Abstract
Generation of the auditory steady state responses (ASSR) is commonly explained by the linear combination of random background noise activity and the stationary response. Based on this model, the decrease of amplitude that occurs over the sequential averaging of epochs of the raw data has been exclusively linked to the cancelation of noise. Nevertheless, this behavior might also reflect the non-stationary response of the ASSR generators. We tested this hypothesis by characterizing the ASSR time course in rats with different auditory maturational stages. ASSR were evoked by 8-kHz tones of different supra-threshold intensities, modulated in amplitude at 115 Hz. Results show that the ASSR amplitude habituated to the sustained stimulation and that dishabituation occurred when deviant stimuli were presented. ASSR habituation increased as animals became adults, suggesting that the ability to filter acoustic stimuli with no-relevant temporal information increased with age. Results are discussed in terms of the current model of the ASSR generation and analysis procedures. They might have implications for audiometric tests designed to assess hearing in subjects who cannot provide reliable results in the psychophysical trials.
Key words: auditory temporal processing, evoked potential, habituation, stationary response, rats
Introduction
Auditory steady state responses (ASSR) are periodic electrical brain oscillations evoked by acoustic stimuli sinusoidally modulated in amplitude-and/or frequency.1,2 Traditionally, the extraction of these responses from the measured signal rely on averaging across stimulus-locked epochs of the raw data. Although successful, such a manipulation assumes that electrophysiological signals represent a linear superposition between the random ongoing background and the highly stereotyped and repeatable auditory response.
In practice, the amplitude of the ASSR progressively decreases over the sequential averaging of epochs.3,4 This phenomenon has been associated with relatively high contribution of the un-averaged noise to the measurements in the first epochs of the recording, which is gradually attenuated as the electrical signal is averaged in the time-domain.1,3,5-9
Alternatively to the noise cancelation hypothesis, the time-dependent decrease of the ASSR amplitude might represent the non-stationary response of the ASSR generators. Specifically, it could be a consequence of the progressive reduction of the synchronic response of the ASSR neural generators following the high presentation rate of acoustic stimuli. This phenomenon can be referred as habituation of the ASSR.
Habituation of the ASSR might have implications for the clinical practice. So far, methodologies for optimizing the detection of ASSR have included the use of different types of stimulus and averaging procedures.3,10,11 In addition, the use of multichannel electroencephalogram recordings and appropriate statistical tests for different samples of stimulus-related epochs has been evaluated.12-14 Recently, attention has been focused on other factors affecting the detection of the ASSR such as the optimum recording length and the stopping criteria of the recordings.4-8,15 The habituation of the response might be then a source of amplitude variability when different acquisition protocols are applied. The non-stationary behavior of the ASSR would lead to estimate different response amplitudes depending on both the number of averaged epochs and the location of those epochs in the recording. This brings the need of analyzing not just the appropriate length of the recording and averaging stopping criteria but also when the averaging should be started. In other words, it will be crucial to decide whether the epochs at which the ASSR habituation is present should be considered or not for the estimation of the response.
Habituation of the ASSR cannot be distinguished from noise cancelation during the standard sequential averaging of epochs. It is necessary to obtain a reliable response for any given epoch independently of the electrical activity of the preceding segments to test the habituation of the evoked potential. When obtained independently, similar response amplitudes across epochs would strongly support the strict stationary behavior of the ASSR. Alternatively, a time-dependent decrease of amplitude might account for the habituation of the response. The primary aim of current study was thoroughly characterize the time course of the ASSR amplitude, using rats as an experimental model. In addition, experiments were performed to test whether the non-stationarity of the ASSR represents physiological processes involved in the coding of a highly repeatable auditory input rather than the decreased ability to respond to sustained acoustic stimuli. Changes in the habituation of the ASSR during maturation were also investigated. Finally, methodological implications of the ASSR habituation were tested based on detections paradigms proposed for the clinical practice.
Materials and Methods
Experimental subjects
Pregnant Wistar rats were monitored to determine the date of birth (defined as postnatal day 0, P0). Auditory responses were obtained from post-hearing onset animals at 15 P, 20 P, 25 P, 35 P, 50 P or 70 P (N=8). This selection was based on previous studies regarding the maturation of the brainstem auditory system of rats using electrophysiological approaches.16,17 Hearing onset of rats occurs at 14 P approximately and they can be considered adults regarding hearing at 70 P.16
Animals were housed in a standard bio-clean animal room under a 12-h light-dark cycle at 22-24°C, with free access to food and tap water. To perform the recordings, animals were anesthetized with ketamine (75.0 mg/kg, ip) and diazepam (5.0 mg/kg, ip). Supplemental doses of anesthesia were administered during the experiment at a level sufficient to maintain the animal in an areflexic state. Atropine sulfate (0.06 mg/kg; im) was administered to decrease the mucosal secretions. Body temperature was maintained at 37.0±0.1°C by a body temperature control system (Bioseb, model LE-6400).
The present study was performed under approval of the Animal Research and Ethics Committee of the Cuban Neuroscience Center, conformed to the guidelines of the National Center for Animal Breeding of Cuba.
Acoustic stimuli
Sinusoidally amplitude modulated tones were generated using the ASSR software module18 of an AUDIX system (Neuronic S.A., Havana, Cuba) and presented monaurally via an ER 3A Etymotic Research insert earphone. Original foam inserts were replaced by custom-fitted ear molds to permit the earphone to be coupled to the rat s ear. The stimulus signal consisted of a carrier tone of 8 kHz modulated in amplitude (95% depth) at a rate of 115 Hz. This carrier tone was selected since previous rat studies have demonstrated a peak of sensitivity at 8 kHz.19-21 This carrier and others lower than 8 kHz have been used to study the ASSR in rats.22-24 For characterizing the ASSR habituation in adult animals, stimulus of 30, 50 and 70 dB SPL were applied. Furthermore, stimulation with a fixed intensity of 30 dB above the ASSR threshold was applied to individuals of every age bracket. The latter procedure was performed to better characterize the age-related change in the ASSR habituation in animals with different sensitivity to the stimulation. All the acoustic levels are referred to a Brüel & Kjær artificial ear (type 4152). Calibration was performed using a Brüel & Kjær 2250 sound level meter (Brüel & Kjær 4144 microphone).
Recordings
Electrophysiological responses were recorded differentially using stainless-steel needle electrodes inserted subdermally (vertex positive; neck negative; thorax ground). Recordings were amplified with gain 1.2×104 and band-pass filtered (10-300 Hz). Output of the filter was digitized at 16-bit of resolution and sampled at 920 Hz.
For every animal, thirty recordings were obtained for each type of stimulus. Every recording was preceded by a no-stimulation period of five minutes. Recordings consisted of 30 stimulus-related epochs of 8.90 s (8192 time points each). Epochs were divided in 16 segments of 0.88 s (812 points each). Segments with peaks of electrical oscillations exceeding 50 µV were rejected online. Typically, fewer than five segments per recording were rejected and they were randomly distributed across epochs. Data acquisition continued until completing 30 artifact-free epochs.
Estimation of the auditory steady state responses habituation
To test the habituation of the ASSR, the amplitude of the evoked potential was calculated for each epoch, independently of the preceding electrical activity. The data analysis procedure was as follows. A data matrix was constructed offline with the epochs of the 30 recordings. Averaging across epochs of the same recording was not carried out. Instead, epochs corresponding to the same time window in the different recordings were averaged in the time-domain (Figure 1). Since these epochs can be considered as independent events, averaging improved the signal-to-noise ratio without implying a possible loss of information. This procedure allowed a reliable estimation of the ASSR amplitude at any given time-windows independently of the preceding electrical activity. To measure the amplitude of these independent responses, the brain wave activity of the each epoch was transformed into the frequency domain using the fast Fourier transform (FFT). The spectral amplitude obtained at 115 Hz (modulation frequency of the stimulus) was considered as the amplitude of the ASSR. The amplitude of the residual noise level (RNL) was estimated using 60 spectral components at each side of the frequency of the response, corresponding to the frequency range between 111.8 Hz and 118.2 Hz. ASSR and RNL amplitudes were compared by using the Hotelling T2 test, implemented in the AUDIX system.25 To describe the temporal course of the ASSR, the amplitude of the independent responses were plotted as a function of time. Negative exponential functions were fitted to the time courses (requiring r2>0.85, and P<0.05 to consider a valid fitting). The percent of habituation (Phab) of the response was calculated using the equation:
| Phab=100 (Ampmax - Amphab) / Ampmax | (1) |
where:
Ampmax represents the maximum amplitude of the curve; and
Amphab represents the magnitude of the response obtained after the ASSR amplitude reached the asymptotic level (defined as the amplitude estimated when the recording length was three times the time constant of the fitted exponential function).
Figure 1.
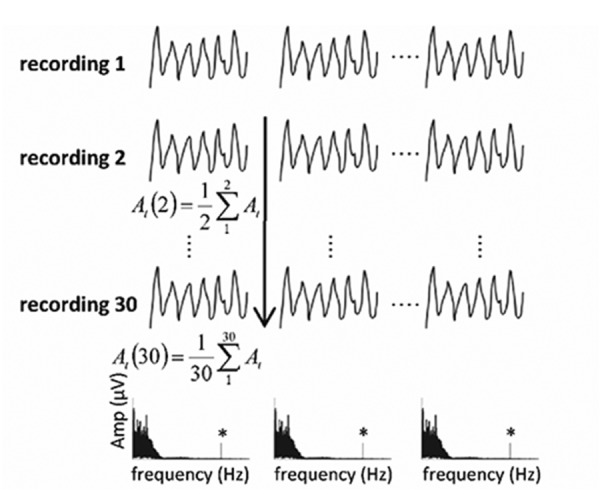
Representative diagram illustrating the analysis of the auditory steady state recordings. A data matrix composed of 30 recordings of 30 epochs each is represented. Fast Fourier transform was applied to the individual epochs obtained after averaging of the epochs corresponding to the same time window across the 30 recordings. The vertical line represents the direction of the averaging.
A one-way ANOVA (P<0.05) and corresponding post-hoc analyses (Tukey test, P<0.05) were performed to analyze the effect of the stimulus intensity on the percent of habituation (Phab) of the ASSR. The same statistical analysis was carried out to study the changes in the Phab during maturation. In addition, a bi-factorial ANOVA was performed to analyze the ASSR amplitude as a function of the type of response (independent vs. averaged responses) and the recording length.
Habituation and deviant stimulation
Two stimulus manipulations were implemented in a customized version of the NEURONIC A 6.0 system26 to test whether the habituation of the ASSR represented a decreased ability to respond to a sustained acoustic stimulation. Adult animals were stimulated with a given combination of stimulus parameters (carrier, intensity and modulation rate). After 133.57-s of stimulation (corresponding to 15 epochs of the recording), either intensity or modulation rate were changed in a single step to a new value. The new stimulation was also maintained over 133.57-s. The intensity was increased from 50 to 60 dB SPL whereas modulation rate was stepped from 93.103 to 108.621 Hz. Recordings parameters of the NEURONIC A 6.0 system were set to the same values as those described for the AUDIX system. Thirty recordings were obtained for each combination of standard-deviant stimuli. The time course of the ASSR independent responses was constructed as described in previous sections.
Auditory steady state responses habituation and amplitude estimation in averaged responses
Furthermore, the influence of ASSR habituation on the amplitude estimated using the classical time-domain averaging of epochs along a recording was analyzed. Based on previous studies,5-8 a fixed number of epochs of every recording obtained when the animals were stimulated with tones of 50 dB SPL were sequentially averaged. The total number of averaged epochs in each recording was 2, 4, 8, 16 and 30. These corresponded to test times of 17.8, 35.6, 71.2, 142.4 and 267.0 s, respectively. In addition, a delay between the stimulus onset and the beginning of the averaging was introduced to exclude from the analysis a predefined number of epochs. The delay was increased from 0 to 124.6 s in steps of 8.90 s. This latter duration corresponded to the duration of one epoch of the recording whereas 124.6 s corresponded to the duration of fourteen epochs. The time series of every epoch was transformed into the frequency domain using the FFT and both the ASSR amplitude and the RNL were estimated as described earlier. This means that statistical testing was repeated after each subsequent epoch was added to the sample. The time course for both the ASSR and the RNL were then constructed. The response amplitude calculated for the first epoch of the recording was statistically analyzed as a function of the delay (one-way ANOVA) when the recording had a fixed length of 16 epochs. Finally, the ASSR estimated after completion of averaging was analyzed as a function of both the delay and the recording length using a bi-factorial ANOVA. Corresponding post-hoc analyses (Tukey test, P<0.05) were performed.
Results
Habituation of the auditory steady state responses
Figure 2 illustrates waveforms and spectrums of different independent epochs of a representative recording, obtained when the ASSR was evoked by 70 dB SPL stimulation. Clearly defined amplitude peak were observed for every epoch. Of note, although the response calculated for a given epoch did not depend on that obtained in previous segments, the ASSR amplitude estimated for the first epoch was systematically higher than the magnitude of the evoke potential calculated in the following segments. No differences in the ASSR amplitude were typically visualized between the fifth and the last epoch. Furthermore, small and relatively constant background noise levels were obtained when different epochs were compared.
Figure 2.
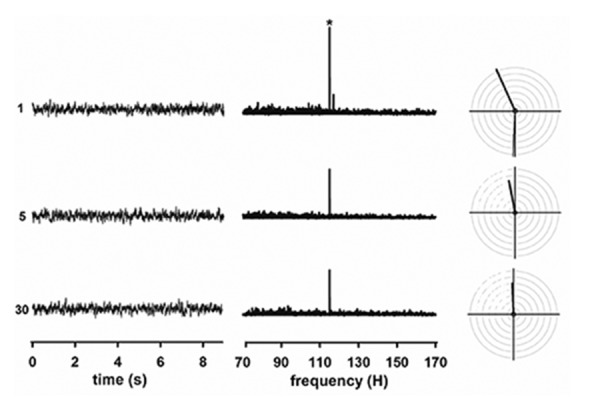
Independent responses evoked by 8-kHz tones of 70 dB SPL in a representative adult rat. Each independent auditory steady state responses (ASSR) was obtained by averaging the 30 epochs corresponding to the same time window across the recordings. Left panels represent the waveform of selected epochs. The spectra obtained after applying the fast Fourier transform are presented in the middle panels. In the polar plots presented in the right panels, the independent ASSR amplitudes are reflected as the length of the vector, while the radius of the small black circle with epicenter in the axes origin corresponds to the mean spectral amplitude of the background noise.
Amplitude of the independent ASSR responses exponentially decreased following the stimulus onset and reached the asymptotic level after 26.7-s of stimulation (corresponding to a recording length of three epochs) (Figure 3 left panels). This behavior was elicited by every stimulus intensity, although statistically significant larger percents of habituation (Phab) were obtained as the level of the tone progressively increased from 30 up to 70 dB SPL (F(2,21)=79.4, P<0.05).
Figure 3.
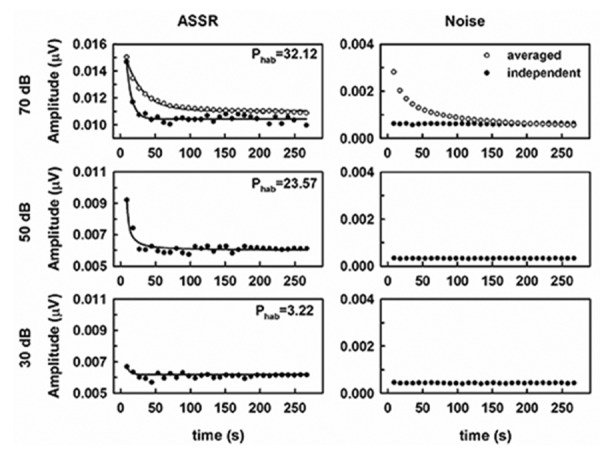
Mean time course of the independent auditory steady state responses (ASSR) amplitude (left panels) and the background noise (right panels) obtained in adults animals (N=8) stimulated with 8-kHz tones of different intensities. The mean percent of habituation (Phab) is represented in the top right corner of each chart. Mean time course of the averaged ASSR is also shown in response to tones of 70 dB SPL. For clarity, error bars of the plots are not shown. Line plots in the left panels represent the decreased exponential fits of the ASSR independent amplitudes versus the stimulation time.
The time course of the independent responses differed from that obtained for the averaged responses. Independent ASSR responses typically showed sharper and more pronounced drop-offs in amplitude than those obtained for averaged responses (Figure 3 left, upper panel). As expected, the differences between independent and averaged amplitudes decreased as the length of the recording increased.
Furthermore, similar time courses of RNL were obtained for the independent responses elicited by the three different stimulus intensities. As expected, the sequential averaging of epochs resulted in the exponential decrease of the background noise (Figure 3 right, upper panel). Therefore, statistically significant differences in the RNL time course between the type of responses were evident (F(1,280)=475.8, P<0.05). However, the RNL of the averaged epochs closely resembled that of the independent responses when the stimulation period exceeded 133.57 s (corresponding to 15 recording epochs) (F(29,280)=37.5 P<0.05). A statistically significant interaction between the type of response and the recording length was found (F(29,280)=37.6 P<0.05).
Dishabituation of the auditory steady state responses to novel acoustic stimuli
When a 10-dB intensity increment was presented following 133.57-s of stimulation with a 50-dB SPL tone, restoration to full strength of the independent ASSR amplitude was observed (Figure 4 upper panel). In other words, changing the stimulation level resulted in the dishabituation of the auditory response. Typically, the higher intensity of the deviant stimulus resulted in higher ASSR amplitudes at any given time point. Although slightly slower than the elicited by the standard stimulation, habituation to the deviant intensity was evident when the new tone was presented steadily.
Figure 4.
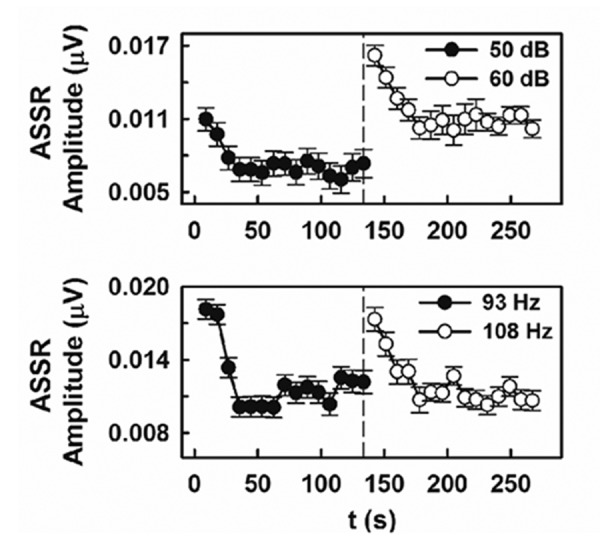
Mean time course of the independent auditory steady state responses (ASSR) amplitude obtained in adult rats when presentation of the standard 8-kHz tone was followed by a deviant stimulus. The deviant stimulus consisted of a 10-dB increase in intensity (upper panel) and a shift in the amplitude modulation frequency of the stimulation (bottom panel). Plots are represented as the mean (symbol)±standard error (vertical bars).
A marked dishabituation also resulted from shifting the modulation rate from 93 to 108 Hz (Figure 4 bottom panel). Similar to the intensity deviant, sustained stimulation with the new modulation frequency elicited the habituation of the response. In this case, however, the rate of the habituation did not differ between the deviant and standard stimuli.
Changes of the auditory steady state responses habituation during maturation
The independent amplitude of the ASSR increased as animals became adults, when the 8 kHz tones were delivered at a level 30 dB above threshold (Figure 5A). Furthermore, the exponential decrease of the independent ASSR amplitude previously described for adult animals was also evident in the rest of the age brackets. However, the habituation magnitude of the evoked potential depended on the maturational stage of the animals (Figure 5B). Habituation significantly increased within the 35 days that followed the hearing onset (F(5,45)= 7.87, P <0.05), whereas changes obtained between 50 P and adult animals were not statistically significant (Figure 5C).
Figure 5.
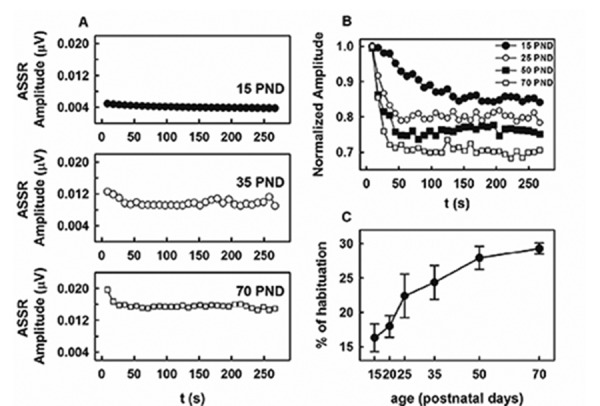
Age-related changes of the auditory steady state responses (ASSR) habituation of rats. (A) Typical time course of the ASSR independent amplitude evoked to stimulus of 30 dB above the ASSR threshold in animals of different ages. (B) Time course of the normalized amplitudes. (C) Changes in the magnitude of the ASSR habituation during maturation. Plot represents the mean (symbol)±standard error (vertical bars) for N=8.
Habituatiovnersusaveraged auditory steady state responses amplitudes
As noted earlier, the time course of the ASSR obtained during the typical sequential averaging of consecutive epochs of the recording was characterized by the progressive decrease of the response amplitude, mainly during the first few epochs (Figure 6A). However, the time course of the response dramatically changed as the beginning of the averaging was progressively delayed.
Figure 6.
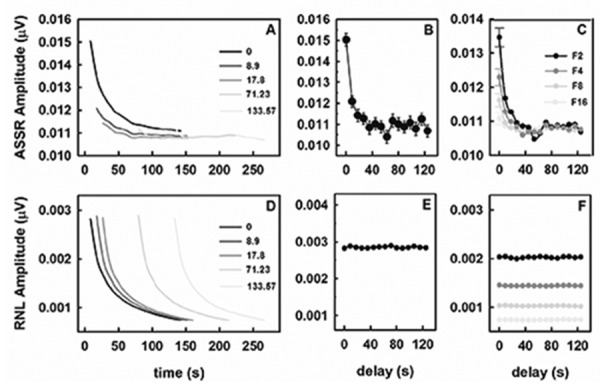
Auditory steady state responses (ASSR) of rats as a function of the delay of the standard time-domain averaging onset. All responses were evoked by 8-kHz tones of 50 dB SPL. The time course of the ASSR amplitude and the residual noise level (RNL) obtained for different delays in a representative adult animal are shown in (A) and (D), respectively. (B) and (E) present the effect of the delay on the ASSR amplitude and the RNL estimated in the first epoch included in the analysis, respectively. (C) and (F) show the effect of the delay on the ASSR amplitude and the RNL obtained after completing the averaging, respectively. The plot in (B) and (C) represents the mean (symbol)±standard error (vertical bar) across the eight animals. For clarity, vertical bars are only represented in (C) for a delay=0 s.
Delaying the beginning of the sequential averaging resulted in statistically significant changes in the amplitude of the response calculated for the first epoch included in the analysis (F(14,105)=64.9, P<0.001) (Figure 6B). The ASSR amplitude estimated for this epoch significantly decreased when the time between the stimulus onset and the beginning of the averaging was increased from 0 s to 8.9 to 17.8 s. When these delays were applied, the recording contained 1, 2 and 3 epoch, respectively. The response amplitudes did not varied significantly for longer delays. More importantly, the ASSR amplitude obtained after completing the averaging depended on both the delay and the recording length (F(14,420)=37.4, P<0.001 and F(3,420)=17.1, P<0.001 for the effect of the delay and the recording length, respectively). In addition, interactions between these parameters were statistically significant (F(42,420)=5.8, P<0.001). Typically, the amplitude of the evoked potential estimated after completing the averaging decreased as the beginning of the averaging was delayed (Figure 6C). At very short delays, significantly smaller ASSR amplitudes were obtained as the recording length was increased. Amplitude of the response did not significantly depend on the recording length for delays longer than 26.7s, a time equivalent to reject the three initial epochs from the averaging.
Unlike the ASSR amplitude, similar time courses of the RNL were obtained independently of the averaging delay when the recordings had equal length (Figure 6D). This result was evident analyzing the RNL of both the first epoch included in the averaging (Figure 6E) and that obtained after completing the analysis (Figure 6F). As expected, markedly lower RNL were obtained as the recording length increased.
Discussion
The main goal of the present study was testing one of the major theoretical principles for the acquisition of the ASSR: the stationarity of the response. Using rats as an experimental model, we found that: i) the ASSR habituate to on-going stimulation; ii) dishabituation occurs in response to deviant acoustic stimuli; and iii) the magnitude of the ASSR habituation depends on the maturational stage of the individual. From a methodological point of view, our findings demonstrate that different ASSR amplitudes can result in clinical practice depending on whether epochs at which habituation occurs are included or excluded from the analysis of the electrophysiological signal.
Auditory steady state responses habituation and auditory steady state responses generators
Evidence to support the habituation of auditory evoked potentials mainly arises from analyzing the effect of the stimulation rate on the amplitude and latency of transient evoked responses, including the auditory brainstem responses (ABR).27-30 In fact, the analysis of ABR elicited by high rate presentation of acoustic stimuli has been proposed as a tool for improving the detection of central neurological disorders.31-34 in that sense, several methods have been implemented to improve the resolution and interpretability of the response.35,36 These studies, however, have mainly focused on the averaged response and not on what we have defined here as independent response. Regarding ASSR, only few studies have analyzed on the time course of the recording.3,4,6,8 They have demonstrated the exponential decrease of the ASSR amplitude during the sequential averaging; a finding which has been explained by the reduction of noise across the averaged epochs. The aforementioned processes are also evident from our data (Figure 3 right, upper panel). However, our findings cannot be taken as a proof of the linear relation between the background noise and the ASSR amplitude. If the contribution of the un-averaged background noise could completely explain the progressive decrease of the ASSR, a relatively flat time course of independent response amplitudes would be expected. Yet, our findings show that a negative exponential decrease of the ASSR independent amplitude occurred (Figure 3 left panels) while the RNL did not vary among independent epochs (Figure 3 right panels). Taken together, these results demonstrate that the amplitude of the electrophysiological response decreased during the first seconds of stimulation independently of the noise cancelation. Therefore, more realistic models of the ASSR need to incorporate the non-stationary evoked activity of the neural generators. The fall of the ASSR amplitude observed during the standard sequential averaging of epoch should not be exclusively interpreted as a lower contribution of the background noise in the subsequent segments but a combination of both noise cancelation and habituation of the neural response. As proposed for other physiological systems,37-39 the habituation of the ASSR might protect the higher cortical centers from being flooded with irrelevant information and prevent stimulus overload by filtering redundant sensory input. Sub-cortical structures are the main generators of the ASSR evoked by acoustic stimuli modulated in amplitude in the range of 90-190 Hz. This is true for rats23,40 and other mammalian species including humans.41-44 Considering that the ASSR amplitude is related to the number of neurons with phase-locked responses to the stimulation, it can be hypothesized that the habituation of the ASSR represents a decrease in the response of the neural population responding synchronously to the repetitive stimulation in the auditory brainstem.
It is important to note that the ASSR amplitude dishabituates in response to deviant stimuli (Figure 4). This suggests that the non-stationary behavior of the ASSR amplitude might reflect the activity of negative feedback loops involved in the coding of the repetitive stimulation rather than a decreased ability of the system to respond to the long-term acoustic stimulation. This idea is supported by results that demonstrate that habituation of the sub-cortical auditory neurons is part of intrinsic mechanisms for detecting novel sounds in the acoustic environment.44-47 Although tentative, the aforementioned hypothesis needs further testing. From one side, the increase of amplitude elicited by increasing-intensity deviants may imply the recruitment of neurons, which were likely inactive during the presentation of the standard stimulus. From the other side, the ASSR amplitude elicited by 8-kHz tones modulated in amplitude at 93 Hz differs from that obtained when the modulation frequency is 108 Hz.23 Therefore, different neural populations might generate the responses to the standard and deviant stimuli described in the current research. Simultaneous local field recordings from auditory brainstem and thalamic nuclei are then needed to gain insight into how ASSR generations recover from habituation.
Auditory steady state responses habituation during maturation
The increase of the ASSR amplitude with age described in the current study (Figure 5A) is in agreement with previous findings in rats23,24 and humans.48-50 Developmental changes in ASSR amplitudes can reflect an increase in the number of responsive auditory brainstem and thalamic neurons to high frequency modulation. This process is likely complemented by the increased ability of the neurons to phase lock to the stimulus envelope. This idea is supported by results demonstrating that the range of best modulation frequency of single neurons in the inferior colliculus of gerbils expands to higher modulation frequencies during maturation and that the modulation frequency for which the greatest number of neurons is tuned increases as animals mature.51 In the medial geniculate body of rats, neurons better synchronize to high frequency current pulse stimulation as animals become adults.24
Because stimuli with equal intensity relative to an individual subject’s ASSR threshold were used, the greater percent of habituation obtained as animals matured (Figure 5B and C) is not likely associated with changes in sensitivity to the carrier tone during maturation. According to the ASSR habituation hypothesis stated earlier, greater habituation during maturation might reflect the increased ability of the sub-cortical neural circuits to filter acoustic stimuli with no-relevant temporal information. Similar to what has been described for the developing of tonotopic maps in central structures,51-53 this process might result from the refinement of the inhibitory connections present in these negative feedback loops.
Methodological implications
The habituation of the ASSR might have important implications for the use of this kind of response for both research and medical purposes. Recent studies focused on the influence of the recording protocol on the detection of the ASSR8 have suggested that a minimum of eight epochs should be recorded to rule out the unpredictable behavior of ASSRs in the beginning of a recording. In addition, it has been described that increasing the duration of the test improves the detection of the ASSR since a better signal to noise ratio is obtained as averaging occurs.1,4,7,9
Our findings contribute to a better understanding of the behavior of the ASSR during the first seconds of stimulation. In the early parts of the recording, in contrast to the RNL, the ASSR time course changes as the averaging of epochs is delayed (Figure 5A and D). In addition, the ASSR amplitude estimated for the first epoch included in the analysis decreases while the RNL does not vary (Figure 5B and E). Taken together, these results demonstrate that the ASSR behavior during the first epochs of a typical recording in a clinical setting is a consequence of the habituation of the neural response.
On the other hand, our findings further demonstrate that the amplitude of the ASSR became independent of the recording length when averaging starts approximately 30 s after the stimulus onset - time equivalent to reject the first three epochs in our recording procedure (Figure 5C). For shorter delays, the effect of the habituation on the ASSR amplitude decreases as the recording length is prolonged. Thereby, the introduction of a delay between the stimulus onset and the beginning of the sequential averaging could be methodologically convenient for the detection of the ASSR. This procedure implies that the estimation of the response would be performed by analyzing only the region of the recording for which the response can be strictly considered as stationary. However, to delay the beginning of the sequential averaging implies estimating the ASSR amplitude based exclusively on the habituated response. It also implies excluding the time window for which the psychophysical auditory response is analyzed in the audiology practice. Alternatively, estimations of the ASSR might be performed by implementing recording procedures that avoid the habituation of the neural response (Appendix Figure 1).
Conclusions
The decrease of the ASSR amplitude along the stimulation period is related to both the cancelation of the background noise of the recording and the habituation of the neural population generating the response. Habituation of the ASSR increase during the maturation of the auditory pathway, suggesting that the ability to filter acoustic stimuli with norelevant temporal information increase with age. From a clinical point of view, to delay the beginning of the signal averaging might be methodologically convenient to rule out the period at which habituation occurs. The latter could be relevant for developing objective audiometric tests designed to assess the supra-threshold hearing, especially those aimed to estimate subjective parameters necessary for adjusting or monitoring the performance of hearing aids in newborns, infants and other populations who cannot actively cooperate in the behavioral procedures.
Acknowledgements
The authors are especially thankful to Teresa Diez, Alejandro Torres-Fortuny, Jose A. Gaya and Maria C. Perez-Abalo, who made important contributions to this work.
Funding Statement
Funding: this work was partially supported by Centro Interdisciplinario de Neurociencia de Valparaíso CONICYT ICM- P09-022-F, ANILLO-ACT1104 and FONDECYT 1130855 to Agustín D. Martínez and Pavel Prado-Gutierrez. Funding was also provided by Proyecto PNCT, Cuban Neuroscience Center.
References
- 1.Picton TW, John MS, Dimitrijevic A, Purcell D. Human auditory steady-state responses. Int J Audiol 2003;42:177-219. [DOI] [PubMed] [Google Scholar]
- 2.Regan D. Human brain electrophysiology: evoked potentials and evoked magnetic fields in science and medicine. Amsterdam: Elsevier; 1989. pp 35. [Google Scholar]
- 3.John MS, Picton TW. MASTER: a Windows program for recording multiple auditory steady-state responses. Comput Methods Programs Biomed 2000;61:125-50. [DOI] [PubMed] [Google Scholar]
- 4.Torres-Fortuny A, Perez-Abalo MC, Sotero-Díaz RC, Rioja Rodríguez L, Rodríguez Dávila E, Galán García L, et al. Stopping criteria for averaging the multiple auditory steady-state response. Acta Otorrinolaringol Esp 2011;62:173-80. [DOI] [PubMed] [Google Scholar]
- 5.Choi JM, Purcell DW, John MS. Phase stability of auditory steady state responses in newborn infants. Ear Hear 2011;32:593-604. [DOI] [PubMed] [Google Scholar]
- 6.John S, Purcell D, Dimitrijevic J, Picton TW. Advantages and caveats when recording steady-state responses to multiple simultaneous stimuli. J Am Acad Audiol 2002;13:246-59. [PubMed] [Google Scholar]
- 7.Luts H, Desloovere C, Kumar A, Vandermeersch E, Wouters J. Objective assessment of frequency-specific hearing thresholds in babies. Int J Pediatr Otorhinolaryngol 2004;68:915-26. [DOI] [PubMed] [Google Scholar]
- 8.Luts H, Van Dun B, Alaerts J, Wouters J. The influence of the detection paradigm in recording auditory steady-state responses. Ear Hear 2008;29:638-50. [DOI] [PubMed] [Google Scholar]
- 9.Luts H, Wouters J. Hearing assessment by recording multiple auditory steady-state responses: the influence of test duration. Int J Audiol 2004;43:471-78. [DOI] [PubMed] [Google Scholar]
- 10.Cebulla M, Sturzebecher E, Elberling C. Objective detection of auditory steady-state responses: comparison of one-sample and q-sample tests. J Am Acad Audiol 2006;17:93-103. [DOI] [PubMed] [Google Scholar]
- 11.Elberling C, Don M, Cebulla M, Stürzebecher E. Auditory steady-state responses to chirp stimuli based on cochlear traveling wave delay. J Acoust Soc Am 2007;122:2772-85. [DOI] [PubMed] [Google Scholar]
- 12.Stürzebecher E, Cebulla M. Automated auditory response detection: Improvement of the statistical test strategy. Int J Audiol 2013;52:861-4. [DOI] [PubMed] [Google Scholar]
- 13.Stürzebecher E, Cebulla M, Elberling C. Automated auditory response detection: Statistical problems with repeated testing. Int J Audiol 2005;44:110-7. [DOI] [PubMed] [Google Scholar]
- 14.Van Dun B, Wouters J, Moonen M. Improving auditory steady-state response detection using independent component analysis on multi-channel EEG data. IEEE Trans Biomed Eng 2007;54:1220-30. [DOI] [PubMed] [Google Scholar]
- 15.D’haenens W, Vinck BM, Maes L, Bockstael A, Keppler H, Philips B, et al. Determination and evaluation of clinically efficient stopping criteria for the multiple auditory steady-state response technique. Clin Neurophysiol 2010;121:1267-78. [DOI] [PubMed] [Google Scholar]
- 16.Blatchley BJ, Cooper WA, Coleman JR. Development of auditory brainstem response to tone pip stimuli in the rat. Dev Brain Res 1987;32:75-84. [DOI] [PubMed] [Google Scholar]
- 17.Church MW, Williams HL, Holloway JA. Postnatal development of the brainstem auditory evoked potential and far-field cochlear microphonic in non-sedated rat pups. Brain Res 1984;316:23-31. [DOI] [PubMed] [Google Scholar]
- 18.Perez-Avalo MC, Savio G, Torres A, Martín V, Rodríguez E, Galán L. An optimized method to test frequency specific thresholds in hearing impaired children and normal subjects. Ear Hear 2001;22:200-11. [DOI] [PubMed] [Google Scholar]
- 19.Heffern H, Masterton B. Hearing in glires: domestic rabbit, cotton rat, feral house mouse, and kangaroo rat. J Acoust Soc Am 1980;68:1584-99. [Google Scholar]
- 20.Heffner HE, Heffner RS, Contos C, Ott T. Audiogram of the hooded Norway rat. Hear Res 1994;73:244-7. [DOI] [PubMed] [Google Scholar]
- 21.Kelly JB, Masterton B. Auditory sensitivity of the albino rat. J Comp Physiol Psychol 1977;91:930-6. [DOI] [PubMed] [Google Scholar]
- 22.Perez-Alcazar M, Nicolas MJ, Valencia M, Alegre M, Iriarte J, Artieda J. Chirp-evoked potentials in the awake and anesthetized rat. A procedure to assess changes in cortical oscillatory activity. Exp Neurol 2008;210:144-53. [DOI] [PubMed] [Google Scholar]
- 23.Prado-Gutierrez P, Mijares E, Savio G, Borrego M, Martínez-Montes E, Torres A. Maturational time course of the Envelope Following Response to amplitude-modulated acoustic signals in rats. Int J Audiol 2012;51:309-16. [DOI] [PubMed] [Google Scholar]
- 24.Venkataraman Y, Bartlett EL. Postnatal development of auditory central evoked responses and thalamic cellular properties. Dev Neurobiol 2014;74:541-55. [DOI] [PubMed] [Google Scholar]
- 25.Valdes JL, Perez-Abalo MC, Martin V, Savio G, Sierra C, Rodriguez E, et al. Comparison of statistical indicators for the automatic detection of 80 Hz auditory steady-state response. Ear Hear 1997;18:420-9. [DOI] [PubMed] [Google Scholar]
- 26.Pérez-Abalo MC, Rodríguez E, Sánchez M, Santos E, Torres-Fortuny A. New system for neonatal hearing screening based on auditory steady state responses. J Med Eng Technol 2013;37:368-74. [DOI] [PubMed] [Google Scholar]
- 27.Budda TW, Nakamuraa T, Fulhamb WR, Todd J, Schall U, Hunter M, et al. Repetition suppression of the rat auditory evoked potential at brief stimulus intervals. Brain Res 2013;1498:59-68. [DOI] [PubMed] [Google Scholar]
- 28.Hecox K, Cone B, Blaw M. Brainstem auditory evoked response in the diagnosis of pediatric neurologic diseases. Neurology 1981;31:832-40. [DOI] [PubMed] [Google Scholar]
- 29.Jiang Z, Brosi D, Wilkinson A. Auditory neural responses to click stimuli of different rates in the brainstem of very preterm babies at term. Pediatr Res 2002;51:454-9. [DOI] [PubMed] [Google Scholar]
- 30.Westenberg IS, Weinberger NM. Evoked potential decrements in auditory cortex. ii. Critical test for habituation. Electroencephalogr Clin Neurophysiol 1976;40:356-69. [DOI] [PubMed] [Google Scholar]
- 31.Debruyne F. Influence of age and hearing loss on the latency shifts of the auditory brainstem response as a result of increased stimulus rate. Audiology 1986;25:101-6. [DOI] [PubMed] [Google Scholar]
- 32.Gerling I, Finitzo H. Auditory brainstem response with high stimulus rates in normal and patient populations. Ann Otol Rhinol Laryngol 1983;92:119-23. [DOI] [PubMed] [Google Scholar]
- 33.Shanon E, Gold S, Himmelfarb M. Assessment of functional integrity of brain stem auditory pathways by stimulus stress. Audiology 1981;20:65-71. [DOI] [PubMed] [Google Scholar]
- 34.Stockard J, Sharbrough F. Detection and localization of occult lesions with brainstem auditory responses. Mayo Clin Proc 1977;52:761-9. [PubMed] [Google Scholar]
- 35.Jewett DL, Caplovitz G, Baird B, Trumpis M, Olson MP, Larson-Prior LJ. The use of QSD (q-sequence deconvolution) to recover superposed, transient evoked-responses. Clin Neurophysiol 2004;115:2754-75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Valderrama JT, Alvarez I, de la Torre A, Segura JC, Sainz M, Vargas JL. Recording of auditory brainstem response at high stimulation rates using randomized stimulation and averaging. J Acoust Soc Am 2012;132:3856-65. [DOI] [PubMed] [Google Scholar]
- 37.Boutros NN, Korzyukov O, Jansen B, Feingold A, Bell M. Sensory gating deficits during the mid-latency phase of information processing in medicated schizophrenia patients. Psychiatry Res 2004;126:203-15. [DOI] [PubMed] [Google Scholar]
- 38.Edgar JC, Huang MX, Weisend MP, Sherwood A, Miller GA, Adler LE, et al. Interpreting abnormality: an EEG and MEG study of P50 and the auditory paired-stimulus paradigm. Biol Psychol 2005;65:1-20. [DOI] [PubMed] [Google Scholar]
- 39.Thoma RJ, Hanlon FM, Huang M, Miller GA, Moses SN, Weisend MP, et al. Impaired secondary somatosensory gating in patients with schizophrenia. Psychiatry Res 2007;151:189-99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Parthasarathy A, Bartlett E. Two-channel recording of auditoryevoked potentials to detect age-related deficits in temporal processing. Hear Res 2012;289:52-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kuwada S, Anderson JS, Batra R, Fitzpatrick DC, Teissier N, D’Angelo WR. Sources of the scalp-recorded amplitude-modulation following response. J Am Acad Audiol 2002;13:188-204. [PubMed] [Google Scholar]
- 42.Pauli-Magnus D, Hoch G, Strenzke N, Anderson S, Jentsch TJ, Moser T. Detection and differentiation of sensorineural hearing loss in mice using auditory steady-state responses and transient auditory brainstem responses. Neuroscience 2007;149:673-84. [DOI] [PubMed] [Google Scholar]
- 43.Purcell DW, John MS, Schneider BA, Picton TW. Human temporal auditory acuity as assessed by envelope following responses. J Acoust Soc Am 2004;116:3581-93. [DOI] [PubMed] [Google Scholar]
- 44.Szalda K, Burkard R. The effects of nembutal anesthesia on the auditory steady-state response (ASSR) from the inferior colliculus and auditory cortex of the chinchilla. Hear Res 2005;203:32-44. [DOI] [PubMed] [Google Scholar]
- 45.Bäuerle P, von der Behrens W, Kössl M, Gaese BH. Stimulus-specific adaptation in the gerbil primary auditory thalamus is the result of a fast frequency-specific habituation and is regulated by the corticofugal system. J Neurosci 2011;31:9708-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Malmierca MS, Cristaudo S, Pérez-González D, Covey E. Stimulus-specific adaptation in the inferior colliculus of the anesthetized rat. J Neurosci 2009;29:5483-93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Patel CR, Redhead C, Cervi AL, Zhang H. Neural sensitivity to novel sounds in the rat’s dorsal cortex of the inferior colliculus as revealed by evoked local field potentials. Hear Res 2012;286:41-54. [DOI] [PubMed] [Google Scholar]
- 48.Mijares-Nodarse E, Pérez-Abalo MC, Torres-Fortuny A, Vega Hernández M, Lage Castellanos A. Maturational changes in the human envelope-following responses. Acta Otorrinolaringol Esp 2012;63:258-64. [DOI] [PubMed] [Google Scholar]
- 49.Pethe J, Mühler R, Siewert K, von Specht H. Nearthreshold recordings of amplitude modulation following responses (AMFR) in children of different ages. Int J Audiol 2004;43:339-45. [DOI] [PubMed] [Google Scholar]
- 50.Savio G, Cardenas J, Perez-Abalo M, González A, Valdés J. The low and high frequency auditory steady-state responses mature at different rates. Audiol Neurotol 2001;6:279-87. [DOI] [PubMed] [Google Scholar]
- 51.Heil P, Schulze H, Langner G. Ontogenetic development of periodicity coding in the inferior colliculus of the Mongolian gerbil. Audit Neurosci 1995;1:363-83. [Google Scholar]
- 52.Kandler K, Clause A, Noh J. Tonotopic reorganization of developing auditory brainstem circuits. Nat Neurosci 2009;12:711-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kotak VC, Sanes DH. Gain adjustment of inhibitory synapses in the auditory system. Biol Cybern 2003;89:363-70. [DOI] [PubMed] [Google Scholar]


