Abstract
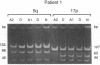
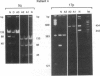
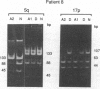
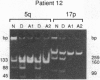
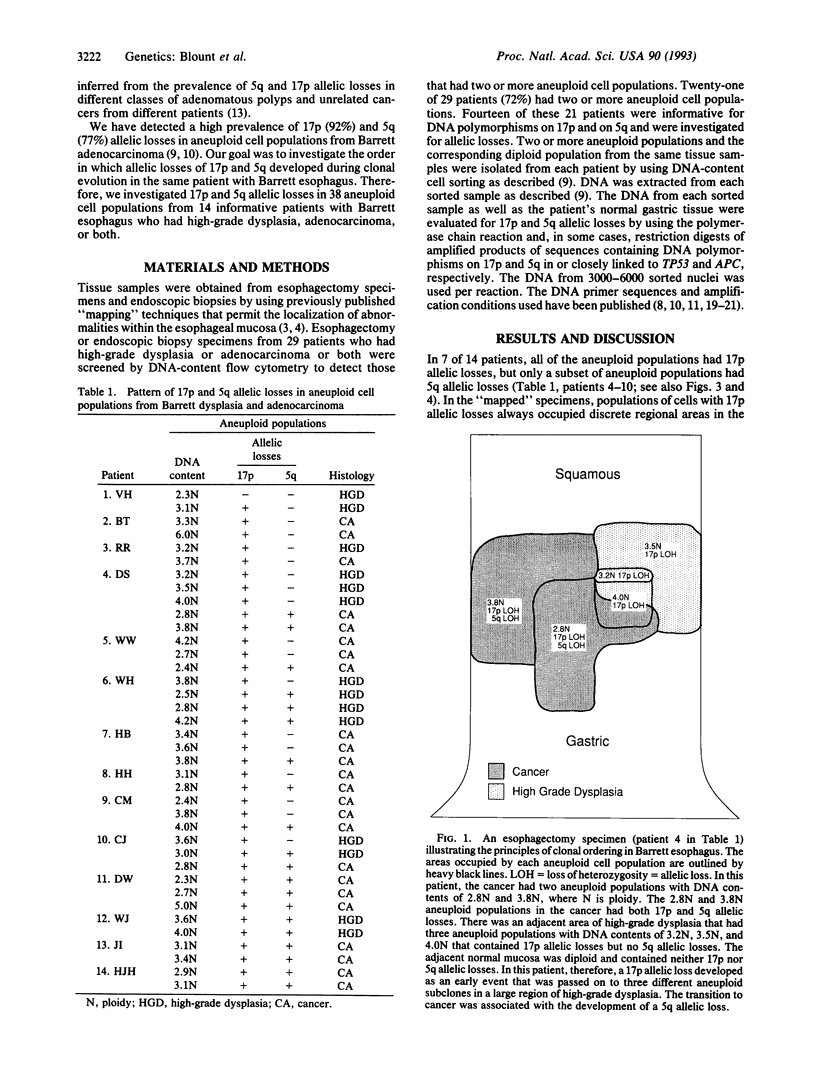
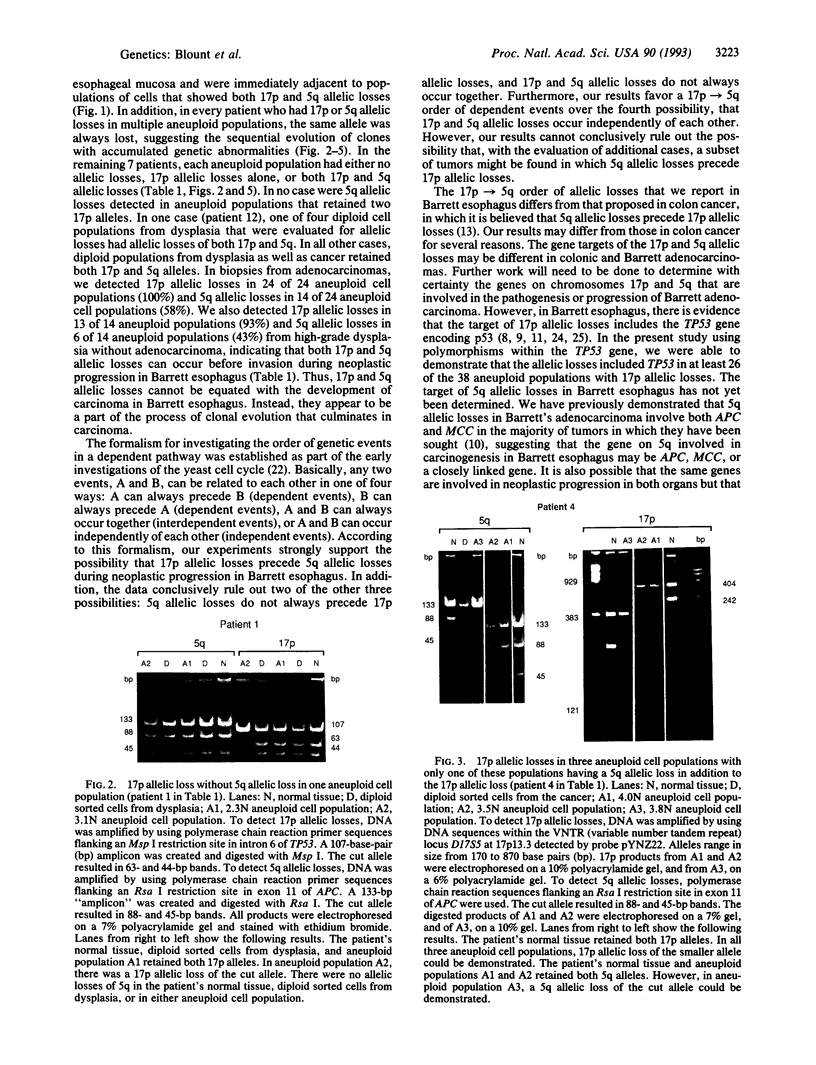
Both 17p and 5q allelic losses appear to be involved in the pathogenesis or progression of many human solid tumors. In colon carcinogenesis, there is strong evidence that the targets of the 17p and 5q allelic losses are TP53, the gene encoding p53, and APC, respectively. It is widely accepted that 5q allelic losses precede 17p allelic losses in the progression to colonic carcinoma. The data, however, supporting this proposed order are largely based on the prevalence of 17p and 5q allelic losses in adenomas and unrelated adenocarcinomas from different patients. We investigated the order in which 17p and 5q allelic losses developed during neoplastic progression in Barrett esophagus by evaluating multiple aneuploid cell populations from the same patient. Using DNA content flow cytometric cell sorting and polymerase chain reaction, 38 aneuploid cell populations from 14 patients with Barrett esophagus who had high grade dysplasia, cancer or both were evaluated for 17p and 5q allelic losses. 17p allelic losses preceded 5q allelic losses in 7 patients, both 17p and 5q allelic losses were present in all aneuploid populations of 4 patients, and only 17p (without 5q) allelic losses were present in the aneuploid populations of 3 patients. In no patient did we find that a 5q allelic loss preceded a 17p allelic loss. Our data suggest that 17p allelic losses typically occur before 5q allelic losses during neoplastic progression in Barrett esophagus.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Blount P. L., Ramel S., Raskind W. H., Haggitt R. C., Sanchez C. A., Dean P. J., Rabinovitch P. S., Reid B. J. 17p allelic deletions and p53 protein overexpression in Barrett's adenocarcinoma. Cancer Res. 1991 Oct 15;51(20):5482–5486. [PubMed] [Google Scholar]
- Boynton R. F., Blount P. L., Yin J., Brown V. L., Huang Y., Tong Y., McDaniel T., Newkirk C., Resau J. H., Raskind W. H. Loss of heterozygosity involving the APC and MCC genetic loci occurs in the majority of human esophageal cancers. Proc Natl Acad Sci U S A. 1992 Apr 15;89(8):3385–3388. doi: 10.1073/pnas.89.8.3385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casson A. G., Mukhopadhyay T., Cleary K. R., Ro J. Y., Levin B., Roth J. A. p53 gene mutations in Barrett's epithelium and esophageal cancer. Cancer Res. 1991 Aug 15;51(16):4495–4499. [PubMed] [Google Scholar]
- Davidoff A. M., Kerns B. J., Iglehart J. D., Marks J. R. Maintenance of p53 alterations throughout breast cancer progression. Cancer Res. 1991 May 15;51(10):2605–2610. [PubMed] [Google Scholar]
- Futreal P. A., Barrett J. C., Wiseman R. W. An Alu polymorphism intragenic to the TP53 gene. Nucleic Acids Res. 1991 Dec 25;19(24):6977–6977. doi: 10.1093/nar/19.24.6977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartwell L. Defects in a cell cycle checkpoint may be responsible for the genomic instability of cancer cells. Cell. 1992 Nov 13;71(4):543–546. doi: 10.1016/0092-8674(92)90586-2. [DOI] [PubMed] [Google Scholar]
- Hereford L. M., Hartwell L. H. Sequential gene function in the initiation of Saccharomyces cerevisiae DNA synthesis. J Mol Biol. 1974 Apr 15;84(3):445–461. doi: 10.1016/0022-2836(74)90451-3. [DOI] [PubMed] [Google Scholar]
- Hollstein M., Sidransky D., Vogelstein B., Harris C. C. p53 mutations in human cancers. Science. 1991 Jul 5;253(5015):49–53. doi: 10.1126/science.1905840. [DOI] [PubMed] [Google Scholar]
- Huang Y., Boynton R. F., Blount P. L., Silverstein R. J., Yin J., Tong Y., McDaniel T. K., Newkirk C., Resau J. H., Sridhara R. Loss of heterozygosity involves multiple tumor suppressor genes in human esophageal cancers. Cancer Res. 1992 Dec 1;52(23):6525–6530. [PubMed] [Google Scholar]
- Joslyn G., Carlson M., Thliveris A., Albertsen H., Gelbert L., Samowitz W., Groden J., Stevens J., Spirio L., Robertson M. Identification of deletion mutations and three new genes at the familial polyposis locus. Cell. 1991 Aug 9;66(3):601–613. doi: 10.1016/0092-8674(81)90022-2. [DOI] [PubMed] [Google Scholar]
- Kastan M. B., Onyekwere O., Sidransky D., Vogelstein B., Craig R. W. Participation of p53 protein in the cellular response to DNA damage. Cancer Res. 1991 Dec 1;51(23 Pt 1):6304–6311. [PubMed] [Google Scholar]
- Kinzler K. W., Nilbert M. C., Su L. K., Vogelstein B., Bryan T. M., Levy D. B., Smith K. J., Preisinger A. C., Hedge P., McKechnie D. Identification of FAP locus genes from chromosome 5q21. Science. 1991 Aug 9;253(5020):661–665. doi: 10.1126/science.1651562. [DOI] [PubMed] [Google Scholar]
- Kinzler K. W., Nilbert M. C., Vogelstein B., Bryan T. M., Levy D. B., Smith K. J., Preisinger A. C., Hamilton S. R., Hedge P., Markham A. Identification of a gene located at chromosome 5q21 that is mutated in colorectal cancers. Science. 1991 Mar 15;251(4999):1366–1370. doi: 10.1126/science.1848370. [DOI] [PubMed] [Google Scholar]
- Levine A. J., Momand J., Finlay C. A. The p53 tumour suppressor gene. Nature. 1991 Jun 6;351(6326):453–456. doi: 10.1038/351453a0. [DOI] [PubMed] [Google Scholar]
- Li F. P., Fraumeni J. F., Jr, Mulvihill J. J., Blattner W. A., Dreyfus M. G., Tucker M. A., Miller R. W. A cancer family syndrome in twenty-four kindreds. Cancer Res. 1988 Sep 15;48(18):5358–5362. [PubMed] [Google Scholar]
- Li F. P., Fraumeni J. F., Jr Soft-tissue sarcomas, breast cancer, and other neoplasms. A familial syndrome? Ann Intern Med. 1969 Oct;71(4):747–752. doi: 10.7326/0003-4819-71-4-747. [DOI] [PubMed] [Google Scholar]
- Livingstone L. R., White A., Sprouse J., Livanos E., Jacks T., Tlsty T. D. Altered cell cycle arrest and gene amplification potential accompany loss of wild-type p53. Cell. 1992 Sep 18;70(6):923–935. doi: 10.1016/0092-8674(92)90243-6. [DOI] [PubMed] [Google Scholar]
- Malkin D., Li F. P., Strong L. C., Fraumeni J. F., Jr, Nelson C. E., Kim D. H., Kassel J., Gryka M. A., Bischoff F. Z., Tainsky M. A. Germ line p53 mutations in a familial syndrome of breast cancer, sarcomas, and other neoplasms. Science. 1990 Nov 30;250(4985):1233–1238. doi: 10.1126/science.1978757. [DOI] [PubMed] [Google Scholar]
- Meltzer S. J., Yin J., Huang Y., McDaniel T. K., Newkirk C., Iseri O., Vogelstein B., Resau J. H. Reduction to homozygosity involving p53 in esophageal cancers demonstrated by the polymerase chain reaction. Proc Natl Acad Sci U S A. 1991 Jun 1;88(11):4976–4980. doi: 10.1073/pnas.88.11.4976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nowell P. C. The clonal evolution of tumor cell populations. Science. 1976 Oct 1;194(4260):23–28. doi: 10.1126/science.959840. [DOI] [PubMed] [Google Scholar]
- Powell S. M., Zilz N., Beazer-Barclay Y., Bryan T. M., Hamilton S. R., Thibodeau S. N., Vogelstein B., Kinzler K. W. APC mutations occur early during colorectal tumorigenesis. Nature. 1992 Sep 17;359(6392):235–237. doi: 10.1038/359235a0. [DOI] [PubMed] [Google Scholar]
- Rabinovitch P. S., Reid B. J., Haggitt R. C., Norwood T. H., Rubin C. E. Progression to cancer in Barrett's esophagus is associated with genomic instability. Lab Invest. 1989 Jan;60(1):65–71. [PubMed] [Google Scholar]
- Ramel S., Reid B. J., Sanchez C. A., Blount P. L., Levine D. S., Neshat K., Haggitt R. C., Dean P. J., Thor K., Rabinovitch P. S. Evaluation of p53 protein expression in Barrett's esophagus by two-parameter flow cytometry. Gastroenterology. 1992 Apr;102(4 Pt 1):1220–1228. [PubMed] [Google Scholar]
- Raskind W. H., Norwood T., Levine D. S., Haggitt R. C., Rabinovitch P. S., Reid B. J. Persistent clonal areas and clonal expansion in Barrett's esophagus. Cancer Res. 1992 May 15;52(10):2946–2950. [PubMed] [Google Scholar]
- Reid B. J., Blount P. L., Rubin C. E., Levine D. S., Haggitt R. C., Rabinovitch P. S. Flow-cytometric and histological progression to malignancy in Barrett's esophagus: prospective endoscopic surveillance of a cohort. Gastroenterology. 1992 Apr;102(4 Pt 1):1212–1219. [PubMed] [Google Scholar]
- Sajantila A., Puomilahti S., Johnsson V., Ehnholm C. Amplification of reproducible allele markers for amplified fragment length polymorphism analysis. Biotechniques. 1992 Jan;12(1):16, 18, 20-2. [PubMed] [Google Scholar]
- Sidransky D., Mikkelsen T., Schwechheimer K., Rosenblum M. L., Cavanee W., Vogelstein B. Clonal expansion of p53 mutant cells is associated with brain tumour progression. Nature. 1992 Feb 27;355(6363):846–847. doi: 10.1038/355846a0. [DOI] [PubMed] [Google Scholar]
- Vogelstein B., Fearon E. R., Hamilton S. R., Kern S. E., Preisinger A. C., Leppert M., Nakamura Y., White R., Smits A. M., Bos J. L. Genetic alterations during colorectal-tumor development. N Engl J Med. 1988 Sep 1;319(9):525–532. doi: 10.1056/NEJM198809013190901. [DOI] [PubMed] [Google Scholar]
- Yin Y., Tainsky M. A., Bischoff F. Z., Strong L. C., Wahl G. M. Wild-type p53 restores cell cycle control and inhibits gene amplification in cells with mutant p53 alleles. Cell. 1992 Sep 18;70(6):937–948. doi: 10.1016/0092-8674(92)90244-7. [DOI] [PubMed] [Google Scholar]
- van Leeuwen C., Tops C., Breukel C., van der Klift H., Fodde R., Khan P. M. CA repeat polymorphism at the D5S299 locus linked to adenomatous polyposis coli (APC). Nucleic Acids Res. 1991 Oct 25;19(20):5805–5805. doi: 10.1093/nar/19.20.5805-a. [DOI] [PMC free article] [PubMed] [Google Scholar]