in situ hybridization assay for the detection of urothelial carcinoma in urine. J Mol Diagn. 2000;2:116–23. doi: 10.1016/S1525-1578(10)60625-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hajdinjak T. UroVysion FISH test for detecting urothelial cancers: Meta-analysis of diagnostic accuracy and comparison with urinary cytology testing. Urol Oncol. 2008;26:646–51. doi: 10.1016/j.urolonc.2007.06.002. [DOI] [PubMed] [Google Scholar]
- 44.Dimashkieh H, Wolff DJ, Smith TM, Houser PM, Nietert PJ, Yang J. Evaluation of urovysion and cytology for bladder cancer detection: A study of 1835 paired urine samples with clinical and histologic correlation. Cancer Cytopathol. 2013;121:591–7. doi: 10.1002/cncy.21327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bonberg N, Pesch B, Behrens T, Johnen G, Taeger D, Gawrych K, et al. Chromosomal alterations in exfoliated urothelial cells from bladder cancer cases and healthy men: A prospective screening study. BMCCancer. 2014;14:854. doi: 10.1186/1471-2407-14-854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bonberg N, Taeger D, Gawrych K, Johnen G, Banek S, Schwentner C, et al. UroScreen Study Group. Chromosomal instability and bladder cancer: The UroVysion™ test in the UroScreen study. BJU Int. 2013;112:E372–82. doi: 10.1111/j.1464-410X.2012.11666.x. [DOI] [PubMed] [Google Scholar]
- 47.Kipp BR, Karnes RJ, Brankley SM, Harwood AR, Pankratz VS, Sebo TJ, et al. Monitoring intravesical therapy for superficial bladder cancer using fluorescence in situ hybridization. J Urol. 2005;173:401–4. doi: 10.1097/01.ju.0000149825.83180.a4. [DOI] [PubMed] [Google Scholar]
- 48.Savic S, Zlobec I, Thalmann GN, Engeler D, Schmauss M, Lehmann K, et al. The prognostic value of cytology and fluorescence in situ hybridization in the follow-up of nonmuscle-invasive bladder cancer after intravesical Bacillus Calmette-Guerin therapy. Int J Cancer. 2009;124:2899–904. doi: 10.1002/ijc.24258. [DOI] [PubMed] [Google Scholar]
- 49.Whitson J, Berry A, Carroll P, Konety B. A multicolour fluorescence in situ hybridization test predicts recurrence in patients with high-risk superficial bladder tumours undergoing intravesical therapy. BJU Int. 2009;104:336–9. doi: 10.1111/j.1464-410X.2009.08375.x. [DOI] [PubMed] [Google Scholar]
- 50.Yoder BJ, Skacel M, Hedgepeth R, Babineau D, Ulchaker JC, Liou LS, et al. Reflex UroVysion testing of bladder cancer surveillance patients with equivocal or negative urine cytology: A prospective study with focus on the natural history of anticipatory positive findings. Am J Clin Pathol. 2007;127:295–301. doi: 10.1309/ADJL7E810U1H42BJ. [DOI] [PubMed] [Google Scholar]
- 51.Kipp BR, Halling KC, Campion MB, Wendel AJ, Karnes RJ, Zhang J, et al. Assessing the value of reflex fluorescence in situ hybridization testing in the diagnosis of bladder cancer when routine urine cytological examination is equivocal. J Urol. 2008;179:1296–301. doi: 10.1016/j.juro.2007.11.082. [DOI] [PubMed] [Google Scholar]
- 52.Gayed BA, Seideman C, Lotan Y. Cost-effectiveness of fluorescence in situ hybridization in patients with atypical cytology for the detection of urothelial carcinoma. J Urol. 2013;190:1181–6. doi: 10.1016/j.juro.2013.03.117. [DOI] [PubMed] [Google Scholar]
- 53.Kim PH, Sukhu R, Cordon BH, Sfakianos JP, Sjoberg DD, Hakimi AA, et al. Reflex fluorescence in situ hybridization assay for suspicious urinary cytology in patients with bladder cancer with negative surveillance cystoscopy. BJU Int. 2014;114:354–9. doi: 10.1111/bju.12516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Halling KC, King W, Sokolova IA, Karnes RJ, Meyer RG, Powell EL, et al. A comparison of BTA stat, hemoglobin dipstick, telomerase and Vysis UroVysion assays for the detection of urothelial carcinoma in urine. J Urol. 2002;167:2001–6. [PubMed] [Google Scholar]
- 55.Reid-Nicholson MD, Ramalingam P, Adeagbo B, Cheng N, Peiper SC, Terris MK. The use of Urovysion fluorescence in situ hybridization in the diagnosis and surveillance of non-urothelial carcinoma of the bladder. Mod Pathol. 2009;22:119–27. doi: 10.1038/modpathol.2008.179. [DOI] [PubMed] [Google Scholar]
- 56.Lodde M, Mian C, Mayr R, Comploj E, Trenti E, Melotti R, et al. Recurrence and progression in patients with non-muscle invasive bladder cancer: Prognostic models including multicolor fluorescence in situ hybridization molecular grading. Int J Urol. 2014;21:968–72. doi: 10.1111/iju.12509. [DOI] [PubMed] [Google Scholar]
- 57.Lokeshwar V.B, Habuchi T., Grossman H.B, Habuchi T, Grossman HB, et al. Bladder tumor markers beyond cytology: international consensus panel on bladder tumor markers. Urology. 2005;66:35–63. doi: 10.1016/j.urology.2005.08.064. [DOI] [PubMed] [Google Scholar]
- 58.Sánchez-Carbayo M, Herrero E, Megías J, Mira A, Soria F. Initial evaluation of the new urinary bladder cancer rapid test in the detection of transitional cell carcinoma of the bladder. Urology. 1999;54:656–61. doi: 10.1016/s0090-4295(99)00195-8. [DOI] [PubMed] [Google Scholar]
- 59.Ritter R, Hennenlotter J, Kühs U, Hofmann U, Aufderklamm S, Blutbacher P, et al. Evaluation of a new quantitative point-of-care test platform for urine-based detection of bladder cancer. Urol Oncol. 2014;32:337–44. doi: 10.1016/j.urolonc.2013.09.024. [DOI] [PubMed] [Google Scholar]
- 60.Mian C, Lodde M, Haitel A, Egarter Vigl E, Marberger M, Pycha A. Comparison of two qualitative assays, the UBC rapid test and the BTA stat test, in the diagnosis of urothelial cell carcinoma of the bladder. Urology. 2000;56:228–31. doi: 10.1016/s0090-4295(00)00664-6. [DOI] [PubMed] [Google Scholar]
- 61.Hakenberg OW, Fuessel S, Richter K, Froehner M, Oehlschlaeger S, Rathert P, et al. Qualitative and quantitative assessment of urinary cytokeratin 8 and 18 fragments compared with voided urine cytology in diagnosis of bladder carcinoma. Urology. 2004;64:1121–6. doi: 10.1016/j.urology.2004.08.019. [DOI] [PubMed] [Google Scholar]
- 62.May M, Hakenberg OW, Gunia S, Pohling P, Helke C, Lübbe L, et al. Comparative diagnostic value of urine cytology, UBC-ELISA, and fluorescence in situ hybridization for detection of transitional cell carcinoma of urinary bladder in routine clinical practice. Urology. 2007;70:449–53. doi: 10.1016/j.urology.2007.04.023. [DOI] [PubMed] [Google Scholar]
- 63.Alvarez A, Lokeshwar VB. Bladder cancer biomarkers: Current developments and future implementation. Curr Opin Urol. 2007;17:341–6. doi: 10.1097/MOU.0b013e3282c8c72b. [DOI] [PubMed] [Google Scholar]
- 64.Nisman B, Barak V, Shapiro A, Golijanin D, Peretz T, Pode D. Evaluation of urine CYFRA 21-1 for the detection of primary and recurrent bladder carcinoma. Cancer. 2002;94:2914–22. doi: 10.1002/cncr.10565. [DOI] [PubMed] [Google Scholar]
- 65.Gkialas I, Papadopoulos G, Iordanidou L, Stathouros G, Tzavara C, Gregorakis A, et al. Evaluation of urine tumor-associated trypsin inhibitor, CYFRA 21-1, and urinary bladder cancer antigen for detection of high-grade bladder carcinoma. Urology. 2008;72:1159–63. doi: 10.1016/j.urology.2008.04.009. [DOI] [PubMed] [Google Scholar]
- 66.Jeong S, Park Y, Cho Y, Kim YR, Kim HS. Diagnostic values of urine CYFRA21-1, NMP22, UBC, and FDP for the detection of bladder cancer. Clin Chim Acta. 2012;414:93–100. doi: 10.1016/j.cca.2012.08.018. [DOI] [PubMed] [Google Scholar]
- 67.Santoni M, Catanzariti F, Minardi D, Burattini L, Nabissi M, Muzzonigro G, et al. Pathogenic and diagnostic potential of BLCA-1 and BLCA-4 nuclear proteins in urothelial cell carcinoma of human bladder. Adv Urol 2012. 2012 doi: 10.1155/2012/397412. 397412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Nielsen ME, Gonzalgo ML, Schoenberg MP, Getzenberg RH. Toward critical evaluation of the role(s) of molecular biomarkers in the management of bladder cancer. World J Urol. 2006;24:499–508. doi: 10.1007/s00345-006-0116-9. [DOI] [PubMed] [Google Scholar]
- 69.Myers-Irvin JM, Landsittel D, Getzenberg RH. Use of the novel marker BLCA-1 for the detection of bladder cancer. J Urol. 2005;174:64–8. doi: 10.1097/01.ju.0000162022.36772.a4. [DOI] [PubMed] [Google Scholar]
- 70.Konety BR, Nguyen TS, Dhir R, Day RS, Becich MJ, Stadler WM, et al. Detection of bladder cancer using a novel nuclear matrix protein, BLCA-4. Clin Cancer Res. 2000;6:2618–25. [PubMed] [Google Scholar]
- 71.Van Le TS, Miller R, Barder T, Babjuk M, Potter DM, Getzenberg RH. Highly specific urine-based marker of bladder cancer. Urology. 2005;66:1256–60. doi: 10.1016/j.urology.2005.07.010. [DOI] [PubMed] [Google Scholar]
- 72.Van Le TS, Myers J, Konety BR, Barder T, Getzenberg RH. Functional characterization of the bladder cancer marker, BLCA-4. ClinCancer Res. 2004;10:1384–91. doi: 10.1158/1078-0432.ccr-0455-03. [DOI] [PubMed] [Google Scholar]
- 73.Konety BR, Nguyen TS, Brenes G, Sholder A, Lewis N, Bastacky S, et al. Clinical usefulness of the novel marker BLCA-4 for the detection of bladder cancer. J Urol. 2000;164:634–9. doi: 10.1097/00005392-200009010-00004. [DOI] [PubMed] [Google Scholar]
- 74.Vlahou A, Schellhammer PF, Mendrinos S, Patel K, Kondylis FI, Gong L, et al. Development of a novel proteomic approach for the detection of transitional cell carcinoma of the bladder in urine. Am J Pathol. 2001;158:1491–502. doi: 10.1016/S0002-9440(10)64100-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Holterman DA, Diaz JI, Blackmore PF, Davis JW, Schellhammer PF, Corica A, et al. Overexpression of alpha-defensin is associated with bladder cancer invasiveness. Urol Oncol. 2006;24:97–108. doi: 10.1016/j.urolonc.2005.07.010. [DOI] [PubMed] [Google Scholar]
- 76.Theodorescu D, Wittke S, Ross MM, Walden M, Conaway M, Just I, et al. Discovery and validation of new protein biomarkers for urothelial cancer: A prospective analysis. Lancet Oncol. 2006;7:230–40. doi: 10.1016/S1470-2045(06)70584-8. [DOI] [PubMed] [Google Scholar]
- 77.Chen YT, Chen CL, Chen HW, Wu CC, Chen CD, Hsu CW, et al. Discovery of novel bladder cancer biomarkers by comparative urine proteomics using iTRAQ technology. J Proteome Res. 2010;9:5803–15. doi: 10.1021/pr100576x. [DOI] [PubMed] [Google Scholar]
- 78.Yang N, Feng S, Shedden K, Xie X, Liu Y, Rosser CJ, et al. Urinary glycoprotein biomarker discovery for bladder cancer detection using LC/MS-MS and label-free quantification. Clin Cancer Res. 2011;17:3349–59. doi: 10.1158/1078-0432.CCR-10-3121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Goodison S, Chang M, Dai Y, Urquidi V, Rosser CJ. A multianalyte assay for the non-invasive detection of bladder cancer. PLoS One. 2012;7:e47469. doi: 10.1371/journal.pone.0047469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Wu XR. Urothelial tumorigenesis: A tale of divergent pathways. Nat Rev Cancer. 2005;5:713–25. doi: 10.1038/nrc1697. [DOI] [PubMed] [Google Scholar]
- 81.Guo G, Sun X, Chen C, Wu S, Huang P, Li Z, et al. Whole-genome and whole-exome sequencing of bladder cancer identifies frequent alterations in genes involved in sister chromatid cohesion and segregation. Nat Genet. 2013;45:1459–63. doi: 10.1038/ng.2798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Cancer Genome Atlas Research Network. Comprehensive molecular characterization of urothelial bladder carcinoma. Nature. 2014;507:315–22. doi: 10.1038/nature12965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Kompier LC, Lurkin I, van der Aa MN, van Rhijn BW, van der Kwast TH, Zwarthoff EC. FGFR3, HRAS, KRAS, NRAS and PIK3CA mutations in bladder cancer and their potential as biomarkers for surveillance and therapy. PLoS One. 2010;5:e13821. doi: 10.1371/journal.pone.0013821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.van der Aa MN, Zwarthoff EC, Steyerberg EW, Boogaard MW, Nijsen Y, van der Keur KA, et al. Microsatellite analysis of voided-urine samples for surveillance of low-grade non-muscle invasive urothelial carcinoma: Feasibility and clinical utility in a prospective multicenter study (Cost-Effectiveness of Follow-Up of Urinary Bladder Cancer trial [CEFUB]) Eur Urol. 2009;55:659–67. doi: 10.1016/j.eururo.2008.05.001. [DOI] [PubMed] [Google Scholar]
- 85.Zuiverloon TC, van der Aa MN, van der Kwast TH, Steyerberg EW, Lingsma HF, Bangma CH, et al. Fibroblast growth factor receptor 3 mutation analysis on voided urine for surveillance of patients with low-grade non-muscle-invasive bladder cancer. Clin Cancer Res. 2010;16:3011–8. doi: 10.1158/1078-0432.CCR-09-3013. [DOI] [PubMed] [Google Scholar]
- 86.Esteller M. Epigenetics in cancer. N Engl J Med. 2008;358:1148–59. doi: 10.1056/NEJMra072067. [DOI] [PubMed] [Google Scholar]
- 87.Sánchez-Carbayo M. Hypermethylation in bladder cancer: Biological pathways and translational applications. Tumour Biol. 2012;33:347–61. doi: 10.1007/s13277-011-0310-2. [DOI] [PubMed] [Google Scholar]
- 88.Chung W, Bondaruk J, Jelinek J, Lotan Y, Liang S, Czerniak B, et al. Detection of bladder cancer using novel DNA methylation biomarkers in urine sediments. Cancer Epidemiol Biomarkers Prev. 2011;20:1483–91. doi: 10.1158/1055-9965.EPI-11-0067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Reinert T, Modin C, Castano FM, Lamy P, Wojdacz TK, Hansen LL, et al. Comprehensive genome methylation analysis in bladder cancer: Identification and validation of novel methylated genes and application of these as urinary tumor markers. Clin Cancer Res. 2011;17:5582–92. doi: 10.1158/1078-0432.CCR-10-2659. [DOI] [PubMed] [Google Scholar]
- 90.Kandimalla R, van Tilborg AA, Zwarthoff EC. DNA methylation-based biomarkers in bladder cancer. Nat Rev Urol. 2013;10:327–35. doi: 10.1038/nrurol.2013.89. [DOI] [PubMed] [Google Scholar]
- 91.Marsit CJ, Houseman EA, Christensen BC, Gagne L, Wrensch MR, Nelson HH, et al. Identification of methylated genes associated with aggressive bladder cancer. PLoS One. 2010;5:e12334. doi: 10.1371/journal.pone.0012334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Kim JS, Chae Y, Ha YS, Kim IY, Byun SS, Yun SJ, et al. Ras association domain family 1A: A promising prognostic marker in recurrent nonmuscle invasive bladder cancer. Clin GenitourinCancer. 2012;10:114–20. doi: 10.1016/j.clgc.2011.12.003. [DOI] [PubMed] [Google Scholar]
- 93.Yan C, Kim YW, Ha YS, Kim IY, Kim YJ, Yun SJ, et al. RUNX3 methylation as a predictor for disease progression in patients with non-muscle-invasive bladder cancer. J Surg Oncol. 2012;105:425–30. doi: 10.1002/jso.22087. [DOI] [PubMed] [Google Scholar]
- 94.Zhao Y, Guo S, Sun J, Huang Z, Zhu T, Zhang H, et al. Methylcap-seq reveals novel DNA methylation markers for the diagnosis and recurrence prediction of bladder cancer in a Chinese population. PLoS One. 2012;7:e35175. doi: 10.1371/journal.pone.0035175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Zuiverloon TC, Beukers W, van der Keur KA, Munoz JR, Bangma CH, Lingsma HF, et al. A methylation assay for the detection of non-muscle-invasive bladder cancer (NMIBC) recurrences in voided urine. BJU Int. 2012;109:941–8. doi: 10.1111/j.1464-410X.2011.10428.x. [DOI] [PubMed] [Google Scholar]
- 96.Reinert T. Methylation markers for urine-based detection of bladder cancer: The next generation of urinary markers for diagnosis and surveillance of bladder cancer. Adv Urol 2012. 2012 doi: 10.1155/2012/503271. 503271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Hanke M, Kausch I, Dahmen G, Jocham D, Warnecke JM. Detailed technical analysis of urine RNA-based tumor diagnostics reveals ETS2/urokinase plasminogen activator to be a novel marker for bladder cancer. Clin Chem. 2007;53:2070–7. doi: 10.1373/clinchem.2007.091363. [DOI] [PubMed] [Google Scholar]
- 98.Margulis V, Lotan Y, Shariat SF. Survivin: A promising biomarker for detection and prognosis of bladder cancer. World J Urol. 2008;26:59–65. doi: 10.1007/s00345-007-0219-y. [DOI] [PubMed] [Google Scholar]
- 99.Shariat SF, Casella R, Khoddami SM, Hernandez G, Sulser T, Gasser TC, et al. Urine detection of Survivin is a sensitive marker for the noninvasive diagnosis of bladder cancer. J Urol. 2004;171:626–30. doi: 10.1097/01.ju.0000107826.78479.90. [DOI] [PubMed] [Google Scholar]
- 100.Horstmann M, Bontrup H, Hennenlotter J, Taeger D, Weber A, Pesch B, et al. Clinical experience with surviving as a biomarker for urothelial bladder cancer. World J Urol. 2010;28:399–404. doi: 10.1007/s00345-010-0538-2. [DOI] [PubMed] [Google Scholar]
- 101.Srivastava AK, Singh PK, Srivastava K, Singh D, Dalela D, Rath SK, et al. Diagnostic role of survivin in urinary bladder cancer. Asian Pac J Cancer Prev. 2013;14:81–5. doi: 10.7314/apjcp.2013.14.1.81. [DOI] [PubMed] [Google Scholar]
- 102.Johnen G, Gawrych K, Bontrup H, Pesch B, Taeger D, Banek S, et al. Performance of survivin mRNA as a biomarker for bladder cancer in the prospective study UroScreen. PLoS One. 2012;7:e35363. doi: 10.1371/journal.pone.0035363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Sanchini MA, Gunelli R, Nanni O, Bravaccini S, Fabbri C, Sermasi A, et al. Relevance of urine telomerase in the diagnosis of bladder cancer. JAMA. 2005;294:2052–6. doi: 10.1001/jama.294.16.2052. [DOI] [PubMed] [Google Scholar]
- 104.Brems-Eskildsen AS, Zieger K, Toldbod H, Holcomb C, Higuchi R, Mansilla F, et al. Prediction and diagnosis of bladder cancer recurrence based on urinary content of hTERT, SENP1, PPP1CA, and MCM5 transcripts. BMC Cancer. 2010;10:646. doi: 10.1186/1471-2407-10-646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Sartini D, Muzzonigro G, Milanese G, Pozzi V, Vici A, Morganti S, et al. Upregulation of tissue and urinary nicotinamide N methyltransferase in bladder cancer: Potential for the development of a urine-based diagnostic test. Cell Biochem Biophys. 2013;65:473–83. doi: 10.1007/s12013-012-9451-1. [DOI] [PubMed] [Google Scholar]
- 106.Holyoake A, O’Sullivan P, Pollock R, Best T, Watanabe J, Kajita Y, et al. Development of a multiplex RNA urine test for the detection and stratification of transitional cell carcinoma of the bladder. Clin Cancer Res. 2008;14:742–9. doi: 10.1158/1078-0432.CCR-07-1672. [DOI] [PubMed] [Google Scholar]
- 107.O’Sullivan P, Sharples K, Dalphin M, Davidson P, Gilling P, Cambridge L, et al. A multigene urine test for the detection and stratification of bladder cancer in patients presenting with hematuria. J Urol. 2012;188:741–7. doi: 10.1016/j.juro.2012.05.003. [DOI] [PubMed] [Google Scholar]
- 108.Bartel DP. MicroRNAs: Genomics, biogenesis, mechanism, and function. Cell. 2004;116:281–97. doi: 10.1016/s0092-8674(04)00045-5. [DOI] [PubMed] [Google Scholar]
- 109.Alvarez-Garcia I, Miska EA. MicroRNA functions in animal development and human disease. Development. 2005;132:4653–62. doi: 10.1242/dev.02073. [DOI] [PubMed] [Google Scholar]
- 110.Catto JW, Miah S, Owen HC, Bryant H, Myers K, Dudziec E, et al. Distinct micro RNA alterations characterize high and low grade bladder cancer. Cancer Res. 2009;69:8472–81. doi: 10.1158/0008-5472.CAN-09-0744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Guancial EA, Bellmunt J, Yeh S, Rosenberg JE, Berman DM. The evolving understanding of microRNA in bladder cancer. Urol Oncol. 2014;32:41.e31–40. doi: 10.1016/j.urolonc.2013.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Ratert N, Meyer HA, Jung M, Lioudmer P, Mollenkopf HJ, Wagner I, et al. miRNA profiling identifies candidate miRNAs for bladder cancer diagnosis and clinical outcome. J Mol Diagn. 2013;15:695–705. doi: 10.1016/j.jmoldx.2013.05.008. [DOI] [PubMed] [Google Scholar]
- 113.Rosenberg E, Baniel J, Spector Y, Faerman A, Meiri E, Aharonov R, et al. Predicting progression of bladder urothelial carcinoma using microRNA expression. BJU Int. 2013;112:1027–34. doi: 10.1111/j.1464-410X.2012.11748.x. [DOI] [PubMed] [Google Scholar]
- 114.Mengual L, Lozano JJ, Ingelmo-Torres M, Gazquez C, Ribal MJ, Alcaraz A. Using microRNA profiling in urine samples to develop a non-invasive test for bladder cancer. Int J Cancer. 2013;133:2631–41. doi: 10.1002/ijc.28274. [DOI] [PubMed] [Google Scholar]
- 115.Dorn GW, 2nd, Matkovich SJ. Menage a trois: Intimate relationship between microRNA, long noncoding RNA, and mRNA. Circ Res. 2014;114:1362–5. doi: 10.1161/CIRCRESAHA.114.303786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Köhler CU, Bryk O, Meier S, Lang K, Rozynek P, Brüning T, et al. Analyses in human urothelial cells identify methylation of miR-152, miR-200b and miR-10a genes as candidate bladder cancer biomarkers. Biochem Biophys Res Commun. 2013;438:48–53. doi: 10.1016/j.bbrc.2013.07.021. [DOI] [PubMed] [Google Scholar]
- 117.Yun SJ, Jeong P, Kim WT, Kim TH, Lee YS, Song PH, et al. Cell-free microRNAs in urine as diagnostic and prognostic biomarkers of bladder cancer. Int J Oncol. 2012;41:1871–8. doi: 10.3892/ijo.2012.1622. [DOI] [PubMed] [Google Scholar]
- 118.Wszolek MF, Rieger-Christ KM, Kenney PA, Gould JJ, Silva Neto B, Lavoie AK, et al. A MicroRNA expression profile defining the invasive bladder tumor phenotype. Urol Oncol. 2011;29:794–801. doi: 10.1016/j.urolonc.2009.08.024. [DOI] [PubMed] [Google Scholar]
- 119.Nana-Sinkam SP, Croce CM. Clinical applications for microRNAs in cancer. Clin Pharmacol Ther. 2013;93:98–104. doi: 10.1038/clpt.2012.192. [DOI] [PubMed] [Google Scholar]
- 120.Hanke M, Hoefig K, Merz H, Feller AC, Kausch I, Jocham D, et al. A robust methodology to study urine microRNA as tumor marker: MicroRNA-126 and microRNA-182 are related to urinary bladder cancer. Urol Oncol. 2010;28:655–61. doi: 10.1016/j.urolonc.2009.01.027. [DOI] [PubMed] [Google Scholar]
- 121.Miah S, Dudziec E, Drayton RM, Zlotta AR, Morgan SL, Rosario DJ, et al. An evaluation of urinary microRNA reveals a high sensitivity for bladder cancer. Br J Cancer. 2012;107:123–8. doi: 10.1038/bjc.2012.221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Frantzi M, Makridakis M, Vlahou A. Biomarkers for bladder cancer aggressiveness. Curr Opin Urol. 2012;22:390–6. doi: 10.1097/MOU.0b013e328356ad0e. [DOI] [PubMed] [Google Scholar]
- 123.Bangma CH, Loeb S, Busstra M, Zhu X, El Bouazzaoui S, Refos J, et al. Outcomes of a bladder cancer screening program using home hematuria testing and molecular markers. Eur Urol. 2013;64:41–7. doi: 10.1016/j.eururo.2013.02.036. [DOI] [PubMed] [Google Scholar]
- 124.Kamat AM, Hegarty PK, Gee JR, Clark PE, Svatek RS, Hegarty N, et al. InternationalConsultationon Urologic Disease-European Association of Urology Consultationon Bladder Cancer2012. ICUD-EAU International Consultation on Bladder Cancer 2012: Screening, diagnosis, and molecular markers. Eur Urol. 2013;63:4–15. doi: 10.1016/j.eururo.2012.09.057. [DOI] [PubMed] [Google Scholar]


