Abstract
Tympanic membrane perforation is a common problem leading to hearing loss. Despite the autoregenerative activity of the eardrum, chronic perforations require surgery using different materials, from autologous tissue - fascia, cartilage, fat or perichondrium - to paper patch. However, both, surgical procedures (myringoplasty or tympanoplasty) and the materials employed, have a number of limitations. Therefore, the advances in this field are incorporating the principles of tissue engineering, which includes the use of scaffolds, biomolecules and cells. This discipline allows the development of new biocompatible materials that reproduce the structure and mechanical properties of the native tympanic membrane, while it seeks to implement new therapeutic approaches that can be performed in an outpatient setting. Moreover, the creation of an artificial tympanic membrane commercially available would reduce the duration of the surgery and costs. The present review analyzes the current treatment of tympanic perforations and examines the techniques of tissue engineering, either to develop bioartificial constructs, or for tympanic regeneration by using different scaffold materials, bioactive molecules and cells. Finally, it considers the aspects regarding the design of scaffolds, release of biomolecules and use of cells that must be taken into account in the tissue engineering of the eardrum. The possibility of developing new biomaterials, as well as constructs commercially available, makes tissue engineering a discipline with great potential, capable of overcoming the drawbacks of current surgical procedures.
Key words: tympanic membrane perforation, myringoplasty, scaffold material, growth factors, cells, regenerative medicine
Introduction
The hearing process consists on the transformation of mechanical energy of sound waves in a biochemical signal that stimulates specific receptors, which trigger a nerve impulse. This process starts by the transmission of the acoustic pressure at the tympanic membrane (TM). This membrane separates the outer ear from the middle ear and plays a crucial role in sound perception and in the protection of the medium ear.1-4 Vibrations of the TM are transmitted and amplified through a chain of mobile ossicles. Finally, the movement of the inner ear fluids stimulates the mechanoreceptors at the hair cells of the cochlea, allowing the perception of sound.1,4-6
The TM has a trilaminar structure, with an outer layer of stratified squamous epithelium composed by keratinocytes; a middle layer consisting of fibroblasts and type II and type III collagen (lamina propria), whose function is to provide mechanical strength, consistency and elasticity; and an inner non-keratinized mucosal epithelium (Figure 1).2,3,5,7-9 The eardrum consists of two parts: pars tensa (PT), where majority of perforations occur, and pars flaccida (PF), and the main difference between them is the composition of the lamina propria, which is perfectly adapted to the specific role of the TM.
Figure 1.
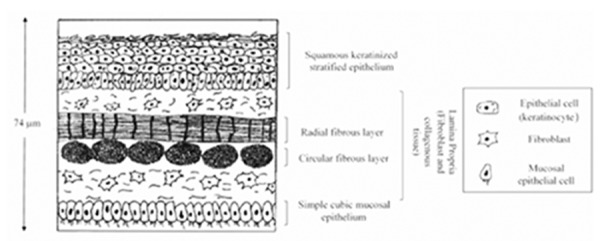
Schematic structure of the tympanic membrane. The tympanic membrane is trilaminar, consisting of an outer epithelial layer formed by keratinocytes; a fibrous middle layer consisting of fibroblasts and collagen - mainly type II and III - (lamina propria); and an inner mucosal non-keratinized epithelium layer. The thickness of the eardrum is heterogeneous in different locations, ranging from 30 to 150 µm. Most authors adopt a mean value of 74 µm. Modified from Teh et al., 2013.2
Thus, the lamina propria of the PT consists mainly of type III collagen in the inner layer (but also of type II and I), while the external layer consists predominantly of type II collagen and, to a lesser degree, type III and I collagen. Furthermore, the lamina propria of the PF is constituted by loose connective tissue and a few elastic fibrils.5,10-14 The eardrum’s shape is oval, and the collagen fibrils are arranged in a radial and circular direction in the outer and inner layer, respectively.
Despite the current data on the TM thickness being limited, it is commonly accepted that it is not homogeneous, varying from 30 to 150 µm, and with a mean value of 74 µm.5,9 The functionality of the TM depends upon its specific structure. Therefore, if the TM is altered (such as in a tympanic perforation), a transmission hearing loss may occur.
TM perforations are a common problem.2,15 The most frequent causes are middle ear infections, traumatic rupture caused by an increased pressure and postoperative complications. These injuries can cause hearing loss and recurrent infections.2,4,15
The perforations of the TM can be classified according to their duration, in acute and chronic (more than three months), and by the presence or absence of drainage, in wet and dry perforations.4 The time of healing and rate of TM perforations closure strongly depends on the type of perforation. Thus, acute and wet perforations close spontaneously after a few weeks in about 76-94% of cases, by using only a topical antibiotic therapy.2,15 However, the closure of chronic perforations needs surgical intervention (myringoplasty) to restore the function of the membrane.2,7
The closure of TM perforations has been investigated for centuries, in order not only to avoid hearing loss, but also to prevent recurrent infections.2 The first approaches to repair the TM date from 1640, when Banzer used pig’s bladder to cover these injuries. In 1848, Yearsley and Toynbee proposed two artificial TMs consisting of a cotton pellet hydrated with glycerol and a disc of natural rubber, respectively. Previously, many authors had used a number of materials to restore tympanic perforations and several patents of TM were issued, but the first TMs were just instruments with a protective function, rather than with a regenerative capacity, and they were unable to improve hearing. However, these early works laid the background for the development of a surgical treatment of tympanic perforations.
Berthold performed the first myringoplasty in 1878, in which he achieved the complete closure of the TM by using a skin graft.16,17 In the 1950s, Hagerman and Ortegen introduced the autologous temporal fascia for myringoplasty, and this graft became the most used in otologic surgery.17,18
Currently there are two therapeutic procedures - myringoplasty and tympanoplasty - to close TM perforations and restore the TM integrity and function.2 Although both techniques have a high success rate, both of them require surgery under general or local anesthesia and an incision to obtain the graft material. Moreover, surgery complications can result and multiple interventions are sometimes required to achieve a complete closure of the perforation.2,4
Therefore, there is a current need of cost-effective, non-surgical and safer alternatives.4 So, tissue engineering focuses on three basic principles to restore the anatomy and functionality of the TM: scaffolds made from various materials, growth factors and cells. However, the studies conducted so far are inconclusive and they have proven their efficacy only in animal models or in reduced groups of patients.2,7 Nevertheless, tissue engineering is a new and promising interdisciplinary field that can overcome the drawbacks and limitations of current surgical techniques.
This systematic review aims to analyze the regeneration of the TM by using tissue engineering as an alternative to conventional surgical procedures. It will focus on the use of different scaffold materials, cells types and growth factors.
Materials and Methods
The sources of information used to develop this systematic review were:
- A search of the online database of the National Library of Medicine (PubMed) performed from January to June 2014. The key terms tympanic membrane repair, tissue engineering of the tympanic membrane, tympanic membrane regeneration were searched. The abstracts of the previous search were examined and included for the current review according to their usefulness to the topic being reviewed. Date of publication, presence of experimental data and the reference to the use of scaffolds, growth factors and/or cells were considered.
- Hand search of the reference lists of these articles was performed. The total articles included for full review was 79. Of those, 33 corresponded to experimental studies performed in animals, 21 were carried out in humans and 8 were in vitro studies. Among the included articles, 35 corresponded to studies of scaffolds, 32 to biomolecules research and 9 to studies with cells. Some works, such as reviews or patents, were not classified in any of these categories.
- The origin of the included articles was as follows: Europe (15), USA (14), Japan (8), Korea (8), Turkey (7), China (6), Australia (3), Canada (2), Brazil (1) and India (1). Some works, such as reviews or patents, were not included in this distribution.
- Personal contacts with specialists of tissue engineering of the TM.
- Specialized textbooks of ear histology and otology for the management of tympanic perforations.
Current treatment of tympanic membrane perforations
Most of TM perforations close spontaneously in few weeks and require only a treatment to prevent water from entering to the ear and infections. However, if the lesion does persist, a surgical intervention is needed.
After an eardrum injury, an exudate composed by lymph, interstitial fluid and /or blood clots is secreted around the edges of the perforation. This secretion forms a layer that protects the underlying tissue from dehydration and provides a support for cell migration. Within days, the proliferation of the squamous epithelial layer occurs and the produced keratin migrates towards the centre of the perforation. Finally, the layer of connective tissue closes the perforation.19
Thus, TM repair occurs mainly by epithelial migration and the function of the different materials used is to act as scaffolds to guide the cell migration from the edges of the perforation. Nowadays there are two clinical treatments to repair the eardrum: myringoplasty (also known as type I tympanoplasty) and tympanoplasty. Both procedures use a material situated in the tympanic cavity, under the perforation, whose function is to act as a support for the regeneration of the TM. The main difference between these techniques is that tympanoplasty usually involves the repair not only of the TM, but also of the mobile ossicles that transmit sound from the eardrum to the inner ear. Tympanoplasty is also the surgery used in large or recurrent perforations. Both techniques use resorbable materials, such as Spongostan® or Gelita® and patient’s own tissue.7 In both cases, the surgical procedure consists in replacing the perforated eardrum by an artificial one, facilitating the perforation closure by providing a patch on which the neomembrane grows.20
Myringoplasty consists of the debridement of the perforation’s edge to provide cells and on the use of a graft that is placed under the remnants of the TM. This graft is inserted through a retroauricular, endoaural or endomeatal incision and it is supported with a resorbable material.21 The graft can be placed under the margins of the perforation with a small part extending over the posterior canal wall (underlay technique) or upon the outer surface of the tympanic membrane, with a slit to tuck it under the handle of the malleus (overlay technique). The 10-year graft success rate is around 80%, with a recurrence rate for chronic otitis media of 15% and 26%, using overlay or underlay technique.22 There are several materials currently used in the clinic, such as perichondrium, vein, cartilage - from the concha or tragus -, fascia and fat, and numerous studies have been conducted to assess their efficacy.16,21,23 Thus, the cartilage-perichondrium graft has proved to produce better results than temporal fascia and perichondrium of the tragus, regarding hearing improvement and TM morphology.24
Despite the wide range of available grafts, the temporal fascia remains the gold standard in the clinical practice.2 However, this graft has some disadvantages such as infection and autolysis. Therefore, the usefulness of other materials for TM closure has been extensively investigated.17,25 The autologous fat is one of the grafts on which most attention has been focused in recent years. By using this material, the rate of success in TM closure ranges between 76-92%, while this rate is 83% in paper myringoplasty.16,17 The adipose tissue graft has the advantages that it is easily and quickly obtained with a low morbidity and it also has a rate of success similar to that of the temporal fascia.25 Additionally, fat grafts secrete angiogenic and growth factors that promote neovascularization and tissue repair, thus increasing the poor blood supply around the TM perforation.17,25
Normally, the fat graft is limited because it is extracted from the earlobe or post-auricular subcutaneous tissue. Therefore, alternative sources of fat tissue have been explored, such as abdominal fat, which has been used in maxillofacial plastic surgery.16 The umbilical region allows the extraction of large amounts of fat tissue without cosmetic problems, which are common in areas where the fat supply is poor or there is scar tissue from previous injuries.16
Furthermore, adipocytes from the abdominal fat are less compact and have less fibrous tissue than the earlobe adipocytes, thus promoting angiogenesis.16 Therefore, abdominal fat is a safe and effective alternative for the closure of the TM.
The selection of the ideal material for TM repair is still under research and there are many types of grafts successfully used in various myringoplasty approaches. Even if temporal fascia or perichondrium are the most frequent choice, adipose tissue grafts have been used for the repair of small eardrum injuries with similar success rate to temporal fascia.25
Despite the large variety of scaffolds and surgical techniques available, there is still no consensus on the optimal alternative for the TM repair and the current techniques have numerous limitations (Table 1).2,7,21 As a result, there is a current need of new therapeutic approaches that overcome these drawbacks. Tissue engineering is an alternative with huge potential for TM repair, as it aims to generate a trilaminar membrane that reproduces the structure, mechanical properties and function of the native membrane.
Table 1.
Comparison between current surgical techniques and tissue engineering for the regeneration of the tympanic membrane.
| Advantages | Disadvantages |
|---|---|
| Myringoplasty/Tympanoplasty | |
| High success rate | Required anesthesia |
| Good outcome in small perforations | Greater surgery time |
| Minimally invasive technique | Open surgical procedure (associated risks) |
| Routine clinical practice | Incision to take the graft and remove squamous epithelium |
| Limited availability of autologous graft in revision cases | |
| Failure of perforation closure due to the deficient regenerative activity at the edges of the injury | |
| Frequent re-perforation | |
| Bilaminar neomembrane: flaccid and acoustically suboptimal | |
| Side effects: retraction pockets, tympanosclerotic mass, rejection | |
| Tissue engineering | |
| Surgery simplification | Mostly animal studies (acute perforations, which would spontaneously close in most cases) |
| Cost savings | |
| Improve outcome in chronic perforations | Lack of a standard animal model |
| Growth factors improve tympanic closure | Scarce human clinical trials |
| Specific design of scaffold materials that reproduce the mechanical properties of the eardrum | Possible side effects of scaffold materials, biomolecules and cells |
| Ethical and legal issues concerning the use of xenografts | |
| Possibility of generating a commercially available tympanic membrane | Complex manufacture of the artificial construct (storage, biopreservatives, quality controls, production and transportation costs) |
Tissue engineering of the tympanic membrane
There are three main problems to achieve the repair of TM perforations: i) the lack of a structural support; ii) the scarce angiogenesis and growth factors; and iii) a deficient extracellular matrix leading to a weak cell adhesion in the neomembrane.2
Tissue engineering has been applied by two approaches: in vitro - constructs generated in tissue cultures or bioreactors -, and in vivo. This discipline employs three main elements: cells, scaffold materials - which serve as a mechanical support for cell proliferation, migration and differentiation - and biomolecules - which provide a suitable biochemical microenvironment.2,21,26 TM perforations can be repair in three ways: i) creating an artificial TM by combining a scaffold material, biomolecules and cells, and applying this membrane to the perforation; ii) applying a scaffold to the perforation and then supplying biomolecules by drops; and iii) placing a scaffold previously moistened with bioactive molecules.2 Tissue engineering methods for TM regeneration has been investigated for a few years, as several authors have utilized scaffolds with exogenous biomolecules in order to repair tympanic perforations. Nevertheless, nowadays this term is being used to refer to the design of biomaterials with different size and shapes. This review will consider separately the three elements of tissue engineering (scaffold materials, biomolecules and cells), although these three factors can be applied alone or in combination.2,7
Scaffold materials
The TM has an intrinsic regenerative capacity. However, the repair process of this structure differs from other tissues, because there is not a support under the epithelial layer. For this reason, the current clinical procedures use different scaffolds or grafts that act as a support to facilitate cells and nutrients migration towards the perforation.19
The most used grafts are fascia (temporal, lata or muscle), fat, cartilage and perichondrium, but tissue engineering is focusing on the research of a large number of novel materials.7,15,17,27 The ideal scaffold should have certain characteristics, but a material that meets all this criteria does not exist yet. Therefore, the new discipline of tissue engineering aims to improve the properties of the current scaffolds to achieve most suitable materials and better results in the TM closure.2
Scaffold materials are generally classified into decellularized tissue and polymers.2
Decellularized tissue
The decellularized tissue is obtained after cell removal of allografts or xenografts. It preserves most of the biological and mechanical properties of the original extracellular matrix. Thus, this type of scaffolds serve as templates for reconstruction and tissue remodeling, as they retain the native structure and contain functional proteins, such as collagen and proteoglycans.2,7,28
Some of the materials that have been studied are: acellular collagen from cadavers or porcine peritoneum,29,30 AlloDerm® - a cryo-dried acellular dermal tissue matrix from human donors -2,7 Sttratice® - obtained from porcine skin -,31 decellularized urinary bladder, Surgisis® - porcine small intestinal submucosa -2 and Tutopatch® and Audiomesh® - bovine and equine pericardium, respectively.2,7,27,31
Most of these materials have managed to produce a trilaminar neomembrane and improve the rate of success and hearing recovery. However, decellularized tissue has been used mainly in animal models and human clinical trials have been only conducted with AlloDerm®, Tutopatch® and Audiomesh®. Moreover, the human application of decellularized tissue is difficult, due to ethical and biosafety issues, as it derives from tissue of cadavers or other species.
Polymers
Polymers have many advantages over the above scaffolds, mainly because their shape, size and porosity can be changed with great ease to better fit their future application. Furthermore, they are biocompatible, biodegradable, easy to synthesize and handle, can be produced on a large-scale basis and their degradation and mechanical properties can be controlled. Nonetheless, polymers may not be able to reproduce the native structure of the membrane. Some of the polymers investigated to achieve the repair of the TM are listed in Table 2.2,18,22,29,31-46
Table 2.
Polymers used for tympanic membrane regeneration by tissue engineering.
| Polymer | Model | Properties and main findings |
|---|---|---|
| Gelfoam®31 | Human | Hemostatic absorbable material used to hold the graft and as a scaffold to growth factors delivery Biocompatibility Faster tympanic closure Increased tympanic closure rate |
| Polylysine polymerized with latex32 | Human | Induction of greater tissue vascularization (possible presence of vascular growth factor, which improves vascularization of the fascia graft by promoting angiogenesis) Biocompatibility (no toxicity or allergic reaction) No significant changes in hearing function or rate of healing |
| Silk fibroin29, 33-35 | In vitro | Maintenance of keratinocyte growth and cell adhesion and integrity Facilitation of continuous epithelium growth without deforming the membranous contour Supplying continuously a hydrated surface |
| Animal | Transparent and trilaminar structure of the neomembrane similar to the native membrane More organized growth of epithelial cells and connective tissue Faster tympanic closure and hearing recovery |
|
| Chitosan18,36,37 | ||
| Water-soluble | Animal | Healing rate comparable to natural healing rate Higher collagen density and more organized structure than in natural healing |
| Water-insoluble | Animal | Better proliferation of tympanic membrane cells Biocompatibility Better quality neomembrane than that obtained with paper patches |
| 3D scaffold | Animal | Good cell proliferation Porous structure that ease cell infiltration Trilaminar structure of the neomembrane similar to the native membrane |
| Calcium alginate38 | Animal | Growth promotion of mucosal and keratinized epithelium Cross-linking with calcium facilitates its manipulation Greater rate of tympanic closure than with paper patches Possible human risk of ototoxicity |
| Hyaluronic acid39-42 | Animal | Reduced time and increased rate of tympanic closure Better quality of the neomembrane |
| Human | Its application on its own (Epifilm®) does not improve perforation closure rate | |
| Epidisc™ application with a fat graft showed a closure rate similar to fat or cartilage myringoplasty and reduced surgery time | ||
| Poly (glicerol sebacate)43 | Animal | Biodegradability Promotion of neovascularization Suitable histological structure of the neomembrane Need to accelerate degradation rate to promote optimal regeneration of the tympanic membrane |
| Hydrogels and sponges derived from glycosaminoglycans44 | Animal | Increased re-epithelialization Reduced time of tympanic closure (Carbylan-GSX) Variable results depending on the specific type of hydrogel Possible inflammatory reaction (CS-SX) |
| Bilaminar atelocollagen- silicone membrane22,45,46 | Human | Biocompatibility Silicone retains collagen configuration and dampness Its application with bFGF improves the results of tympanic closure (success rate, time and hearing recovery) Often need to repeat the procedure to achieve complete perforation closure |
Carbylan-GSX, Carbylan-S/Gelatin-DTPH; CS-SX, cross-linked thiolated chondroitin sulfate [CS-DTPH-PEGDA (CS-SX)]; bFGF, basic fibroblast growth factor.
Gelfoam® is a gelatinous sponge obtained from denatured swine skin. It has been used as a scaffold for tympanic and ossicular grafts and in traumatic perforations.2,32 Another researched material is polylysine polymerized with latex from Hevea brasiliensis tree, which induce a greater vascularization when externally applied to a fascia graft.33
Silk fibroin is also a useful support for TM regeneration. It is obtained after the removal of the antigenic component - sericin - of Bombyx mori silk and presents numerous advantages, such as elasticity, biodegradability and biocompatibility. In fact, it has been used for years as a suture material. It also presents ease to bind peptides, thus facilitating cell adhesion, and it can be processed in a large number of structures (fibers and sponges).2,34,35 Some studies have shown that human keratinocytes from the TM have a better growth in silk patches than in other scaffolds. These results are supported by in vivo studies in which, by using a support made from silk fibroin, tympanic perforation closure was achieved in less time and in a more organized way and with a faster hearing improvement.7,30,35,36 An artificial TM whose main component is silk fibroin has recently been patented.20
Chitosan is a N-deacetylated form of chitin, which is a mucopolysaccharide with anti-bacterial properties present in the exoskeleton of arthropods. Chitosan promotes healing of skin, bone and liver wounds and has been successfully tested as water-soluble and water-insoluble patches. Three-dimensional chitosan patch proved to have advantages with respect to other support materials.7,18,37,38
Alginate, a natural polymer derived from seaweed, serves as support to the growth of mucosa and keratinized epithelium. It is also used as a delivery system for tissue regeneration, although further research is needed to assess human safety and efficacy in the long term.2,7,39
Moreover, hyaluronic acid, which can be used as a solid scaffold (with different chemical modifications) or as a topical biomolecule, has shown its usefulness in tympanic repair.2,40-43
Synthetic materials, such as polyglycerol sebacate (PGS), have also been tested. PGS is a biodegradable polymer obtained by polycondensation of glycerol and sebacic acid that has many advantages.2,7,44 Hydrogels and sponges derived from glycosaminoglycans could be also useful in tissue engineering of the TM. Among them, Carbylan-GSX promoted TM closure in less time and without inflammatory reaction.7,47
Finally, a bilaminar atelocollagen-silicone membrane soaked with fibroblast growth factor is one of the most recent and promising inventions, which will be explained later.
Biomolecules
Growth factors stimulate wound healing in many tissues. These biomolecules and their receptors are expressed during the regeneration process of the TM, as shown in animal trials.2,7
Perforation closure occurs by epithelial proliferation and migration. Therefore, molecules, such as exogenous growth factors, that enhance regenerative processes could help to close TM perforations.19,48
There are five main groups of growth factors: epithelial growth factor (EGF), fibroblast growth factor (FGF), platelet-derived growth factor (PDGF), transforming growth factor β (TGF-β) and insulin-like growth factor. Regarding TM regeneration, most of the research has focused in biomolecules of the first two groups. These molecules can be applied either in drops or in a scaffold soaked with them.2,7,49
EGF stimulates the synthesis of DNA, RNA, proteins and hyaluronic acid. Moreover, there are high affinity EGF receptors in tympanic epithelial and stromal cells, and after an injury in this structure, the EGF expression is parallel to the reparative process. Although the number of EGF receptors does not increase after a perforation, their inhibition delay TM closure.2 This growth factor is naturally expressed after damage, but its topical application achieved positive results in acute and chronic perforation animal models (Table 3).19,21,48-72
Table 3.
Biomolecules used for tympanic membrane regeneration by tissue engineering.
| Biomolecule | Properties | Model | Main findings |
|---|---|---|---|
| EGFl9,48-52 | Mitogenic effect | Animal | Greater tympanic closure in less time Neomembrane thickness and histology similar to the native membrane; the thickness achieved in spontaneous closure is less than half the normal thickness of the TM Stimulated neovascularization and fibroblast number Stimulated proliferation (mainly of the squamous layer) Long-term application leads to re-perforation and cholesteatoma formation |
| Human | No improvement of tympanic closure No toxicity |
||
| TGF-α53,54,60 | In vitro | More effective than EGF in promoting colony dispersion and injuries healing Greater pro motility activity than EGF |
|
| Animal | TGF-α was not observed in normal TM, but it was expressed after a perforation | ||
| TGF-β65 | Chemotaxis induction Extracellular matrix production Angiogenesis stimulation Possible excess of scar tissue Thicker TM |
Animal | Reduction of perforation closure time Need to repeated application to achieve the above beneficial effects Possible formation of a disorganized fibrous scar |
| PGF45,46,55-59 | Stimulation of fibroblast, endothelial cells and keratinocytes proliferation and differentiation Stimulation of collagen fibrils growth Vasodilation promoting Stimulation of protease production |
Animal | Epithelial and/or connective tissue hiperplasia Increased success of tympanic closure when applied directly to the perforation; if applied with Gelfoam®, it forms a voluminous scar that protrudes from the middle ear cavity and ossicles (use of Gelfoam® inadvisable) |
| Human | Increased tympanic closure rate and reduced time of TM closure Enhanced hearing recovery Possible epithelial pearl formation Reduction of middle ear infections Hyperplasia of granulation tissue - which disappears in 5-7 days |
||
| KGF60,61 | Reactive oxygen species detoxyfication Promotion of re-epithelialization Keratinocytes proliferation and migration |
Animal | Enhanced epithelial migration and proliferation in the first steps No increase in tympanic closure rate More organized repair process |
| PDGF62,63 | Fibroblast mitogen | Animal | Increased tympanic closure rate Reduction of perforation closure time More abundant connective fibrous tissue layer |
| Human | No increase in tympanic closure | ||
| VEGF64 | Fibroblast mitogen Angiogenesis stimulation Induction of collagen deposition Induction of epithelialization |
Animal | VEGF is more specific and important than bFGF in acute perforation closure |
| Autologous serum from peripheral blood21 | Promotion of wound healing Lack of antigenicity Large quantity of growth factors |
Human | ASET does not require anesthesia, reduces or completely closes chronic perforations and implies a continuous supply of growth factors by the own patient Further studies are required to conclude whether the beneficial effect is due to the serum or to the scaffold used for the tympanic membrane closure |
| Human umbilical cord serum68 | Large quantity of growth factors (greater concentration of EGF, NGF and TGF-αβ than in autologous serum from peripheral blood) | Animal | Applied with a 3D collagen scaffold, it enhances significantly chronic perforation closure and hearing capacity from early stages than with paper patches Neomembrane thickness similar to the native membrane Further studies are required to conclude whether the beneficial effect is due to the serum or to the scaffold used for the tympanic membrane closure |
| Hyaluronic acid19,69 | Viscoelastic properties It can be used as a scaffold or as a biomolecule administered in drops Increased motility and phagocytic activity of polimorfonuclear leucocytes |
Animal | Reduced time in tympanic perforation closure Increased closure success rate than natural closure Increased levels of FGF and VEGF |
| Human | No increased tympanic closure success rate | ||
| Human insulin66,70 | Neovascularization Increased fibroblast growth rate Increased keratinocytes proliferation, migration and differentiation from the perforation edges Activation of insulin receptor and IGF 1 receptor |
Animal | Beneficial effect in perforation epithelialization Neovascularization Formation of finger-like projections Fibroblast activity Presence of inflammatory cells in the lamina propria |
| Stimulation of keratyn migration | Human | Increased micro-vascularization from the remnants of tympanic membrane or graft towards the perforation, with the induction of inflammation and epithelialization from the perforation edges Halfen the perforation size after its forth or fifth application |
|
| Platelet-rich plasma67 | α granules of platelets contain growth factors | Animal | Reduction of the average time of tympanic closure |
| Plasminogen71,72 | Degradation of fibrin and extracellular matrix proteins | Animal | Reduced time of tympanic closure |
EGF, epidermic growth factor; TM, tympanic membrane; TGF-α, -β, transformant growth factor type α, -β; FGF, fibroblast growth factor; KGF, keratinocyte growth factor; PDGF, platelet-derived growth factor; VEGF, vascular endothelial growth factor; bFGF, basic fibroblast growth factor; ASET, autologous serum eardrop therapy; NGF, nerve growth factor; IGF-1, insulin-like growth factor type I.
TGF-α is another molecule from EGF family used in tympanic regeneration, as its expression correlates with epidermal cells proliferation in the TM.2,54,55
Within the FGF family, basic fibroblast growth factor (bFGF) has been the most investigated. This factor is produced after TM injury and it facilitates the perforation closure through various mechanisms.2 bFGF acts mainly in the epithelial layer, where there are more specific receptors for it, although these receptors are also present in the mucosal layer.56 The application of bFGF in animal models increases the success rate of tympanic closure, both in acute and chronic perforations.45,57,58 In both cases, the beneficial effect of bFGF also lies in the induction of rapid proliferation of the subepithelial connective tissue.58
On the other hand, several studies in humans showed that the application of different scaffold materials soaked with bFGF achieved a higher and faster healing rate, but these results were not significant when using only the support material. This fact suggests that it is not the scaffold itself, but its combination with growth factors what is crucial in the closure of the TM.48
One of the most recent approaches with better results in human clinical trials is the application of a bilayer membrane soaked with Trafermin® (human recombinant bFGF). This membrane is composed of atelocollagen - a low-antigenic collagen obtained from calfskin collagen treated with protease - and silicone, which prevents the membrane from its introduction in the middle ear. Collagen is an excellent material to close perforations, since it is resorbable and compatible with the surrounding tissue. Moreover, it acts as a support for fibroblast growth, which infiltrate from the perforation edges, so that the bilaminar membrane is replaced by connective tissue. bFGF aids the remodelling of TM by different mechanisms.22,46,59 The atelocollagen-silicone membrane is placed with the collagen layer in the inner side. Subsequently, this layer is soaked with bFGF and the silicone layer, which acts only as a support, is removed after 2-3 weeks after surgery. This bilayer achieved a significant improvement in tympanic closure and hearing recovery, and it also implies a shorter surgical time.22,46
Another successful approach tested in human chronic perforations is the regenerative treatment with bFGF and a gelatin sponge, which is sealed with fibrin glue.60 In addition to the growth factors mentioned above, other factors61-66 and other biomolecules, such as autologous serum, hyaluronic acid, human insulin,67 platelet-rich plasma68 and plasminogen have been used with different efficacy.
Autologous serum contains a variety of growth factors, vitamins and immunoglobulins capable of modulating the proliferation of various tissues to promote wound healing. Its administration in drops (autologous serum eardrop therapy) is beneficial in human chronic perforations. Furthermore, it eliminates the surgery procedure of refreshing the perforation edges. However, more studies are needed to clarify if the results are due to the autologous serum, the chitin support employed or the combination of both.21
Studies with human umbilical cord serum have also been conducted, as it achieves a faster healing of corneal wounds than autologous serum. The effectiveness of serum is due to the presence of molecules capable of accelerating tissue regeneration, such as growth factors [EGF, FGF, TGF-β, PDGF, nerve growth factor (NGF), insulin-like growth factor type 1], vitamin A, fibronectin, antiproteases and P substance. Umbilical cord serum has a higher concentration of NGF, EGF and TGF-β than peripheral blood serum, thus it might be more useful for TM repair.69
Hyaluronic acid is a natural high molecular weight polysaccharide that regulates the recovery of the fibrous layer, as it prevents the perforation edges from dehydration.2,19,70
Human insulin has been used to the epithelialization of tympanic perforations. The beneficial effects led to a pilot study in humans, which confirmed the usefulness of insulin in the closure of this type of injury. The joint application of insulin and TGF-β obtained a faster healing than if these molecules were used separately, so the combined administration could reduce the dose of growth factors.71
Platelet-rich plasma has been tested for its ability to increase the local concentration of growth factors.7 Plasminogen, a zymogen able to degrade fibrin and extracellular matrix proteins, plays an important role in tissue remodelling. Thus, it could be an alternative to repair the TM.72,73
Nonetheless, most studies have been conducted in experimental animal models. The therapeutic potential in humans has only been assessed for EGF, hyaluronic acid, PDGF, bFGF and autologous serum, and the beneficial effects were found only for bFGF and autologous serum.2,74
Cells
Scaffolds, biomolecules and cells interact to achieve the regeneration of the TM. However, most studies do not employ cells, because of the natural repair capacity of the TM and the endogenous cellular source from the excoriated edges of the perforation. Thus, normally only scaffolds and active molecules that are able to recruit cells for the TM are used.7 Cells can be applied by injection or integrated within a support material. Although there are various theoretical cell sources that could be used for tympanic regeneration, only a few animal studies have been realized so far (Table 4).74-78
Table 4.
Cells used for tympanic membrane regeneration in animals.
| Cells | Model | Main findings |
|---|---|---|
| Autologous fibroblasts74 | Guinea pigs | Accelerated tissue regeneration Production of new extracellular matrix |
| Mouse embryonic stem cells75,76 | Gerbil | Greater tympanic closure success rate than in controls Strengthened tympanic structure (resistance of higher pressure) |
| Rat | No enhanced tympanic closure with respect to controls Thicker lamina propria After 6 months:
|
|
| Human mesenchymal stem cells77,78 | In vitro | MSCs are able to grow and differentiate to fibroblast in the different biomaterials Synthesis of appropriate molecules of the extracellular matrix Type II collagen production |
| Rat | Greater tympanic closure success rate than in controls Granulation tissue formation |
TM, tympanic membrane.
Autologous fibroblasts seeded on acellular swine dermis or dura mater is the most studied cell source.2,7,75 Stem cells (SCs) have also been studied, given their regenerative capacity. Von Unge et al. used embryonic SCs from mice with promising results, due to the differentiation and integration of these cells in the TM.77,78 Application of human mesenchymal SCs showed inconclusive results.78,79
Stem cells have the crucial feature that they differentiate depending on the signals received from their environment. Hence, the ear microenvironment may influence the maturation of SCs. The aim to repair the TM is that most of the cells become fibroblasts of the intermediate layer, whose regeneration is most difficult to achieve. Thus, SCs could be used in combination with FGF to stimulate cell differentiation into fibroblasts.
Tissue engineering is also used to obtain artificial tympanic substitutes. To this purpose, biocompatible and resorbable polymeric matrices were seeded with bone marrow-derived mesenchymal stem cells, previously differentiated in vitro into fibroblasts by the addition of growth factors. Moreover, these bioconstructs experienced mechanical stress during culture to promote fibroblast differentiation. This process increased the production of type II collagen, which is a constitutive protein of the intermediate layer of the human TM.78
However, the use of SCs is linked to safety issues (infection, rejection and tumor formation), not to mention the ethical and legal issues. Therefore, studies in this field are scarce. Additionally, human progenitor cells of the TM were found in the umbo, annulus and handle of the malleus, which suggests the possibility that there are regenerative SCs in the TM itself.80 Thus, if the remaining membrane have its own progenitor cells, it would not be necessary to incorporate external cell sources to achieve the TM repair.2 Despite the usefulness of SCs in pilot studies, this last fact and the problems of using SCs, suggest that it could be difficult the application of SCs for tympanic perforation closure or to search other cell sources, such as autologous keratinocytes.7
Considerations for clinical application
Scaffold materials
Most scaffold materials achieved favorable results in the repair of tympanic perforations. However, almost all novel materials are compared with autologous tissue (temporal fascia, tragus perichondrium or auricular or tragus cartilage), which remains the gold standard, because of the advantages of employing the patient’s own tissue. In addition, the time to collect the autologous tissue and the associated morbidity do not justify the need of a bioartificial membrane commercially available.
Therefore, novel scaffold material will become an alternative to autologous tissue, if they are available in different sizes and thicknesses that allow them to fit in the different perforations. In that sense, computer-aid design and mold injection are being useful.2,7 However, there is a current need to optimize biomaterials that reproduce biomechanical and vibroacustic properties of the TM.
Biomolecules
Normally, growth factors improve the healing of tympanic perforations. Nevertheless, there are still doubts concerning the release vector, type of molecule, dose and duration of the therapy to get the maximum benefit. Some studies showed that these factors should be administered until the perforation is completely closed, while others showed that one single application was enough to achieve tympanic closure. Moreover, current research aims to obtain the release of biomolecule drops in a specific area, thus facilitating the administration by the own patient. If biomolecules are applied within a scaffold, it should also be considered the loading capacity, release kinetics and binding affinity.2
Furthermore, most of trials tested the effect of biomolecules independently, but the synergistic effect of various growth factors should be considered. Growth factors stimulate the proliferation of epidermal and connective tissue. Thus, its use is linked to epithelial pearl and cholesteatoma formation. Therefore, more long-term studies are needed to assess their efficacy and safety before their clinical application.2,7,59
Cells
So far, the use of cells in tissue engineering of the TM is scarce and all the research has been conducted in animals. This is due to the autoregenerative capacity of the eardrum and to the fact that there is an endogenous cellular source provided by the excoriation process. Even though the application of SCs achieved a neomembrane structure similar to the native TM, their clinical use is limited due to the possible side effects, high cost and ethical and legal issues.
Furthermore, the application of cells to the constructs is difficult in a clinical setting, given the complex structure of the TM. Cell culture and seeding require strict biosafety controls and it is difficult to seed different cells in the different layers of the TM, since each cell type needs specific conditions. It is also necessary to avoid epithelial cell infiltration into the middle ear, because it could lead to iatrogenic cholesteatoma formation. So, the application of cells might not be necessary if the remnants of the TM have useful progenitor cells for the regeneration process.2,7
The main challenge to regenerate the TM is to translate the results obtained in animal experimental models to human models. Moreover, the choice of the optimal biomolecules and scaffold materials is still not resolved, and the incorporation of cells is controversial.
Conclusions
The following key points should be considered: i) tissue engineering is an alternative for tympanic closure that combines the use of scaffolds, cells and biomolecules. It focuses on the development of novel materials that reproduce the mechanical and vibroacustic properties of the native tympanic structure; ii) current techniques and scaffold materials used for epithelial proliferation and migration produce a bilaminar - and therefore, acoustically suboptimal - neomembrane; iii) several biomolecules with proliferative and angiogenic effects such as EGF, bFGF, insulin and serum can improve the outcome in TM perforations; iv) the development of new biomaterials and commercially available constructs for the TM makes tissue engineering a discipline with great potential in otology surgery.
References
- 1.Mallo M. Formation of the middle ear: recent progress on the developmental and molecular mechanisms. Dev Biol 2001;231:410-9. [DOI] [PubMed] [Google Scholar]
- 2.Teh BM, Marano RJ, Shen Y, Friedland PL. Tissue engineering of the tympanic membrane. Tissue Eng B 2013;19:116-32. [DOI] [PubMed] [Google Scholar]
- 3.Stevens A LJ. Histology. Hong Kong: Gower Medical Publishing; 1992. [Google Scholar]
- 4.De Juan J, et al. Tympanic membrane prosthesis with mechanical fixation. US Patent 6309419; 2001.
- 5.Volandri G, Di Puccio F, Forte P, Carmignani C. Biomechanics of the tympanic membrane. J Biomech 2011;44:1219-36. [DOI] [PubMed] [Google Scholar]
- 6.Aernouts J, Soons J a M, Dirckx JJJ. Quantification of tympanic membrane elasticity parameters from in situ point indentation measurements: validation and preliminary study. Hear. Res. 2010;263:177-82. [DOI] [PubMed] [Google Scholar]
- 7.Hong P, Bance M, Gratzer PF. Repair of tympanic membrane perforation using novel adjuvant therapies: a contemporary review of experimental and tissue engineering studies. Int J Pediatr Otorhinolaryngol 2013;77:3-12. [DOI] [PubMed] [Google Scholar]
- 8.Tringali S, Dubreuil C, Bordure P. Tympanic membrane perforation and tympanoplasty. Ann Otolaryngol Chir Cervicofac 2008;125:261-72. [DOI] [PubMed] [Google Scholar]
- 9.Cheng T, Dai C, Gan RZ. Viscoelastic properties of human tympanic membrane. Ann Biomed Eng 2007;35:305-14. [DOI] [PubMed] [Google Scholar]
- 10.Gil-Carcedo L, Vallejo L, Gil-Carcedo E. Otología. Madrid: Editorial Médica Panamericana; 2011. pp 23, 229. [Google Scholar]
- 11.Knutsson J, Bagger-Sjöbäck D, Von Unge M. Collagen type distribution in the healthy human tympanic membrane. Otol Neurotol 2009;30:1225-29. [DOI] [PubMed] [Google Scholar]
- 12.Knutsson J, Bagger-Sjöbäck D, Von Unge M. Different kinds of collagens in tympanic membranes. Hear Res 2010;263:233-52. [Google Scholar]
- 13.Stenfeldt K, Johansson C, Hellström S. The collagen structure of the tympanic membrane. Arch Otolaryngol Head Neck Surg 2006;132:293-8. [DOI] [PubMed] [Google Scholar]
- 14.Young B, Lowe J, Stevens A, Heath J. Wheater’s functional histology: a text and colour atlas. (Young B., Ed.). Shanghai: Churchill Livingstone-Elsevier; 2006. pp 417. [Google Scholar]
- 15.Zhang Q, Lou Z. Impact of basic fibroblast growth factor on healing of tympanic membrane perforations due to direct penetrating trauma: a prospective non-blinded/controlled study. Clin Otolaryngol 2012;37:446-51. [DOI] [PubMed] [Google Scholar]
- 16.Kwong KM, Smith MM, Coticchia JM. Fat graft myringoplasty using umbilical fat. Int J Pediatr Otorhinolaryngol 2012;76:1098-101. [DOI] [PubMed] [Google Scholar]
- 17.Acar M, Yazıcı D, San T, Muluk NB, Muluk NB, Cingi C. Fat-plug myringoplasty of ear lobule vs abdominal donor sites. Eur Arch Otorhinolaryngol;2014. [Google Scholar]
- 18.Kim JH, Bae J-H, Lim KT, Choung PH, Park JS, Choi SJ, et al. Development of water-insoluble chitosan patch scaffold to repair traumatic tympanic membrane perforations. J Biomed Mater Res A 2009;90:446-55. [DOI] [PubMed] [Google Scholar]
- 19.Güneri EA, Tekin S, Yilmaz O, Ozkara E, Erda TK, Ikiz AO, et al. The effects of hyaluronic acid, epidermal growth factor, and mitomycin in an experimental model of acute traumatic tympanic membrane perforation. Otol Neurotol 2003;24:371-6. [DOI] [PubMed] [Google Scholar]
- 20.Kang W, Kr H, Kr S, et al. Artificial eardrum using silk. US Patent 8500808; 2013.
- 21.Kakehata S, Hirose Y, Kitani R, Futai K, Maruya S, Ishii K, et al. Autologous serum eardrops therapy with a chitin membrane for closing tympanic membrane perforations. Otol Neurotol 2008;29:791-5. [DOI] [PubMed] [Google Scholar]
- 22.Nardone M, Sommerville R, Bowman J, Danesi G. Myringoplasty in simple chronic otitis media: critical analysis of long-term results in a 1,000-adult patient series. Otol Neurotol 2012;33:48-53. [DOI] [PubMed] [Google Scholar]
- 23.Hakuba N, Taniguchi M, Shimizu Y, Futai K, Maruya S, Ishii K, et al. A new method for closing tympanic membrane perforations using basic fibroblast growth factor. Laryngoscope 2003;113:1352-5. [DOI] [PubMed] [Google Scholar]
- 24.Zhang Z, Huang Q, Zheng Y, Sun W, Chen YB, Si Y, et al. Three autologous substitutes for myringoplasty: a comparative study. Otol Neurotol 2011;32:1234-8. [DOI] [PubMed] [Google Scholar]
- 25.Ozgursoy OB, Yorulmaz I. Fat graft myringoplasty: a cost-effective but underused procedure. J Laryngol Otol 2005;119:277-9. [DOI] [PubMed] [Google Scholar]
- 26.Harrison R, St-Pierre J, Stevens M. Tissue Engineering and regenerative medicine : a year in review. Tissue Eng B 2014;20:1-16. [DOI] [PubMed] [Google Scholar]
- 27.Kaftan H. Trommelfellrekonstruktion mit nicht-autogenen Transplantaten und alloplastischen Materialien Autogenous Transplants and Alloplastic Materials. Laryngorhinootologie 2010;89:562-8; quiz 569-70. [DOI] [PubMed] [Google Scholar]
- 28.Parekh A, Mantle B, Banks J, Swarts JD, Badylak SF, Dohar JE, et al. Repair of the tympanic membrane with urinary bladder matrix. Laryngoscope 2009;119:1206-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Perkins. Synthetic tympanic membrane US Patent 4077069; 1978.
- 30.Shen Y, Redmond SL, Teh BM, Yan S, Wang Y, Atlas MD, et al. Tympanic membrane repair using silk fibroin and acellular collagen scaffolds. Laryngoscope 2013;123:1976-82. [DOI] [PubMed] [Google Scholar]
- 31.Albera R, Dagna F, Lacilla M, Canale A. Equine versus bovine pericardium in transmeatal underlay myringoplasty. Ann Otol Rhinol Laryngol 2009;118:287-91. [DOI] [PubMed] [Google Scholar]
- 32.Lou Z-C, He J-G. A randomised controlled trial comparing spontaneous healing, gelfoam patching and edge-approximation plus gelfoam patching in traumatic tympanic membrane perforation with inverted or everted edges. Clin Otolaryngol 2011;36:221-6. [DOI] [PubMed] [Google Scholar]
- 33.Miranda Araujo M, Tanaka Massuda E, Hyppolito M. Anatomical and functional evaluation of tympanoplasty using a transitory natural latex biomembrane implant from the rubber tree Hevea brasiliensis. Acta Cirúrgica Bras 2012;27:566-71. [DOI] [PubMed] [Google Scholar]
- 34.Kim J, Kim CH, Park CH, Seo JN, Kweon H, Kang SW, et al. Comparison of methods for the repair of acute tympanic membrane perforations: Silk patch vs. paper patch. Wound Repair Regen 2010;18:132-8. [DOI] [PubMed] [Google Scholar]
- 35.Levin B, Redmond SL, Rajkhowa R, Eikelboom RH, Atlas MD, Marano RJ. Utilising silk fibroin membranes as scaffolds for the growth of tympanic membrane keratinocytes and application to myringoplasty surgery. J Laryngol Otol 2013;127:S13-20. [DOI] [PubMed] [Google Scholar]
- 36.Ghassemifar R, Redmond S, Zainuddin S, Chirila T. Advancing towards a tissue-engineered tympanic membrane: silk fibroin as substratum for growing human eardrum keratinocytes. Appl J Biomater 2010;24:591-606. [DOI] [PubMed] [Google Scholar]
- 37.Kim J, Choi S, Park J, Lim KT, Choung PH, Kim SW, et al. Tympanic membrane regeneration using a water-soluble chitosan patch. Tissue Eng A 2010;16:225-32. [DOI] [PubMed] [Google Scholar]
- 38.Kim J, Kim S, Choi S, Lim KT, Lee JB, Seonwoo H, et al. A healing method of tympanic membrane perforations using three-dimensional porous chitosan scaffolds. Tissue Eng A 2011;17:2763-72. [DOI] [PubMed] [Google Scholar]
- 39.Weber DE, Semaan MT, Wasman JK, Beane R, Bonassar LJ, Megerian CA. Tissue-engineered calcium alginate patches in the repair of chronic chinchilla tympanic membrane perforations. Laryngoscope. 2006;116:700-4. [DOI] [PubMed] [Google Scholar]
- 40.Konakçi E, Koyuncu M, Ünal R, Tekat A, Uyar M. Repair of subtotal tympanic membrane perforations with Seprafilm. J Laryngol Otol 2004;118:862-5. [DOI] [PubMed] [Google Scholar]
- 41.Prior M, Gibbins N, John G, Rhys-Williams S, Scott P. Hyaluronic acid ester in myringoplasty. J Laryngol Otol 2007;122:e3. [DOI] [PubMed] [Google Scholar]
- 42.Saliba I, Woods O. Hyaluronic acid fat graft myringoplasty: a minimally invasive technique. Laryngoscope 2011;121:375-80. [DOI] [PubMed] [Google Scholar]
- 43.Saliba I, Froehlich P. Hyaluronic acid fat graft myringoplasty: an office-based technique adapted to children. Arch Otolaryngol Head Neck Surg 2011;137:1203-9. [DOI] [PubMed] [Google Scholar]
- 44.Wieland A, Sundback C, Hart A, Kulig K, Masiakos PT, Hartnick CJ. Poly (glicerol sebacate)-engineered plugs to repair chronic tympanic membrane perforations in a chinchilla model. Otolaryngol Neck Surg 2010;143:127-33. [DOI] [PubMed] [Google Scholar]
- 45.Fina M, Baird A, Ryan A. Direct application of basic fibroblast growth factor improves tympanic membrane perforation healing. Laryngoscope 1993;103:804-9. [DOI] [PubMed] [Google Scholar]
- 46.Hakuba N, Iwanaga M, Tanaka S, Hiratsuka Y, Kumabe Y, Konishi M, et al. Basic fibroblast growth factor combined with atelocollagen for closing chronic tympanic membrane perforations in 87 patients. Otol Neurotol 2010;31:118-21. [DOI] [PubMed] [Google Scholar]
- 47.Park A. Crosslinked hydrogels for tympanic membrane repair. Otolaryngol Neck Surg 2006;135:877-83. [DOI] [PubMed] [Google Scholar]
- 48.Lou Z, Xu L, Yang J, Wu X. Outcome of children with edge-everted traumatic tympanic membrane perforations following spontaneous healing versus fibroblast growth factor-containing gelfoam patching with or without edge repair. Int J Pediatr Otorhinolaryngol 2011;75:1285-8. [DOI] [PubMed] [Google Scholar]
- 49.O’Daniel T, Petitjean M, Jones SC, Zogg J, Martinez SA, Nolph MB, et al. Epidermal growth factor binding and action on tympanic membranes. Ann Otol Rhinol Laryngol 1990;99:80-4. [DOI] [PubMed] [Google Scholar]
- 50.Amoils C, Jackler R, Lustig L. Repair of chronic tympanic membrane perforations using epidermal growth factor. Arch Otolaryngol Head Neck Surg 1992;107:669-83. [DOI] [PubMed] [Google Scholar]
- 51.Lee A, Jackler R, Kato B, Scott N. Repair of chronic tympanic membrane perforations using epidermal growth factor: progress towards clinical application. Otol Neurotol 1994;15:10-8. [PubMed] [Google Scholar]
- 52.Dvorak D, Abbas G, Ali T, Stevenson S, Welling DB. Repair of chronic tympanic membrane perforations with long-term epidermal growth factor. Laryngoscope 1995;105:1300-4. [DOI] [PubMed] [Google Scholar]
- 53.Ramsay H, Heikkonen E, Laurila P. Effect of epidermal growth factor on tympanic membranes with chronic perforations: a clinical trial. Otolaryngol. Neck Surg 1995;113:375-9. [DOI] [PubMed] [Google Scholar]
- 54.Cha D, O’Brien P, O’Toole E, Woodley DT, Hudson LG. Enhanced modulation of keratinocyte motility by transforming growth factor alpha (TGF-a) relative to epidermal growth factor (EGF). J Invest Dermatol 1996;106:590-7. [DOI] [PubMed] [Google Scholar]
- 55.Koba R, Kawabata I. Immunohistochemical study of transforming growth factor-expression in normal and perforated tympanic membrane. Ann Otol Rhinol Laryngol 1995;104:793-7. [DOI] [PubMed] [Google Scholar]
- 56.Kakigi A, Uchida A, Nishimura M, Takeda T, Takeda S, Nakatani H. Expression of fibroblast growth factor receptors 1-4 in human chronic tympanic membrane perforation. J Otorhinolaryngol Relat Spec 2010;71:67-70. [DOI] [PubMed] [Google Scholar]
- 57.Kato M, Jackler R. Repair of chronic tympanic membrane perforations with fibroblast growth factor. Otolaryngol Neck Surg 1996;115:538-47. [DOI] [PubMed] [Google Scholar]
- 58.Fina M, Bresnick S, Baird A, Ryan A. Improved healing of tympanic membrane perforations with basic fibroblast growth factor. Growth Factors 1991;5:265-72. [DOI] [PubMed] [Google Scholar]
- 59.Hakuba N, Hato N, Omotehara Y, Okada M, Gyo K. Epithelial pearl formation following tympanic membrane regeneration therapy using an atelocollagen/silicone membrane and basic fibroblast growth factor: our experience from a retrospective study of one hundred sixteen patients. Clin Otolaryngol 2013;38:394-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kanemaru S-I, Umeda H, Kitani Y, Nakamura T, Hirano S, Ito J. Regenerative treatment for tympanic membrane perforation. Otol Neurotol 2011;32:1218-23. [DOI] [PubMed] [Google Scholar]
- 61.Ishibashi T, Shinogami M, Ishimoto S, Yoshida K, Kaga K. Induction of KGF, basic FGF, and TGF alpha mRNA expression during healing of experimental TM perforations. Acta Otolaryngol 1998;118:701-4. [DOI] [PubMed] [Google Scholar]
- 62.Clymer M, Schwaber M, Davidson J. The effects of keratinocyte growth factor on healing of tympanic membrane perforations. Laryngoscope 1996;106:280-5. [DOI] [PubMed] [Google Scholar]
- 63.Yeo S, Kim S, Suh B, Cho S. Effects of platelet-derived growth factor-AA on the healing process of tympanic membrane perforation. Am J Otolaryngol 2000;21:153-60. [DOI] [PubMed] [Google Scholar]
- 64.Röösli C, Von Büren T, Gassmann N, Huber A. The impact of platelet-derived growth factor on closure of chronic tympanic membrane perforations: a randomized, double-blind, placebo-controlled study. Otol Neurotol 2011;32:1224-9. [DOI] [PubMed] [Google Scholar]
- 65.Cho K, Lee D, Shin D, Park YD, Chon KM. The importance of vascular endothelial growth factor in the healing of acute tympanic membrane perforation. Am J Otolaryngol 2009;31:309-14. [DOI] [PubMed] [Google Scholar]
- 66.Kaftan H, Herzog M, Miehe B, Hosemann W. Topical application of transforming growth factor beta-1 in acute traumatic tympanic membrane perforations: an experimental study in rats. Wound Repair Regen 2006;14:453-6. [DOI] [PubMed] [Google Scholar]
- 67.Eken M, Ates G, Sanli A, Evren C, Bozkurt S. The effect of topical insulin on the healing of acute tympanic membrane perforations: a histopathologic study. Eur Arch Otorhinolaryngol 2007;264:999-1002. [DOI] [PubMed] [Google Scholar]
- 68.Erkilet E, Koyuncu M, Atmaca S, Yarim M. Platelet-rich plasma improves healing of tympanic membrane perforations: experimental study. J Laryngol Otol 2009;123:482-7. [DOI] [PubMed] [Google Scholar]
- 69.Ho C, Beom Y, Yeo M, Lee H, Min EJ, Lee BH, et al. Regeneration of chronic tympanic membrane perforation using 3D collagen with topical umbilical cord serum. Int J Biol Macromol 2013;62:232-40. [DOI] [PubMed] [Google Scholar]
- 70.Ozturk K, Yaman H, Avunduk M, Arbag H, Keles B, Uyar Y. Effectiveness of MeroGel hyaluronic acid on tympanic membrane perforations. Acta Otolaryngol 2006;126:1158-63. [DOI] [PubMed] [Google Scholar]
- 71.Pujary P, Pujary K, Ramawamy B, Kanth S. Topical insulin for treatment of small central perforations - a pilot study. Int Adv Otol 2011;7:317-22. [Google Scholar]
- 72.Wang AY, Shen Y, Wang JT, Eikelboom RH, Dilley RJ. Animal models of chronic tympanic membrane perforation: in response to plasminogen initiates and potentiates the healing of acute and chronic tympanic membrane perforations in mice. Clin Transl Med 2014;3:5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Shen Y, Guo Y, Wilczynska M, Li J, Hellström S, Ny T. Plasminogen initiates and potentiates the healing of acute and chronic tympanic membrane perforations in mice. J Transl Med 2014;12:5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Stenfors L. Treatment of tympanic membrane perforations with hyaluronan in an open pilot study of unselected patients. Acta Otolaryngol 1987;104:81-7. [DOI] [PubMed] [Google Scholar]
- 75.Deng Z, Wu J, Qiu J, Wang J, Tian Y, Li Y, et al. Comparison of porcine acellular dermis and dura mater as natural scaffolds for bioengineering tympanic membrane. Tissue Eng A 2009;15:3729-39. [DOI] [PubMed] [Google Scholar]
- 76.Von Unge M, Dirckx JJJ, Olivius NP. Embryonic stem cells enhance the healing of tympanic membrane perforations. Int J Pediatr Otorhinolaryngol 2003;67:215-9. [DOI] [PubMed] [Google Scholar]
- 77.Rahman A, Von Unge M, Olivius P, Dirckx J, Hultcrantz M. Healing time, long-term result and effects of stem cell treatment in acute tympanic membrane perforation. Int J Pediatr Otorhinolaryngol 2007;71:1129-37. [DOI] [PubMed] [Google Scholar]
- 78.Rocca A, D’Alessandro D, Chiellini F, Dinucci D, Puppi D, Trombi L, et al. In vitro study on the generation of tympanic membrane substitutes via tissue engineering. Ital J Anat Embriol 2012;117:2012. [Google Scholar]
- 79.Rahman A, Olivius P, Dirckx J, Von Unge M, Hultcrantz M, et al. Stem cells and enhanced healing of chronic tympanic membrane perforation. Stem Cells 2008;128:352-9. [DOI] [PubMed] [Google Scholar]
- 80.Knutsson J, Von Unge M, Rask-Andersen H. Localization of progenitor/stem cells in the human tympanic membrane. Audiol Neurootol 2011;16:263-9. [DOI] [PubMed] [Google Scholar]


