Abstract
Introduction:
Nonmuscle invasive bladder cancer (NMIBC) is the most common presentation of bladder cancer and is often treatable with endoscopic resection and intravesical therapies. Cystoscopy and urine cytology are the gold standard in diagnosis and surveillance but are limited by their sensitivity in some situations. We seek to provide an overview of recent additions to the diagnostic armamentarium for urologists treating this disease.
Methods:
Articles were identified through a literature review of articles obtained through PubMed searches including the terms “bladder cancer” and various diagnostic techniques described in the article.
Results:
A variety of urinary biomarkers are available to assist the diagnosis and management of patients with NMIBC. Many have improved sensitivity over urine cytology, but less specificity. There are certain situations in which this has proved valuable, but as yet these are not part of the standard guidelines for NMIBC. Fluorescence cystoscopy has level 1 evidence demonstrating increased rates of tumor detection and prolonged recurrence-free survival when utilized for transurethral resection. Other technologies seeking to enhance cystoscopy, such as narrow band imaging, confocal laser endomicroscopy, and optical coherence tomography are still under evaluation.
Conclusions:
A variety of urine biomarker and adjunctive endoscopic technologies have been developed to assist the management of NMIBC. While some, such as fluorescence cystoscopy, have demonstrated a definite benefit in this disease, others are still finding their place in the diagnosis and treatment of this disease. Future studies should shed light on how these can be incorporated to improve outcomes in NMIBC.
Keywords: Biological markers, cystoscopy, cytology, diagnostic techniques, urinary bladder neoplasms, urological
INTRODUCTION
Bladder cancer is the second most common genitourinary malignancy after prostate cancer and the ninth most common cancer in the world. In 2012, there were 430,000 new cases diagnosed worldwide with a male to female ratio of 3:1.[1] At presentation, 85% of patients have nonmuscle invasive bladder cancer (NMIBC) which compromises stages Tis, Ta and T1.[2] Despite complete gross resection, bladder cancer has a high rate of recurrence (50–70%) within 5 years, and up to 20% of NMIBC will progress to muscle-invasive disease and require radical treatment.[3] Thus, early diagnosis, complete resection, and close surveillance are essential to reduce disease progression and morbidity from radical treatment.
Cystoscopy and urine cytology are the gold standard in the diagnostic assessment of patients with suspicion for bladder cancer (e.g., hematuria),[4,5,6] as well as surveillance in those with a history of NMIBC. The standard approach for cystoscopy is white light cystoscopy (WLC), which has undergone significant technological advances with improved image quality in modern WLC. Despite this, WLC has numerous limitations that affect its accuracy to detect and stage bladder cancer. These limitations include the inability to accurately detect flat carcinoma in situ (CIS), which is missed in up to 20%, and difficulty in distinguishing benign reactive lesions from malignancy, particularly in those with prior transurethral resection (TUR) or intravesical therapy.[7] To address these shortcomings, complementary endoscopic technologies have been developed to improve the detection rate, and include fluorescence cystoscopy (FC), narrow band imaging (NBI), confocal laser endomicroscopy (CLE) and optical coherence tomography (OCT). Although these methods can improve detection and/or accuracy, they are invasive, expensive, and time consuming. Urine cytology and other urinary biomarkers, on the other hand, offer a noninvasive way to detect bladder cancer. Though urine cytology is a part of the standard evaluation for those at risk of bladder cancer, its utility is limited by its low sensitivity, particularly for low grade tumors. To attempt to address this low sensitivity, a variety of U.S. Food and Drug Administration-approved urinary biomarkers have been developed to improve the diagnosis and monitoring of patients with bladder cancer.
METHODS
Using the PubMed/Medline search engine, we conducted a computer search with the term bladder cancer in combination with one of the following: Photodynamic diagnosis (PDD); fluorescent cystoscopy; NBI; CLE; OCT; biomarkers; and cytology. The search was limited to articles in English but not to any time period or article type. Focus was mainly on original studies with large cohorts and systematic reviews with meta-analysis to report the pooled data. We also used the references of the retrieved articles, when relevant, to conduct a manual search of additional studies.
Laboratory diagnostics
For the sake of brevity, a summary related to urine testing for the detection of bladder cancer is provided here as a more thorough review is contained elsewhere within this issue. Urine cytology is the mainstay in the diagnosis of bladder cancer due to its high specificity for high grade urothelial carcinoma though it is limited by its low sensitivity. A systematic review of urinary markers in 2005 found cytology had a median sensitivity and specificity of 35% and 94%, respectively, however in grade 1 tumors the sensitivity was only 17% compared with 58% in grade 3 tumors.[8] Other urinary tests are currently available which have improved sensitivity, and include NMP22, bladder tumor antigen (BTA) stat/BTA TRAK, ImmunoCyt, and UroVysion fluorescence in situ hybridization (FISH). These tests often sacrifice specificity for the increase in sensitivity, though have some unique characteristics which can make them useful to the practicing urologist. Some of the clinical characteristics of these assays are shown in Table 1, with data obtained from two recent prospective trials which directly compared a variety of assays on the same population.[9,10]
Table 1.
Comparing single test and combination test sensitivities and specificities from 2 recent prospective studies which compared all the various markers on the same samples[9,10]
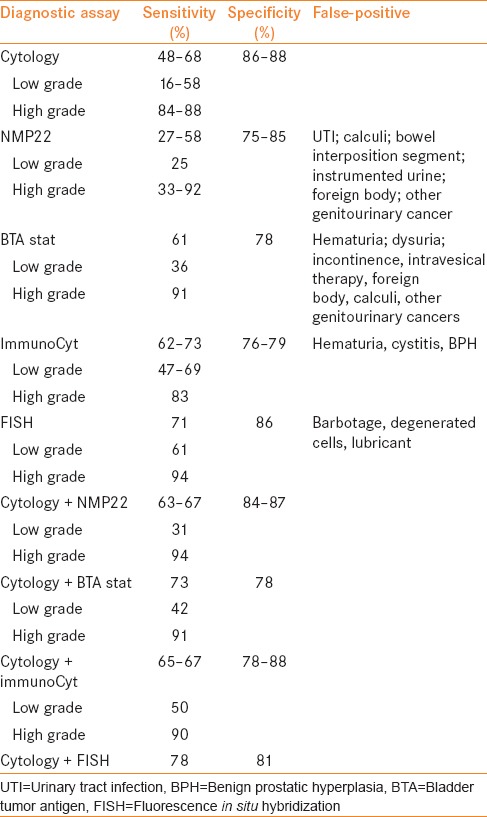
In two prospective trials, NMP22 was found to have a sensitivity around 50%,[11,12] and there is some evidence that NMP22 has a sensitivity even higher for low grade disease,[13] where cytology performs poorly. The BTA tests similarly have higher sensitivity than cytology,[14] though appears to perform better in high grade tumors. A drawback of both the NMP22 and BTA tests are limitations due to false-positives in a variety of clinical settings as shown in Table 1. ImmunoCyt combines an immunoassay for 3 specific antigens with cytology and has a sensitivity and specificity in the range of 70–75%. One potential benefit of this assay is its ability to predict future recurrence in those with a negative cystoscopy,[15] and the FISH assay has been shown to have the same capability.[16] FISH also is useful in the setting of an atypical cytology and negative/equivocal cystoscopy, with an overall negative predictive value of 98.7% in two prospective series.[17,18] Additionally, a number of studies have demonstrated that FISH can be used to stratify patients following bacillus Calmette-Guérin (BCG) treatment and identify those at higher risk of recurrence and progression.[19,20,21] Thus, some difficult clinical scenarios certainly can benefit from the addition of one of these markers to the standard cystoscopic and cytologic evaluation, though urine cytology remains the standard test for the detection of initial and recurrent bladder cancer according to all current guidelines.[4,5,6]
ENDOSCOPIC EVALUATION
White light cystoscopy
WLC is an endoscopic technique to visualize the urethra, bladder, and ureteric orifices. It is the gold standard for the examination and diagnosis of cancer of the lower urinary tract, using either flexible or rigid cystoscopy.[4,5,6] WLC has a sensitivity of 85–90% for detecting papillary tumors,[22] and lower sensitivity (up to 67%) to detect CIS. Flexible cystoscopy is often performed for initial diagnosis and surveillance though it can miss up to 10% of papillary tumors when compared to rigid cystoscopy.[22] While flexible cystoscopy is more comfortable and convenient for the patient, the diagnostic yield of endoscopic removal of the tumor using this approach is limited, which can potentially compromise grading and staging of the tumor.[23] Flexible cystoscopy thus can be used in the primary evaluation or surveillance of BC patients, and TUR of bladder tumor (TURBT) can be then conducted using rigid cystoscopy when needed. WLC has the advantage of being widely available and has lower cost than all the newer endoscopic techniques; yet WLC has lower sensitivity to detect flat and CIS lesions, has limited ability to differentiate benign from malignant lesions, and is operator dependent. Even when all visible tumor is removed, there appears to be remaining cancer in 21% of single tumor cases and 46% of multiple tumor cases.[24]
Fluorescent cystoscopy
FC, also known PDD, is a modification of WLC where an intravesical agent is instilled, and blue light (375–440 nm) is used for visualization. The instilled agents are photoactive porphyrin analogs, such as a 5-aminolevulinic acid (5-ALA) and hexaminolevulinate, which are taken up by epithelial cells and used in the formation of intermediate photoactive porphyrins. Intermediate porphyrins accumulate preferentially in neoplastic cells because of the accelerated enzymatic activity, and after excitation with blue light will return to lower energy levels and fluoresce. Tumor tissues will thus appear as well demarcated bright red lesions against a dark blue background [Figure 1].[25] In a meta-analysis of studies comparing PDD to WLC, PDD was found to have higher sensitivity (92% [confidence interval [CI]: 80–100]) than WLC (71% [CI: 49–93%]), but lower specificity (57% [CI: 36–79] vs. 72% [CI: 47–96]).[26] PDD has a higher sensitivity for CIS (92.4%) compared to WLC (60.5%),[27] but less pronounced differences for other lesions: 94–97% for PDD compared with 83–88% for WLC in the detection of Ta lesions,[28,29] and 10% more T1 lesions detected by PDD compared to WLC.[29,30] No difference was detected for MIBC,[30] thus its main advantage lies in the detection of NMIBC. Moreover, on repeat TUR, significantly fewer lesions were found when the initial TURBT was done using PDD compared to conventional WLC: 4% compared to 28% for CIS lesions, 15% compared to 35% for pT1 lesions, and 17% compared to 37% for high grade lesions.[31] There are several phase III trials which have demonstrated the utility of PDD in tumor detection and recurrence prevention. A meta-analysis of 12 randomized clinical trials, comparing the outcome of TUR using FC vs. WLC, showed that the FC decreases recurrence (odds ratio [OR] 0.5; P < 0.0001).[32] Moreover, the recurrence-free survival at 1 year (OR 0.69; P < 0.0001) and 2 years (OR 0.65; P < 0.0004) were improved in the group who underwent resection with FC compared to the group who had TUR with WLC, but there was no effect on the rate of progression to MIBC. One caveat is that PDD should be postponed 9–12 weeks post-BCG treatment to avoid false-positive results.
Figure 1.
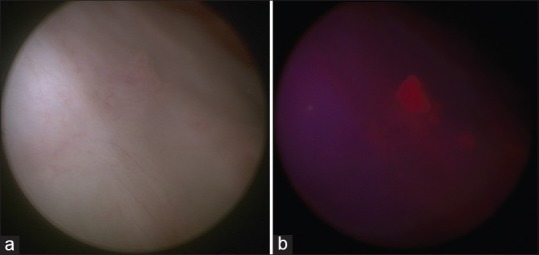
(a) White light cystoscopic image of a small bladder tumor. (b) Blue light cystoscopic image of the same small bladder tumor
Narrow band imaging
NBI is another modification to conventional WLC, where discrete blue (415 nm) and green (540 nm) light bands are used instead of the entire visible light spectrum. These two discrete light bands are strongly absorbed by hemoglobin, hence enhancing the visualization of the mucosal vascular structures. While the blue light gets reflected by the mucosal capillaries, the green light gets reflected by the deeper submucosal vessels. Papillary tumors that typically have an underlying vascular stalk that appears dark green and flat CIS lesions that have dense capillaries will be distinguishable from normal mucosa. In a meta-analysis of all prospective studies, 17% more patients and 24% more cancerous lesions were detected when NBI versus WLC was used. Moreover, 28% of CIS lesions that were missed by WLC were detected by NBI.[33] In a prospective randomized clinical trial to study the recurrence of bladder cancer, the 1 year recurrence rate was 32.9% in those who underwent TURBT using NBI, compared to 51.4% in those who underwent TURBT using WLC (OR = 0.62, P = 0.0141).[34] A second look using NBI, 1 month after TURBT using WLC, identified 13% patients with residual/recurrent tumor.[35]
Confocal laser endomicroscopy
CLE is based on optical biopsy and high resolution in vivo subsurface imaging that enables the visualization of tissue microarchitecture and cellular features. It utilizes a 488 nm laser as the light source and fluorescein as an exogenous contrast agent. The fluorescein may be administered either intravenous or intravesical.[36] The system currently available clinically (Cellvizio, Mauna Kea Technologies) utilizes miniaturized fiberoptic imaging probes that can be passed through the working channels of standard endoscopes.[37] The images are acquired as video sequences at 12 frames/s, which also enables dynamic imaging of physiologic parameters (e.g., vascular flow) besides the tissue microarchitecture. Real time microscopy of normal urothelium, inflammation, CIS, low grade cancer (presence of fibrovascular stalks bordered by monomorphic cells) and high grade urothelial carcinoma (where the microarchitecture is distorted) have been demonstrated with images comparable to conventional histopathology, and is the basis of this technology.[38] However, since CLE has a limited visual field, it cannot serve as tool to survey the whole bladder. It has been investigated as an adjunct to PDD to reduce the false-positive rate, which is higher than that of WLC,[39] providing histologic information about the tumor area and edges to be resected, and visualization of the deep muscular layer only after the lesion has been resected.[36] The optical depth of CLE is limited to lamina propria, and hence the assessment of muscularis propria cannot be performed prior to resection.
Optical coherence tomography
OCT is an optical equivalent of ultrasound that enables cross-sectional imaging of tissue, but different from ultrasound by using infrared light instead of sound waves, and having 10 times higher resolution.[40] It enables the evaluation of luminal surfaces of biological tissue with spatial resolution close to the cellular level at 15–20 μm, visualization of subsurface structures in vivo deeper than what can be seen by CLE. Using this technology, the urothelium appears as an area of low intensity, followed by high intensity lamina propria, and low intensity muscularis propria. While these three layers are well distinguished in a normal bladder, the contrast is lost in MIBC wherein distinct layers or boundaries are disrupted.[41,42] OCT also appears to be helpful in detecting NMIBC and CIS on diagnostic cystoscopy as well as recurrent tumors.[40] The sensitivity of OCT is 98%, and specificity is 72% for the detection of pathologically confirmed tumors.[42] Moreover, OCT was able to discriminate, in an ex vivo study, between normal, CIS and invasive urothelial cell carcinoma (UCC) with a sensitivity of 83.8%, specificity of 78.1% and a false-positive rate 5.7%.[43] For different stages of bladder UCC, OCT had 90% sensitivity, 89% specificity, for Ta lesions; and 75% sensitivity, 97% specificity for T1 lesions; and 100% sensitivity, 90% specificity for T2 lesions.[44] OCT also reliably identifies the accumulation of inflammatory exudate under the epithelium, such in chronic cystitis, and thus differentiates malignant lesions from inflammatory ones.[45]
CONCLUSIONS
The standard evaluation of those with suspicion for new or recurrent bladder cancer is WLC and urine cytology, and this has not changed despite the development of these new technologies. Urine cytology is useful mainly due to its high specificity and PPV though overall its sensitivity is low. A number of newer urinary markers are available which offer improved sensitivity, often at the cost of specificity. There are clinical settings in which these characteristics may be useful, such as those in which the cytology and/or cystoscopy is difficult to interpret. Other roles will likely be determined in future prospective studies. PDD appears to improve on WLC in terms of identifying more cancerous lesions and decreasing recurrence as determined in multiple randomized trials and is being used with increasing frequency due to these benefits. Further similar studies on NBI, CLE, and OCT will likely determine the role of these technologies in NMIBC diagnosis and management.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Ferlay J, Soerjomataram I, Ervik M, Dikshit R, Eser S, Mathers C, et al. Lyon, France: International Agency for Research on Cancer; 2013. GLOBOCAN 2012 v1.0, Cancer Incidence and Mortality Worldwide: IARC Cancer Base No. 11. [Google Scholar]
- 2.Babjuk M, Oosterlinck W, Sylvester R, Kaasinen E, Böhle A, Palou-Redorta J, et al. EAU guidelines on non-muscle-invasive urothelial carcinoma of the bladder, the 2011 update. Actas Urol Esp. 2012;36:389–402. doi: 10.1016/j.acuro.2011.12.001. [DOI] [PubMed] [Google Scholar]
- 3.Holmäng S, Hedelin H, Anderström C, Holmberg E, Busch C, Johansson SL. Recurrence and progression in low grade papillary urothelial tumors. J Urol. 1999;162:702–7. doi: 10.1097/00005392-199909010-00019. [DOI] [PubMed] [Google Scholar]
- 4.NCCN Clinical Practice Guidelines in Oncology. Bladder cancer. [Last accessed on 2015 July 4]. Available from: http://www.tri-kobe.org/nccn/guideline/urological/english/bladder.pdf .
- 5.Babjuk M, Burger M, Zigeuner R, Shariat SF, van Rhijn BW, Compérat E, et al. EAU guidelines on non-muscle-invasive urothelial carcinoma of the bladder: Update 2013. Eur Urol. 2013;64:639–53. doi: 10.1016/j.eururo.2013.06.003. [DOI] [PubMed] [Google Scholar]
- 6.American Urological Association. Bladder Cancer: Guideline for the Management of Nonmuscle Invasive Bladder Cancer: (Stages Ta, T1, and Tis); 2007 update. [Last accessed on 2015 July 4]. Available from: http://www.auanet.org/common/pdf/education/clinical-guidance/Bladder-Cancer.pdf .
- 7.Jichlinski P, Leisinger HJ. Fluorescence cystoscopy in the management of bladder cancer: Help for the urologist! Urol Int. 2005;74:97–101. doi: 10.1159/000083277. [DOI] [PubMed] [Google Scholar]
- 8.van Rhijn BW, van der Poel HG, van der Kwast TH. Urine markers for bladder cancer surveillance: A systematic review. Eur Urol. 2005;47:736–48. doi: 10.1016/j.eururo.2005.03.014. [DOI] [PubMed] [Google Scholar]
- 9.Yafi FA, Brimo F, Steinberg J, Aprikian AG, Tanguay S, Kassouf W. Prospective analysis of sensitivity and specificity of urinary cytology and other urinary biomarkers for bladder cancer. Urol Oncol. 2015;33:66.e25–31. doi: 10.1016/j.urolonc.2014.06.008. [DOI] [PubMed] [Google Scholar]
- 10.Todenhöfer T, Hennenlotter J, Esser M, Mohrhardt S, Tews V, Aufderklamm S, et al. Combined application of cytology and molecular urine markers to improve the detection of urothelial carcinoma. Cancer Cytopathol. 2013;121:252–60. doi: 10.1002/cncy.21247. [DOI] [PubMed] [Google Scholar]
- 11.Grossman HB, Soloway M, Messing E, Katz G, Stein B, Kassabian V, et al. Surveillance for recurrent bladder cancer using a point-of-care proteomic assay. JAMA. 2006;295:299–305. doi: 10.1001/jama.295.3.299. [DOI] [PubMed] [Google Scholar]
- 12.Grossman HB, Messing E, Soloway M, Tomera K, Katz G, Berger Y, et al. Detection of bladder cancer using a point-of-care proteomic assay. JAMA. 2005;293:810–6. doi: 10.1001/jama.293.7.810. [DOI] [PubMed] [Google Scholar]
- 13.Choi HS, Lee SI, Kim DJ, Jeong TY. Usefulness of the NMP22BladderChek Test for Screening and Follow-up of Bladder Cancer. Korean J Urol. 2010;51:88–93. doi: 10.4111/kju.2010.51.2.88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Malkowicz SB. The application of human complement factor H-related protein (BTA TRAK) in monitoring patients with bladder cancer. Urol Clin North Am. 2000;27:63–73. doi: 10.1016/s0094-0143(05)70235-4. ix. [DOI] [PubMed] [Google Scholar]
- 15.Piaton E, Daniel L, Verriele V, Dalifard I, Zimmermann U, Renaudin K, et al. Improved detection of urothelial carcinomas with fluorescence immunocytochemistry (uCyt assay) and urinary cytology: Results of a French Prospective Multicenter Study. Lab Invest. 2003;83:845–52. doi: 10.1097/01.lab.0000074893.70675.2e. [DOI] [PubMed] [Google Scholar]
- 16.Gofrit ON, Zorn KC, Silvestre J, Shalhav AL, Zagaja GP, Msezane LP, et al. The predictive value of multi-targeted fluorescent in-situ hybridization in patients with history of bladder cancer. Urol Oncol. 2008;26:246–9. doi: 10.1016/j.urolonc.2007.02.011. [DOI] [PubMed] [Google Scholar]
- 17.Lotan Y, Bensalah K, Ruddell T, Shariat SF, Sagalowsky AI, Ashfaq R. Prospective evaluation of the clinical usefulness of reflex fluorescence in situ hybridization assay in patients with atypical cytology for the detection of urothelial carcinoma of the bladder. J Urol. 2008;179:2164–9. doi: 10.1016/j.juro.2008.01.105. [DOI] [PubMed] [Google Scholar]
- 18.Schlomer BJ, Ho R, Sagalowsky A, Ashfaq R, Lotan Y. Prospective validation of the clinical usefulness of reflex fluorescence in situ hybridization assay in patients with atypical cytology for the detection of urothelial carcinoma of the bladder. J Urol. 2010;183:62–7. doi: 10.1016/j.juro.2009.08.157. [DOI] [PubMed] [Google Scholar]
- 19.Kipp BR, Karnes RJ, Brankley SM, Harwood AR, Pankratz VS, Sebo TJ, et al. Monitoring intravesical therapy for superficial bladder cancer using fluorescence in situ hybridization. J Urol. 2005;173:401–4. doi: 10.1097/01.ju.0000149825.83180.a4. [DOI] [PubMed] [Google Scholar]
- 20.Kamat AM, Dickstein RJ, Messetti F, Anderson R, Pretzsch SM, Gonzalez GN, et al. Use of fluorescence in situ hybridization to predict response to bacillus Calmette-Guérin therapy for bladder cancer: Results of a prospective trial. J Urol. 2012;187:862–7. doi: 10.1016/j.juro.2011.10.144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Whitson J, Berry A, Carroll P, Konety B. A multicolour fluorescence in situ hybridization test predicts recurrence in patients with high-risk superficial bladder tumours undergoing intravesical therapy. BJU Int. 2009;104:336–9. doi: 10.1111/j.1464-410X.2009.08375.x. [DOI] [PubMed] [Google Scholar]
- 22.Loidl W, Schmidbauer J, Susani M, Marberger M. Flexible cystoscopy assisted by hexaminolevulinate induced fluorescence: A new approach for bladder cancer detection and surveillance? Eur Urol. 2005;47:323–6. doi: 10.1016/j.eururo.2004.10.025. [DOI] [PubMed] [Google Scholar]
- 23.Nargund VH, Tanabalan CK, Kabir MN. Management of non-muscle-invasive (superficial) bladder cancer. Semin Oncol. 2012;39:559–72. doi: 10.1053/j.seminoncol.2012.08.001. [DOI] [PubMed] [Google Scholar]
- 24.Brausi M, Collette L, Kurth K, van der Meijden AP, Oosterlinck W, Witjes JA, et al. Variability in the recurrence rate at first follow-up cystoscopy after TUR in stage Ta T1 transitional cell carcinoma of the bladder: A combined analysis of seven EORTC studies. Eur Urol. 2002;41:523–31. doi: 10.1016/s0302-2838(02)00068-4. [DOI] [PubMed] [Google Scholar]
- 25.Cysview (Hexaminolevulinate HCL); 2011. Photoculture: Package Insert. [Last accessed on 2015 July 4]. Available from: http://www.cysview.com/wp-content/uploads/2013/08/Prescribing-Information.pdf .
- 26.Liu JJ, Droller MJ, Liao JC. New optical imaging technologies for bladder cancer: Considerations and perspectives. J Urol. 2012;188:361–8. doi: 10.1016/j.juro.2012.03.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Isfoss BL. The sensitivity of fluorescent-light cystoscopy for the detection of carcinoma in situ (CIS) of the bladder: A meta-analysis with comments on gold standard. BJU Int. 2011;108:1703–7. doi: 10.1111/j.1464-410X.2011.10485.x. [DOI] [PubMed] [Google Scholar]
- 28.Geavlete B, Jecu M, Multescu R, Georgescu D, Geavlete P. HAL blue-light cystoscopy in high-risk nonmuscle-invasive bladder cancer – Re-TURBT recurrence rates in a prospective, randomized study. Urology. 2010;76:664–9. doi: 10.1016/j.urology.2010.02.067. [DOI] [PubMed] [Google Scholar]
- 29.Schmidbauer J, Witjes F, Schmeller N, Donat R, Susani M, Marberger M Hexvix PCB/Study Group. Improved detection of urothelial carcinoma in situ with hexaminolevulinate fluorescence cystoscopy. J Urol. 2004;171:135–8. doi: 10.1097/01.ju.0000100480.70769.0e. [DOI] [PubMed] [Google Scholar]
- 30.Schumacher MC, Holmäng S, Davidsson T, Friedrich B, Pedersen J, Wiklund NP. Transurethral resection of non-muscle-invasive bladder transitional cell cancers with or without 5-aminolevulinic Acid under visible and fluorescent light: Results of a prospective, randomised, multicentre study. Eur Urol. 2010;57:293–9. doi: 10.1016/j.eururo.2009.10.030. [DOI] [PubMed] [Google Scholar]
- 31.Filbeck T, Roessler W, Knuechel R, Straub M, Kiel HJ, Wieland WF. Clinical results of the transurethreal resection and evaluation of superficial bladder carcinomas by means of fluorescence diagnosis after intravesical instillation of 5-aminolevulinic acid. J Endourol. 1999;13:117–21. doi: 10.1089/end.1999.13.117. [DOI] [PubMed] [Google Scholar]
- 32.Yuan H, Qiu J, Liu L, Zheng S, Yang L, Liu Z, et al. Therapeutic outcome of fluorescence cystoscopy guided transurethral resection in patients with non-muscle invasive bladder cancer: A meta-analysis of randomized controlled trials. PLoS One. 2013;8:e74142. doi: 10.1371/journal.pone.0074142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chiu HM, Chang CY, Chen CC, Lee YC, Wu MS, Lin JT, et al. A prospective comparative study of narrow-band imaging, chromoendoscopy, and conventional colonoscopy in the diagnosis of colorectal neoplasia. Gut. 2007;56:373–9. doi: 10.1136/gut.2006.099614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Naselli A, Introini C, Timossi L, Spina B, Fontana V, Pezzi R, et al. A randomized prospective trial to assess the impact of transurethral resection in narrow band imaging modality on non-muscle-invasive bladder cancer recurrence. Eur Urol. 2012;61:908–13. doi: 10.1016/j.eururo.2012.01.018. [DOI] [PubMed] [Google Scholar]
- 35.Naselli A, Introini C, Bertolotto F, Spina B, Puppo P. Narrow band imaging for detecting residual/recurrent cancerous tissue during second transurethral resection of newly diagnosed non-muscle-invasive high-grade bladder cancer. BJU Int. 2010;105:208–11. doi: 10.1111/j.1464-410X.2009.08701.x. [DOI] [PubMed] [Google Scholar]
- 36.Sonn GA, Jones SN, Tarin TV, Du CB, Mach KE, Jensen KC, et al. Optical biopsy of human bladder neoplasia with in vivo confocal laser endomicroscopy. J Urol. 2009;182:1299–305. doi: 10.1016/j.juro.2009.06.039. [DOI] [PubMed] [Google Scholar]
- 37.Adams W, Wu K, Liu JJ, Hsiao ST, Jensen KC, Liao JC. Comparison of 2.6. and 1.4-mm imaging probes for confocal laser endomicroscopy of the urinary tract. J Endourol. 2011;25:917–21. doi: 10.1089/end.2010.0686. [DOI] [PubMed] [Google Scholar]
- 38.Wu K, Liu JJ, Adams W, Sonn GA, Mach KE, Pan Y, et al. Dynamic real-time microscopy of the urinary tract using confocal laser endomicroscopy. Urology. 2011;78:225–31. doi: 10.1016/j.urology.2011.02.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bonnal JL, Rock A, Jr, Gagnat A, Papadopoulos S, Filoche B, Mauroy B. Confocal laser endomicroscopy of bladder tumors associated with photodynamic diagnosis: An ex vivo pilot study. Urology. 2012;80:1162.e1–5. doi: 10.1016/j.urology.2012.06.035. [DOI] [PubMed] [Google Scholar]
- 40.Ren H, Waltzer WC, Bhalla R, Liu J, Yuan Z, Lee CS, et al. Diagnosis of bladder cancer with microelectromechanical systems-based cystoscopic optical coherence tomography. Urology. 2009;74:1351–7. doi: 10.1016/j.urology.2009.04.090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Jesser CA, Boppart SA, Pitris C, Stamper DL, Nielsen GP, Brezinski ME, et al. High resolution imaging of transitional cell carcinoma with optical coherence tomography: Feasibility for the evaluation of bladder pathology. Br J Radiol. 1999;72:1170–6. doi: 10.1259/bjr.72.864.10703474. [DOI] [PubMed] [Google Scholar]
- 42.Zagayanova EV, Streltsova OS, Gladkova ND, Shakova NM, Feldchtein FI, Kamensky VA, et al. Optical coherence tomography in diagnostics of precancer and cancer of human bladder. Proc SPIE. 2004;5312:75–81. [Google Scholar]
- 43.Hermes B, Spöler F, Naami A, Bornemann J, Först M, Grosse J, et al. Visualization of the basement membrane zone of the bladder by optical coherence tomography: Feasibility of noninvasive evaluation of tumor invasion. Urology. 2008;72:677–81. doi: 10.1016/j.urology.2008.02.062. [DOI] [PubMed] [Google Scholar]
- 44.Goh AC, Tresser NJ, Shen SS, Lerner SP. Optical coherence tomography as an adjunct to white light cystoscopy for intravesical real-time imaging and staging of bladder cancer. Urology. 2008;72:133–7. doi: 10.1016/j.urology.2008.02.002. [DOI] [PubMed] [Google Scholar]
- 45.Zagaynova EV, Streltsova OS, Gladkova ND, Snopova LB, Gelikonov GV, Feldchtein FI, et al. In vivo optical coherence tomography feasibility for bladder disease. J Urol. 2002;167:1492–6. [PubMed] [Google Scholar]


