Abstract
Bladder cancer is the ninth-most prevalent cancer worldwide. Most patients with urothelial cell carcinoma of the bladder present with non-muscle-invasive disease and are treated with bacillus Calmette-Guérin (BCG) intravesical therapy. Many of these patients experience disease recurrence after BCG failure. Radical cystectomy is the recommended treatment for high-risk patients failing BCG. However, many patients are unfit for or unwilling to undergo this procedure. We searched the published literature on the treatment of non-muscle-invasive bladder cancer (NMIBC) after BCG failure. We review current evidence regarding intravesical therapy with gemcitabine, mitomycin combined with thermo-chemotherapy, docetaxel, nab-paclitaxel, photodynamic therapy (PDT), BCG with interferon (IFN), and combination sequentially administered chemotherapy.
Keywords: Chemotherapy, interferon, photodynamic therapy, thermochemotherapy, urinary bladder neoplasms
INTRODUCTION
Urothelial cell carcinoma of the bladder is the ninth-most common cancer worldwide. At the time of diagnosis, 75-80% cases of bladder cancer are classified as non-muscle-invasive bladder cancer (NMIBC). This grouping consists of the pathologic stages Ta (papillary), T1 (invading into the lamina propria), and carcinoma in situ (CIS). The pathologic stages are further subclassified as low- or high-grade based on the 2004 World Health Organization (WHO) classification and as grade 1, 2, or 3 based on the 1973 WHO classification.[1,2] The European Organization for Research and Treatment of Cancer (EORTC) has produced a risk calculator to stratify patients into low-, intermediate-, and high-risk categories for recurrence and progression of disease [Table 1].[3] Following transurethral resection, adjuvant induction therapy followed by maintenance treatment with bacillus Calmette-Guérin (BCG) is the standard of care for the treatment of some cases of intermediate and all cases of high-risk NMIBC.[4,5] Despite the promises of BCG, long-term disease-free and progression-free survival is difficult to achieve.[6] BCG failures are further classified into BCG-refractory, BCG-resistant, BCG-relapsing, and BCG-intolerant disease [Table 2].[7]
Table 1.
Risk stratification of NMIBC
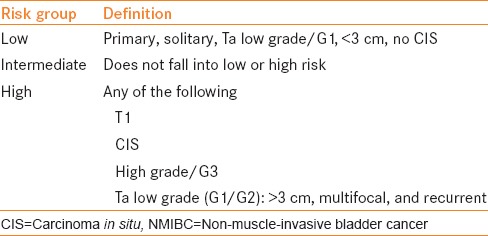
Table 2.
Definition of BCG failure

Currently, cystectomy is recommended as the standard of care after BCG failure in patients with high-risk disease. If cystectomy is performed before progression to muscle-invasive disease, cancer-specific survival has been reported to be over 90%.[8] However, other studies have compared early cystectomy with delayed cystectomy in patients failing BCG and shown a 10-year cancer-specific survival rate of 78% in the early cystectomy group versus 51% in a deferred cystectomy group, with upstaging in 30% of the cystectomy cases.[9] A recent review of the Surveillance, Epidemiology and End Results (SEER) Medicare data suggests that most recurrences occur within the first 2 years from initial therapy.[10] Because NMIBC is generally a diagnosis of the elderly, many patients are unfit or unwilling to undergo radical cystectomy as recommended. Such high rates of progression and recurrence demanding intervention pose significant morbidity and distress for the patients. We present a review of the literature regarding treatment options for patients who have failed BCG therapy.
MATERIALS AND METHODS
We performed a literature search using standard search engines with the last search in March 2015. Articles were identified for review regarding NMIBC and intravesical treatment after BCG failure. The references of these articles were then reviewed to find any article left out of the initial literature search for further review.
Valrubicin
Valrubicin is the only therapy approved by the United Stated Food and Drug Administration (U.S. FDA) for BCG-refractory CIS. This is based on an open-label, phase III trial performed in 90 patients, all of whom had CIS.[11] All patients experienced recurrence after receiving at least one prior intravesical therapy. The complete response (CR) rate to a 6-week induction course of valrubicin was 32%.[12] These data were updated recently, and the updated CR rate was 18% in two separate trials of valrubicin, while 4% of patients were expected to be disease-free at 2-year follow-up. The rate of progression was low. Therefore, despite FDA approval, long-term disease-free survival (DFS) remains poor with valrubicin and highlights the need for development of additional bladder-conserving therapies.
Gemcitabine
Gemcitabine is a deoxycytidine analogue that inhibits DNA synthesis.[13] When combined with cisplatin, gemcitabine is administered systemically as neoadjuvant chemotherapy in patients with muscle-invasive bladder cancer.[14] The use of intravesical gemcitabine for salvage therapy after BCG failure was first explored by Dalbagni et al. in 2002 [Table 3–5].[15] The results of their phase I trial demonstrated no drug-limiting toxicities at the maximum dose of 2000 mg/100 cc bicarbonate buffered saline administered twice weekly for 3 weeks with a 1-week break and then a second 3-week cycle of twice weekly instillation. In this phase I study, 39% of the patients experienced a CR at the 8-week follow-up. Adverse reactions included nausea, frequency of urination, intermittent gross hematuria, fatigue, and skin reaction. The initial results of this trial were then carried into a phase II trial in 30 patients given a 6-week course of induction therapy.[16] CR was assessed by visually negative cystoscopy, no tumor on biopsy, and negative cytology at 8 weeks, and then serial cystoscopy every 3 months. All patients had intermediate- to high-risk disease, including 76.6% of patients with CIS. Fifty percent of patients had a CR with median DFS of 3.6 months. At 1 year, 10% of patients experienced DFS, while two of the 30 patients had disease progression, including one with initial CR. All patients were BCG-refractory or -intolerant.[17] The follow-up of this study was then published by Sternberg et al. in 2013.[18] They followed 69 patients with intermediate- and high-risk disease who received induction therapy with gemcitabine after failing BCG, and found that 39.1% had a CR. Of the 69 patients in the study cohort, 37 patients had BCG-refractory disease, while only five were BCG-intolerant, 65 had high-grade disease, and 52 had either T1 or CIS, or both, demonstrating a good response in high-risk patients.
Table 3.
The use of intravesical gemcitabine for BCG failure
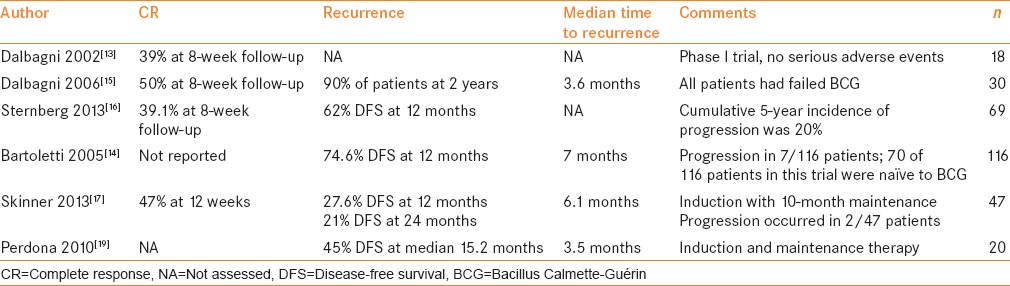
Table 5.
The use of intravesical PDT for BCG failure
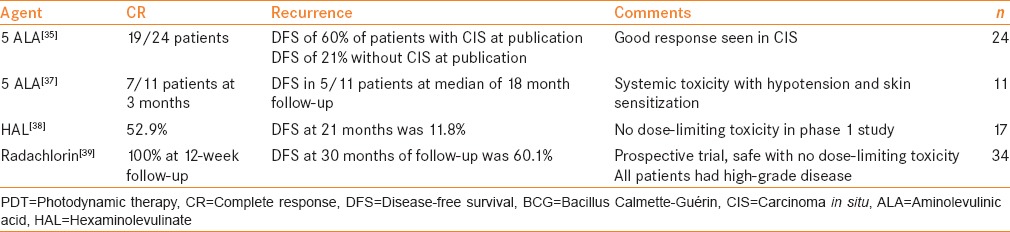
Table 4.
The use of intravesical docetaxel for BCG failure

A multicenter phase II trial evaluated gemcitabine in 116 patients at European centers in patients with intermediate- and high-risk disease.[16] Seventy patients in this trial were naïve to intravesical therapy, while 46 had received at least one prior treatment, most with BCG. In this trial, 2000 mg in 50 cc of diluent was administered over 6 consecutive weekly treatments. Patients were followed with cystoscopy and cytology every 3 months for recurrence or progression. At 1 year, 74.6% of patients were disease-free. Progression occurred in seven patients. Multivariate analysis did not reveal significant differences in patient characteristics; however, this trial was conducted in more intravesical treatment-naïve patients than BCG failure patients, and the results for BCG failure must be interpreted with caution. A SWOG trial was conducted in 47 patients with intermediate- to high-risk NMIBC (42 of 47 were high-risk) who had failed two prior induction cycles of BCG or BCG/interferon (IFN), with the most recent case being within at least 36 months of the trial.[19] Patients received 2000 mg gemcitabine in 100 mL for a 6-week induction, followed by 10 months of maintenance therapy. The study demonstrated a 47% CR rate at 3 months, 27.6% DFS at 12 months, 21% DFS at 24 months, and progression in two of 47 patients. This high-risk patient population likely accurately reflects the course of typical early BCG failure in that there is a substantial subset of patients who will respond to gemcitabine treatment, with a significant number of those patients achieving prolonged DFS.
BCG versus gemcitabine as second-line therapy has also been compared in patients with high-risk disease and first-time BCG failure. DiLorenzo et al. randomized 40 patients to transurethral resection (TUR) and then either 2000 mg/50 mL of gemcitabine instilled twice weekly for 6 weeks with 3 weeks of maintenance therapy at 3 months, 6 months, and 12 months, versus TUR followed by 6 weeks of induction with 81 mg/50 mL BCG and 3 weeks of maintenance therapy at 3 months, 6 months, and 12 months.[20] There was no difference between groups in terms of grade, stage, or concomitant CIS. Recurrence was evaluated by cystoscopy, cytology, and confirmatory biopsy every 3 months. At a median follow-up of 15.2 months, 47.5% of patients in the gemcitabine arm were disease-free, compared to 12.5% in the BCG arm. The poor response to BCG was unexpected given that both groups had similar disease characteristics; however, the type of BCG failure was not reported for either group and might have contributed to this. Progression was similar in both groups, with 33% and 37% progression in the gemcitabine and BCG arms, respectively. In a smaller cohort of 20 patients treated with gemcitabine with the same protocol, at 15.2 months, 45% of patients experienced DFS, while five of 20 patients experienced disease progression.[21]
In a phase III randomized trial, 109 patients with intermediate-risk NMIBC who had received prior treatment with BCG or epirubicin were randomized to receive five doses of 40 mg mitomycin C (MMC) or 6-weekly doses of 2000 mg/50 mL gemcitabine with responders receiving 10-monthly maintenance doses.[22] At a median follow-up of 36 months, 72% of patients in the gemcitabine and 61% of patients in the MMC arm experienced DFS. No patient had CIS. Median time to recurrence in the mitomycin arm was 15 months, while in the gemcitabine arm, it had not yet been reached. Ten patients in the MMC and six patients in the gemcitabine arm had progression, which suggests that gemcitabine might be a viable treatment option in patients with intermediate-risk disease in place of MMC. These results are promising. The data suggest that gemcitabine is an emerging therapy for the treatment of NIMBC after BCG failure and is less toxic than MMC, which is in agreement with a recent Cochrane review.[23] It provides some evidence for the efficacy and importance of maintenance regimens for intravesical chemotherapy in maintaining and prolonging DFS.
Taxanes (paclitaxel and docetaxel)
Taxanes exert their action via the inhibition of microtubule depolymerization, leading to cell cycle arrest and cell death.[24] A phase I trial in 18 patients failing two prior courses of BCG found no dose-limiting toxicity to docetaxel at 75 mg.[25] Adverse drug reactions included dysuria, hematuria, and urgency without systemic toxicity. All patients could hold the medication for 2 h in the bladder. Fifty-five percent of patients had a CR at 4-week follow-up.[26] Long-term follow-up of the phase I trial at a median of 48 months confirmed DFS in 22.2% and progression in 11.1% of the patients. This cohort of 18 patients and 15 additional patients were followed up.[27] Sixty-one percent of patients were found to have a CR. Half of these patients received maintenance therapy with docetaxel for up to 9 months. At the median follow-up of 29 months, 1-year DFS was 45% and 2-year DFS was 32%. This cohort was expanded to 13 patients with intermediate- and high-risk NMIBC having received at least one prior induction cycle of BCG, who received 75 mg docetaxel and monthly maintenance therapy for 9 months. The CR rate was 76.9% and the DFS was 46.2% at the median follow-up of 13 months.[28] Follow-up on 54 patients treated by the same group showed that 59% of patients experienced a CR. DFS at 1 year was 40% and at 3 years was 25%. When analyzed for those who received maintenance docetaxel and those who did not, median time to recurrence was 39.3 months versus 19 months, respectively.[29] Findings were not statistically significant, and the patient sample size was limited in these cohorts. However, the CR rate to docetaxel in BCG-refractory NMIBC was initially high (55-75%). These studies underscore the importance of maintenance therapy after CR.
Paclitaxel bound to albumin (nab-paclitaxel), a second taxane with similar mechanism of action of docetaxel, is approved for use in breast cancer, with increased prolongation of progression-free survival in patients with metastatic disease compared to docetaxel.[30] Given these findings, nab-paclitaxel was evaluated in 18 intermediate- and high-risk patients with NMIBC who had been treated with BCG/IFN and MMC.[31] There were no dose-limiting toxicities in this phase I trial and 28% of patients experienced a CR after 6 weeks of inductions. This was then evaluated in a phase II trial of 28 patients.[32] Thirty-five percent of patients experienced a CR, and eight of 10 of these patients went on to receive 6 months of monthly maintenance therapy. Seven of these eight patients were free of disease at a mean follow-up of 21.
MMC and thermochemotherapy
MMC cross-links DNA and inhibits DNA synthesis.[33] It is the standard of care to instill MMC after transurethral resection of bladder tumor (TURBT). This has been combined with a local microwave hyperthermia device (Synergo, Medical Enterprises, Amsterdam, the Netherlands). A multicenter trial was conducted in 83 patients with intermediate- and high-risk recurrent NMIBC that compared MMC to MMC plus local microwave hyperthermia.[34] In this study, 42 patients were assigned to receive microwave hyperthermia with MMC, with 41 patients receiving MMC alone. In each group, 42% of patients had received prior therapy with BCG, MMC, or epirubicin. Induction was 8 weekly treatments, and then monthly maintenance was performed for 4 months. The study found that 17.1% of patients in the MMC plus microwave hyperthermia group versus 57.5% in the MMC-only group experienced recurrence of disease. This difference was found to be statistically significant. A long-term, intention-to-treat analysis at a median follow-up of 90 months of 65 of the initial 75 patients demonstrated that 40% of patients in the MMC plus microwave hyperthermia group and 80% of patients in the MMC-only group experienced recurrence of disease.[35] These data should be interpreted with caution, as over half the patients in each group were naïve to intravesical therapy. These data do, however, support the superiority of MMC plus microwave hyperthermia to MMC alone.
Witjes analyzed 49 patients receiving MMC plus microwave hyperthermia for 6 weeks followed by 6 maintenance treatments at 6-week intervals. Thirty-four of the patients in this cohort had failed BCG and all had CIS. At initial evaluation, 92% of patients were disease-free. Fifty-one percent of patients at the median of 22-month follow-up remained free of disease, while four of the patients were found to have disease progression after cystectomy. These results were not dependent on prior BCG treatment and were similar in both groups.[36] In a more recent series of 21 patients receiving MMC plus microwave hyperthermia, MMC plus microwave hyperthermia was poorly tolerated, requiring cessation of therapy in 38% of patients. At a median of 50 months, 29% of patients remained recurrence-free.[37] Further evaluation of MMC plus microwave hyperthermia to improve tolerance and in cohorts composed solely of patients with BCG-refractory NMIBC are needed.
Photodynamic therapy
PDT involves the administration of a photosensitizing agent with activation of the agent by light at the appropriate wavelength. This has been investigated for use in BCG-refractory NMIBC using 5-aminolevulinic acid (5-ALA). The first report of PDT using oral 5-ALA was published by Waidelich et al.[38] In a cohort of 24 patients with median follow-up of 36 months and at least two prior courses of BCG, 19 of 24 patients achieved a CR to therapy, including all patients with CIS. This response was durable at publication in 60% of patients with CIS and 21% of patients without CIS. Noted side effects included nausea, hypotension, tachycardia, and some skin sensitivity. These resolved within 24 h but were noted to require intervention to maintain hemodynamic stability in some patients with oral administration of 5-ALA. An additional study was performed in 31 patients, 10 of whom had received BCG, while 21 were intravesical therapy-naïve, which demonstrated a good tolerability of PDT.[39] In a group of 11 patients, 10 with prior therapy, 7 of 11 patients treated with PTD and ALA experienced a CR at 3 months and 5 of the 11 patients experienced DFS at median of 18 month follow-up.[40] In patients unfit for radical cystectomy for cardiovascular reasons, it should be noted that systemic ALA can cause hemodynamic instability.
PDT with intravesical hexaminolevulinate (HAL) was investigated in a phase I trial of 17 patients with intermediate or high risk NMIBC, of whom 15 had received prior intravesical therapy.[41] These patients received three treatments, 6 weeks apart, with varying energy levels and differing concentrations. Adverse events were severe, irritative voiding complaints without any noted hemodynamic instability. Initial CR rate was 52.9%, with 21 months’ DFS achieved in 11.8% of patients.
Radachlorin, a third photosensitizer has also been investigated in a prospective trial of 34 patients with high-risk NMIBC and BCG failure.[42] This trial demonstrated that radachlorin was also safe without significant adverse events. The authors also demonstrated a CR rate of 100% at 12-week follow-up and 60.1% DFS at 30-month follow-up. These studies are small and underpowered to assess efficacy; however, PDT with photosensitizing agents, especially HAL and radachlorin, appears to be safe and well-tolerated. Future investigations into radachlorin are warranted to confirm these promising results. PDT is only available in highly specialized centers.
BCG/IFN
IFN is an immunostimulant that has been evaluated in patients with BCG failure and been found to have some efficacy as a single agent in BCG failure.[43] However, IFN has most thoroughly been evaluated in combination with BCG. An induction and maintenance course of one-third dose BCG/IFN therapy was described in 42 patients with recurrence after BCG failure.[44] It was found that 63% and 53% of patients were free from recurrence at 12 months and 24 months, respectively. All patients who completed maintenance therapy were disease-free at median 30-month follow-up. A second cohort of BCG-naïve and BCG-refractory patients demonstrated an initial CR rate in 60% of the BCG-refractory patients, and DFS at median 22 months in 95% of patients.[45] A large, multicenter trial of 1,007 patients both naïve to BCG and having failed BCG received full-dose BCG/IFN or one-third dose BCG/INF respectively with induction, and then three maintenance courses.[46] At 24 months’ median follow-up, 45% of patients in the BCG failure arm were disease-free. Multivariate analysis revealed that stage T1 tumors had a lower response than stage Ta, that tumors larger than 5 cm had a worse outcome than those less than 1 cm, and that multifocal disease and more than two BCG failures predicted a worse outcome.
Sequential gemcitabine and MMC
Both gemcitabine and MMC have shown promise in NMIBC. A phase I trial of 10 patients who underwent a 6-week course of induction therapy with instillation of gemcitabine followed immediately by MMC in the same sitting and then 12 monthly maintenance treatments was performed.[47] In this study, nine of 10 patients had failed BCG, eight of 10 had failed BCG/IFN, and one patient had failed MMC. Patients were evaluated by biopsy at first cystoscopy. Six of the 10 patients had a CR, which was durable at median 14 months of follow-up. One patient progressed. No major adverse drug reactions were reported. In a larger study of 27 patients, 23 of whom had recurrent high-risk disease, all of whom had received at least one course of intravesical therapy, 63% of patients experienced recurrence at a median of 15.2 months[48] Induction therapy was 6-8 weeks and maintenance therapy was not standardized, as only three patients received maintenance. The largest multi-institutional study was conducted in 47 patients having failed a median of two intravesical treatments.[49] Ten of the 47 patients were naïve to prior therapy. Patients were given a 6-week induction course and 12 months of maintenance therapy. The CR rate was 68%. DFS at 1 and 2 years was 48% and 38%, respectively. The data from these studies are promising and support intravesical sequential therapy; however, the cohorts are small and prospective data are missing.
Radical cystectomy
Limited prospective trials are available to assess the timing of radical cystectomy for NMIBC and no trials could be found comparing radical cystectomy and intravesical chemotherapy after BCG failure. A retrospective comparison of two cohorts, of 307 patients studied from 1980 to 1989 and 589 patients treated between 1992 and 2004, with BCG for NMIBC examined T1 disease specifically.[50] In the cohort of 307 patients, the authors found that of 85 patients with T1 disease at re-TUR who received a second course of BCG, 60 (70%) of the patients had progression to muscle-invasive disease at a median of 4.3 years of follow-up. In the 589-patient cohort, 129 patients had T1 disease at recurrence. At a median follow-up of 2.2 years, 77 of the 129 patients (60%) experienced progression of disease. In the 307-patient cohort, 42 patients died from bladder cancer at a median of 2.5 years from recurrence and nine died of other causes. In the larger cohort, 30 patients died from disease and 31 of other causes at median of 2.7 years of follow-up. The difference noted between these two groups was that cystectomy was offered earlier in the larger, more contemporary cohort. This study does highlight a reasonably high rate of progression in patients with T1 disease after BCG treatment.
A second similar, retrospective comparison of a historic cohort (77 patients treated 1993-2002) and a contemporary cohort (75 patients treated 2003-2007) was performed in patients who had failed BCG induction and underwent radical cystectomy.[51] There were no differences between the groups including time to cystectomy, progression at cystectomy, final disease stage, or prior BCG treatment. In the first and second groups, 43% and 52% of patients, respectively had muscle-invasive disease at cystectomy. In these studies, patients with recurrence after BCG failure who underwent cystectomy were noted to have progression of disease in 43% to 70% of cases, with high-grade T1 tumors posing the largest risk. Herr et al. demonstrated that patients who undergo cystectomy within 2 years from BCG failure have better outcomes than those who undergo cystectomy more than 2 years after failure.[8] This suggests that, despite the risk of progression, there is still a window in which patients have the opportunity to receive bladder-conserving therapy.
DISCUSSION
The current standard of care for recurrent, high-grade NMIBC BCG failure is cystectomy. However, the evidence for cystectomy is mostly retrospective in nature, and many patients are either unfit or unwilling to undergo cystectomy. In addition, radical cystectomy has been associated with significant morbidity and mortality.[52] Despite this, quality of life after cystectomy is largely unchanged,[53] while surveillance and intravesical treatments can impact the quality of life.[54] The healthcare costs associated with bladder cancer are significant, with increased costs in patients with muscle-invasive disease compared to NMIBC; however, the cost of progression is significantly higher than either group.[55] As up to 70% of patients recur and up to 30% progress after BCG therapy, it is of vital importance to define appropriate treatment options for these patients.[3,5,10]
Evidence for most therapies is from small, retrospective cohorts, with heterogeneous patient populations, which are likely biased owing to poor patient fitness for radical cystectomy. In terms of nonchemotherapeutic therapies, results of recent PDTs, especially with radachlorin, for CIS, are promising. Single-agent chemotherapy can achieve a CR rate of up to 50% for gemcitabine, 77% for docetaxel, 35% with nab-paclitaxel, and 32% for valrubicin,[11,12] with a trend for improvement when maintenance therapy is given. Long-term DFS is still difficult to achieve despite these CR rates. MMC plus microwave hyperthermia has been shown to be superior to MMC alone, with CR rates of up to 92% having been reported. These must be interpreted with caution, as current studies include BCG-naïve and BCG failure cohorts and there is some evidence that microwave hyperthermia might not be well tolerated.
As systemic chemotherapy is generally given in combination with multiple agents, it would make sense that combination therapy might be more efficacious in bladder cancer. Combination therapy with BCG/IFN is promising and represents the largest prospectively studied cohort of patients to date with BCG failure in NMIBC. A CR rate of up to 60% has been described, and with maintenance therapy, 24-month DFS was noted in 45% of patients.[46] In addition, the combination of gemcitabine and mitomycin given sequentially has been shown to be well tolerated, with CRs in up to 68% of patients, with up to 38% DFS at 2 years with maintenance therapy.
It is possible that BCG-refractory disease might represent a more aggressive or treatment-resistant form of NMIBC. In addition, cystectomy studies suggest that many of these patients, especially those greater than 2 years from BCG failure, will have already had progression of disease at the time of initiation of second-line intravesical treatment. Further studies are needed to best define treatment options in BCG failure, including possible prospective trials comparing intravesical treatments with early cystectomy. The classification of failure, individual tumor characteristics, and comorbid conditions of those treated should be addressed for analysis. In addition to DFS and progression, overall survival and cancer-specific survival should also be considered, as NMIBC is often a disease of the elderly patient with multiple comorbid conditions. PDT also shows promise, and future studies might consider combining PDT with other intravesical therapy. Currently, the combination of multiple, sequentially administered chemotherapeutic agents with a maintenance protocol or therapy with hyperthermia appears to provide improved outcomes over single-agent trials and should be considered for further investigation.
Financial support and sponsorship
Nil.
Conflicts of interest
Nathan A. Brooks: None. Michael A. O’Donnell: Consultant/Advisor for the following: Cytologics, Sanofi-Aventis, Viventia, Merck, BioNiche, Sonacare, BioCencell, Theralase.
REFERENCES
- 1.Colombel M, Soloway M, Akaza H, Böhle A, Palou J, Buckley R, et al. Epidemiology, staging, grading, and risk stratification of bladder cancer. Eur Urol Suppl. 2008;7:618–26. [Google Scholar]
- 2.Ferlay J, Shin HR, Bray F, Forman D, Mathers C, Parkin DM. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int J Cancer. 2010;127:2893–917. doi: 10.1002/ijc.25516. [DOI] [PubMed] [Google Scholar]
- 3.Sylvester RJ, van der Meijden AP, Oosterlinck W, Witjes JA, Bouffioux C, Denis L, et al. Predicting recurrence and progression in individual patients with stage Ta T1 bladder cancer using EORTC risk tables: A combined analysis of 2596 patients from seven EORTC trials. Eur Urol. 2006;49:466–77. doi: 10.1016/j.eururo.2005.12.031. [DOI] [PubMed] [Google Scholar]
- 4.Babjuk M, Burger M, Zigeuner R, Shariat SF, van Rhijn BW, Compérat E, et al. European Association of Urology. EAU guidelines on non-muscle-invasive urothelial carcinoma of the bladder: Update 2013. Eur Urol. 2013;64:639–53. doi: 10.1016/j.eururo.2013.06.003. [DOI] [PubMed] [Google Scholar]
- 5.Brausi M, Witjes JA, Lamm D, Perdas J, Palou J, Colombel M, et al. A review of current guidelines and best practice recommendations for the management of nonmuscle invasive bladder cancer by the International Bladder Cancer Group. J Urol. 2011;186:2158–67. doi: 10.1016/j.juro.2011.07.076. [DOI] [PubMed] [Google Scholar]
- 6.Logan C, Brown M, Hayne D. Intravesical therapies for bladder cancer - indications and limitations. BJU Int. 2012;110(Suppl 4):12–21. doi: 10.1111/j.1464-410X.2012.11619.x. [DOI] [PubMed] [Google Scholar]
- 7.O’Donnell M, Boehle A. Treatment options for BCG failures. World J Urol. 2006;24:481–7. doi: 10.1007/s00345-006-0112-0. [DOI] [PubMed] [Google Scholar]
- 8.Herr HW, Sogani PC. Does early cystectomy improve the survival of patients with high risk superficial bladder tumors? J Urol. 2001;166:1296–9. [PubMed] [Google Scholar]
- 9.Denzinger S, Fritsche HM, Otto W, Blana A, Wieland WF, Burger M. Early versus deferred cystectomy for initial high-risk pt1g3 urothelial carcinoma of the bladder: Do risk factors define feasibility of bladder-sparing approach? Eur Urol. 2008;53:146–52. doi: 10.1016/j.eururo.2007.06.030. [DOI] [PubMed] [Google Scholar]
- 10.Chamie K, Litwin MS, Bassett JC, Daskivich TJ, Lai J, Hanley JM, et al. Recurrence of high-risk bladder cancer: A population-based analysis. Cancer. 2013;119:3219–27. doi: 10.1002/cncr.28147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Steinberg G, Bahnson R, Brosman S, Middleton R, Wajsman Z, Wehle M. Efficacy and safety of valrubicin for the treatment of Bacillus Calmette-Guerin refractory carcinoma in situ of the bladder. The Valrubicin Study Group. J Urol. 2000;163:761–7. [PubMed] [Google Scholar]
- 12.Dinney CP, Greenberg RE, Steinberg GD. Intravesical valrubicin in patients with bladder carcinoma in situ and contraindication to or failure after bacillus Calmette-Guérin. Urol Oncol. 2013;31:1635–42. doi: 10.1016/j.urolonc.2012.04.010. [DOI] [PubMed] [Google Scholar]
- 13.Plunkett W, Huang P, Searcy CE, Gandhi V. Gemcitabine: Preclinical pharmacology and mechanisms of action. Semin Oncol. 1996;23(Suppl 10):3–15. [PubMed] [Google Scholar]
- 14.Dash A, Pettus JA, 4th, Herr HW, Bochner BH, Dalbagni G, Donat SM, et al. A role for neoadjuvant gemcitabine plus cisplatin in muscle-invasive urothelial carcinoma of the bladder: A retrospective experience. Cancer. 2008;113:2471–7. doi: 10.1002/cncr.23848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dalbagni G, Russo P, Sheinfeld J, Mazumdar M, Tong W, Rabbani F, et al. Phase 1 trial of intravesical gemcitabine in bacillus Calmette-Guérin-refractory transitional-cell carcinoma of the bladder. J Clin Oncol. 2002;20:3193–8. doi: 10.1200/JCO.2002.02.066. [DOI] [PubMed] [Google Scholar]
- 16.Bartoletti R, Cai T, Gacci M, Giubilei G, Viggiani F, Santelli G, et al. TUR (Toscana Urologia) Group. Intravesical gemcitabine therapy for superficial transitional cell carcinoma: Results of a phase II prospective multicenter study. Urology. 2005;66:726–31. doi: 10.1016/j.urology.2005.04.062. [DOI] [PubMed] [Google Scholar]
- 17.Dalbagni G, Russo P, Bochner B, Ben-Porat L, Sheinfeld J, Sogani P, et al. Phase II trial of intravesical gemcitabine in bacille Calmette-Guérin-refractory transitional cell carcinoma of the bladder. J Clin Oncol. 2006;24:2729–34. doi: 10.1200/JCO.2005.05.2720. [DOI] [PubMed] [Google Scholar]
- 18.Sternberg IA, Dalbagni G, Chen LY, Donat SM, Bochner BH, Herr HW. Intravesical gemcitabine for high risk, nonmuscle invasive bladder cancer after bacillus Calmette-Guérin treatment failure. J Urol. 2013;190:1686–91. doi: 10.1016/j.juro.2013.04.120. [DOI] [PubMed] [Google Scholar]
- 19.Skinner EC, Goldman B, Sakr WA, Petrylak DP, Lenz HF, Lee CT, et al. SWOG S0353: Phase II trial of intravesical gemcitabine in patients with nonmuscle invasive bladder cancer and recurrence after 2 prior courses of intravesical bacillus Calmette-Guérin. J Urol. 2013;190:1200–4. doi: 10.1016/j.juro.2013.04.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Di Lorenzo G, Perdonà S, Damiano R, Faiella A, Cantiello F, Pignata S, et al. Gemcitabine versus bacille Calmette-Guérin after initial bacille Calmette-Guérin failure in non-muscle-invasive bladder cancer. A multicenter prospective randomized trial. Cancer. 2010;116:1893–900. doi: 10.1002/cncr.24914. [DOI] [PubMed] [Google Scholar]
- 21.Perdonà S, Di Lorenzo G, Cantiello F, Damiano R, De Sio M, Masala D, et al. Is gemcitabine an option in BCG-refractory nonmuscle-invasive bladder cancer? A single-arm prospective trial. Anticancer Drugs. 2010;21:101–6. doi: 10.1097/CAD.0b013e3283324d83. [DOI] [PubMed] [Google Scholar]
- 22.Addeo R, Caraglia M, Bellini S, Abbruzzese A, Vincenzi B, Montella L, et al. Randomized phase III trial on gemcitabine versus mytomicin in recurrent superficial bladder cancer: Evaluation of efficacy and tolerance. J Clin Oncol. 2010;28:543–8. doi: 10.1200/JCO.2008.20.8199. [DOI] [PubMed] [Google Scholar]
- 23.Jones G, Cleves A, Wilt TJ, Mason M, Kynaston HG, Shelley M. Intravesical gemcitabine for non-muscle invasive bladder cancer. Cochrane Database Syst Rev. 2012;1:CD009294. doi: 10.1002/14651858.CD009294.pub2. [DOI] [PubMed] [Google Scholar]
- 24.Herbst RS, Khuri FR. Mode of action of docetaxel - a basis for combination with novel anticancer agents. Cancer Treat Rev. 2003;29:407–15. doi: 10.1016/s0305-7372(03)00097-5. [DOI] [PubMed] [Google Scholar]
- 25.McKiernan JM, Masson P, Murphy AM, Goetzl M, Olsson CA, Petylak DP, et al. Phase I trial of intravesical docetaxel in the management of superficial bladder cancer refractory to standard intravesical therapy. J Clin Oncol. 2006;24:3075–80. doi: 10.1200/JCO.2005.03.1161. [DOI] [PubMed] [Google Scholar]
- 26.Laudano MA, Barlow LJ, Murphy AM, Petrylak DP, Desai M, Benson MC, et al. Long-term clinical outcomes of a phase I trial of intravesical docetaxel in the management of non-muscle-invasive bladder cancer refractory to standard intravesical therapy. Urology. 2010;75:134–7. doi: 10.1016/j.urology.2009.06.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Barlow L, McKiernan J, Benson MC. The novel use of intravesical docetaxel for the treatment of non-muscle invasive bladder cancer refractory to BCG therapy: A single institution experience. World J Urol. 2009;27:331–5. doi: 10.1007/s00345-009-0377-1. [DOI] [PubMed] [Google Scholar]
- 28.Barlow L, McKiernan J, Sawczuk I, Benson M. A single-institution experience with induction and maintenance intravesical docetaxel in the management of non-muscle-invasive bladder cancer refractory to bacille Calmette-Guérin therapy. BJU Int. 2009;104:1098–102. doi: 10.1111/j.1464-410X.2009.08543.x. [DOI] [PubMed] [Google Scholar]
- 29.Barlow LJ, McKiernan JM, Benson MC. Long-term survival outcomes with intravesical docetaxel for recurrent nonmuscle invasive bladder cancer after previous bacillus Calmette-Guérin therapy. J Urol. 2013;189:834–9. doi: 10.1016/j.juro.2012.10.068. [DOI] [PubMed] [Google Scholar]
- 30.Gradishar WJ, Krasnojon D, Cheporov S, Makhson AN, Manikhas GM, Clawson A, et al. Significantly longer progression-free survival with nab-paclitaxel compared with docetaxel as first-line therapy for metastatic breast cancer. J Clin Oncol. 2009;27:3611–9. doi: 10.1200/JCO.2008.18.5397. [DOI] [PubMed] [Google Scholar]
- 31.McKiernan JM, Barlow LJ, Laudano MA, Mann MJ, Petrylak DP, Benson MC. A Phase I trial of intravesical nanoparticle albumin-bound paclitaxel in the treatment of bacillus Calmette-Guérin refractory nonmuscle invasive bladder cancer. J Urol. 2011;186:448–51. doi: 10.1016/j.juro.2011.03.129. [DOI] [PubMed] [Google Scholar]
- 32.McKiernan JM, Holder DD, Ghandour RA, Barlow LJ, Ahn JJ, Kates M, et al. Phase II trial of intravesical nanoparticle albumin bound paclitaxel for the treatment of nonmuscle invasive urothelial carcinoma of the bladder after bacillus Calmette-Guérin treatment failure. J Urol. 2014;192:1633–8. doi: 10.1016/j.juro.2014.06.084. [DOI] [PubMed] [Google Scholar]
- 33.Verweij J, Pinedo HM. Mitomycin C: Mechanism of action, usefulness and limitations. Anticancer Drugs. 1990;1:5–13. [PubMed] [Google Scholar]
- 34.Colombo R, Da Pozzo LF, Salonia A, Rigatti P, Leib Z, Baniel J, et al. Multicentric study comparing intravesical chemotherapy alone and with local microwave hyperthermia for prophylaxis of recurrence of superficial transitional cell carcinoma. J Clin Oncol. 2003;21:4270–6. doi: 10.1200/JCO.2003.01.089. [DOI] [PubMed] [Google Scholar]
- 35.Colombo R, Salonia A, Leib Z, Pavone-Macaluso M, Engelstein D. Long-term outcomes of a randomized controlled trial comparing thermochemotherapy with mitomycin-C alone as adjuvant treatment for non-muscle-invasive bladder cancer (NMIBC) BJU Int. 2011;107:912–8. doi: 10.1111/j.1464-410X.2010.09654.x. [DOI] [PubMed] [Google Scholar]
- 36.Alfred Witjes J, Hendricksen K, Gofrit O, Risi O, Nativ O. Intravesical hyperthermia and mitomycin-C for carcinoma in situ of the urinary bladder: Experience of the European Synergo working party. World J Urol. 2009;27:319–24. doi: 10.1007/s00345-009-0384-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kiss B, Schneider S, Thalmann GN, Roth B. Is thermochemotherapy with the Synergo system a viable treatment option in patients with recurrent non-muscle-invasive bladder cancer? Int J Urol. 2015;22:158–62. doi: 10.1111/iju.12639. [DOI] [PubMed] [Google Scholar]
- 38.Waidelich R, Stepp H, Baumgartner R, Weninger E, Hofstetter A, Kriegmair M. Clinical experience with 5-aminolevulinic acid and photodynamic therapy for refractory superficial bladder cancer. J Urol. 2001;165:1904–7. doi: 10.1097/00005392-200106000-00015. [DOI] [PubMed] [Google Scholar]
- 39.Berger AP, Steiner H, Stenzl A, Akkad T, Bartsch G, Holtl L. Photodynamic therapy with intravesical instillation of 5-aminolevulinic acid for patients with recurrent superficial bladder cancer: A single-center study. Urology. 2003;61:338–41. doi: 10.1016/s0090-4295(02)02123-4. [DOI] [PubMed] [Google Scholar]
- 40.Waidelich R, Beyer W, Knchel R, Stepp H, Baumgartner R, Schröder J, et al. Whole bladder photodynamic therapy with 5-aminolevulinic acid using a white light source. Urology. 2003;61:332–7. doi: 10.1016/s0090-4295(02)02164-7. [DOI] [PubMed] [Google Scholar]
- 41.Bader MJ, Stepp H, Beyer W, Pongratz T, Sroka R, Kriegmair M, et al. Photodynamic therapy of bladder cancer - a phase I study using hexaminolevulinate (HAL) Urol Oncol. 2013;31:1178–83. doi: 10.1016/j.urolonc.2012.02.007. [DOI] [PubMed] [Google Scholar]
- 42.Lee JY, Diaz RR, Cho KS, Lim Ms, Chung JS, Kim WT, et al. Efficacy and safety of photodynamic therapy for recurrent, high grade nonmuscle invasive bladder cancer refractory or intolerant to bacille Calmette-Guérin immunotherapy. J Urol. 2013;190:1192–9. doi: 10.1016/j.juro.2013.04.077. [DOI] [PubMed] [Google Scholar]
- 43.Askeland EJ, Newton MR, O’Donnell MA, Luo Y. Bladder cancer immunotherapy: BCG and beyond. Adv Urol 2012. 2012 doi: 10.1155/2012/181987. 181987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.O’Donnell MA, Krohn J, DeWolf WC. Salvage intravesical therapy with interferon-alpha 2b plus low dose bacillus Calmette-Guerin is effective in patients with superficial bladder cancer in whom bacillus Calmette-Guerin alone previously failed. J Urol. 2001;166:1300–5. [PubMed] [Google Scholar]
- 45.Lam JS, Benson MC, O’Donnell MA, Sawczuk A, Gavazzi A, Wechsler MH, et al. Bacillus Calmete-Guérin plus interferon-alpha2B intravesical therapy maintains an extended treatment plan for superficial bladder cancer with minimal toxicity. Urol Oncol. 2003;21:354–60. doi: 10.1016/s1078-1439(03)00012-7. [DOI] [PubMed] [Google Scholar]
- 46.Joudi FN, Smith BJ, O’Donnell MA National BCG-Interferon Phase 2 Investigator Group. Final results from a national multicenter phase II trial of combination bacillus Calmette-Guérin plus interferon alpha-2B for reducing recurrence of superficial bladder cancer. Urol Oncol. 2006;24:344–8. doi: 10.1016/j.urolonc.2005.11.026. [DOI] [PubMed] [Google Scholar]
- 47.Breyer BN, Whitson JM, Carroll PR, Konety BR. Sequential intravesical gemcitabine and mitomycin C chemotherapy regimen in patients with non-muscle invasive bladder cancer. Urol Oncol. 2010;28:510–4. doi: 10.1016/j.urolonc.2008.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Cockerill PA, Knoedler JJ, Frank I, Tarrell R, Karnes RJ. Intravesical gemcitabine in combination with mitomycin C as salvage treatment in recurrent non-muscle-invasive bladder cancer. BJU Int. 2015 doi: 10.1111/bju.13088. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 49.Lightfoot AJ, Breyer BN, Rosevear HM, Erickson BA, Konety BR, O’Donnell MA. Multi-institutional analysis of sequential intravesical gemcitabine and mitomycin C chemotherapy for non-muscle invasive bladder cancer. Urol Oncol. 2014;32:35.e15–9. doi: 10.1016/j.urolonc.2013.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Raj GV, Herr H, Serio AM, Donat SM, Bochner BH, Vickers AJ, et al. Treatment paradigm shift may improve survival of patients with high risk superficial bladder cancer. J Urol. 2007;177:1283–6. doi: 10.1016/j.juro.2006.11.090. [DOI] [PubMed] [Google Scholar]
- 51.Soloway MS, Hepps D, Katkoori D, Ayyathurai R, Manoharan M. Radical cystectomy for BCG failure: Has the timing improved in recent years? BJU Int. 2011;108:182–5. doi: 10.1111/j.1464-410X.2010.09830.x. [DOI] [PubMed] [Google Scholar]
- 52.Stein JP, Lieskovsky G, Cote R, Groshen S, Feng AC, Boyd S, et al. Radical cystectomy in the treatment of invasive bladder cancer: Long-term results in 1,054 patients. J Clin Oncol. 2001;19:666–75. doi: 10.1200/JCO.2001.19.3.666. [DOI] [PubMed] [Google Scholar]
- 53.Somani BK, Gimlin D, Fayers P, N’Dow J. Quality of life and body image for bladder cancer patients undergoing radical cystectomy and urinary diversion-a prospective cohort study with a systematic review of literature. Urology. 2009;74:1138–43. doi: 10.1016/j.urology.2009.05.087. [DOI] [PubMed] [Google Scholar]
- 54.van der Aa MN, Steyerberg EW, Sen EF, Zwarthoff EC, Kirkels WJ, van der Kwast TH, et al. Patients’ perceived burden of cystoscopic and urinary surveillance of bladder cancer: A randomized comparison. BJU Int. 2008;101:1106–10. doi: 10.1111/j.1464-410X.2007.07224.x. [DOI] [PubMed] [Google Scholar]
- 55.Sievert KD, Amend B, Nagele U, Schilling D, Bedke J, Horstmann M, et al. Economic aspects of bladder cancer: What are the benefits and costs? World J Urol. 2009;27:295–300. doi: 10.1007/s00345-009-0395-z. [DOI] [PMC free article] [PubMed] [Google Scholar]


