Abstract
Introduction:
Non-muscle invasive bladder cancer (NMIBC) represents a broad spectrum of disease, the hallmarks of which include disease recurrence and progression. Clinicians have a number of surgical and therapeutic options at their disposal when treating this disease, and the underlying evidence continues to evolve. A number of professional organizations have invested in the development of clinical practice guidelines to guide patient management.
Materials and Methods:
We review and summarize four major guidelines, the American Urological Association, the European Association of Urology, the International Consultation on Urological Disease and the National Comprehensive Cancer Network.
Results:
Guideline panels differed in their composition, methodological approach and structure of recommendations. Despite this, many recommendations were similar between various panels, although differences are present in panel recommendations related to initial diagnosis and treatment, adjuvant therapy and disease surveillance.
Conclusions:
Guideline recommendations are similar at many decision points that clinicians face when managing NMIBC, although they are far from uniform. While future prospective, well-designed studies will hopefully clarify NMIBC management, urologists ultimately must rely on a combination of evidence-based recommendations, which they should seek to integrate with patients’ values and preferences and the individual circumstances to provide the best possible patient care.
Keywords: Bladder cancer, non-muscle invasive, practice guidelines
INTRODUCTION
Bladder cancer is a common worldwide malignancy, with an estimated 330,400 new cases diagnosed and 123,100 attributable deaths worldwide in 2012.[1] It is more common in men as the cumulative risk of being diagnosed with bladder cancer before the age of 75 years is 2.0% in men and 0.4% in women in developed areas. Bladder cancer is less common in developing areas, with 0.6% of men and 0.2% of women diagnosed before the age of 75 years.[1]
At diagnosis, 70–85% of bladder cancers are not invasive into the detrusor muscle of the bladder. This is a critical branch point in the treatment of bladder cancer, as patients with non-muscle invasive bladder cancer (NMIBC) may frequently be managed with bladder-sparing therapies. NMIBC is notable for frequent recurrence (50–80%) and progression to muscle-invasive disease (up to 30%), necessitating close patient follow-up and, at times, consideration of aggressive management.[2] Multiple risk factors contribute to disease recurrence and progression, including tumor size, grade, multifocality and the presence of carcinoma in situ (CIS). To combat the disease, urologists utilize endoscopy to resect and monitor tumor, intravesical therapies to prevent recurrence and progression and, at times, highly invasive extirpative surgery.[3]
Because of the complexity of NMIBC management, multiple groups have convened panels of international experts to develop clinical practice guidelines. The purpose of these guidelines is to provide concrete management guidance to clinicians at the point of care that are based on the current best evidence. Such guidelines should be developed using transparent and rigorous methodology as reflected in the recently published Institute of Medicine standards.[4] Hallmarks of such methodology include an assessment of the quality of evidence, broad stakeholder involvement as relevant to the disease entity, effective conflict of interest management and a distinction between the quality of evidence rating and the strength of recommendations. A recent study of clinical practice guidelines for prostate cancer suggests that many urology guidelines fall short of these standards.[5]
These limitations notwithstanding, the purpose of this review is to summarize the currently available guidelines for NMIBC, briefly describing their methodological approach and highlighting their recommendations.
GUIDELINE SUMMARY AND METHODOLOGY
Four major evidence-based guidelines were chosen for review, those published by the American Urological Association (AUA), the European Association of Urology (EAU), the International Consultation on Urological Disease (ICUD) and the National Comprehensive Cancer Network (NCCN) [Table 1].[2,6,7,8,9] Each guideline panel performed a literature review and sought to determine the available quality of evidence supporting a given management option, usually based on tumor staging [Table 2]. Guidelines panels varied based on their members, article inclusion criteria and methodological approach. These differences likely contributed to dissimilarities observed between panel recommendations.
Table 1.
Guideline panel organization, methods and recommendation categories
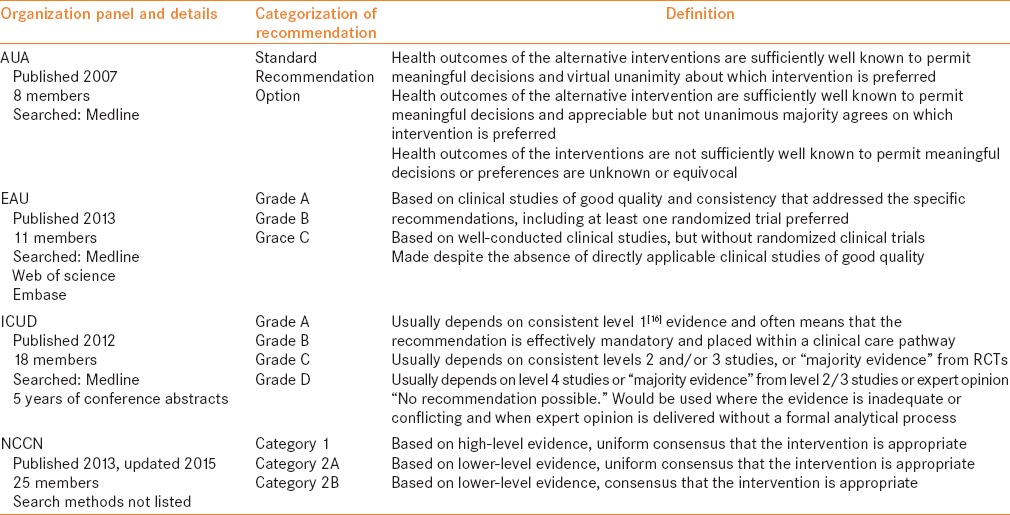
Table 2.
2010 AJCC TNM bladder cancer classification by tumor stage[17]
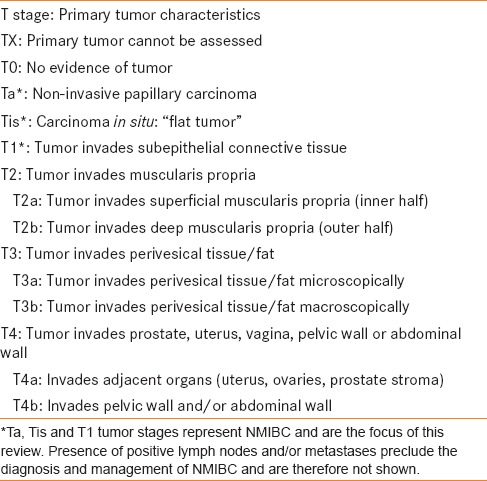
Table 1 summarizes the terminology used by each professional organization with regard to its guidance statements and the underlying quality of evidence. Unfortunately, there is no “cross-walk” to translate a given recommendation from one professional organization to that of another, thereby requiring the reader to interpret these in the context of the corresponding methodological framework. It should be noted that while all four are considered guideline documents, only that of the EAU meets minimal criteria to be listed by the National Guideline Clearing House[10] as an electronic depository for evidence-based clinical practice guidelines.[11] Adoption of a more rigorous methodological approach now places the AUA in a position to meet the guideline quality criteria recently defined by the Institute of Medicine. However, the guideline on NMIBC dates back to 2007 (with a revision in 2010). Despite their widespread appeal and dissemination, the ICUD and NCCN guidelines are not considered evidence based. They should be considered expert consensus-based documents and therefore interpreted with caution. A more in-depth discussion of guideline methodology is beyond the scope of this review and can be found elsewhere.[12,13] Efforts to raise methodological standards for all guideline developers in urology, ideally using a unifying, methodologically rigorous system are underway.[14]
INITIAL DIAGNOSIS AND STAGING
Initial trans-urethral resection of bladder tumor (TURBT)
For any abnormal urothelial growth, the AUA guidelines recommend that a biopsy is completed and “under most circumstances,” complete eradication of all visible tumors be performed (standard). Staging and clinical exam are mentioned as “universally accepted.” The EAU, ICUD and NCCN guidelines recommend TURBT with bimanual exam for initial staging (Grade C, not formally graded, and 2a, respectively). The latter three panels additionally have a number of recommendations related to TURBT technique and other perioperative issues, which are beyond the scope of this review.
Peri-procedural intravesical chemotherapy
All guidelines recommend some form of single-dose intravesical chemotherapy to selected patients after initial TURBT. The AUA guidelines recommend that an immediate, single-dose intravesical chemotherapy for all non-muscle invasive bladder tumors may be administered (recommendation). The EAU panel recommends a single post-operative dose of intravesical chemotherapy only in patients with presumed low- or intermediate-risk tumors (Grade A). The ICUD recommends one immediate instillation of chemotherapy for patients with low-grade Ta disease (Grade A) and otherwise considers immediate post-operative chemotherapy to be an option (Grade A). Of note, the panel also states that the use of single-dose perioperative intravesical chemotherapy in T1 disease is not supported by consistent data and should “not be recommended for standard practice” (Grade A). The NCCN panel recommends consideration of a single dose of chemotherapy (most commonly mitomycin) within 24 h of resection only for low-grade Ta lesions (2a).
Thus, for low-grade Ta lesions, all groups recommend perioperative intravesical chemotherapy, while groups differ in recommendations for other NMIBC stages. Notably, clinicians usually do not have the benefit of pathological review immediately after TURBT, making recommendations for use of chemotherapy based on results logistically difficult.
Imaging
Peri-operative imaging is used to aid workup and staging of NMIBC. All guidelines mention the use of upper tract imaging in the workup of NMIBC, although they differ in terms of modality and application.
The AUA guidelines do not have recommendations related to initial imaging, although they acknowledge the need to work up gross hematuria that often leads to the detection of NMIBC.[15] They also state that upper tract imaging “may be useful” in some cases. The EAU guidelines state that computed tomography (CT), urography (CTU), or intravenous urography (IVU) should be performed in select cases (an example being a trigonal tumor) (Grade B). The ICUD guidelines state that routine upper tract studies are not recommended for patients with low-grade Ta tumors at diagnosis, although they are recommended when gross hematuria or unexplained positive cytology is present (Grade B). They also acknowledge that CTU has become the “de facto” standard for imaging in patients with bladder cancer, although IVU, CT abdomen/pelvis, ultrasound and magnetic resonance imaging (MRI) are listed as options (Grade C). The NCCN guidelines recommend CT scan prior to TURBT if cystoscopy reveals a tumor that appears solid, high grade or muscle invasive (2a). Tumors without these features can have upper tract imaging “…deferred until after surgery.” Imaging modalities recommended are renal ultrasound or non-contrasted CT with retrograde pyelogram, CTU or MRI urogram, omitting IVU as an option (2a). Therefore, no guideline specifically recommends the use of imaging for disease that appears low grade or is shown to be low-grade Ta, although guidelines differ as to the timing of imaging and the usefulness of IVU.
Repeat TURBT
Incomplete initial resection
The AUA guidelines do not explicitly recommend re-resection for incomplete TURBT, although this can likely be inferred based on the “standard” to eradicate all tumors, when possible, on initial TURBT. The EUA (Grade A), ICUD (Grade A) and NCCN (2a) guidelines advocate for re-resection in cases of incomplete initial resection.
Low-grade Ta
No guideline recommends re-resection for low-grade Ta disease alone. The EAU guidelines specifically list low-grade Ta tumors without muscularis propria in the specimen as an exception to their recommendation involving re-resection.
High-grade Ta, T1 and CIS
Repeat resection is not recommended for isolated CIS by any guideline panel. Recommendations vary as to the need for additional TURBT in high-grade Ta, although repeat TURBT for T1 tumors is almost uniformly recommended.
The AUA guidelines recommend repeat TURBT as a standard in cases of T1 disease when muscularis propria is not present in the specimen. The panel states that repeat resection “may be appropriate” in cases of high-grade Ta or T1 tumors, even if muscularis is identified in the specimen.
The EAU panel recommends re-resection in all patients with “grade 3” (high grade) tumors (excluding CIS), all T1 tumors and any tumor with no muscle in the initial specimen (aside from CIS and low-grade Ta) (Grade A). This should occur within 2–6 weeks (Grade C) of the initial TURBT.
The ICUD recommends a second TURBT with high-grade Ta lesions or any T1 lesions (Grade B), stating that the optimal timing is within 1–4 weeks after the initial TURBT (Grade C).
The NCCN guidelines recommend to strongly consider repeat resection if no muscle is present in a specimen with high-grade Ta tumor (2a) and for low-grade or high-grade T1 disease if immediate cystectomy is not undertaken (discussed below) (2a).
SUBSEQUENT TREATMENT AND FOLLOW-UP
Adjuvant intravesical therapy
Low-grade Ta
Substantial discrepancy exists in the guidelines as to the use of adjuvant treatment for isolated low-grade Ta disease.
The AUA guidelines recommend induction intravesical therapy with BCG (full-dose BCG once weekly for 6 weeks) or mitomycin C for patients with multifocal and/or large low-grade Ta tumors (recommendation) and also consideration of maintenance therapy (option).
The EAU guidelines do not recommend intravesical therapy or BCG beyond peri-operative chemotherapy for low-risk tumors. For intermediate-risk tumors, the EAU guidelines recommend either chemotherapy or full-dose BCG for 1 year (Grade A). Intravesical BCG for 1–3 years is indicated in patients with high-risk tumors (Grade A). Of note, the EAU panel offers additional recommendations related to intravesical chemotherapy administration, including pH optimization (Grade B), limiting patient fluid intake (Grade B) and instilling intravesical therapy for 1–2 h (Grade C).
The ICUD panel states that induction intravesical chemotherapy with or without maintenance has unclear but potential benefit (Grade B). They also state that intravesical BCG is not appropriate as initial therapy for low-grade Ta tumors (Grade B).
The NCCN recommends considering induction intravesical chemotherapy (2a) and using tumor size, number and grade to help guide decisions based on probability of recurrence and progression.
High-grade Ta, T1 and CIS
All panels advocate the use of BCG in high-grade Ta, T1 and CIS tumors, although they disagree regarding length of treatment, use of other agents and utility of maintenance BCG therapy.
The AUA guidelines recommend an induction course followed by maintenance therapy (3 weeks of full-dose BCG at 3 and 6 months, then every 6 months up to 36 months if tolerated by the patient) for patients with high-grade Ta, T1 and/or CIS (recommendation).
The EAU guidelines recommend full-dose intravesical BCG for 1–3 years after re-resection of high-risk tumors (Grade A).
The ICUD recommends initiation of BCG in patients with high-grade Ta NMIBC (Grade A), although duration is not specified. The panel also recommends intravesical BCG for patients with T1 disease who desire a bladder-sparing approach (Grade B). For CIS, the panel recommends induction BCG (Grade A) and maintenance BCG (Grade A), although note that the optimal length of treatment is not known (at least 1 year of treatment is recommended [Grade A]).
The NCCN recommends induction intravesical therapy, either BCG or mitomycin, for high-grade Ta tumors, with BCG as the preferred treatment (2a). Observation is also listed as an option (2a). For T1 tumors, the NCCN panel recommends BCG for both high-grade and low-grade T1 (category 1), with mitomycin as an alternative in patients with no residual disease after re-resection (BCG preferred) (2a). Observation is also a potential option for patients with no residual disease on re-resection (2a) “in highly select cases” (limited lamina propria invasion and no CIS). The panel also recommends BCG for any CIS after resection (2a). The panel does not specifically advocate for maintenance therapy, although it lists it as optional for those who have received BCG (2a).
Initial cystectomy
All guidelines recommend either consideration or discussion of immediate cystectomy after TURBT in patients with high-risk disease.
Low-grade Ta
No guideline panel recommended consideration of cystectomy after diagnosis of low-grade Ta disease.
High-grade Ta, T1 and CIS
The AUA guidelines state that cystectomy should be “considered” for initial therapy in select patients (option). They note that factors increasing the risk of progression should be considered along with possible morbidities and complications associated with cystectomy.
The EAU panel recommends consideration of immediate cystectomy for tumors in the highest risk category (Grade C).
The ICUD recommends offering cystectomy to high-risk patients based on assessment of grade, multiplicity, size, concomitant CIS and prostatic urethral involvement (Grade A). The panel notes that radical cystectomy at the time of CIS diagnosis provides excellent disease-free survival but is overtreatment in over 50% of patients (Grade A).
The NCCN lists cystectomy as an option immediately following the diagnosis of high-grade T1 disease (2a). Cystectomy is also listed as an option if residual disease is present on re-resection of either low-grade T1 or high-grade T1 disease (2a), although BCG is preferred in this setting given its category 1 rating. The panel mentions that in patients with multifocal T1 disease or pathologic vascular invasion, cystectomy is preferred (not graded).
Surveillance
All guidelines advocate the use of cystoscopy during follow-up of NMIBC. The AUA guidelines state that pathologically confirmed bladder cancer should be followed with “periodic” cystoscopy (standard). The EUA guidelines state that the follow-up of Ta and T1 tumors is based on regular cystoscopy (Grade A). The ICUD guidelines state that cystoscopy alone is the most cost-effective method to detect recurrence of bladder cancer (Grade B). The NCCN guidelines recommend cystoscopy for all diagnoses of NMIBC (2a). Guidelines differ in the frequency of cystoscopy as well as the use of imaging and cytology.
Low-grade Ta
The AUA guidelines do not make recommendations regarding the timing of surveillance or the use of imaging, cystoscopy or cytology during follow-up.
The EAU guidelines recommend that patients should have cystoscopy at 3 months. If negative, subsequent cystoscopy is advised 9 months later and then yearly for 5 years (Grade C). No recommendations are made about cytology or imaging.
The ICUD panel offers no recommendations about timing of surveillance cystoscopy. Routine upper tract studies are not recommended as surveillance for low-grade Ta tumors by the panel (Grade B).
The NCCN panel recommends cystoscopy at 3 months and then at “increasing intervals as appropriate” (2a). Neither cytology nor upper tract imaging are advocated.
High-grade Ta, T1 and CIS
As stated above, the AUA guidelines do not recommend specifics around follow-up of NMIBC.
The EAU recommendations state that high-risk tumors should have cystoscopy and urine cytology at 3 months. If negative, subsequent cystoscopy and cytology should be repeated every 3 months for 2 years, then every 6 months until 5 years and then yearly (Grade C). Patients with intermediate-risk Ta and T1 tumors should have an in-between follow-up scheme using cystoscopy and cytology (Grade C). Yearly upper tract imaging (using CT-IVU or IVU) is recommended for high-risk tumors (Grade C).
The ICUD guidelines do not make specific recommendations regarding follow-up of high-grade Ta lesions, although note that schedules resemble those for CIS. For CIS, the panel recommends cystoscopy and cytology every 3 months for 2 years, every 4 months in the third year and every 6 months in the fourth and fifth years, then yearly (Grade C). No schedule is given for follow-up of T1 disease. The ICUD does recommend “periodic” imaging of the upper urinary tract using ultrasound, IVU or CT for patients with CIS (Grade C), although it does not create an explicit schedule for other tumors.
For high-grade Ta and T1 tumors and/or CIS, the NCCN panel recommends cystoscopy and urine cytology every 3–6 months for 2 years, followed by increasing intervals “as appropriate” (2a). They also recommend “consideration” of upper tract imaging every 1–2 years for high-grade Ta, T1 tumors and CIS (2a).
Recurrent disease
Low-grade Ta
The AUA guidelines recommend that patients with recurrent low-grade Ta receive a course of BCG or mitomycin (recommendation) and may subsequently consider maintenance therapy (option).
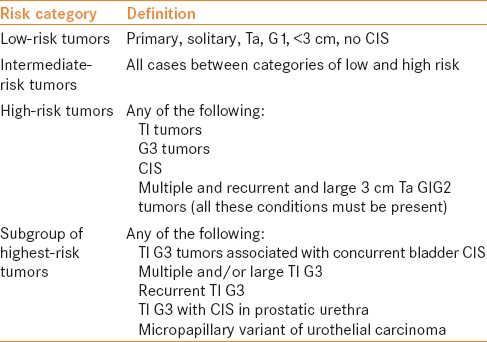
The EUA guidelines categorize recurrent low-grade Ta as either intermediate or high risk based on tumor size and multiplicity [Table 3]. For intermediate-risk disease, either chemotherapy or full-dose BCG for 1 year (Grade A) is advocated. Intravesical BCG for 1–3 years is recommended for patients with high-risk tumors (Grade A). If BCG is given for an intermediate-risk tumor and a “non-high-grade” recurrence subsequently occurs, the panel recommends either repeat BCG or intravesical chemotherapy or radical cystectomy (Grade C). Of note, the panel states that if intravesical chemotherapy was initially given and has failed, BCG is recommended (grade not given).
Table 3.
EAU risk group stratification
The ICUD panel recommends intravesical BCG for patients who have suffered a low-grade recurrence after use of intravesical chemotherapy (Grade B). Interestingly, the ICUD states that “expectant management” (observation of a visualized tumor) can be pursued in patients with an established history of low-grade Ta with recurrence (Grade B). The panel states that, optimally, patients offered observation would have a low tumor burden (Grade B). Additionally, observation is “particularly applicable” to those with advanced age or comorbidity, but can be offered to younger patients after careful and thorough discussion (Grade B). They recommend “periodic” surveillance with cystoscopy and cytology (Grade B) and that patients who demonstrate increase in size, number or appearance of tumor(s), or develop positive urine cytology, should undergo TURBT (Grade B). Office fulgaration with or without intravesical chemotherapy is also listed as an option for these patients (Grade B).
The NCCN guidelines recommend consideration of post-resection adjuvant treatment with BCG or mitomycin, depending on risk of further recurrence and progression (2a). Treatments may be pursued with a single agent for no more than two consecutive 3-month cycles (2a). If a patient has been treated with intravesical BCG or mitomycin prior to the recurrence, the panel recommends changing the intravesical agent or cystectomy (2a).
High-grade Ta, T1 and CIS
The AUA guidelines recommend consideration of cystectomy as a therapeutic alternative to patients with recurrent high-grade Ta, T1 or CIS (recommendation) and state that this is the “preferred treatment” given the “substantial risk of progression to muscle invasive cancer in these patients.” The panel also states that further intravesical therapy may be considered in this group (option).
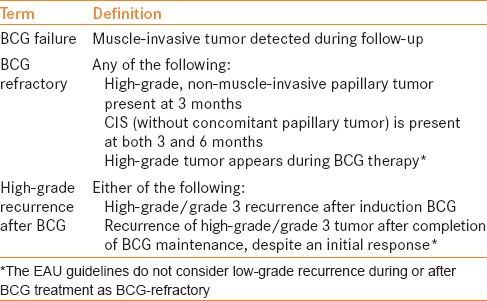
The EAU guidelines categorize patients with recurrent high-risk disease as “BCG failure,” “High grade recurrence” or “BCG refractory” [Table 4]. BCG failure represents muscle-invasive bladder cancer for which cystectomy is recommended, if possible. For BCG-refractory tumor, radical cystectomy (or bladder-preserving strategies for patients not suitable for cystectomy) is recommended (Grade B). For high-grade recurrence after BCG, the panel recommends radical cystectomy or repeat induction BCG (Grade C). “Bladder-preserving strategy” is also an option (Grade C), although the panel admits that this recommendation “must be considered oncologically inferior at this time.”
Table 4.
EAU categories of post-intravesical BCG recurrence
The ICUD panel recommends offering cystectomy to patients with recurrent or persistent high-grade T1 (Grade A). They recommend cystectomy for any case of BCG non-response or failure (defined as any recurrent disease after initiation of BCG therapy) (Grade A), noting that this is the “gold standard.” They specifically recommend offering a patient with CIS who did not respond to induction BCG cystectomy (with additional BCG being the other option) (Grade B). Panelists also state that the threat of progression after BCG failure remains real but comfortably low enough within the first 6 months of BCG initiation to consider cystectomy alternatives for patients unfit or unwilling to undergo the procedure (Grade B). Repeat resection with adjuvant BCG (Grade A) and possible interferon (Grade C) is the recommended option for these patients.
The NCCN guidelines for recurrent high-grade Ta, T1 and CIS disease are similar to those pertaining to low-risk Ta; however, they differ in that patients found to have high-risk Ta or T1 disease after TURBT for recurrence should be offered cystectomy (2a). If no residual disease is found on TURBT, they recommend maintenance BCG (2a). If Tis or Ta disease is present, they recommend changing the intravesical agent or cystectomy (2a). If a patient is not a cystectomy candidate, they should “consider” concurrent chemotherapy and radiation, change of intravesical agent or clinical trial (2a).
This review is limited in that published recommendations related to other aspects of NMIBC care, including those pertaining to pathologic processing and reporting, the role of random bladder and prostatic urethral biopsies, and molecular markers and tests used to detect recurrent disease and prognosis, are not included. We encourage readers to view these topics within the individual guidelines.
CONCLUSION
Current clinical practice guidelines offer a broad consensus on a number of basic management points, including the effectiveness of TURBT, peri-operative intravesical chemotherapy for low-grade disease and efficacy of BCG in patients at high risk of recurrence/progression. However, despite a thorough literature review and expert consultation, guideline panel recommendations often disagree, a fact that may be related to differing methodologies, evidence and panel composition.
ACKNOWLEDGEMENTS
We would like to acknowledge Jeffrey Bassett, M.D. and Peter Clark, M.D. for their help in preparing this manuscript.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics, 2012: Global Cancer Statistics, 2012. CA Cancer J Clin. 2015 doi: 10.3322/caac.21262. In Press. [DOI] [PubMed] [Google Scholar]
- 2.Hall MC, Chang SS, Dalbagni G, Pruthi RS, Seigne JD, Skinner EC, et al. Guideline for the Management of Nonmuscle Invasive Bladder Cancer (Stages Ta, T1, and Tis): 2007 Update. J Urol. 2007;178:2314–30. doi: 10.1016/j.juro.2007.09.003. [DOI] [PubMed] [Google Scholar]
- 3.Jones J, larchian W. Campbell-Walsh Urology. 10th ed. Philadelphia, PA: Elsevier Saunders; 2012. Non-Muscle-Invasive Bladder Cancer (Ta, T1, and CIS) pp. 2335–54. [Google Scholar]
- 4.Graham R, Mancher M, Wolman DM, Greenfield S, Steinberg E. Washington, DC: The National Academies Press; 2011. [Last accessed on 2 March, 2015]. Clinical Practice Guidelines We Can Trust; p. 266. Available from: http://www.iom.edu/Reports/2011/Clinical-Practice-Guidelines-We-Can-Trust.aspx . [PubMed] [Google Scholar]
- 5.Gupta M, McCauley J, Farkas A, Gudeloglu A, Neuberger MM, Ho YY, et al. Clinical practice guidelines on prostate cancer: A Critical Appraisal. J Urol. 2014;14:S0022–5347. doi: 10.1016/j.juro.2014.10.105. [DOI] [PubMed] [Google Scholar]
- 6.Babjuk M, Burger M, Zigeuner R, Shariat SF, van Rhijn BW, Compérat E, et al. EAU guidelines on non-muscle-invasive urothelial carcinoma of the bladder: Update 2013. Eur Urol. 2013;64:639–53. doi: 10.1016/j.eururo.2013.06.003. [DOI] [PubMed] [Google Scholar]
- 7.Kamat AM, Hegarty PK, Gee JR, Clark PE, Svatek RS, Hegarty N, et al. ICUD-EAU International Consultation on Bladder Cancer 2012: Screening, diagnosis, and molecular markers. Eur Urol. 2013;63:4–15. doi: 10.1016/j.eururo.2012.09.057. [DOI] [PubMed] [Google Scholar]
- 8.Burger M, Oosterlinck W, Konety B, Chang S, Gudjonsson S, Pruthi R, et al. ICUD-EAU International Consultation on Bladder Cancer 2012: Non-muscle-invasive urothelial carcinoma of the bladder. Eur Urol. 2013;63:36–44. doi: 10.1016/j.eururo.2012.08.061. [DOI] [PubMed] [Google Scholar]
- 9.Clark PE, Agarwal N, Biagioli MC, Eisenberger MA, Greenberg RE, Herr HW, et al. Bladder cancer. J Natl Compr Cancer Netw. 2013;11:446–75. doi: 10.6004/jnccn.2013.0059. [DOI] [PubMed] [Google Scholar]
- 10.National Guideline Clearinghouse (NGC). Guidelines on non-muscleinvasive bladder cancer (TaT1 and CIS). Agency for Healthcare Research and Quality (AHRQ) 2013. [Last cited on 2015 Mar 2]. Available from: http://www.guideline.gov .
- 11.Krupski TL, Dahm P, Fesperman SF, Schardt CM. How to Perform a Literature Search. J Urol. 2008;179:1264–70. doi: 10.1016/j.juro.2007.11.087. [DOI] [PubMed] [Google Scholar]
- 12.Dahm P, Clubb A. How to critically appraise a clinical practice guideline. Indian J Urol. 2011;27:498–502. doi: 10.4103/0970-1591.91441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dahm P, Yeung LL, Gallucci M, Simone G, Schünemann HJ. How to use a clinical practice Guideline. J Urol. 2009;181:472–9. doi: 10.1016/j.juro.2008.10.041. [DOI] [PubMed] [Google Scholar]
- 14.Dahm P, Chapple CR, Konety BR, Joyce AD, Parsons K, Wolf JS, et al. The future of clinical practice guidelines in urology. Eur Urol. 2011;60:72–4. doi: 10.1016/j.eururo.2011.04.007. [DOI] [PubMed] [Google Scholar]
- 15.Grossfeld GD, Wolf JS, Litwan MS, Hricak H, Shuler CL, Agerter DC, et al. Asymptomatic microscopic hematuria in adults: Summary of the AUA best practice policy recommendations. Am Fam Physician. 2001;63:1145–54. [PubMed] [Google Scholar]
- 16.OCEBM Levels of Evidence Working Group. Oxford Centre for Evidence-Based Medicine Levels of Evidence. 2009. [Last accessed on 2 March 2015]. Available from: http://www.cebm.net/oxford-centre-evidence-based-medicinelevels-evidence-march-2009/
- 17.Edge S, Byrd D, Compton C. 7th ed. Chicago: Springer-Verlag; 2010. AJCC Cancer Staging Manual. [Google Scholar]


