Abstract
Introduction:
Non-muscle invasive bladder cancer (NMIBC) comprises about 70% of all newly diagnosed bladder cancer, and includes tumors with stage Ta, T1 and carcinoma in situ (CIS.) Since, NMIBC patients with progression to muscle-invasive disease tend to have worse prognosis than with patients with primary muscle-invasive disease, there is a need to significantly improve risk stratification and earlier definitive treatment for high-risk NMIBC.
Materials and Methods:
A detailed Medline search was performed to identify all publications on the topic of prognostic factors and risk predictions for superficial bladder cancer/NMIBC. The manuscripts were reviewed to identify variables that could predict recurrence and progression.
Results:
The most important prognostic factor for progression is grade of tumor. T category, tumor size, number of tumors, concurrent CIS, intravesical therapy, response to bacillus Calmette–Guerin at 3- or 6-month follow-up, prior recurrence rate, age, gender, lymphovascular invasion and depth of lamina propria invasion are other important clinical and pathological parameters to predict recurrence and progression in patients with NMIBC. The European Organization for Research and Treatment of Cancer (EORTC) and the Spanish Club UrológicoEspañol de Tratamiento Oncológico (CUETO) risk tables are the two best-established predictive models for recurrence and progression risk calculation, although they tend to overestimate risk and have poor discrimination for prognostic outcomes in external validation. Molecular biomarkers such as Ki-67, FGFR3 and p53 appear to be promising in predicting recurrence and progression but need further validation prior to using them in clinical practice.
Conclusion:
EORTC and CUETO risk tables are the two best-established models to predict recurrence and progression in patients with NMIBC though they tend to overestimate risk and have poor discrimination for prognostic outcomes in external validation. Future research should focus on enhancing the predictive accuracy of risk assessment tools by incorporating additional prognostic factors such as depth of lamina propria invasion and molecular biomarkers after rigorous validation in multi-institutional cohorts.
Keywords: Non-muscle invasive bladder cancer, superficial bladder cancer, outcome, prediction models, progression, recurrence, risk stratification
INTRODUCTION
Bladder cancer is the second most commonly diagnosed genitourinary malignancy in the USA, with an estimated 74,000 newly diagnosed cases and 16,000 deaths in 2015.[1] The incidence of bladder cancer rises with age, peaking between age 50 and 70 years, and is three times more common in men than in women.[1] Commonly accepted risk factors for bladder cancer include cigarette smoking, occupational exposure to aniline dyes, benzidene compounds, analgesic abuse (phenacetin) and chronic irritation, such as indwelling catheters.[2]
Of all newly diagnosed cases of bladder cancer, about 70% present as non-muscle invasive bladder cancer (NMIBC), also known as superficial bladder cancer.[3,4] It comprises a heterogeneous group of patients and includes pathological stage Ta (confinement to the epithelium or mucosa), T1 (invasion of the subepithelial connective tissue or lamina propria) and CIS (Tis: Flat, high-grade, non-papillary carcinomas confined to the urothelium). Of all newly diagnosed NMIBC, 70% present as stage Ta, 20% as T1 and 10% as CIS.[3,4] Approximately 50-70% of NMIBC will recur and roughly 10–20% will progress to muscle (i.e., muscularis propria) invasive disease.[3,4] In patients with low-grade Ta disease, the 15-year progression-free survival is 95% with no cancer-specific mortality.[5] Patients with high-grade Ta tumors had a progression-free survival of 61% and a disease-specific survival of 74%, whereas patients with T1 disease had a progression-free survival of 44% and a disease-specific survival of 62%, providing support to the view that grouping all patients with NMIBC as one disease is misleading as one patient's prognosis can be quite different from that of another patient.[5] When considering a patient's prognosis with NMIBC, it is necessary to consider not only clinical and pathological factors but also take into account the potential effect of the intravesical treatment received and molecular alterations present in the tumors.
RISK STRATIFICATION BASED ON CLINICAL AND PATHOLOGICAL PARAMETERS
Table 1 summarizes the available predictive models to predict recurrence and progression in patients with NMIBC. The most important risk factor for progression is grade, not stage, because patients with high-grade tumors progress with similar frequency regardless of whether they were invasive (T1) or non-invasive (Ta).[19] Millan-Rodriguez et al.[19] evaluated a cohort of 1529 primary NMIBC patients treated with transurethral resection (TUR) and random bladder biopsy and identified prognostic factors for recurrence, progression and disease-specific mortality (median follow-up: 40 months). Multivariate analysis demonstrated that the main prognostic factors of recurrence were multiplicity, tumor size >3 cm, presence of CIS and treatment with bacillus Calmette–Guerin (BCG). The prognostic factors for progression were grade 3 disease, multiplicity, tumor size >3 cm, CIS and treatment with BCG. Furthermore, the prognostic factors for mortality were presence of grade 3 disease and CIS.
Table 1.
Prediction of disease recurrence and progression in patients with non-muscle invasive bladder cancer
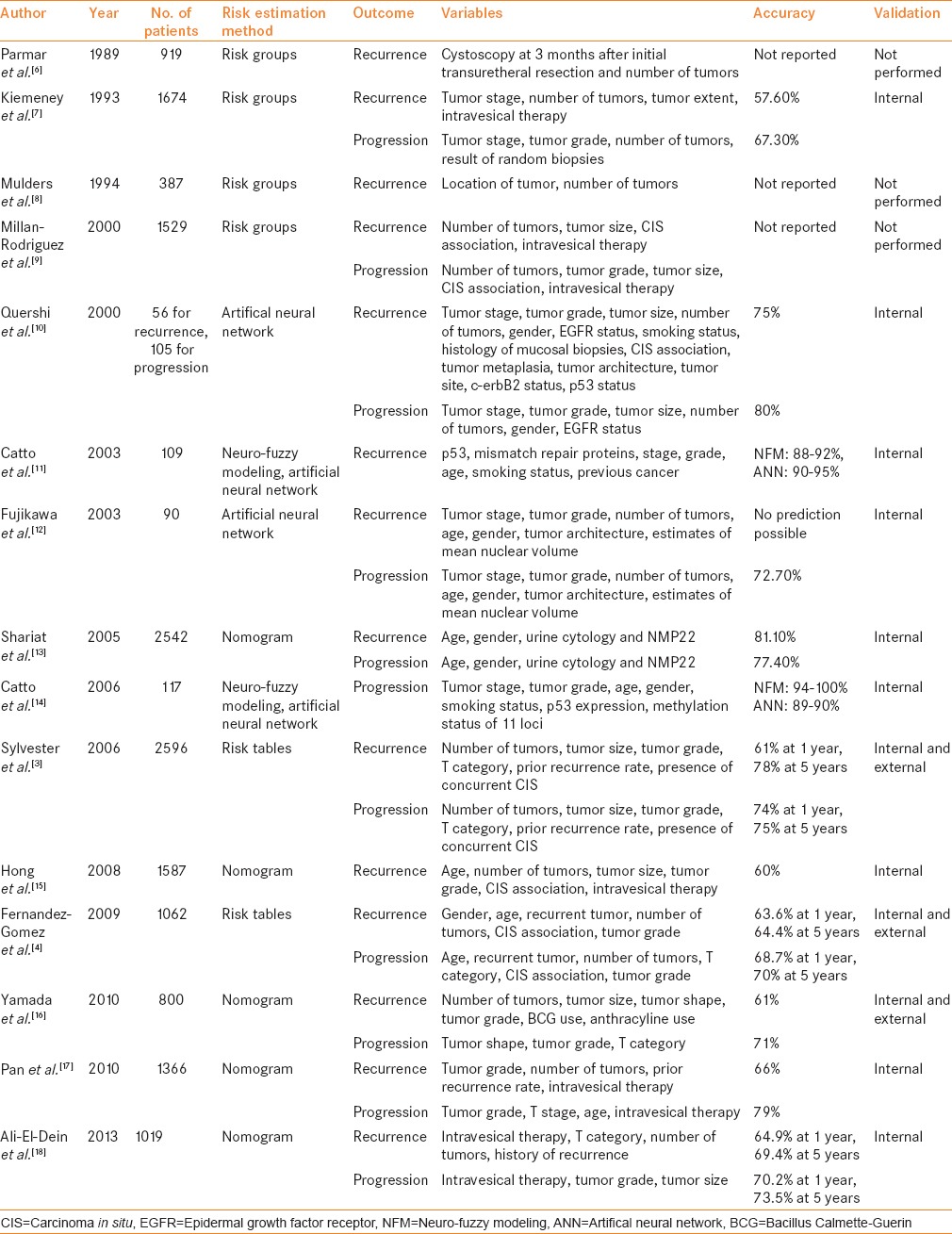
In another study, Millan-Rodriguez et al.[9] stratified NMIBC into three risk groups based on previously identified risk factors.[19] Risk groups were classified as:
Low risk (grade 1 stage Ta disease and a single grade 1 stage T1 tumor)
Intermediate risk (multiple grade 1 stage T1 tumors, grade 2 stage Ta disease and a single grade 2 stage T1 tumor)
High risk (multiple grade 2 stage T1 tumors, grade 3 stages Ta and T1 disease and any stage disease associated with CIS).
The rates of recurrence, progression and mortality were 37%, 0% and 0% in the low-risk group, 45%, 1.8% and 0.73% in the intermediate-risk group and 54%, 15% and 9.5% in the high-risk group. The relative risks of recurrence, progression and mortality in the low-risk versus intermediate-risk and high-risk groups were 1.37, 2.84 and 1, and 1.87, 24.76 and 14.69, respectively.
The European Organization for Research and Treatment of Cancer (EORTC) developed risk tables to predict recurrence and progression in patients with NMIBC using clinical and pathologic information from 2596 patients enrolled in seven clinical trials that utilized prophylactic treatments after TUR.[3] The median follow-up was 3.9 years and 78% of patients had received intravesical treatment, mostly with chemotherapy. In their study cohort, 80% of patients had a maximum tumor diameter < 3 cm and 56% of patients had pTa tumors. The EORTC risk tables [Tables 2 and 3] for recurrence and progression were based on the scoring system derived from the following six clinical and pathological factors:
Table 2.
Weights used to calculate EORTC risk tables’ recurrence and progression scores
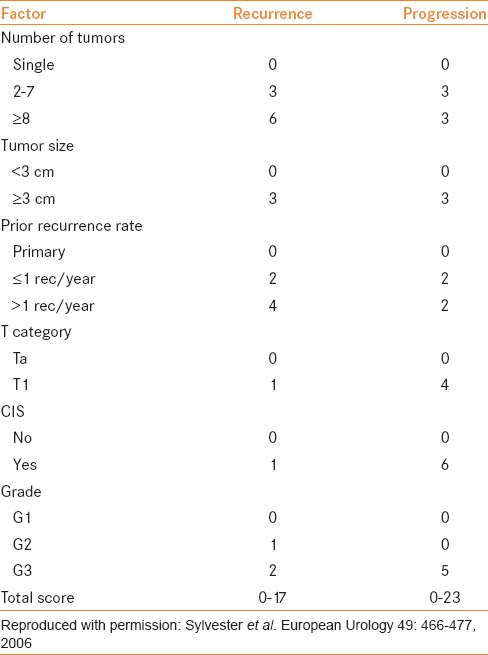
Table 3.
Probability of recurrence and progression according to the EORTC risk tables total score
Number of tumors
Tumor size
T category
Tumor grade (WHO 1973)
Prior recurrence rate
Presence of concurrent CIS.
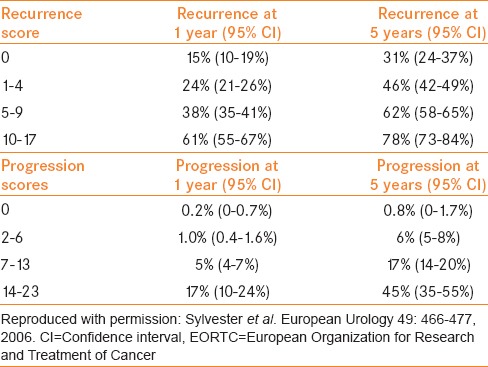
Based on these EORTC risk tables (electronic calculator is available at http://www.eortc.be/tools/bladdercalculator/), the probability of recurrence varied from 15% to 61% at 1 year and from 31% to 78% at 5 years. The probability of progression varied from 0.2% to 17% at 1 year and from 0.8% to 45% at 5 years. For their recurrence and progression models, Harrell's concordance indices at 1 year were 0.66 and 0.74, respectively. Of note, only 6.6% (171 of 2596) patients received BCG for six induction instillations. In addition, patients with high-grade disease did not have a second TUR or receive maintenance BCG therapy.
BCG is currently the most effective intravesical therapy for NMIBC, especially in intermediate- and high-risk tumors. A meta-analysis of 24 randomized trials (n = 4863) showed that BCG significantly reduces the risk of progression to muscle-invasive disease after TUR in patients with NMIBC who receive maintenance BCG.[20] The patient's response to BCG at 3 or 6 months is an important prognostic factor to predict subsequent tumor recurrence and progression.[21,22] Because the EORTC risk tables were generated using NMIBC patient's clinical and pathological information, where majority of the patients did not receive BCG induction and/or BCG maintenance therapy, the EORTC risk tables tend to overestimate patient's risk for recurrence and progression after BCG therapy.[23]
The Spanish Club UrológicoEspañol de TratamientoOncológico (CUETO) group[4] developed a scoring model using information from 1062 patients treated with intravesical adjuvant BCG therapy to predict risk of recurrence and progression. Patients had BCG induction as weekly instillations for 6 weeks and six additional instillations repeated at 2-week intervals as a maintenance therapy. By the end of the study, 4.2% of patients received fewer than six instillations, 22.5% of patients received six to nine instillations and 73.3% of patients received more than 10 instillations. The risk tables for recurrence and progression were based on the scoring systems derived from seven clinical and pathological factors:
Age
Gender
Number of tumors
Recurrent tumor
T category
Tumor grade (WHO 1973)
Presence of concurrent CIS.
Based on the CUETO risk tables, the probability of recurrence varied from 8.24% to 41.79% at 1 year to 20.98% to 67.61% at 5 years. The probability of progression varied from 1.17% to 13.97% at 1 year to 3.76% to 33.57% at 5 years. For their recurrence and progression models, Harrell's concordance indices at 1 year were 0.636 and 0.687, respectively. Using the CUETO risk tables, the calculated risk of recurrence were lower than that obtained by the EORTC risk tables. For progression probabilities, the risk is lower only in high-risk patients. The lower risks in the CUETO tables is likely attributable to using BCG, which is a more effective intravesical therapy.
Although the EORTC and CUETO risk tables are the two best-established predictive multivariate models for recurrence and progression risk calculation in patients with NMIBC, limitations include retrospective analysis, use of the 1987 TNM classification and use of the WHO 1973 grading system. In addition, repeat TUR for high-grade cancer and immediate instillation of chemotherapy, such as mitomycin, was not routinely performed after TUR; hence, both models are prone to overestimate the risk of recurrence and progression in patients treated with current standard of care. A restaging TUR is routinely performed these days in patients with high-grade NMIBC, especially for high-grade T1, as the rate of residual tumor is high and 30% of tumors are upstaged when muscle is absent in the first obtained specimen.[24] Photodynamic diagnosis with blue light and narrow-band imaging have been shown to reduce residual tumor rates by roughly 20% and to improve recurrence-free survival of NMIBC patients compared with white light cystoscopy.[25] A meta-analysis of seven randomized trials (n = 1476) confirmed the effectiveness of single immediate intravesical instillation of chemotherapy in treated patients compared with TUR alone, reporting a 39% decrease in the odds of recurrence.[26] The efficacy of intravesical mitomycin C can be optimized by administering a dose of 40 mg at a concentration of 2 mg/mL in water, complete bladder emptying just before dose administration, fluid restriction and oral bicarbonate to alkalinize the urine.[27] This approach improved the recurrence-free rate at 5 years from 24.6% to 41% and increased the interval to tumor recurrence from 11.9 to 29 months.[27] Using a multi-institutional cohort of 4689 patients with NMIBC, Xylinas et al.[28] showed that both the EORTC and CUETO risk tables exhibit a poor discrimination for disease recurrence and progression. These models overestimated the risk of disease recurrence and progression in high-risk patients.
Additional factors not included in the EORTC or the CUETO models could be added to new prognostic models to enhance their usefulness. Bladder neck involvement,[29] prostatic urethra involvement,[30] lymphovascular invasion[31] and depth of lamina propria invasion, i.e., whether the tumor is superficial to, into or beyond the muscularis mucosae (T1a, T1b, T1c),[32] have been identified as risk factors for progression in patients with NMIBC. In the prognostic factor meta-analysis of 33 studies of high-grade T1 bladder cancer patients (n = 8880), the highest impact risk factor for progression and cancer-specific survival was depth of invasion (T1b/c) into the lamina propria.[33] In this meta-analysis, several other previously proposed factors, i.e. lymphovascular invasion, associated CIS, non-use of BCG, tumor size >3 cm, gender, multiple tumors and older age, also predicted progression.[33] NMIBC patients with progression to muscle-invasive disease tend to have worse prognosis compared with patients with primary muscle-invasive disease, and it underscores the need to significantly improve risk stratification and earlier definitive treatment for high-risk NMIBC.[34]
Risk groupings and risk tables provide average predictions, which may or may not apply to the patient interested in knowing individualized risk. Nomograms have been proposed as a method that avoids the arbitrary division of patients into risk groups.[13] They provide individualized predictions based on the characteristics of individual patients. Shariat et al.[13] developed nomograms to estimate the risk of disease recurrence and progression in patients with NMIBC using a large international cohort. Nomograms based on age, gender, urine cytology and dichotomized NMP22 had accuracy of 0.811 for recurrence of any bladder cancer; 0.772 for recurrence of grade 3 Ta or T1 or CIS and 0.774 for recurrence of stage ≥T2 bladder cancer after bootstrap validation. However, this nomogram had several limitations, including failure to incorporate established predictors, such as pathologic grade and stage, number and pattern of previous recurrences, time since the original diagnosis and prior use of intravesical therapy. Furthermore, the performance of the nomograms varied significantly among the institutions, emphasizing the need for external validation prior to its clinical use.
Artificial neural networks (ANN) are algorithms that can be trained to identify complex patterns between input variables and outcomes in the data sets and then apply these patterns to new cases.[35] They have a theoretical advantage over conventional statistics as they are not limited by predefined mathematical relationships between input variables and outcomes; thus, they are able to model complex non-linear parameters. Qureshi et al.[10] used ANN to predict bladder cancer recurrence and progression within 6 months of diagnosis in a small cohort of about 100 patients with NMIBC. The accuracy of ANN in predicting stage progression and recurrence was 80% and 75%, respectively. However, there was no significant difference between the ANN and the clinicians’ predictions of progression and recurrence in the patients with NMIBC. On restricting the validation subset to patients with T1G3 tumors in relation to stage progression, ANN accuracy was better than predictions of clinicians. Several investigators have compared ANN and other machine learning techniques with standard statistical approaches to predict outcomes and did not find improvement in predictive accuracy with ANN and other machine learning techniques over standard statistical approaches.[36,37]
Most of the currently available predictive tools assume that NMIBC is pure urothelial carcinoma and does not consider the impact of variant histology on prognosis prediction. Variant histology such as micropapillary, sarcomatoid and plasmacytoid has been shown to be associated with poor prognosis, and early cystectomy is generally advocated in such cases.[38,39] Small cell carcinoma of the bladder is considered a systemic disease and chemotherapy followed by tailored local therapy is recommended for patients with non-muscle invasive small cell carcinoma of the bladder.[39] However, Spaliviero et al.[40] reported not significantly worse outcomes in conservatively managed T1 micropapillary bladder cancer patients compared with patients treated with early radical cystectomy. Given the conflicting findings on the impact of variant histology in smaller studies, the association of variant histology with prognosis deserves further evaluation in larger studies.
RISK STRATIFICATION BASED ON MOLECULAR BIOMARKERS
For molecular biomarkers to be of clinical use, they should be able to increase the predictive accuracy beyond the standard clinical and pathological parameter models.[41] Several investigators have attempted to use molecular biomarkers as prognostic factors to predict outcomes in patients with NMIBC.[10,13,42,43,44] However, molecular biomarkers have shown mixed results so far and are not sufficiently validated to be used in clinical practice at this time.[45]
It is becoming clear that superficial low-grade cancers and invasive or high-grade cancers harbor distinctive genetic defects: The low-grade, non-invasive papillary tumors are characterized by activating mutations in the H-Ras gene and fibroblast growth factor receptor 3 (FGFR3) gene and the high-grade invasive tumors are characterized by structural and functional defects in the p53 and retinoblastoma protein (Rb) tumor-suppressor pathways.[46] The deletion of both arms of chromosome 9 occurs frequently in bladder cancer during the earliest stages of tumorigenesis. However, these chromosomal aberrations do not seem to distinguish between the two tumor development pathways.[47] Tumor invasion and progression in the bladder seems to be a multifactorial process, promoted by micro-environmental changes that include the up-regulation of N-cadherin, the down-regulation of E-cadherin, the overexpression of matrix metalloproteinases 2 and 9, an imbalance between angiogenic and anti-angiogenic factors and increased synthesis of prostaglandin.[46]
In patients with T1 bladder cancer, nuclear overexpression of p53 protein has been reported to have a higher probability of disease progression.[43] In a meta-analysis, p53 was a predictor for recurrence, progression and mortality in patients with bladder cancer.[48] However, investigators of this meta-analysis had concerns of overestimating findings because of publication and reporting bias, and suggested that current evidence is not sufficient to conclude whether changes in p53 act as markers of outcome in patients with bladder cancer.[48] Hernandez et al.,[49] in a prospective cohort of 772 patients with NMIBC, showed that FGFR3 mutations are prevalent in low-grade Ta and that these mutations are independent predictors of recurrence in patients with low-grade Ta tumors. Van Rhijn et al.[42] showed that molecular grading, the grading system based on FGFR3 and MIB-1 (Ki-67) status, is an independent predictor for recurrence, progression and disease-specific survival. Van Rhijn et al.[44] validated the utility of molecular grading as a prognostic factor to predict outcomes in patients with NMIBC. The addition of molecular grade to the multivariable model for progression increased the predictive accuracy from 74.9% to 81.7%.[44] Shariat et al.[50] evaluated NMP22 for detecting recurrence and progression in patients with NMIBC in a large multi-institutional international cohort. There was a substantial degree of heterogeneity in the diagnostic performance among institutions. There was no clearly defined NMP22 cut-off that could indicate higher risk disease, but there was a continuum of risk for recurrence and progression.
The international consensus panel on cytology and bladder tumor markers evaluated the prognostic utility of molecular markers for bladder cancer.[45] Molecular markers were classified into six groups, i.e. microsatellite-associated markers (e.g. FISH, LOH), proto-oncogenes/oncogenes (e.g. Her-2/neu, H-Ras, BCL-2, MDM-2, FGFR-3, C-MYC) tumor suppressor genes (e.g., p53, Rb), cell cycle regulators (e.g., p21, p27, Ki-67, Cyclin-D1, Cyclin-E), angiogenesis-related factors (VEGF, COX-2, TSP-1) and extracellular matrix adhesion molecules (e.g. E-cadherin, MMPs, TIMPs, CD44, U-PA). The panel concluded that although certain biomarkers, such as Ki-67 and p53, appear to be promising in predicting recurrence and progression in patients with bladder cancer, the data are still heterogeneous.[45] The panel recommended that identifying definitive criteria for test positivity, a clearly defined patient population, standardization of techniques used to evaluate markers and clearly specified endpoints and statistical methods will help to bring accurate independent prognostic indicators into the clinical management of patients with bladder cancer.[45]
CONCLUSIONS
NMIBC comprises of a heterogeneous group of patients and includes pathological stage Ta, T1 and CIS. Patients with low-grade Ta disease have very low risk of progression while patients with T1 disease with concurrent CIS have a much higher risk of progression, approaching 50%. The EORTC and CUETO risk tables are the two best-established predictive models for recurrence and progression risk calculation in patients with NMIBC. However, both the EORTC and the CUETO risk tables exhibit a poor discrimination for prognostic outcomes and overestimate the risk of disease recurrence and progression in high-risk patients in external validation. Additional prognostic factors such as depth of lamina propria invasion should be added to new prognostic models to enhance their usefulness. Molecular biomarkers such as Ki-67, FGFR3 and p53 appear to be promising in predicting recurrence and progression, but need further validation prior to using them in clinical practice. Future research should focus to enhance the predictive accuracy of the risk assessment tools by incorporating additional prognostic factors such as depth of lamina propria invasion and molecular biomarkers after rigorous validation in multi-institutional cohorts.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2015. CA Cancer J Clin. 2015;65:5–29. doi: 10.3322/caac.21254. [DOI] [PubMed] [Google Scholar]
- 2.Konety BR, Williams RD. Superficial transitional (Ta/T1/CIS) cell carcinoma of the bladder. BJU Int. 2004;94:18–21. doi: 10.1111/j.1464-410X.2003.04894.x. [DOI] [PubMed] [Google Scholar]
- 3.Sylvester RJ, van der Meijden AP, Oosterlinck W, Witjes JA, Bouffioux C, Denis L, et al. Predicting recurrence and progression in individual patients with stage Ta T1 bladder cancer using EORTC risk tables: A combined analysis of 2596 patients from seven EORTC trials. Eur Urol. 2006;49:466–5. doi: 10.1016/j.eururo.2005.12.031. discussion 75-7. [DOI] [PubMed] [Google Scholar]
- 4.Fernandez-Gomez J, Madero R, Solsona E, Unda M, Martinez-Piñeiro L, Gonzalez M, et al. Predicting nonmuscle invasive bladder cancer recurrence and progression in patients treated with bacillus Calmette-Guerin: The CUETO scoring model. J Urol. 2009;182:2195–203. doi: 10.1016/j.juro.2009.07.016. [DOI] [PubMed] [Google Scholar]
- 5.Herr HW. Tumor progression and survival of patients with high grade, noninvasive papillary (TaG3) bladder tumors: 15-year outcome. J Urol. 2000;163:60–2. [PubMed] [Google Scholar]
- 6.Parmar MK, Freedman LS, Hargreave TB, Tolley DA. Prognostic factors for recurrence and followup policies in the treatment of superficial bladder cancer: Report from the British Medical Research Council Subgroup on Superficial Bladder Cancer (Urological Cancer Working Party) J Urol. 1989;142:284–8. doi: 10.1016/s0022-5347(17)38731-1. [DOI] [PubMed] [Google Scholar]
- 7.Kiemeney LA, Witjes JA, Heijbroek RP, Verbeek AL, Debruyne FM. Predictability of recurrent and progressive disease in individual patients with primary superficial bladder cancer. J Urol. 1993;150:60–4. doi: 10.1016/s0022-5347(17)35397-1. [DOI] [PubMed] [Google Scholar]
- 8.Mulders PF, Meyden AP, Doesburg WH, Oosterhof GO, Debruyne FM. Prognostic factors in pTa-pT1 superficial bladder tumours treated with intravesical instillations. The Dutch South-Eastern Urological Collaborative Group. Br J Urol. 1994;73:403–8. doi: 10.1111/j.1464-410x.1994.tb07604.x. [DOI] [PubMed] [Google Scholar]
- 9.Millán-Rodríguez F, Chéchile-Toniolo G, Salvador-Bayarri J, Palou J, Algaba F, Vicente-Rodríguez J. Primary superficial bladder cancer risk groups according to progression, mortality and recurrence. J Urol. 2000;164:680–4. doi: 10.1016/s0022-5347(05)67280-1. [DOI] [PubMed] [Google Scholar]
- 10.Qureshi KN, Naguib RN, Hamdy FC, Neal DE, Mellon JK. Neural network analysis of clinicopathological and molecular markers in bladder cancer. J Urol. 2000;163:630–3. [PubMed] [Google Scholar]
- 11.Catto JW, Linkens DA, Abbod MF, Chen M, Burton JL, Feeley KM, et al. Artificial intelligence in predicting bladder cancer outcome: a comparison of neuro-fuzzy modeling and artificial neural networks. Clinical Cancer Research. 2003;9:4172–77. [PubMed] [Google Scholar]
- 12.Fujikawa K, Matsui Y, Kobayashi T, Miura K, Oka H, Fukuzawa S, et al. Predicting disease outcome of non-invasive transitional cell carcinoma of the urinary bladder using an artificial neural network model: Results of patient follow-up for 15 years or longer. Int J Urol. 2003;10:149–52. doi: 10.1046/j.1442-2042.2003.00589.x. [DOI] [PubMed] [Google Scholar]
- 13.Shariat SF, Zippe C, Lüdecke G, Boman H, Sanchez-Carbayo M, Casella R, et al. Nomograms including nuclear matrix protein 22 for prediction of disease recurrence and progression in patients with Ta, T1 or CIS transitional cell carcinoma of the bladder. J Urol. 2005;173:1518–25. doi: 10.1097/01.ju.0000154696.48217.75. [DOI] [PubMed] [Google Scholar]
- 14.Catto JW, Abbod MF, Linkens DA, Hamdy FC. Neuro-fuzzy modeling: An accurate and interpretable method for predicting bladder cancer progression. J Urol. 2006;175:474–9. doi: 10.1016/S0022-5347(05)00246-6. [DOI] [PubMed] [Google Scholar]
- 15.Hong SJ, Cho KS, Han M, Rhew HY, Kim CS, Ryu SB, et al. Nomograms for prediction of disease recurrence in patients with primary Ta, T1 transitional cell carcinoma of the bladder. J Korean Med Sci. 2008;23:428–33. doi: 10.3346/jkms.2008.23.3.428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yamada T, Tsuchiya K, Kato S, Kamei S, Taniguchi M, Takeuchi T, et al. A pretreatment nomogram predicting recurrence- and progression-free survival for nonmuscle invasive bladder cancer in Japanese patients. Int J Clin Oncol. 2010;15:271–9. doi: 10.1007/s10147-010-0049-6. [DOI] [PubMed] [Google Scholar]
- 17.Pan CC, Chang YH, Chen KK, Yu HJ, Sun CH, Ho DM. Constructing prognostic model incorporating the 2004 WHO/ISUP classification for patients with non-muscle-invasive urothelial tumours of the urinary bladder. J Clin Pathol. 2010;63:910–5. doi: 10.1136/jcp.2010.079764. [DOI] [PubMed] [Google Scholar]
- 18.Ali-El-Dein B, Sooriakumaran P, Trinh QD, Barakat TS, Nabeeh A, Ibrahiem el-HI. Construction of predictive models for recurrence and progression in >1000 patients with non-muscle-invasive bladder cancer (NMIBC) from a single centre. BJU Int. 2013;111:E331–41. doi: 10.1111/bju.12026. [DOI] [PubMed] [Google Scholar]
- 19.Millán-Rodríguez F, Chéchile-Toniolo G, Salvador-Bayarri J, Palou J, Vicente-Rodríguez J. Multivariate analysis of the prognostic factors of primary superficial bladder cancer. J Urol. 2000;163:73–8. doi: 10.1016/s0022-5347(05)67975-x. [DOI] [PubMed] [Google Scholar]
- 20.Sylvester RJ, van der Meijden AP, Lamm DL. Intravesical bacillus Calmette-Guerin reduces the risk of progression in patients with superficial bladder cancer: A meta-analysis of the published results of randomized clinical trials. J Urol. 2002;168:1964–70. doi: 10.1016/S0022-5347(05)64273-5. [DOI] [PubMed] [Google Scholar]
- 21.Herr HW, Dalbagni G. Defining bacillus Calmette-Guerin refractory superficial bladder tumors. J Urol. 2003;169:1706–8. doi: 10.1097/01.ju.0000062605.92268.c6. [DOI] [PubMed] [Google Scholar]
- 22.Solsona E, Iborra I, Dumont R, Rubio-Briones J, Casanova J, Almenar S. The 3-month clinical response to intravesical therapy as a predictive factor for progression in patients with high risk superficial bladder cancer. J Urol. 2000;164:685–9. doi: 10.1097/00005392-200009010-00016. [DOI] [PubMed] [Google Scholar]
- 23.Fernandez-Gomez J, Madero R, Solsona E, Unda M, Martinez-Piñeiro L, Ojea A, et al. Club Urológico Español de Tratamiento Oncológico. The EORTC tables overestimate the risk of recurrence and progression in patients with non-muscle-invasive bladder cancer treated with bacillus Calmette. Guerin: External validation of the EORTC risk tables. Eur Urol. 2011;60:423–30. doi: 10.1016/j.eururo.2011.05.033. [DOI] [PubMed] [Google Scholar]
- 24.Dalbagni G, Vora K, Kaag M, Cronin A, Bochner B, Donat SM, et al. Clinical outcome in a contemporary series of restaged patients with clinical T1 bladder cancer. Eur Urol. 2009;56:903–10. doi: 10.1016/j.eururo.2009.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Witjes JA, Redorta JP, Jacqmin D, Sofras F, Malmström PU, Riedl C, et al. Hexaminolevulinate-guided fluorescence cystoscopy in the diagnosis and follow-up of patients with non-muscle-invasive bladder cancer: Review of the evidence and recommendations. Eur Urol. 2010;57:607–14. doi: 10.1016/j.eururo.2010.01.025. [DOI] [PubMed] [Google Scholar]
- 26.Sylvester RJ, Oosterlinck W, van der Meijden AP. A single immediate postoperative instillation of chemotherapy decreases the risk of recurrence in patients with stage Ta T1 bladder cancer: A meta-analysis of published results of randomized clinical trials. J Urol. 2004;171:2186–90, quiz 2435. doi: 10.1097/01.ju.0000125486.92260.b2. [DOI] [PubMed] [Google Scholar]
- 27.Au JL, Badalament RA, Wientjes MG, Young DC, Warner JA, Venema PL, et al. InternationalMitomycin CConsortium. Methods to improve efficacy of intravesical mitomycin C: Results of a randomized phase III trial. J Natl Cancer Inst. 2001;93:597–604. doi: 10.1093/jnci/93.8.597. [DOI] [PubMed] [Google Scholar]
- 28.Xylinas E, Kent M, Kluth L, Pycha A, Comploj E, Svatek RS, et al. Accuracy of the EORTC risk tables and of the CUETO scoring model to predict outcomes in non-muscle-invasive urothelial carcinoma of the bladder. Br J Cancer. 2013;109:1460–6. doi: 10.1038/bjc.2013.372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fujii Y, Fukui I, Kihara K, Tsujii T, Ishizaka K, Kageyama Y, et al. Significance of bladder neck involvement on progression in superficial bladder cancer. Eur Urol. 1998;33:464–8. doi: 10.1159/000019636. [DOI] [PubMed] [Google Scholar]
- 30.Huguet J, Crego M, Sabaté S, Salvador J, Palou J, Villavicencio H. Cystectomy in patients with high risk superficial bladder tumors who fail intravesical BCG therapy: Pre-cystectomy prostate involvement as a prognostic factor. Eur Urol. 2005;48:53–9. doi: 10.1016/j.eururo.2005.03.021. [DOI] [PubMed] [Google Scholar]
- 31.Cho KS, Seo HK, Joung JY, Park WS, Ro JY, Han KS, et al. Lymphovascular invasion in transurethral resection specimens as predictor of progression and metastasis in patients with newly diagnosed T1 bladder urothelial cancer. J Urol. 2009;182:2625–30. doi: 10.1016/j.juro.2009.08.083. [DOI] [PubMed] [Google Scholar]
- 32.Orsola A, Trias I, Raventós CX, Español I, Cecchini L, Búcar S, et al. Initial high-grade T1 urothelial cell carcinoma: Feasibility and prognostic significance of lamina propria invasion microstaging (T1a/b/c) in BCG-treated and BCG-non-treated patients. Eur Urol. 2005;48:231–8. doi: 10.1016/j.eururo.2005.04.013. [DOI] [PubMed] [Google Scholar]
- 33.Martin-Doyle W, Leow JJ, Orsola A, Chang SL, Bellmunt J. Improving selection criteria for early cystectomy in high-grade T1 bladder cancer: A meta-analysis of 15,215 patients. J Clin Oncol. 2015;33:643–50. doi: 10.1200/JCO.2014.57.6967. [DOI] [PubMed] [Google Scholar]
- 34.Schrier BP, Hollander MP, van Rhijn BW, Kiemeney LA, Witjes JA. Prognosis of muscle-invasive bladder cancer: Difference between primary and progressive tumours and implications for therapy. Eur Urol. 2004;45:292–6. doi: 10.1016/j.eururo.2003.10.006. [DOI] [PubMed] [Google Scholar]
- 35.Naguib RN, Sherbet GV. Artificial neural networks in cancer research. Pathobiology. 1997;65:129–39. doi: 10.1159/000164114. [DOI] [PubMed] [Google Scholar]
- 36.Sargent DJ. Comparison of artificial neural networks with other statistical approaches: Results from medical data sets. Cancer. 2001;91(Suppl):1636–42. doi: 10.1002/1097-0142(20010415)91:8+<1636::aid-cncr1176>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- 37.Kattan MW. Comparison of Cox regression with other methods for determining prediction models and nomograms. J Urol. 2003;170:S6–10. doi: 10.1097/01.ju.0000094764.56269.2d. [DOI] [PubMed] [Google Scholar]
- 38.Kamat AM, Dinney CP, Gee JR, Grossman HB, Siefker-Radtke AO, Tamboli P, et al. Micropapillary bladder cancer: A review of the University of Texas M. D. Anderson Cancer Center experience with 100 consecutive patients. Cancer. 2007;110:62–7. doi: 10.1002/cncr.22756. [DOI] [PubMed] [Google Scholar]
- 39.Porten SP, Willis D, Kamat AM. Variant histology: Role in management and prognosis of nonmuscle invasive bladder cancer. Curr Opin Urol. 2014;24:517–23. doi: 10.1097/MOU.0000000000000089. [DOI] [PubMed] [Google Scholar]
- 40.Spaliviero M, Dalbagni G, Bochner BH, Poon BY, Huang H, Al-Ahmadie HA, et al. Clinical outcome of patients with T1 micropapillary urothelial carcinoma of the bladder. J Urol. 2014;192:702–7. doi: 10.1016/j.juro.2014.02.2565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kattan MW. Judging new markers by their ability to improve predictive accuracy. J Natl Cancer Inst. 2003;95:634–5. doi: 10.1093/jnci/95.9.634. [DOI] [PubMed] [Google Scholar]
- 42.van Rhijn BW, Vis AN, van der Kwast TH, Kirkels WJ, Radvanyi F, Ooms EC, et al. Molecular grading of urothelial cell carcinoma with fibroblast growth factor receptor 3 and MIB-1 is superior to pathologic grade for the prediction of clinical outcome. J Clin Oncol. 2003;21:1912–21. doi: 10.1200/JCO.2003.05.073. [DOI] [PubMed] [Google Scholar]
- 43.Sarkis AS, Dalbagni G, Cordon-Cardo C, Zhang ZF, Sheinfeld J, Fair WR, et al. Nuclear overexpression of p53 protein in transitional cell bladder carcinoma: A marker for disease progression. J Natl Cancer Inst. 1993;85:53–9. doi: 10.1093/jnci/85.1.53. [DOI] [PubMed] [Google Scholar]
- 44.van Rhijn BW, Zuiverloon TC, Vis AN, Radvanyi F, van Leenders GJ, Ooms BC, et al. Molecular grade (FGFR3/MIB-1) and EORTC risk scores are predictive in primary non-muscle-invasive bladder cancer. Eur Urol. 2010;58:433–41. doi: 10.1016/j.eururo.2010.05.043. [DOI] [PubMed] [Google Scholar]
- 45.Habuchi T, Marberger M, Droller MJ, Hemstreet GP, 3rd, Grossman HB, Schalken JA, et al. Prognostic markers for bladder cancer: International Consensus Panel on bladder tumor markers. Urology. 2005;66(Suppl 1):64–74. doi: 10.1016/j.urology.2005.08.065. [DOI] [PubMed] [Google Scholar]
- 46.Wu XR. Urothelial tumorigenesis: A tale of divergent pathways. Nat Rev Cancer. 2005;5:713–25. doi: 10.1038/nrc1697. [DOI] [PubMed] [Google Scholar]
- 47.Hartmann A, Schlake G, Zaak D, Hungerhuber E, Hofstetter A, Hofstaedter F, et al. Occurrence of chromosome 9 and p53 alterations in multifocal dysplasia and carcinoma in situ of human urinary bladder. Cancer Res. 2002;62:809–18. [PubMed] [Google Scholar]
- 48.Malats N, Bustos A, Nascimento CM, Fernandez F, Rivas M, Puente D, et al. P53 as a prognostic marker for bladder cancer: A meta-analysis and review. Lancet Oncol. 2005;6:678–86. doi: 10.1016/S1470-2045(05)70315-6. [DOI] [PubMed] [Google Scholar]
- 49.Hernández S, López-Knowles E, Lloreta J, Kogevinas M, Amorós A, Tardón A, et al. Prospective study of FGFR3 mutations as a prognostic factor in nonmuscle invasive urothelial bladder carcinomas. J Clin Oncol. 2006;24:3664–71. doi: 10.1200/JCO.2005.05.1771. [DOI] [PubMed] [Google Scholar]
- 50.Shariat SF, Marberger MJ, Lotan Y, Sanchez-Carbayo M, Zippe C, Lüdecke G, et al. Variability in the performance of nuclear matrix protein 22 for the detection of bladder cancer. J Urol. 2006;176:919–26. doi: 10.1016/j.juro.2006.04.017. [DOI] [PubMed] [Google Scholar]


