Abstract
Mediator is a large multiprotein complex conserved in all eukaryotes, which has a crucial coregulator function in transcription by RNA polymerase II (Pol II). However, the molecular mechanisms of its action in vivo remain to be understood. Med17 is an essential and central component of the Mediator head module. In this work, we utilised our large collection of conditional temperature-sensitive med17 mutants to investigate Mediator's role in coordinating preinitiation complex (PIC) formation in vivo at the genome level after a transfer to a non-permissive temperature for 45 minutes. The effect of a yeast mutation proposed to be equivalent to the human Med17-L371P responsible for infantile cerebral atrophy was also analyzed. The ChIP-seq results demonstrate that med17 mutations differentially affected the global presence of several PIC components including Mediator, TBP, TFIIH modules and Pol II. Our data show that Mediator stabilizes TFIIK kinase and TFIIH core modules independently, suggesting that the recruitment or the stability of TFIIH modules is regulated independently on yeast genome. We demonstrate that Mediator selectively contributes to TBP recruitment or stabilization to chromatin. This study provides an extensive genome-wide view of Mediator's role in PIC formation, suggesting that Mediator coordinates multiple steps of a PIC assembly pathway.
INTRODUCTION
In eukaryotes, the synthesis of mRNAs and a large number of small non-coding RNAs requires RNA polymerase II (Pol II) and the general transcription factors (GTFs) TFIIA, B, D, E, F and H that assemble into a large complex on the promoter DNA. This transcription preinitiation complex (PIC) is a key intermediate in Pol II transcription. In-vitro reconstitution studies of transcription initiation suggested a model in which PIC components assemble in a linear sequence (1,2). The first GTF that binds to the promoter is TFIID. TFIIA and TFIIB are then recruited followed by Pol II in association with TFIIF. Finally, TFIIE and TFIIH complete the formation of a PIC that is sufficient for basal in-vitro transcription but unable to drive activated transcription in response to specific activators. Important insights have been made on PIC architecture in yeast and human systems by biochemical and structural studies (3). A precise map of PIC locations across the yeast genome, including Pol II and GTFs, has been recently obtained, enabling the identification of TATA-like elements bound by TBP on TATA-less promoters and distinct PICs for divergent transcription (4,5).
Pol II transcriptional regulation requires additional multiprotein complexes, coactivators and corepressors, which modify the chromatin structure or directly contribute to PIC formation. Mediator of transcription regulation is one of these coregulators. While Mediator has been studied intensively, its complexity has precluded a detailed understanding of the molecular mechanisms of its action in vivo. Mediator is a huge complex (1.5 MDa) composed of 25–30 subunits depending on the organism that are organized into four structural modules: head, middle, tail and Cdk8 kinase modules. Recent electron microscopy studies redefined the modular organization of Mediator complex (6,7). Mediator subunits are engaged in numerous contacts within the complex (8–10). The large size, complexity and extreme flexibility have precluded the determination of a complete crystallographic structure of Mediator. Recently, structural data have been obtained for the yeast Mediator head module, the largest Mediator subcomplex characterized to date at high resolution (10,11).
Mediator transmits regulatory signals from specific transcription factors to the Pol II transcription machinery (12,13). This coregulator is generally required for Pol II transcription (14–18) and is present at most Pol II-transcribed gene promoters (19,20). Together with the GTFs, it promotes PIC formation and transcription initiation. Mediator also stimulates the phosphorylation of the Pol II carboxy-terminal domain (CTD) by TFIIH (21,22). Mediator cooperates with TFIID in yeast and human cells to stabilize the PIC (23–26). Previously, we have identified a direct contact between the Med11 Mediator head subunit and the Rad3 TFIIH subunit and showed that this contact is essential for the recruitment of the GTF to the PIC independently of Pol II (27). Recently, we showed that the Rpb3 Pol II subunit and the Med17 Mediator subunit are in direct contact to position Pol II on promoters and activate transcription, which strongly supports the notion that the Mediator–Pol II interaction is generally required for Pol II recruitment and transcription of class II genes in eukaryotes (28). Mediator was found to play an essential role in PIC assembly at the level of Pol II, TFIIH and TFIIE recruitment (27,28). Based on our results, we proposed a model of PIC assembly through multiple pathways (27).
Mediator is conserved in all eukaryotes (29) supporting a very ancient eukaryotic origin for this four-module complex. Given its central role in transcription regulation, it is not surprising that Mediator has been implicated in numerous developmental processes in animals, and mutations in Mediator subunits are involved in a number of pathologies (30,31). For example, a mutation in the Med23 subunit cosegregates with intellectual disability (32). In line with the fact that oncogenesis results from gene disregulation, Mediator has been found to be involved in several cancers (33–38).
Med17 is one of the ten essential yeast Mediator subunits. MED17 was initially isolated as SRB4, one of the SRB genes whose mutations suppressed the growth phenotype of truncations of the Pol II Rpb1 CTD (39). The general requirement of Mediator for Pol II transcription has been shown using the med17-138 mutant that generally affects Pol II transcription in a manner comparable with that of the rpb1-1 Pol II mutant (14). This classical med17 temperature-sensitive (ts) allele causes dissociation of the head module from the rest of Mediator leading to a loss of Mediator function at the restrictive temperature (40–42). The central role of the Med17 subunit in Mediator's function is also consistent with a central and extended positioning of this subunit within the Mediator head structural model (10). The importance of the Med17 subunit's role has been highlighted by the fact that a mutation in this subunit has been associated with infantile cerebral atrophy (43) and because of this subunit's involvement in cancer (37).
We aimed to enhance our understanding of the key mechanisms that allow Mediator to stimulate PIC formation in vivo at the genomic scale. We chose a strategy using temperature-sensitive mutants that allow the study of essential Mediator subunits providing specific changes in Mediator function without a complete loss or disassembly of Mediator. In this work, we have obtained a detailed genome-wide view of Mediator's role in PIC assembly in vivo by characterizing our large collection of conditional ts mutants in the Med17 Mediator head subunit. A mutant of yeast Med17 proposed to be equivalent to the human Med17-L371P responsible for infantile cerebral atrophy, which has a severe ts phenotype, was also included in our analysis. We show that med17 mutations, which do not have a major effect on Mediator stability, differentially affect the genome-wide occupancy of PIC components. This work suggests that Mediator independently orchestrates multiple steps of the PIC assembly pathway.
MATERIALS AND METHODS
Strains and plasmids
All S. cerevisiae strains are described in Supplementary Table S1. All plasmids are listed in Supplementary Table S2. The oligonucleotides used in this study can be found in Supplementary Table S3.
ChIP and ChIP-seq
ChIP experiments were performed as described previously (44). Cell cultures (100 ml) were grown to exponential phase in YPD medium at 30°C, transferred for 45 minutes to a shaking incubator set at 37°C to allow gradual warming and cross-linked with 1% formaldehyde for 10 min. The 3HA-tagged proteins were immunoprecipitated with 12CA5 antibody and Pol II with 8WG16 anti-CTD antibody (Covance), bound to IgG magnetic beads (Dynabead). ChIP-seq experiments were performed as described previously (45). Chromatin preparation for ChIP-seq experiments was performed as described previously for conventional ChIP, except that an additional sonication step with Bioruptor (Diagenode, 6 cycles of 30s with medium intensity setting) was included to generate DNA fragments of ∼200 bp mean size. DNA sequencing of 40 nt-tags was performed on a Solexa genome analyzer GA-IIx or Hi-Seq using the procedures recommended by the manufacturer (Illumina). Input DNA and DNA from ChIP with an untagged strain were used as negative controls. Mediator ChIP-seq analysis was performed only for the Med15 subunit. Low ChIP signals were obtained for the Med8-HA subunit. High background levels in the ChIP-seq experiments for Med5-HA and Med6-HA subunits precluded accurate enrichment detection. It should be noted that Mediator enrichment levels in ChIP experiments are relatively low, depending on the Mediator subunit used, possibly due to the low efficiency of formaldehyde crosslinking and/or the dynamic association of Mediator to chromatin.
The ChIP-seq data have been deposited to the Array Express under accession numbers E-MTAB-2961 for the mutant strains and E-MTAB-1595 for the wild-type (WT) strain.
Coimmunoprecipitation experiments
Whole yeast extracts were prepared from 100 ml of cells grown to exponential phase in YPD medium at 30°C. When indicated, the cells were then transferred for 45 min to 37°C. Whole yeast extract preparation, immunoprecipitation (IP) in IP buffer (50 mM HEPES, pH 7.5, 100 mM NaCl, 20% glycerol, 1 mM dithiothreitol, 0.5 mM EDTA, 0.05% NP-40) supplemented with a protease inhibitor cocktail (Complete, Roche) and 1 mM PMSF and western blotting were performed as described previously (46). The 12CA5 anti-HA antibodies were used against HA-tagged proteins; rabbit polyclonal anti-Med14 and anti-Med17 antibodies were used against corresponding N-terminal peptides to detect Med14 and Med17 Mediator subunits, respectively.
Formaldehyde cross-linking experiments were performed as described previously (28). Briefly, cells were cross-linked with 1% formaldehyde for 10 min, as described for ChIP. Lysis was performed in 0.5 ml FA/SDS buffer in the presence of glass beads (0.75 ml, 425 to 600 μm) by vortexing for 45 min at 4°C. Crude extract preparation and IP were performed as described for conventional CoIP except that the immune complexes were washed as described for ChIP. A sonication step was added to recover chromatin-associated proteins. Denaturing conditions (8 M urea for 20 min at room temperature, cooled to 4°C and diluted with lysis buffer to a final urea concentration of 2 M) were used before IP to dissociate the subunits of Pol II and Mediator.
Mediator purification
Mediator purification was performed following a procedure adapted from Cai et al. (47) with some modifications. Med17 mutant and WT strains derived from CA001 protease-deficient background were grown at 30°C in 1 liter of 2×YPD medium overnight to late exponential phase. Cells were collected by centrifugation, washed twice in distillated water, resuspended in a buffer (1 ml/g of cells) containing 0.25 M Tris–HCl, pH7.6, 0.3 M ammonium sulfate, 2 mM EDTA, 30% glycerol, 5 mM 2-mercapthoethanol supplemented with 2× protease inhibitor cocktail (Complete, Roche) and frozen with liquid nitrogen. To prepare whole cell extract, cells were disrupted in an Eaton-Press. After thawing, the extract was clarified by ultracentrifugation (4°C, 1 h, 40 000g). The clear extract was submitted to a selective ammonium sulfate precipitation to isolate the 30–55% fraction. All buffers used for purification were supplemented with a protease inhibitor cocktail except for TEV digestion buffer. The precipitate was resuspended in IgG binding buffer (25 mM sodium phosphate buffer, pH7.5, 200 mM potassium acetate, 1 mM EDTA, 10% glycerol, 5 mM 2-mercapthoethanol) and incubated for 3 h with 100 μl of IgG-coated beads (GE Healthcare) in poly-prep columns (BioRad) at 4°C on a rotator. Beads were drained by gravity and washed with 10 ml of IgG wash buffer I (50 mM sodium phosphate buffer, pH 7.5, 500 mM ammonium sulfate, 1 mM EDTA, 10% glycerol, 5 mM 2-mercapthoethanol), followed by a wash with 10 ml of IgG wash buffer II (50 mM sodium phosphate buffer, pH 7.5, 100 mM ammonium sulfate, 1 mM EDTA, 10% glycerol, 5 mM 2-mercapthoethanol) and 10 ml of TEV digestion buffer (50 mM sodium phosphate buffer, pH 7.5, 100 mM potassium acetate, 1 mM EDTA, 10% glycerol, 5 mM 2-mercapthoethanol). Beads were incubated overnight with 20 U of TEV protease (Life Technologies) in 400 μl of TEV digestion buffer. The eluate was recovered, diluted 6-fold in Ni-NTA binding buffer (50 mM sodium phosphate buffer, pH 7.5, 0.01% NP-40, 10% glycerol, 500 mM potassium acetate, 10 mM imidazole) and incubated for 3 h on a rotator at 4°C with 100 μl of Ni-NTA sepharose beads (GE Healthcare) on poly-prep columns. Beads were drained by gravity and washed with 10 ml of Ni-NTA wash buffer (50 mM sodium phosphate buffer, pH 7.5, 0.01% NP-40, 10% glycerol, 500 mM potassium acetate, 40 mM imidazole). The Mediator complex was eluted with 200 μl of Ni-NTA elution buffer (50 mM sodium phosphate buffer, pH 7.5, 0.01% NP-40, 10% glycerol, 500 mM potassium acetate, 200 mM imidazole), and flash-frozen after addition of 10% glycerol to a final glycerol concentration of 20%.
The presence of selected Mediator subunits (Med8-HA, Med14, Med17 and Med21) was verified by western blotting with anti-HA antibody (12CA5), polyclonal rabbit anti-Med14, anti-Med17 or anti-Med21 antibodies. Purified Mediator complex was then analyzed using a mass-spectrometry approach.
Mass spectrometry analysis
To analyze Mediator integrity in med17 mutants, Mediator was immunoprecipitated from crude extracts of Med5-HA tagged strains as described for CoIP experiments, except that protein G magnetic beads were used instead of IgG magnetic beads (Dynabead) and the elution was performed for 20 min at 65°C instead of 2 min at 85°C. The MED17 strain carrying the non-tagged Mediator subunit was used as a negative control. Proteins from each eluate were separated by SDS-PAGE to fractionate the protein samples into three fractions and exclude the regions corresponding to heavy and light IgG chains. The gels were stained with Coomassie Blue and each fraction was cut into bands of ∼2 mm and subjected to in-gel trypsin digestion with the Progest robot (Genomic Solutions, Chelmsford, MA) using standard conditions including reduction and alkylation. To analyse the purified Mediator complex, a short migration in NuPage 4–12% gel (Life Technologies) was performed before mass spectrometry analysis. After overnight tryptic digestion, peptides were extracted with 60% acetonitrile and 0.1% (v/v) formic acid. Tryptic peptides extracted from different bands of three gel regions were pooled, vacuum dried and resuspended in 0.1% (v/v) formic acid prior to nanoLC–MS/MS mass spectrometry analyses. The same cutting pattern of the SDS-PAGE lane was performed for each eluate.
The nanoLC–MS/MS analyses were performed with the Triple-TOF 4600 mass spectrometer (AB Sciex, Framingham, MA, USA) coupled to the nanoRSLC ultra performance liquid chromatography (UPLC) system (Thermo Scientific) equipped with a trap column (Acclaim PepMap100C18, 75 μm i.d. × 2 cm, 3 μm) and an analytical column (Acclaim PepMapRSLCC18, 75 μm i.d. × 15 cm, 2 μm, 100 Å). Peptide separation was performed with a 5–35% solvent B gradient for 40 min. Solvent A was 0.1% formic acid in water, and solvent B was 0.1% formic acid in 100% acetonitrile. The nanoLC–MS/MS experiments were conducted using the data-dependent acquisition method by selecting the 20 most intense precursors for CID fragmentation with Q1 quadrupole set at low resolution for better sensitivity. Raw data were processed using MS Data Converter software (AB Sciex) for generating .mgf data files and protein identification was performed using the MASCOT search engine (Matrix science, London, UK) against the Swissprot database (release 2014_08) with carbamidomethylation of cysteines and oxidation of methionines set as fixed and variable modifications, respectively. Peptide and fragment tolerance were respectively set at 20 ppm and 0.05 Da. Results were analyzed using Scaffold 3.6.5 software (Proteome Software). Only proteins with at least two unique peptides and a 95% probability for both peptide and protein identifications were considered.
Quantitative real-time PCR (qRT-PCR) analysis
RNA was extracted with hot acidic phenol following a protocol derived from Schmitt et al. (48). Reverse transcription of 0.5 μg RNA samples was performed using an iScript cDNA synthesis kit (BIO-RAD) with a mixture of oligo(dT) and random hexamers for priming. qRT-PCR results were normalized using 25S rRNA, 18S rRNA and SCR1 Pol III-synthesized RNA as internal controls. Values represent the average of three independent experiments, and error bars indicate standard deviations.
Data analysis
ChIP-seq data were analyzed as described previously (45). The sequences were aligned on the S. cerevisiae genome (UCSC version sacCer3) using Bowtie version 0.12.7 (49). Only uniquely mapped tags were used and a maximum of two mismatches was allowed. Conversions to different file formats were performed using Samtools version 0.1.16. To avoid possible sequencing artifacts, reads with the best-quality scores were conserved for each position. The number of mappable tags for each ChIP-seq experiment is indicated in Supplementary Table S4.
To calculate ChIP-seq density, reads were extended to an assumed fragment length of 150–180 nt, and a count of reads was determined per 1-bp bin using Bedtools version 2.15.0. ChIP-seq density profiles were displayed using the Integrated Genome Browser (IGB) yeast genome browser. Input DNA and DNA from ChIP with an untagged strain were used as negative controls. The ChIP sample from the untagged strain generally shows very low tag density on the genome, except for some regions representing <1% of the genome that displays an apparent enrichment. It should be noted that the majority of these regions were located inside the highly transcribed class II genes. To correct for this non-random background distribution, we subtracted the normalized signal of the untagged strain sample from the ChIP samples for each protein. The ChIP signals of the untagged strain have different impacts on the ChIP signals for different proteins. For this reason, the subtraction step was preceded by a normalization of the ChIP signal of an untagged strain compared with the corresponding ChIP sample based on the qPCR analyses of nontranscribed control regions. The normalization values for untagged strain sample before subtraction are indicated in Supplementary Table S5.
The significantly enriched regions were identified using peak calling MACS2 software version 2.0.10.20120703 (50). To avoid possible sequencing artifacts, reads beyond five repetitions at the same position of the genome were removed. A minimum false discovery rate (q-value) cutoff peak detection of 0.01 was used for Med15 and 0.05 for Pol II, Rad3, Kin28 and TBP. This step was followed using the PeakSplitter step of PeakAnalyzer software for the subdivision of ChIP-seq regions into discrete peaks (51). The significantly enriched regions were also identified in the control ChIP sample with an untagged strain using Bayespeak version 1.10.0 (52) with a threshold of 0.9. Around 200 peaks were identified by this method in the control sample and they were used to correct peakcalling data from Med15, Rad3, Kin28 and TBP ChIP-seq.
GTFs, Mediator and Pol II significantly enriched peaks were annotated relative to the intergenic region or the ORF in which they were detected using BedTools version 2.15.0. The nomenclature used for the intergenic regions was as follows: tandem regions were named relative to the downstream-located gene, divergent and convergent regions were named relative to the two genes surrounding the region.
To compare read counts in WT and med17 mutant ChIP-seq data, a count of reads was determined on promoter regions of Pol II-transcribed genes for Rad3, Kin28, TBP and Med15, and on Pol II-transcribed gene ORFs for Pol II, using Bedtools. For ChIPseq data analysis, promoter regions were defined as corresponding intergenic regions on the yeast genome. For this step, all mapped reads were conserved to allow quantitative comparison between the different samples. Read numbers were normalized relative to qPCR data on a set of selected regions (Supplementary Table S6) using the ratio between WT and mutant strains. The ratio of ChIP-qPCR values (V) between WT and mutant strains was calculated for each selected region (RqPCR = VqPCRmut/VqPCRwt). In parallel, the ratio of ChIP-seq reads (N) between WT and mutant strains was determined for the same region (Rseq = Nseqmut/Nseqwt). The normalization coefficient between ChIP-qPCR and read ratios was then calculated for each region (Q = RqPCR/Rseq). The median of normalization coefficients between ChIP-qPCR and read ratios was used for ChIPseq data normalization (Supplementary Table S7).
RESULTS
Collection of conditional med17 Mediator mutants
Previously, to investigate the in-vivo role of Med17 in Pol II recruitment, we selected a large collection of conditional med17 mutants (28). The med17 mutants with different thermosensitive phenotypes, from slow growth to lethal at non-permissive temperature (37°C), were generated by random mutagenesis (Supplementary Figure S1, Table S8). A number of multiple mutations were separated to obtain 5 additional point mutants by site-directed mutagenesis. An L371P mutation in the Med17 subunit has been associated with infantile cerebral atrophy (43). A corresponding M504P mutation in the homologous S. cerevisiae Med17 (Supplementary Figure S2A) localized within one of the conserved domain based on multiple sequence alignments and secondary structure features (29) inactivated the protein at 37°C. To study the molecular consequences of this med17 mutation, a med17-504 yeast strain carrying a med17-M504P mutation was constructed and the growth phenotype of this mutant was investigated. Figure 1A shows that med17-504 leads to a slow growth phenotype at 30°C and a strong thermosensitive phenotype at 37°C. In total, a collection of 30 conditional mutants in the Med17 subunit was obtained. The integrity of the main Mediator modules in all med17 mutants has been verified by CoIP experiments (Supplementary Table S9). We decided to use this collection of Med17 Mediator mutants to investigate the in-vivo action of Mediator in general, and of the Med17 subunit in particular in PIC formation.
Figure 1.
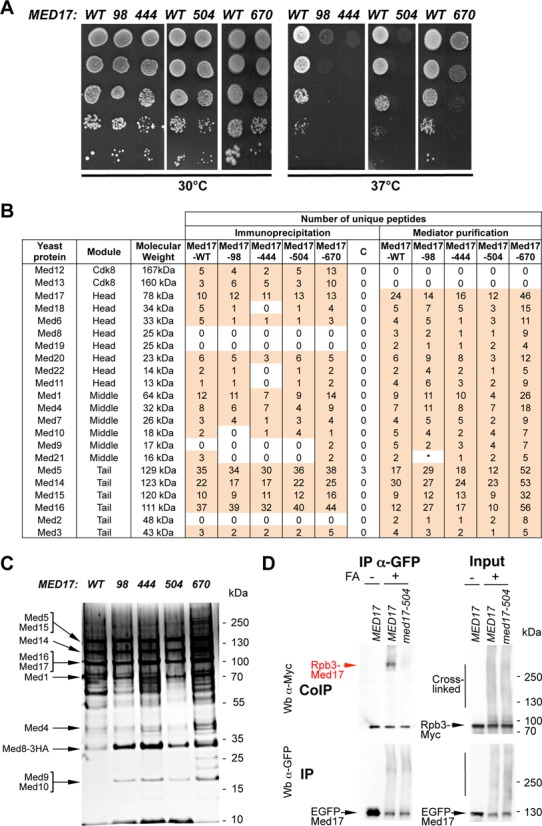
med17 thermosensitive mutants. (A) Med17 mutant phenotypes. Cultures of WT and mutant med17 yeast strains were serially diluted, spotted on YPD agar plates and incubated for 3 days at permissive (30°C) or non-permissive (37°C) temperatures. (B) Mass spectrometry analysis of Mediator integrity in med17 mutants. Mediator subunits identified by mass spectrometry analysis in WT and med17 mutants are indicated. (Immunoprecipitation) Mediator was immunoprecipitated through Med5-HA from crude extracts using magnetic protein G beads coupled to anti-HA antibodies. The MED17 strain carrying a non-tagged Mediator subunit was used as a negative control (C for Control IP). (Mediator purification) Core Mediator complex containing head, middle and tail modules was purified from med17 mutants and a wild-type strain. An asterisk marks Med21 subunit in med17-98 mutant that was detected in a second data acquisition identifying two unique peptides and also by western blotting with anti-Med21 antibody (Supplementary Figure S3B). (C) Silver-stain SDS-PAGE analysis of purified Mediator complex from the WT strain and med17 mutants. (D) Interaction between Pol II and Mediator in med17-504 mutant. Rpb3-Myc Med17-EGFP strains with WT MED17 or a med17 mutation were grown at 30°C in YPD medium and cross-linked or not with formaldehyde (FA), as indicated. Med17-EGFP was immunoprecipitated (IP) with anti-EGFP antibody from crude extracts (Input) and analyzed by western blotting with anti-Myc antibody (CoIP) against Rpb3. The cross-linked Rpb3-Med17 band is indicated in red. The position of unidentified cross-linked proteins with the tagged Med17 or Rpb3 subunits is indicated by a vertical bar.
Effects of med17 mutations on Mediator stability, interaction with Pol II and mRNA level
Four mutants were selected for further study based on their growth phenotypes (normal or slightly slower growth at 30°C combined with pronounced ts phenotype at 37°C), Mediator integrity and low mutation number: med17-98 (L86Q, E186G, E448Q), med17-444 (Q444P), med17-504 (M504P) and med17-670 (V670E) (Figure 1A). These four med17 mutants all have mutations localized within or near the conserved domains of this subunit defined by a primary sequence comparison and predicted secondary structure (Supplementary Figure S2B) (29). The mutated amino acid residues of med17 mutants were mapped on a partial structural model of the Mediator head module if the corresponding residues were present on the model (10) (Supplementary Figure S2C) and were found to often be located close to the module surface.
Since Mediator mutations can lead to dissociation of Mediator modules and a loss of Mediator function, the effect of med17 mutations on the integrity of the main Mediator modules was first verified by CoIP experiments (Supplementary Figure S3A and Table S9). The HA-tagged Med5 Mediator subunit was immunoprecipitated with an anti-HA antibody. The coimmunoprecipitated Med17 Mediator head subunit and Med14 Mediator subunit bridging tail and middle modules were detected by western blotting with antibodies produced in house. Mediator head, middle and tail modules coimmunoprecipitated with the Med5 tail module subunit in all four mutants without any significant difference compared to the WT strain. We also performed CoIP experiments with whole yeast extracts prepared from cells grown to exponential phase in YPD medium at 30°C and then transferred for 45 min to 37°C (Supplementary Figure S3A). Similar to cells grown at 30°C, no significant differences were observed in med17 mutants compared to the WT strain.
To investigate Mediator integrity in the selected med17 mutants in more detail, Mediator was immunoprecipitated from crude extracts of Med5-HA tagged strains and immunoprecipitated proteins were analyzed using a mass spectrometry approach (Figure 1B, immunoprecipitation). To improve Mediator subunit identification using mass spectrometry, the immunoprecipitated proteins were separated on gel and the bands corresponding to IgG heavy and light chains were cut out. This procedure excluded some Mediator subunits with molecular weights close to those of the IgG chains. A strain carrying an untagged Med5 subunit was used as a negative IP control. Up to 19 Mediator subunits were identified belonging to all four Mediator modules. Thirteen Mediator subunits of head, middle, tail and Cdk8 modules (Med12, 13, 17, 6, 20, 1, 4, 7, 5, 14, 15, 16, 3) were present for all med17 mutants and the WT strain. Mediator subunits with low molecular weight (Med9, 10, 11, 21, 22) were not detected in some samples. Mass spectrometry analysis thus showed the presence of Mediator head, middle, tail and Cdk8 modules for all med17 mutants, indicating that mutations did not have a major effect on Mediator stability.
To improve our analysis of Mediator integrity in med17 mutants, we purified Mediator complex by a two-step affinity purification method that allows to prepare core Mediator containing head, middle and tail modules but lacking Cdk8 module (47). Purified Mediator complex from the four med17 mutants and a WT strain was assayed by SDS-PAGE (Figure 1C) and analyzed using a mass spectrometry approach. Yeast core Mediator is composed of 21 subunits since it lacks the 4 Cdk8 module subunits. The Med31 Mediator subunit escaped mass spectrometric identification (8), leading to 20 core Mediator subunits that could be detected by this approach. Our mass spectrometry analysis showed that all 20 Mediator subunits were present in Mediator complex purified from med17 mutants and a WT strain (Figure 1B, Mediator purification). Even though no peptides were initially detected for Med21 subunit (a small Mediator subunit of 16 kDa) in med17-98 mutant, the presence of this subunit in med17-98 Mediator complex was confirmed by a second data acquisition identifying 2 unique peptides and our western blotting analysis with anti-Med21 antibody (Supplementary Figure S3B). Taken together, our data strongly suggest that med17-98, 444, 504 and 670 mutations did not compromise Mediator integrity.
The Med17 Mediator subunit directly interacts in vivo with the Rpb3 Pol II subunit (28). As described previously (28), CoIP experiments for Mediator–Pol II interaction are not appropriate to investigate the effect of mutations since their effects depend on the buffer conditions. Hence the need to determine how Mediator Med17 mutations affect the specific Med17–Rpb3 contact using a formaldehyde-crosslinking approach with IP under denaturing conditions. This method was used in our previous work to show an impaired Med17–Rpb3 contact in an rpb3-2 mutant (28). We examined whether this contact was modified in med17 mutants. For these experiments, yeast strains were constructed carrying an EGFP-tagged Med17 subunit and an Myc-tagged Rpb3 subunit in WT and med17 mutant contexts. Proteins were cross-linked with formaldehyde, the Med17-EGFP-tagged subunit was immunoprecipitated under denaturing conditions and the cross-linked Rpb3-Med17 band was detected with an anti-Myc antibody (Figure 1D, red arrow). The results demonstrated that Rpb3–Med17 contact was strongly reduced in the med17-504 mutant (Figure 1D), but remained largely unchanged in med17-98, 444 and 670 compared to the wild type (Supplementary Figure S4).
The effect of med17 mutations after a transfer to 37°C on mRNA abundance of ADH1, PYK1 and PMA1 genes was investigated (Supplementary Figure S5). The med17-98, 444 and 670 mutants were found to affect the level of these mRNAs. The effect of med17-98 was accentuated at 37°C and that of other mutations was essentially similar at 30°C and 37°C. It should be noted that med17-504 shows little, if any, effect on the steady-state levels of the three mRNAs. As the measurement of mRNA levels is a cumulative result of RNA synthesis and RNA turnover, mRNA levels do not fully reflect the effects of med17 mutations on transcription. Moreover, it has been shown recently that cells can compensate for global transcription changes by RNA degradation changes to buffer cellular mRNA levels (53,54). We measured Pol II occupancy on transcribed regions by ChIP since it indicates transcriptional activity in yeast (55,56).
Mutations in Med17 differentially affect chromatin association of PIC components on selected constitutively expressed genes
To analyse the molecular effect of med17 mutations on PIC formation, occupancy of Pol II, GTFs and Mediator was determined by ChIP on selected constitutively expressed genes. Yeast strains grown in YPD-rich medium and transferred to a non-permissive temperature (37°C) for 45 min were used for chromatin preparation. The association of Mediator was measured by ChIP experiments with three different Mediator subunits: Med6-HA (head), Med15-HA (tail) and Med5-HA (tail) (Figure 2A–C). The Mediator occupancy of the ADH1, PYK1 and PMA1 promoter regions remained largely unchanged except for Med15 Mediator occupancy that was reduced in the med17-444 mutant and to some extent for other mutants on the PMA1 promoter region. In contrast, all examined mutations led to a decrease in Pol II association with the promoter and transcribed regions of class II genes compared to the WT (Figure 2D), with med17-444 and med17-504 showing the most pronounced effect, reflecting the effects on Pol II transcription.
Figure 2.
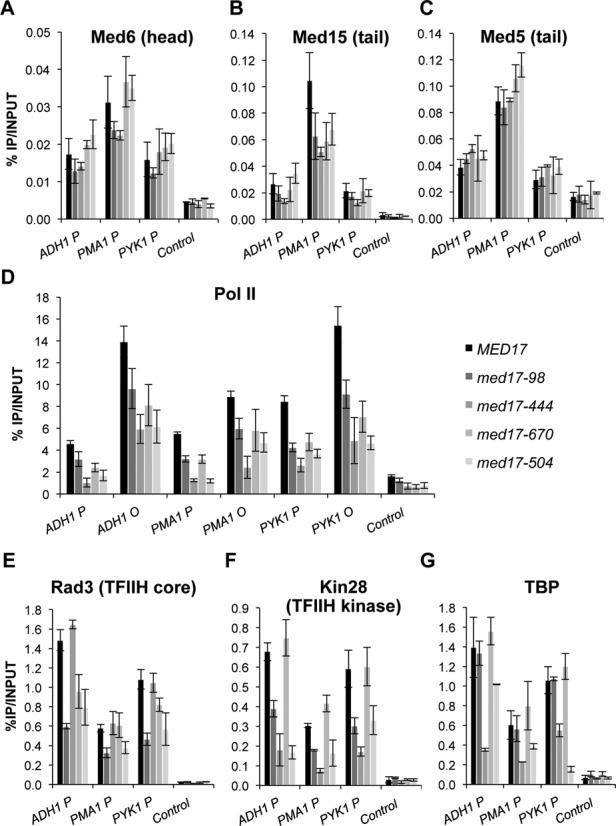
Effect of med17 mutations on Mediator, Pol II, TFIIH and TBP occupancy. Cells were grown to exponential phase at 30°C on YPD medium and then transferred for 45 min to 37°C. Quantitative ChIP experiments were performed using anti-HA antibody (12CA5) against Mediator subunits Med6-HA (A), Med15-HA (B) or Med5-HA (C), Rad3-HA (E), Kin28-HA (F), TBP-HA (G) or anti-Rpb1 Pol II antibody (8WG16) (D). Immunoprecipitated DNA was amplified with primers corresponding to ADH1, PMA1 and PYK1 ORF (O) or promoters (P). P1 primers listed in Supplementary Table S3 located close to upstream regulatory regions were used for Mediator ChIP experiments and P2 primers located close to core promoters were used for Pol II, Rad3, Kin28 and TBP ChIP experiments. GAL1 ORF was used as a negative control. The mean values and standard deviation (indicated by error bars) of three independent experiments are shown.
With the Mediator head module directly interacting with TFIIH and TBP, the ability of med17 mutations to influence the occupancy of these GTFs was assessed in vivo. Two TFIIH modules, TFIIH core (Rad3-HA subunit) and TFIIK kinase module (Kin28-HA) were ChIPed (Figure 2E and F). Interestingly, mutations in the Med17 Mediator subunit were found to differentially affect the presence of the two TFIIH modules on the ADH1, PYK1 and PMA1 promoters. In the med17-98 and med17-504 mutants, Med17 mutations led to a decrease in the occupancy of both TFIIH modules. The med17-444 mutation specifically affected Kin28 occupancy. More unexpectedly, a decrease in Rad3 occupancy without any effect on Kin28 occupancy was observed in med17-670, suggesting that TFIIH modules can behave independently in vivo.
TBP occupancy in med17 mutants was also analyzed (Figure 2G). As previously observed for med11 Mediator mutants, TBP occupancy was similar to that of the WT strain in med17-98 and 670 mutants. In contrast, TBP association to the ADH1, PYK1 and PMA1 gene promoters was reduced in med17-444 and med17-504 indicating that Mediator could affect the recruitment and/or stability of this GTF in vivo.
Effects of med17 mutations on chromatin occupancy of PIC components on the inducible GAL1 gene
To address the recruitment of PIC components to an inducible yeast gene, ChIP experiments were performed with Mediator, Pol II and GTFs in med17 mutants on the GAL1 gene. The association of Mediator (Med5 subunit), Pol II, TFIIH core (Rad3 subunit), TFIIH kinase module (Kin28 subunit) and TBP were analyzed by ChIP at 45 min of galactose induction (data not shown). The association of PIC components was generally affected in the same manner as for the three constitutively expressed genes, with med17-504 being an exception with reduced Mediator recruitment and unmodified Rad3 and TBP recruitment to the GAL1 promoter region at 45 min of galactose induction.
Two med17 mutants were chosen for further experiments to address the kinetics of the GAL1 gene induction: med17-444, since it is the only mutant that affected TBP recruitment to the GAL1 promoter and med17-504, since its effects on the GAL1 gene differ from those on the ADH1, PYK1 and PMA1 genes for several PIC components. Yeast cells were grown in YP raffinose medium at 30°C, then galactose was added at the 0 time point and cultures were transferred to 37°C. Samples were collected at 0, 20, 40 and 60 min of galactose induction and transfer to 37°C. The kinetics of Mediator, Pol II and TBP recruitment upon galactose induction were analyzed in med17-444 and -504 mutants compared to the wild type (Figure 3). Mediator recruitment was decreased in med17-504 for all kinetics points, showing a particular Mediator behavior on the GAL1 gene in this mutant (Figure 3A). In the med17-444 mutant, Mediator occupancy of the GAL1 gene promoter reached a level similar to that in the wild type, even though the kinetics of Mediator recruitment were slower (20-min point). Pol II occupancy generally followed that of Mediator (Figure 3B). The med17-444 mutation was found to slow TBP recruitment during galactose induction and med17-504 showed TBP recruitment kinetics similar to that of the WT strain (Figure 3C).
Figure 3.
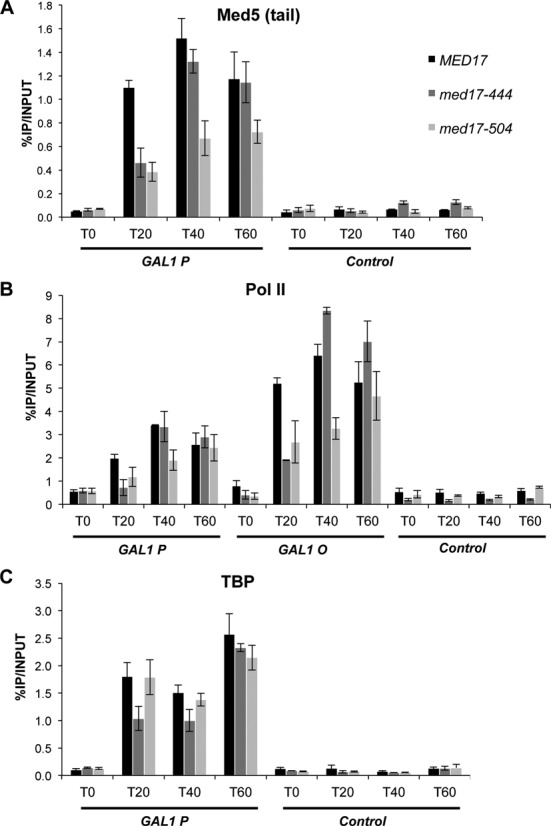
Kinetics of galactose induction in med17-444, -504 and WT strains. Yeast strains were grown in raffinose-supplemented medium at 30°C, then galactose was added, cultures were transferred at the same time to 37°C and samples were collected for ChIP experiments at indicated time points upon galactose induction (T0, T20, T40 and T60 min). Quantitative ChIP experiments were performed using anti-HA antibody (12CA5) against Mediator subunit Med5-HA (A), TBP-HA (C) or anti-Rpb1 Pol II antibody (8WG16) (B). The mean values and standard deviation (indicated by error bars) of three independent experiments are shown. Immunoprecipitated fragments from ChIP experiments were amplified with primers corresponding to the GAL1 gene promoter (GAL1 P) or ORF (GAL1 O). A nontranscribed region on chromosome V was used as a negative control.
Taken together, the ChIP results demonstrate that mutations in the Med17 Mediator subunit could differentially affect the recruitment of PIC components and also highlight some gene-specific effects suggesting that regulatory mechanisms might differ depending on particular Pol II-transcribed genes.
Genome-wide location analysis of Mediator, Pol II, TBP and TFIIH modules
To extend the ChIP analysis of PIC assembly to the whole yeast genome, ChIP-seq experiments were performed for Mediator (Med15 subunit), Pol II and GTFs (TBP and two TFIIH subunits: Kin28 and Rad3). Input DNA and DNA from ChIP with an untagged strain were used as negative controls. Figure 4A shows examples of ChIP-seq density distributions using the IGB for all proteins on selected class II genes in WT strains. To correct for chromatin-state bias corresponding to the highly expressed genomic loci observed for control samples (15,45,57–59), the normalized signals of an untagged strain sample were subtracted from the ChIP samples for each protein as described previously (45) and in the ‘Materials and Methods’ section. The ChIP signals of Mediator inside ORFs most likely resulted from chromatin-state bias and are mostly corrected by untagged strain signal subtraction.
Figure 4.
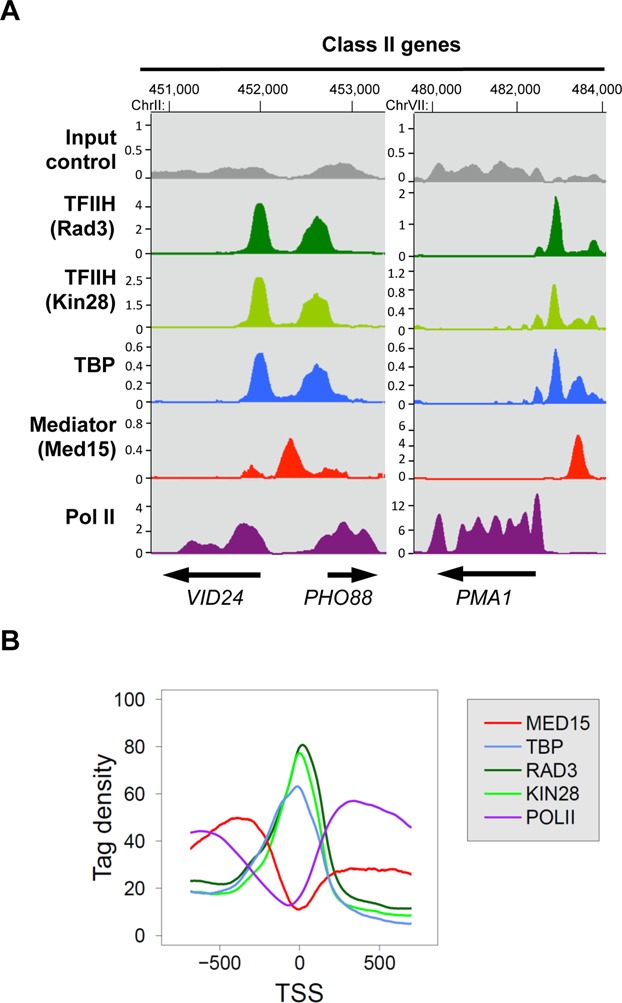
Enrichment profiles of Mediator, Pol II, TFIIH and TBP on yeast genome. Cells were grown at 30°C in YPD medium and then transferred for 45 minutes to 37°C. (A) Examples of Mediator, Pol II, TBP, and TFIIH ChIP-seq enrichment profiles on selected class II genes. Densities of sequence tags were assessed from ChIP-seq experiments performed with Med15-HA (Mediator), TBP-HA, Rad3-HA (TFIIH core), and Kin28-HA (TFIIK) strains using anti-HA antibody. Pol II was immunoprecipitated using anti-Rpb1 antibody. ChIP-seq density profiles are displayed using the IGB yeast genome browser. Input DNA and DNA from ChIP with an untagged strain were used as negative controls. Densities of sequence tags were displayed after subtraction of the normalized control of an untagged strain. The scales in kiloreads are indicated for each profile. (B) Distribution of Mediator, TBP, TFIIH and Pol II ChIP-seq densities around TSS. Intergenic regions encompassing Pol III-transcribed genes and divergent genes were excluded. The tag density was determined for each protein in a 1600-bp window centred on the TSS. The TSS positions were provided from previous studies (4,5). Mean tag density for each nucleotide position was then calculated and plotted over the window.
The location of Mediator, Pol II and GTF peaks were compared around transcription start sites (TSS) in WT strains in a metagene analysis (Figure 4B). To consider only Pol II transcribed gene promoters, intergenic regions encompassing Pol III-transcribed genes were excluded. Divergent genes with double peaks for GTFs were also excluded from the analysis. Mean tag density (1 bp bin) was determined for each protein in a 1600-bp window centred on the TSS. These analyses show that the ChIP-seq distributions of TFIIH subunits and TBP superposed with each other close to TSS. As expected, Pol II is distributed inside transcribed regions. Mediator distribution was located upstream of TSS on regulatory regions.
med17 mutations can globally destabilize the PIC in vivo
To investigate whether PIC assembly is generally affected in med17 mutants or in a gene-specific manner, the genome-wide occupancy of Mediator, Pol II and GTFs were analyzed using ChIP-seq experiments in mutants to compare them with the WT strains. Cells were grown at 30°C in YPD medium and then transferred for 45 min to 37°C. The normalization step is essential for ChIP-seq comparative analysis, but widely used normalization methods like a quantile normalization step cannot be applied in situations where occupancy increases or decreases genome-wide. To detect the potential global effects of Mediator mutations on PIC component occupancy, we performed the following normalization step. A count of reads was determined on the promoter regions of Pol II-transcribed genes for Med15, Rad3, Kin28 and TBP, and on Pol II-transcribed gene ORFs for Pol II and then normalized relative to qPCR data on a set of selected regions, as described in the ‘Materials and Methods’ section. Regression analysis of PIC component binding in each med17 mutant versus WT was systematically performed.
Genome-wide Mediator occupancy was analyzed for the Med15 Mediator subunit. It was not possible to obtain good quality ChIP-seq results for the Med5 Mediator tail module subunit or the Med6 and Med8 Mediator head module subunits (see ‘Materials and Methods’ section). Chromatin associated with the Med17 Mediator head subunit can be ChIPed efficiently. However, the addition of tags to mutant subunits has been observed to potentially lead to an aggravation of growth phenotypes. For this reason, Med17 occupancy was not investigated in mutant strains.
The ChIP results on the selected class II genes showed the most pronounced effects on Pol II, TBP and Kin28 occupancy for the med17-444 mutant whereas Rad3 occupancy remained unaffected in this mutant (Figures 2 and 3). Genome-wide analysis of the med17-444 mutant revealed a global 1.8-fold decrease (the slope of regression line, shown in red, is equal to 0.55) in Med15 Mediator occupancy with a high correlation coefficient (R2 equal to 0.93) (Figure 5A). As a consequence, the presence of other PIC components like Pol II, TFIIH and TBP was also globally reduced to a different extent. A global decrease in genome-wide occupancy for Pol II and the Kin28 TFIIK subunit (2.6- and 2.7-fold, respectively) was observed. The TBP association to chromatin was also globally affected with a 1.6-fold decrease. The Rad3 TFIIH core subunit binding was slightly affected (1.2-fold decrease), consistent with the ChIP results on the ADH1, PYK1 and PMA1 gene promoters. Taken together, the genome-wide results on the med17-444 mutant confirmed a central role for the Med17 Mediator subunit in PIC formation and suggested that the Mediator tail module destabilization on the chromatin could lead to a global change in the in-vivo binding of several PIC components but only marginally affected Rad3 occupancy.
Figure 5.
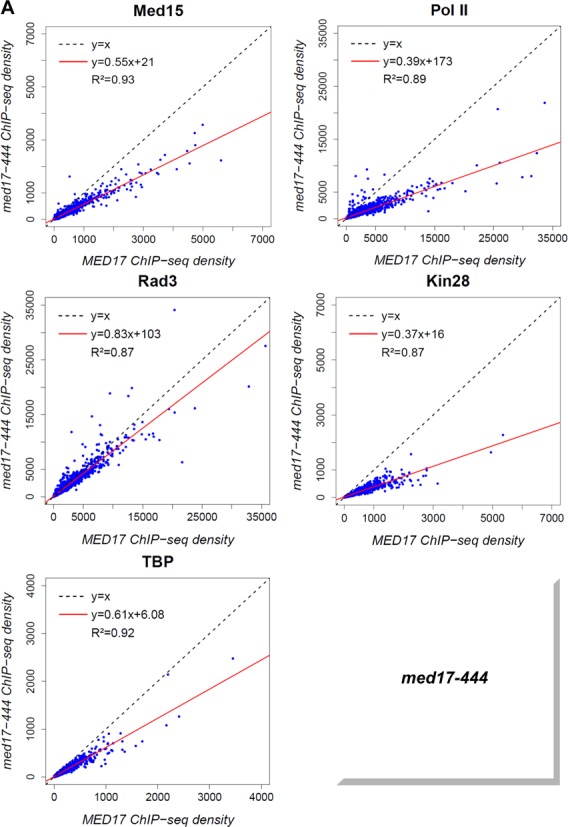
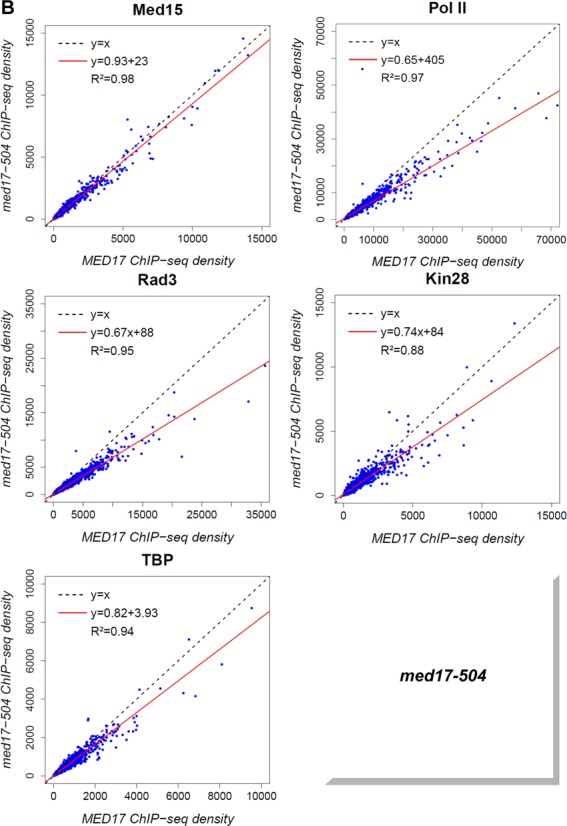
Global destabilization of PIC assembly in med17-444 and -504 mutant strains. The density of sequence tags in Med15, Rad3, Kin28 and TBP ChIP-seq experiments was calculated for promoter regions of Pol II-transcribed genes. Cells were grown at 30°C in YPD medium and then transferred for 45 minutes to 37°C. The density of sequence tags in the Pol II ChIP-seq experiments was calculated for the Pol II-transcribed genes. In all, 3852 Pol II-enriched genes were used for these analyses. Tag densities were normalized relative to qPCR data on a set of selected genes. Each point on the plot corresponds to one promoter region or one ORF. Promoter regions correspond to intergenic regions in tandem or in divergent orientation, excluding intergenic regions encompassing Pol III-transcribed genes. A linear regression (red line) for ChIP-seq density in mutant versus ChIP-seq density in WT and an R2 correlation coefficient are indicated. (A) Med15, Pol II, Rad3, Kin28 and TBP ChIP-seq density in med17-444 versus ChIP-seq density in WT on class II promoter regions or ORFs (for Pol II). (B) Med15, Pol II, Rad3, Kin28 and TBP ChIPseq density in med17-504 versus ChIP-seq density in WT on class II promoter regions or ORFs (for Pol II).
The med17-504 mutation was found to impair Pol II-Mediator interaction (Figure 1D) and led to reduced binding of Pol II and GTFs to the ADH1, PYK1 and PMA1 genes (Figure 2) and Mediator, Pol II and some GTFs on the GAL1 gene (Figure 3 for Mediator, Pol II and TBP, and data not shown for TFIIH). To determine how general the effects of med17-504 were, the genome-wide Mediator, Pol II, Rad3, Kin28 and TBP occupancy were compared in this mutant and the WT (Figure 5B). The genome–wide binding of the Mediator Med15 subunit in med17-504 was similar to that of the WT strain (the slope equal to 0.93 and R2 equal to 0.98). In contrast, the association of Pol II and the GTFs was globally diminished in this mutant compared to the WT, suggesting that the impaired Pol II–Mediator interaction leads to a general PIC assembly defect. The genome-wide analysis of med17-444 and -504 mutants demonstrated that a destabilization of Mediator binding to chromatin or an impaired interaction between Mediator and Pol II globally destabilize the PIC assembly in vivo.
Mutations in the Med17 Mediator subunit specifically affect the genome-wide association of the TFIIH core subcomplex
Previously, we have shown that mutations in the Med11 Mediator subunit affecting its interaction with the Rad3 TFIIH core subunit can selectively impair the binding of the TFIIH kinase module (27). Unexpectedly, the ChIP experiments suggested that some med17 mutations like med17-670 could lead to a selective dissociation of the Rad3 TFIIH core subunit. TFIIH core genome-wide binding in med17 mutants was therefore investigated. The ChIP-seq results revealed that the genome-wide association of this TFIIH subcomplex was affected independently of the TFIIK subcomplex in med17-670 (Figure 6A) and -98 mutants (Figure 6B). A global 2-fold decrease of the Rad3 TFIIH core subunit binding was observed in these mutants with the slope of regression line equal to 0.55 and 0.51 for med17-670 and -98, respectively, with high correlation coefficients. In contrast, the association of the Kin28 TFIIK subunit was similar to the WT level (the slope equal to 1.01 and 0.94, R2 equal to 0.98 and 0.90 for med17-670 and -98, respectively). The presence of the Med15 Mediator subunit was mostly unchanged or only slightly decreased (the slope equal to 0.87 and 0.80, R2 equal to 0.93 and 0.94 for med17-670 and -98, respectively). The genome-wide binding of TBP was not reduced and even showed a slight increase (the slope equal to 1.25 and 1.14, R2 equal to 0.92 and 0.96 for med17-670 and -98, respectively). In the med17-670 mutant, a global 1.4-fold decrease in Pol II occupancy was observed when compared to the WT strain with the slope of regression line equal to 0.72 and a high correlation coefficient equal to 0.97. The Pol II association was also reduced in the med17-98 mutant showing a 1.2-fold global decrease with a reduced correlation coefficient of 0.83 that suggested a more complex transcriptional effect for this mutation. The genome-wide analysis of PIC components of med17-670 and -98 mutants suggests that Mediator can selectively affect the TFIIH core subcomplex binding to the yeast genome without impairing that of the TFIIH kinase module or TBP.
Figure 6.
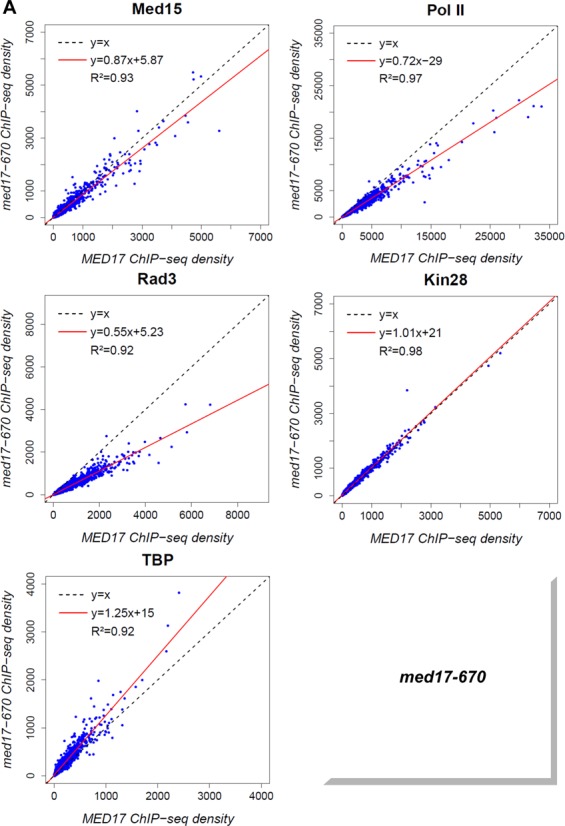
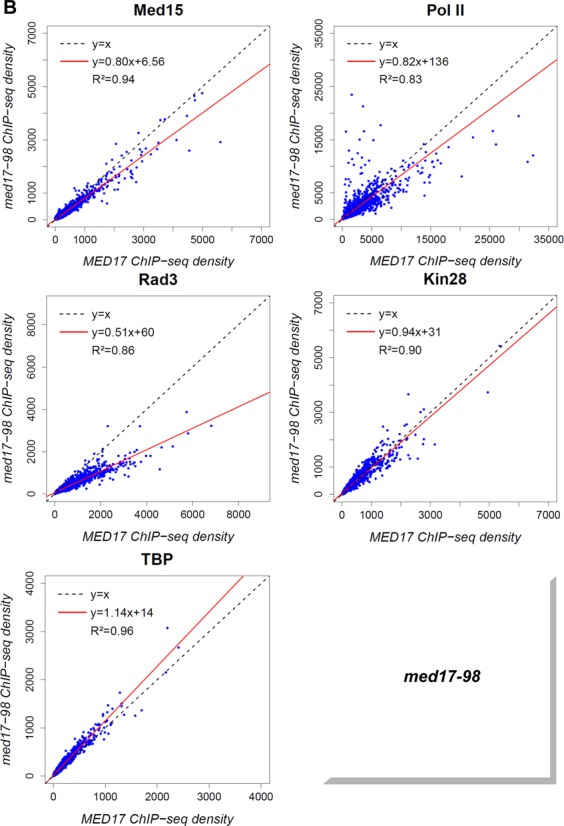
Global decrease of TFIIH core association independent of TFIIK in med17-670 and -98 mutant strains. The density of sequence tags in the Med15, Pol II, Rad3, Kin28 and TBP ChIP-seq experiments was calculated as in Figure 5. (A) Med15, Pol II, Rad3, Kin28 and TBP ChIP-seq density in med17-670 versus ChIP-seq density in WT on class II promoter regions or ORFs (for Pol II). (B) Med15, Pol II, Rad3, Kin28 and TBP ChIPseq density in med17-98 versus ChIP-seq density in WT on class II promoter regions or ORFs (for Pol II).
DISCUSSION
In this study, a genome-wide analysis of PIC assembly in vivo was performed by taking advantage of conditional Mediator mutants of the essential Med17 head module subunit after a transfer to a non-permissive temperature. We propose that the Mediator head module in general, and Med17 in particular, independently coordinate the PIC assembly in vivo at different levels. (i) In accordance with previous studies, the results of this work support a central role for the Med17 Mediator subunit in Mediator organization and function. We demonstrate that med17 mutants globally affect Pol II transcription and specific mutations in Med17 could result in a global destabilization of PIC assembly in vivo. (ii) The ChIP and ChIP-seq results show that Mediator selectively contributes to TBP recruitment or stabilization to chromatin in vivo, supporting Mediator head-TBP functional contact that was previously identified in vitro. (iii) We show that Mediator recruitment stabilizes TFIIK kinase and TFIIH core modules independently since med17 mutations can differentially affect the genome-wide occupancy of the two TFIIH modules. We therefore propose that the recruitment or the stability of TFIIH kinase and core modules is regulated independently on the yeast genome. (iv) This work suggests a possible mechanism for infantile cerebral atrophy caused by a mutation in Med17. We propose that the corresponding mutation in yeast Med17 affects Mediator–Pol II contact that leads to a global destabilization of PICs.
Independent recruitment of TFIIH modules
The Mediator head module directly interacts with the TFIIH complex via Med11-Rad3 contact and this interaction is important for the recruitment of this GTF to promoters (27). Mutations in the Med11 subunit differentially affected the promoter occupancy of the TFIIH core and TFIIK kinase modules. A med11 mutation that impaired Mediator–TFIIH contact destabilized TFIIK on a group of promoters. The genome-wide analysis of med17 mutants performed in this study supports a functional role of Mediator in the independent recruitment of TFIIH core (Rad3 subunit) and TFIIK kinase (Kin28 subunit) modules. Indeed, the med17-504 mutation reduced the global association of Rad3 and Kin28 to a similar extent. On the contrary, the med17-444 mutation, while affecting the occupancy of both TFIIH modules, displayed a particularly pronounced decrease in Kin28 occupancy. These results show that the TFIIK module was further destabilized in the med17-444 mutant, similarly to the med11-T47A mutant. Conversely, we also observed that the genome-wide association of the TFIIH core subcomplex could be affected independently of the TFIIK subcomplex in med17-670 and med17-98 mutants. A large decrease of Rad3 genome-wide occupancy was observed in these mutants, whereas the association of the Kin28 TFIIK subunit remained unchanged. Taken together, these results suggest that Mediator differentially affects the recruitment or the stabilization of TFIIH modules and that the stability of TFIIH modules is regulated independently within the PICs. We show that two biochemically separable TFIIH modules have independent in-vivo behavior. The recruitment of the core TFIIH module can be selectively affected, independently of TFIIK and TBP. In line with the functional links between Mediator and TFIIK, one of the Mediator functions consists in stimulating the CTD phosphorylation by Kin28 TFIIK kinase (22). Moreover, Kin28 was reported to phosphorylate Med4 and Med14 Mediator subunits in vitro (60) and Kin28 phosphorylation of Med4 occurs in vivo (61). Recently, the phosphorylation of the Pol II CTD by Kin28 TFIIK kinase has been reported to regulate the transient association of Mediator to core promoters and a release of Mediator from the PIC and the promoter escape (15,56), highlighting reciprocal links between Mediator and the TFIIK kinase module.
Mediator contribution to TBP recruitment in vivo
TBP directly binds to the Mediator head module via the Med8–Med18–Med20 subcomplex (23,25). Previously, human Mediator and TBP-containing TFIID complexes have been shown to assemble cooperatively on promoter DNA in vitro (24). In accordance with in-vitro data, our in-vivo genome-wide analysis of yeast Mediator mutants showed that TBP chromatin binding could be affected by Mediator mutations (med17-444 and -504). It should be noted that the loss of Mediator function in the med17-138 mutant, in which the Mediator head disassembles, has been previously shown to reduce TBP occupancy of several class II promoters (62,63). TBP association depended on the Mediator mutant examined in this study. The genome-wide binding of this GTF either did not decrease or was found to slightly increase in the med17-670 and med17-98 mutants. In this work, we identified mutations in the Med17 subunit that affected global TBP binding to chromatin, further supporting a coordinated behavior with Mediator. Interestingly, even though the effects of med17 mutations are global, the range of effects can depend on specific genes. For example, the med17-504 mutation resulted in a global decrease in TBP occupancy, but did not affect TBP recruitment to the GAL1 gene promoter upon transcription activation. Conversely, the med17-98 mutation did not reduce genome-wide TBP or TFIIK occupancy. However, in this mutant the TFIIK binding, together with that of Pol II and the core TFIIH module, was reduced on the three model promoters, but not in the case of TBP. These results therefore suggest a selective contribution of Mediator in TBP recruitment or stabilization to chromatin.
Global stabilization of PIC by Mediator
In med17-444 and -504 mutants, PIC assembly was destabilized leading to a decrease of Pol II, Rad3 TFIIH core subunit, Kin28 TFIIK subunit and TBP presence. The following mechanistic explanations specific to each mutant can be proposed. The presence of the Mediator tail module (Med15 subunit) on chromatin was globally affected in the med17-444 mutant. We propose that, as a consequence of this effect on tail module occupancy, overall PIC assembly is destabilized with a large decrease in Pol II, Kin28 and TBP occupancy and a moderate effect on Rad3 occupancy. In contrast, the binding of Mediator remained globally unaffected in the med17-504 mutant. This mutation led also to a global decrease in Pol II and GTF occupancy. We propose that an impaired interaction between Mediator (the Med17 subunit) and Pol II (the Rpb3 subunit) in this mutant destabilized the global PIC assembly in vivo, as was observed for the Pol II rpb3-2 mutant with reduced Mediator–Pol II interaction (28) highlighting the functional importance of Mediator–Pol II contact. Taken together, our results revealed that specific point mutations in the Med17 Mediator head subunit (med17-Q444P, med17-M504P) could result in the global destabilization of PIC assembly in vivo through two different mechanisms, a destabilization of Mediator binding to chromatin or an impaired interaction between Mediator and Pol II, consistent with a central role for the Med17 subunit in Mediator function.
Among the med17 mutants examined, only the med17-444 mutation reduced the global Mediator tail (Med15 subunit) occupancy. Other med17 mutations either did not affect or only slightly reduced the level of genome-wide Med15 binding to chromatin. ChIP experiments on the ADH1, PYK1 and PMA1 genes did not reveal any decrease in Med6 Mediator head or Med5 Mediator tail subunit occupancy in med17 mutants. Moreover, Mediator stability in the med17 mutants examined in this study is supported by mass spectrometry and CoIP experiments indicating the presence of all Mediator modules. A decrease in ChIP signal for the Med15 Mediator tail subunit in med17-444 might reflect a conformational change in Mediator complex bound to chromatin leading to reduced cross-linking efficiency or Mediator tail destabilization on chromatin.
A possible mechanism for infantile cerebral atrophy
Mediator is conserved in all eukaryotes. The mutated residues in the med17 mutants characterized in this study correspond to the same (human Q310 for yeast Q444P in med17-444 and human E314 for yeast E448Q, one of the three mutations in med17-98) or functionally close (human L371 for yeast M504P in med17-504) amino acids in human Med17 protein (Supplementary Figure S6). Given the conservation of the Mediator subunits, the molecular mechanisms of the Mediator function in PIC assembly are probably conserved from yeast to human and might extend to all eukaryotes. We showed that the med17-M504P mutation, proposed to be equivalent to the human mutation responsible for infantile cerebral atrophy, led to a severe temperature-sensitive phenotype in yeast, impaired Pol II–Mediator interaction and resulted in a decrease of genome-wide occupancy of all PIC components tested except Mediator itself. These results suggest that infantile cerebral atrophy might be the direct consequence of a defect in Mediator–Pol II contact and a global destabilization of PICs. Recently, we have shown that Mediator, in addition to its coregulator role, can also serve as a link between transcription and DNA repair (45). Several med17 mutants were UV-sensitive in a global-genome repair-deficient context. Furthermore, med17–504 and the other mutants analyzed in this study do not show any increase in UV sensitivity (data not shown), suggesting that these mutations led to transcriptional defects only.
Specific versus global effects of med17 mutations
One of the questions that we addressed in analysing the impact of conditional mutations in the Med17 Mediator subunit on genome-wide PIC assembly was its gene-specific versus global nature. A high correlation between genome-wide densities of PIC components highlights the global effects of med17 mutations. It should be noted that the range of the effects could still be gene-specific. Consistent with a central role for the Med17 subunit in Mediator function, all med17 mutations reduced the global Pol II chromatin binding, but to a varying extent. The global decrease of Pol II occupancy in med17 mutants compared to the WT was associated with a high correlation coefficient, indicating that most of the genes were affected to a similar extent. The med17-98 mutant was an exception since it showed a global decrease of Pol II presence but with a reduced correlation coefficient (R2 equal to 0.83) suggesting a more complex and gene-specific transcriptional effect for this mutation. The occupancy of the Kin28 TFIIK subunit remained globally unchanged in the med17-98 mutant compared to the WT, but Kin28 ChIP signals were decreased on the ADH1, PYK1 and PMA1 gene promoters. The med17-444 mutation did not modify the presence of the Rad3 TFIIH core subunit on the three constitutively expressed genes or the GAL1 inducible gene, but our genome-wide analysis revealed a slight decrease in global Rad3 occupancy. The med17-504 mutant had a particular effect on the GAL1 gene, showing a reduced Mediator association and unmodified Rad3 and TBP recruitment, whereas the genome-wide Mediator occupancy was similar to that of the WT and the occupancy of all other PIC components was globally reduced.
In conclusion, we suggest that Mediator differentially coordinates the multiple steps of a PIC assembly pathway in vivo at the level of independent recruitment or stabilization of several PIC components including Pol II, TBP and two TFIIH modules. The global effects of Mediator mutations on PIC formation are consistent with a central role for the Med17 subunit in Mediator function and organization. Additional PIC components, for example, GTFs that were not examined in this study, might contribute to PIC assembly. Together with Mediator, other coactivators, such as SAGA or TFIID complexes, modify PIC assembly and activity. In future studies it would be worth considering the dynamics of PIC assembly to improve understanding of in-vivo transcription mechanisms at work in eukaryotes.
Supplementary Material
Acknowledgments
We thank F.J. Asturias for CA001 strain, C. Thermes, E. Van Dijk and Y. Jaszczyszyn for performing the high throughput-sequencing of ChIP samples, the SPI (CEA/Saclay) for monoclonal antibodies and L. Kuras for critical reading of the manuscript. This work has benefited from the facilities and expertise of the high-throughput sequencing and the SICaPS mass spectrometry platforms of IMAGIF (Centre de Recherche de Gif, www.imagif.cnrs.fr).
Footnotes
Present addresses:
Sandra Wydau-Dematteis, EA 4065, CRP2, DHU Risks in pregnancy, Faculté de Pharmacie de Paris, Université Paris Descartes, Sorbonne Paris Cité, France.
Fanny Eyboulet, Institut de recherches cliniques de Montréal, 110 avenue des Pins Ouest, Montréal, Québec H2W 1R7, Canada.
SUPPLEMENTARY DATA
Supplementary Data are available at NAR Online.
FUNDING
Agence Nationale de la Recherche [ANR 11 BSV8 020 01, ANR-14-CE10-0012-01]; Fondation ARC [SL 2201130607079]; Fondation ARC [DOC20130606697 to F.E. ]. Funding for open access charge: Fondation ARC [SL 2201130607079].
Conflict of interest statement. None declared.
REFERENCES
- 1.Buratowski S., Hahn S., Guarente L., Sharp P.A. Five intermediate complexes in transcription initiation by RNA polymerase II. Cell. 1989;56:549–561. doi: 10.1016/0092-8674(89)90578-3. [DOI] [PubMed] [Google Scholar]
- 2.Ranish J.A., Hahn S. Transcription: basal factors and activation. Curr. Opin. Genet. Dev. 1996;6:151–158. doi: 10.1016/s0959-437x(96)80044-x. [DOI] [PubMed] [Google Scholar]
- 3.Grunberg S., Hahn S. Structural insights into transcription initiation by RNA polymerase II. Trends Biochem. Sci. 2013;38:603–611. doi: 10.1016/j.tibs.2013.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rhee H.S., Pugh B.F. Genome-wide structure and organization of eukaryotic pre-initiation complexes. Nature. 2012;483:295–301. doi: 10.1038/nature10799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Xu Z., Wei W., Gagneur J., Perocchi F., Clauder-Munster S., Camblong J., Guffanti E., Stutz F., Huber W., Steinmetz L.M. Bidirectional promoters generate pervasive transcription in yeast. Nature. 2009;457:1033–1037. doi: 10.1038/nature07728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tsai K.L., Tomomori-Sato C., Sato S., Conaway R.C., Conaway J.W., Asturias F.J. Subunit architecture and functional modular rearrangements of the transcriptional mediator complex. Cell. 2014;157:1430–1444. doi: 10.1016/j.cell.2014.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wang X., Sun Q., Ding Z., Ji J., Wang J., Kong X., Yang J., Cai G. Redefining the modular organization of the core Mediator complex. Cell Res. 2014;24:796–808. doi: 10.1038/cr.2014.64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Guglielmi B., van Berkum N.L., Klapholz B., Bijma T., Boube M., Boschiero C., Bourbon H.M., Holstege F.C., Werner M. A high resolution protein interaction map of the yeast Mediator complex. Nucleic Acids Res. 2004;32:5379–5391. doi: 10.1093/nar/gkh878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Koschubs T., Lorenzen K., Baumli S., Sandstrom S., Heck A.J., Cramer P. Preparation and topology of the Mediator middle module. Nucleic Acids Res. 2010;38:3186–3195. doi: 10.1093/nar/gkq029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lariviere L., Plaschka C., Seizl M., Wenzeck L., Kurth F., Cramer P. Structure of the Mediator head module. Nature. 2012;492:448–451. doi: 10.1038/nature11670. [DOI] [PubMed] [Google Scholar]
- 11.Robinson P.J., Bushnell D.A., Trnka M.J., Burlingame A.L., Kornberg R.D. Structure of the Mediator Head module bound to the carboxy-terminal domain of RNA polymerase II. Proc. Natl. Acad. Sci. U.S.A. 2012;109:17931–17935. doi: 10.1073/pnas.1215241109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kornberg R.D. Mediator and the mechanism of transcriptional activation. Trends Biochem. Sci. 2005;30:235–239. doi: 10.1016/j.tibs.2005.03.011. [DOI] [PubMed] [Google Scholar]
- 13.Ries D., Meisterernst M. Control of gene transcription by Mediator in chromatin. Semin. Cell Dev. Biol. 2011;22:735–740. doi: 10.1016/j.semcdb.2011.08.004. [DOI] [PubMed] [Google Scholar]
- 14.Holstege F.C., Jennings E.G., Wyrick J.J., Lee T.I., Hengartner C.J., Green M.R., Golub T.R., Lander E.S., Young R.A. Dissecting the regulatory circuitry of a eukaryotic genome. Cell. 1998;95:717–728. doi: 10.1016/s0092-8674(00)81641-4. [DOI] [PubMed] [Google Scholar]
- 15.Jeronimo C., Robert F. Kin28 regulates the transient association of Mediator with core promoters. Nat. Struct. Mol. Biol. 2014;21:449–455. doi: 10.1038/nsmb.2810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lacombe T., Poh S.L., Barbey R., Kuras L. Mediator is an intrinsic component of the basal RNA polymerase II machinery in vivo. Nucleic Acids Res. 2013;41:9651–9662. doi: 10.1093/nar/gkt701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Paul E., Zhu Z.I., Landsman D., Morse R.H. Genome-wide association of mediator and RNA polymerase II in wild-type and mediator mutant yeast. Mol. Cell. Biol. 2015;35:331–342. doi: 10.1128/MCB.00991-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Thompson C.M., Young R.A. General requirement for RNA polymerase II holoenzymes in vivo. Proc. Natl. Acad. Sci. U.S.A. 1995;92:4587–4590. doi: 10.1073/pnas.92.10.4587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Andrau J.C., van de Pasch L., Lijnzaad P., Bijma T., Koerkamp M.G., van de Peppel J., Werner M., Holstege F.C. Genome-wide location of the coactivator mediator: Binding without activation and transient Cdk8 interaction on DNA. Mol. Cell. 2006;22:179–192. doi: 10.1016/j.molcel.2006.03.023. [DOI] [PubMed] [Google Scholar]
- 20.Zhu X., Wiren M., Sinha I., Rasmussen N.N., Linder T., Holmberg S., Ekwall K., Gustafsson C.M. Genome-wide occupancy profile of mediator and the Srb8–11 module reveals interactions with coding regions. Mol Cell. 2006;22:169–178. doi: 10.1016/j.molcel.2006.03.032. [DOI] [PubMed] [Google Scholar]
- 21.Jiang Y.W., Veschambre P., Erdjument-Bromage H., Tempst P., Conaway J.W., Conaway R.C., Kornberg R.D. Mammalian mediator of transcriptional regulation and its possible role as an end-point of signal transduction pathways. Proc. Natl. Acad. Sci. U.S.A. 1998;95:8538–8543. doi: 10.1073/pnas.95.15.8538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kim Y.J., Bjorklund S., Li Y., Sayre M.H., Kornberg R.D. A multiprotein mediator of transcriptional activation and its interaction with the C-terminal repeat domain of RNA polymerase II. Cell. 1994;77:599–608. doi: 10.1016/0092-8674(94)90221-6. [DOI] [PubMed] [Google Scholar]
- 23.Cai G., Imasaki T., Yamada K., Cardelli F., Takagi Y., Asturias F.J. Mediator head module structure and functional interactions. Nat. Struct. Mol. Biol. 2010;17:273–279. doi: 10.1038/nsmb.1757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Johnson K.M., Wang J., Smallwood A., Arayata C., Carey M. TFIID and human mediator coactivator complexes assemble cooperatively on promoter DNA. Genes Dev. 2002;16:1852–1863. doi: 10.1101/gad.995702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lariviere L., Geiger S., Hoeppner S., Rother S., Strasser K., Cramer P. Structure and TBP binding of the Mediator head subcomplex Med8-Med18-Med20. Nat. Struct. Mol. Biol. 2006;13:895–901. doi: 10.1038/nsmb1143. [DOI] [PubMed] [Google Scholar]
- 26.Takahashi H., Parmely T.J., Sato S., Tomomori-Sato C., Banks C.A., Kong S.E., Szutorisz H., Swanson S.K., Martin-Brown S., Washburn M.P., et al. Human mediator subunit MED26 functions as a docking site for transcription elongation factors. Cell. 2011;146:92–104. doi: 10.1016/j.cell.2011.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Esnault C., Ghavi-Helm Y., Brun S., Soutourina J., Van Berkum N., Boschiero C., Holstege F., Werner M. Mediator-dependent recruitment of TFIIH modules in preinitiation complex. Mol. Cell. 2008;31:337–346. doi: 10.1016/j.molcel.2008.06.021. [DOI] [PubMed] [Google Scholar]
- 28.Soutourina J., Wydau S., Ambroise Y., Boschiero C., Werner M. Direct interaction of RNA polymerase II and mediator required for transcription in vivo. Science. 2011;331:1451–1454. doi: 10.1126/science.1200188. [DOI] [PubMed] [Google Scholar]
- 29.Bourbon H.M. Comparative genomics supports a deep evolutionary origin for the large, four-module transcriptional mediator complex. Nucleic Acids Res. 2008;36:3993–4008. doi: 10.1093/nar/gkn349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Risheg H., Graham J.M. Jr, Clark R.D., Rogers R.C., Opitz J.M., Moeschler J.B., Peiffer A.P., May M., Joseph S.M., Jones J.R., et al. A recurrent mutation in MED12 leading to R961W causes Opitz-Kaveggia syndrome. Nat. Genet. 2007;39:451–453. doi: 10.1038/ng1992. [DOI] [PubMed] [Google Scholar]
- 31.Schwartz C.E., Tarpey P.S., Lubs H.A., Verloes A., May M.M., Risheg H., Friez M.J., Futreal P.A., Edkins S., Teague J., et al. The original Lujan syndrome family has a novel missense mutation (p.N1007S) in the MED12 gene. J. Med. Genet. 2007;44:472–477. doi: 10.1136/jmg.2006.048637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hashimoto S., Boissel S., Zarhrate M., Rio M., Munnich A., Egly J.M., Colleaux L. MED23 mutation links intellectual disability to dysregulation of immediate early gene expression. Science. 2011;333:1161–1163. doi: 10.1126/science.1206638. [DOI] [PubMed] [Google Scholar]
- 33.Firestein R., Bass A.J., Kim S.Y., Dunn I.F., Silver S.J., Guney I., Freed E., Ligon A.H., Vena N., Ogino S., et al. CDK8 is a colorectal cancer oncogene that regulates beta-catenin activity. Nature. 2008;455:547–551. doi: 10.1038/nature07179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gade P., Singh A.K., Roy S.K., Reddy S.P., Kalvakolanu D.V. Down-regulation of the transcriptional mediator subunit Med1 contributes to the loss of expression of metastasis-associated dapk1 in human cancers and cancer cells. Int. J. Cancer. 2009;125:1566–1574. doi: 10.1002/ijc.24493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kuuselo R., Savinainen K., Sandstrom S., Autio R., Kallioniemi A. MED29, a component of the mediator complex, possesses both oncogenic and tumor suppressive characteristics in pancreatic cancer. Int. J. Cancer. 2011;129:2553–2565. doi: 10.1002/ijc.25924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Li L.H., He J., Hua D., Guo Z.J., Gao Q. Lentivirus-mediated inhibition of Med19 suppresses growth of breast cancer cells in vitro. Cancer Chemother. Pharmacol. 2010;68:207–215. doi: 10.1007/s00280-010-1468-9. [DOI] [PubMed] [Google Scholar]
- 37.Vijayvargia R., May M.S., Fondell J.D. A coregulatory role for the mediator complex in prostate cancer cell proliferation and gene expression. Cancer Res. 2007;67:4034–4041. doi: 10.1158/0008-5472.CAN-06-3039. [DOI] [PubMed] [Google Scholar]
- 38.Zhang X., Krutchinsky A., Fukuda A., Chen W., Yamamura S., Chait B.T., Roeder R.G. MED1/TRAP220 exists predominantly in a TRAP/ Mediator subpopulation enriched in RNA polymerase II and is required for ER-mediated transcription. Mol. Cell. 2005;19:89–100. doi: 10.1016/j.molcel.2005.05.015. [DOI] [PubMed] [Google Scholar]
- 39.Thompson C.M., Koleske A.J., Chao D.M., Young R.A. A multisubunit complex associated with the RNA polymerase II CTD and TATA-binding protein in yeast. Cell. 1993;73:1361–1375. doi: 10.1016/0092-8674(93)90362-t. [DOI] [PubMed] [Google Scholar]
- 40.Linder T., Zhu X., Baraznenok V., Gustafsson C.M. The classical srb4–138 mutant allele causes dissociation of yeast Mediator. Biochem. Biophys. Res. Commun. 2006;349:948–953. doi: 10.1016/j.bbrc.2006.08.099. [DOI] [PubMed] [Google Scholar]
- 41.Takagi Y., Calero G., Komori H., Brown J.A., Ehrensberger A.H., Hudmon A., Asturias F., Kornberg R.D. Head module control of mediator interactions. Mol. Cell. 2006;23:355–364. doi: 10.1016/j.molcel.2006.06.007. [DOI] [PubMed] [Google Scholar]
- 42.Takagi Y., Kornberg R.D. Mediator as a general transcription factor. J. Biol. Chem. 2006;281:80–89. doi: 10.1074/jbc.M508253200. [DOI] [PubMed] [Google Scholar]
- 43.Kaufmann R., Straussberg R., Mandel H., Fattal-Valevski A., Ben-Zeev B., Naamati A., Shaag A., Zenvirt S., Konen O., Mimouni-Bloch A., et al. Infantile cerebral and cerebellar atrophy is associated with a mutation in the MED17 subunit of the transcription preinitiation mediator complex. Am. J. Hum. Genet. 2010;87:667–670. doi: 10.1016/j.ajhg.2010.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ghavi-Helm Y., Michaut M., Acker J., Aude J.C., Thuriaux P., Werner M., Soutourina J. Genome-wide location analysis reveals a role of TFIIS in RNA polymerase III transcription. Genes Dev. 2008;22:1934–1947. doi: 10.1101/gad.471908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Eyboulet F., Cibot C., Eychenne T., Neil H., Alibert O., Werner M., Soutourina J. Mediator links transcription and DNA repair by facilitating Rad2/XPG recruitment. Genes Dev. 2013;27:2549–2562. doi: 10.1101/gad.225813.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Soutourina J., Bordas-Le Floch V., Gendrel G., Flores A., Ducrot C., Dumay-Odelot H., Soularue P., Navarro F., Cairns B.R., Lefebvre O., et al. Rsc4 connects the chromatin remodeler RSC to RNA polymerases. Mol. Cell. Biol. 2006;26:4920–4933. doi: 10.1128/MCB.00415-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Cai G., Imasaki T., Takagi Y., Asturias F.J. Mediator structural conservation and implications for the regulation mechanism. Structure. 2009;17:559–567. doi: 10.1016/j.str.2009.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Schmitt M.E., Brown T.A., Trumpower B.L. A rapid and simple method for preparation of RNA from Saccharomyces cerevisiae. Nucleic Acids Res. 1990;18:3091–3092. doi: 10.1093/nar/18.10.3091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Langmead B., Trapnell C., Pop M., Salzberg S.L. Ultrafast and memory-efficient alignment of short DNA sequences to the human genome. Genome Biol. 2009;10:R25. doi: 10.1186/gb-2009-10-3-r25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zhang Y., Liu T., Meyer C.A., Eeckhoute J., Johnson D.S., Bernstein B.E., Nusbaum C., Myers R.M., Brown M., Li W., et al. Model-based analysis of ChIP-Seq (MACS) Genome Biol. 2008;9:R137. doi: 10.1186/gb-2008-9-9-r137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Salmon-Divon M., Dvinge H., Tammoja K., Bertone P. PeakAnalyzer: genome-wide annotation of chromatin binding and modification loci. BMC Bioinformatics. 2010;11:415. doi: 10.1186/1471-2105-11-415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Spyrou C., Stark R., Lynch A.G., Tavare S. BayesPeak: Bayesian analysis of ChIP-seq data. BMC Bioinformatics. 2009;10:299. doi: 10.1186/1471-2105-10-299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Haimovich G., Medina D.A., Causse S.Z., Garber M., Millan-Zambrano G., Barkai O., Chavez S., Perez-Ortin J.E., Darzacq X., Choder M. Gene expression is circular: factors for mRNA degradation also foster mRNA synthesis. Cell. 2013;153:1000–1011. doi: 10.1016/j.cell.2013.05.012. [DOI] [PubMed] [Google Scholar]
- 54.Sun M., Schwalb B., Schulz D., Pirkl N., Etzold S., Lariviere L., Maier K.C., Seizl M., Tresch A., Cramer P. Comparative dynamic transcriptome analysis (cDTA) reveals mutual feedback between mRNA synthesis and degradation. Genome Res. 2012;22:1350–1359. doi: 10.1101/gr.130161.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Bonnet J., Wang C.Y., Baptista T., Vincent S.D., Hsiao W.C., Stierle M., Kao C.F., Tora L., Devys D. The SAGA coactivator complex acts on the whole transcribed genome and is required for RNA polymerase II transcription. Genes Dev. 2014;28:1999–2012. doi: 10.1101/gad.250225.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wong K.H., Jin Y., Struhl K. TFIIH Phosphorylation of the Pol II CTD Stimulates Mediator Dissociation from the Preinitiation Complex and Promoter Escape. Mol. Cell. 2014;54:601–612. doi: 10.1016/j.molcel.2014.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Chen Y., Negre N., Li Q., Mieczkowska J.O., Slattery M., Liu T., Zhang Y., Kim T.K., He H.H., Zieba J., et al. Systematic evaluation of factors influencing ChIP-seq fidelity. Nat. Methods. 2012;9:609–614. doi: 10.1038/nmeth.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Park D., Lee Y., Bhupindersingh G., Iyer V.R. Widespread misinterpretable ChIP-seq bias in yeast. PLoS One. 2013;8:e83506. doi: 10.1371/journal.pone.0083506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Teytelman L., Thurtle D.M., Rine J., van Oudenaarden A. Highly expressed loci are vulnerable to misleading ChIP localization of multiple unrelated proteins. Proc. Natl. Acad. Sci. U.S.A. 2013;110:18602–18607. doi: 10.1073/pnas.1316064110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Liu Y., Kung C., Fishburn J., Ansari A.Z., Shokat K.M., Hahn S. Two cyclin-dependent kinases promote RNA polymerase II transcription and formation of the scaffold complex. Mol. Cell. Biol. 2004;24:1721–1735. doi: 10.1128/MCB.24.4.1721-1735.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Guidi B.W., Bjornsdottir G., Hopkins D.C., Lacomis L., Erdjument-Bromage H., Tempst P., Myers L.C. Mutual targeting of mediator and the TFIIH kinase Kin28. J. Biol. Chem. 2004;279:29114–29120. doi: 10.1074/jbc.M404426200. [DOI] [PubMed] [Google Scholar]
- 62.Kuras L., Struhl K. Binding of TBP to promoters in vivo is stimulated by activators and requires Pol II holoenzyme. Nature. 1999;399:609–613. doi: 10.1038/21239. [DOI] [PubMed] [Google Scholar]
- 63.Li X.Y., Virbasius A., Zhu X., Green M.R. Enhancement of TBP binding by activators and general transcription factors. Nature. 1999;399:605–609. doi: 10.1038/21232. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


