Supplemental Digital Content is Available in the Text.
Key Words: antiretroviral therapy, mortality, lost to follow-up, survival, retention
Abstract:
In resource-limited settings, early mortality on antiretroviral therapy (ART) is approximately 10%; yet, it is unclear how much of that mortality occurs in care or after lost to follow-up. We assessed mortality rates and predictors of death among 12,222 nonpregnant ART-naive adults initiating first-line ART between April 2004 and May 2012 in South Africa, stratified by person-years in care and lost. We found 14.6% of patients died and being lost accounted for a minority of deaths across multiple definitions of loss (population attributable-risk percent ranged from 10.4% to 42.5%). Although mortality rates in patients lost were much higher than in care, most ART-related mortality occurred on treatment.
INTRODUCTION
The rapid scale-up of antiretroviral therapy (ART) HIV treatment in low- and middle-income countries has led to a substantial decrease in morbidity and mortality, averting nearly 4.2 million deaths in the decade from 2002 to 2012.1 Although mortality rates (MRs) remain higher in HIV-positive adults than in those without HIV, this difference is decreasing,2 and data from Rwanda,3 Uganda,4 and South African cohorts5 have shown that HIV-positive adults can achieve near-normal life expectancies with timely ART initiation. In South Africa, substantial gains in population-level adult life expectancy have been attributed to ART rollout since 2004.6,7
These gains are remarkable and attest to the success of one of the most effective public health programs in history. Still, in resource-limited settings, 1-year mortality in patients who start ART averages approximately 10% with decreasing rates thereafter.8–12 Although this has been remarkably consistent across settings, there has been little ability to reduce this early mortality.
Patient attrition and late appearance for treatment are likely contributing to early mortality,13,14 yet record-keeping and mortality assessment from observational cohorts rarely allow differentiating what percentage of mortality occurs in care or among patients lost to follow-up (LTF). With more than 2.2 million people on treatment,1 South Africa provides a unique place to try to answer this question as roughly 94% of all adult deaths among citizens are detected by the National Population Register (NPR).15 This makes it possible to independently estimate mortality in patients who are lost from care16–18 or transferred out.18 In this study, we aimed to compare mortality in patients in care and lost after treatment initiation and assess the sensitivity of estimates to different definitions of LTF.
METHODS
Study Site
We used prospectively collected data from the Themba Lethu Clinic in Johannesburg, South Africa.19 Themba Lethu is a large public-sector ART clinic that has enrolled nearly 38,000 HIV-positive patients in care since April 2004, of whom more than 29,000 have initiated ART. All clinical and demographic data, laboratory test results, medications, and patient visits are captured in real time using an electronic patient management system, TherapyEdge-HIV.
Care at Themba Lethu follows national ART guidelines.20–22 From 2004 until March 2010, adults initiated ART with a CD4 count <200 cells per cubic millimeter or a WHO stage IV condition. In April 2010, treatment eligibility expanded for pregnant women and tuberculosis (TB) coinfected patients to a CD4 count <350 cells per cubic millimeter and in 2011 to all patients with a CD4 count <350 cells per cubic millimeter and patients with multi- or extensively drug-resistant TB regardless of CD4.
Antiretroviral medications are collected at monthly pharmacy visits (2004 guidelines) or monthly for the first 6–12 months and every 2 months thereafter if stable (2010 guidelines). Up to 3 attempts are made by phone to contact patients who miss 2 consecutive scheduled visits to try to return them to care or determine their vital status. To help prevent missed visits, an mHealth solution was implemented at the clinic in April 2008, which provides patients with reminders for the scheduled clinic visits and also enables patients to make contact with the clinic using a free SMS to change their appointment. We estimate that this is used by up to 15% of patients to change their appointment.
Study Population
This cohort analysis assessed mortality among nonpregnant ART-naive adults (≥18 years) initiating first-line ART between April 2004 and May 2012 at Themba Lethu. Only patients with valid national identity numbers (IDs) were included and mortality was ascertained from the NPR. Because deaths take up to 6 months to be recorded in the death register, we closed the data set on December 31, 2012, 6 months before registry linkage.
Study Variables
The exposure variable for the analysis was in care status (either in care or LTF). Our primary outcome was mortality.
We compared MRs after treatment initiation stratified by person-time in care and person-time lost. Although lost is often defined in clinics and research studies as ≥3 months late for a scheduled visit, several authors have shown that the chosen definition of lost can impact results,23–25 so we assessed this by varying the definition of lost from ≥1 day late for a scheduled visit to ≥6 months late. Person-time in care began at ART initiation or restarting ART after loss and ended at the earliest of LTF, death, or censoring (ie, transfer date or data set closure). Person-time lost accrued from date of loss until the earliest of restarting ART, death, or censoring. Using this approach, patients could contribute person-time to both the in care and lost groups, and individual patients could be considered lost on multiple occasions as they leave care temporarily before returning at a later date (see Figure S1, Supplemental Digital Content, http://links.lww.com/QAI/A714).
Statistical Methods
Baseline clinical characteristics and mortality were stratified by in care status and were summarized with descriptive statistics. We calculated MRs in care and lost (presented overall and separately for months 1–12, 13–24, and 1–24 after ART initiation) with corresponding MR differences, attributable-risk percent (AR%) among those lost, and population attributable-risk percent (PAR%) with 95% confidence intervals (CIs). The AR% among the exposed refers to the fraction of deaths among the exposed (lost) that would not have occurred if the exposure had not occurred. In other words, if we assume a causal association between loss and death, then AR% is the proportion of deaths among those lost that could be eliminated if patients always remained in care:
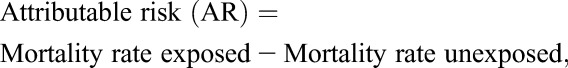 |
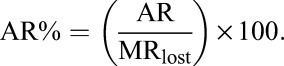 |
By contrast, the PAR% refers to the proportion of all deaths (among those lost and in care) that can be attributed to loss from care and could be prevented if patients always remained in care26:
 |
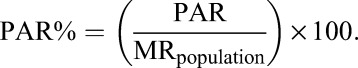 |
The relation between in care status and all-cause mortality was estimated with Cox proportional hazards models. Predictors of mortality were also estimated after stratifying by in care status. Hazard ratios (HRs) were adjusted for baseline covariates, including sex, age, CD4 count, body mass index, hemoglobin level, first-line ART regimen, ART start year, and TB coinfection.
Ethics
Retrospective analysis of the Themba Lethu cohort data set and linkage of these data with the NPR were approved by the Human Research Ethics Committee of the University of the Witwatersrand. Boston University provided permission for analysis of deidentified data.
RESULTS
A total of 18,483 patients initiated ART during the study period, of whom 12,222 (66.1%) had valid national IDs. Of these, 61.9% were female, median age was 37.2 years (interquartile range [IQR]: 31.8–43.8), and median CD4 count was 98 cells per cubic millimeter (IQR: 37–173) (Table 1). Patients were followed for a total of 43,378 person-years (py) (median 3.2 py, IQR 1.4–5.5) during which time 14.6% (1784/12,222) of patients died (Table 2). The overall MR was 41.1 deaths/1000 py (95% CI: 39.3 to 43.1), but it was substantially higher in the first year after ART initiation (96.2 deaths/1000 py, 95% CI: 90.5 to 102.4) than it was in the second year (31.2 deaths/1000 py, 95% CI: 27.6 to 35.2) (Supplementary Appendix Table S1 http://links.lww.com/QAI/A714). High early mortality was observed, with 47% (831/1784) of deaths occurring before a patient's last scheduled visit and another 18% (329/1784) of deaths occurring within 1 month after this date.
TABLE 1.
Baseline Characteristics of 18,483 Adults Initiating ART at Themba Lethu Clinic With and Without a Valid South African National ID Number
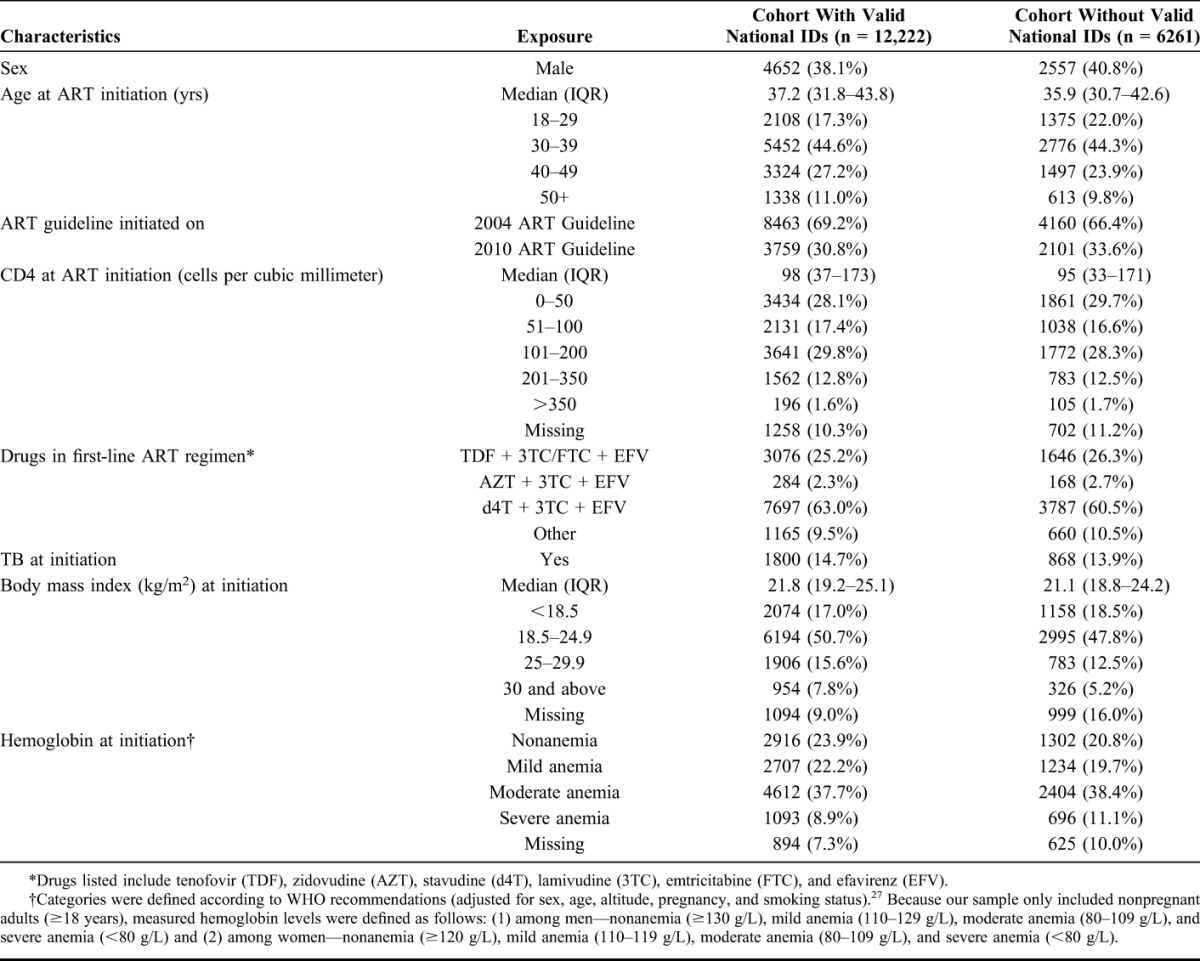
TABLE 2.
Mortality Rates Among 12,222 Adults in Care and LTF at The Themba Lethu HIV Clinic in Johannesburg, South Africa
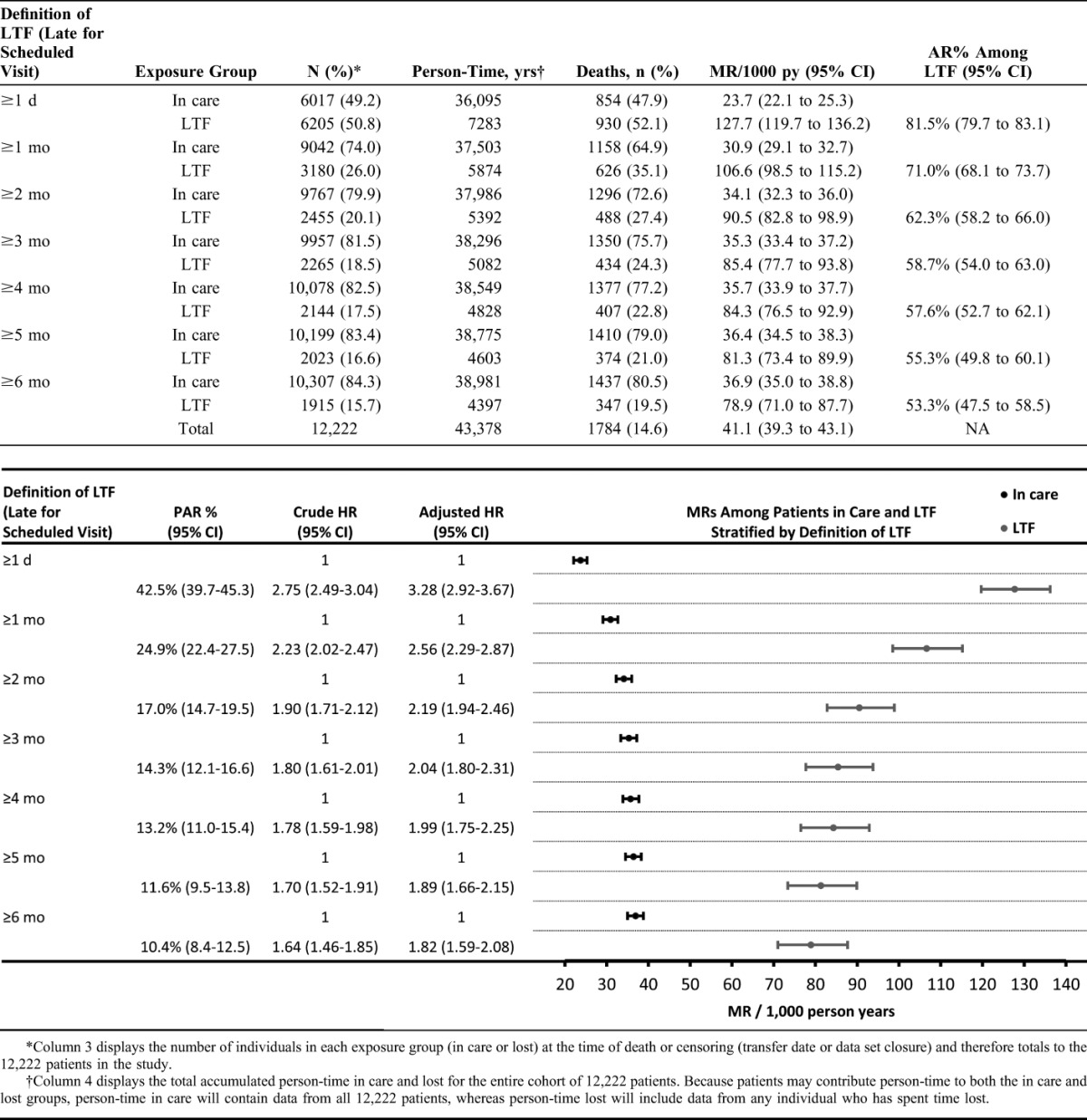
With loss defined as ≥3 months late for a scheduled visit, nearly 76% (1350/1784) of all deaths were considered to have occurred in care. Being lost accounted for a minority of overall deaths (AR%: 58.7%, 95% CI: 54.0 to 63.0 and PAR%: 14.3%, 95% CI: 12.1 to 16.6). However, in terms of rates, mortality was higher in patients lost (85.4 deaths/1000 py, 95% CI: 77.7 to 93.8) than in care (35.3 deaths/1000 py, 95% CI: 33.4 to 37.2). In adjusted analyses, individuals lost had 2.04 times higher mortality than those in care [adjusted HR (aHR): 2.04; 1.80–2.31]. A stratified analysis of predictors of death showed that low CD4 count at baseline was predictive of death among those in care and lost, although several baseline covariates were predictive of death only among those in care, including being male (aHR: 1.40; 1.23–1.61), more than 50 years of age (aHR: 2.07; 1.63–2.62), having a body mass index less than 18.5 kg/m2 (aHR: 1.62; 1.41–1.87) and having severe anemia (aHR: 3.62; 2.83–4.63) (Supplementary Appendix Table S2 http://links.lww.com/QAI/A714). As might be expected, the duration of time spent lost increased the proportion of patients who had died, with nearly 53% of patients lost in 2005 having died compared with just 11% in 2011.
As the definition of loss is varied from ≥1 day late to ≥6 months late for a scheduled visit, we found a shrinking minority of overall deaths could be attributed to loss from ART care (PAR% decreased from 43% to 10%). Additionally, the MR in patients lost decreased and the MR in patients in care increased (Table 2). However, mortality in patients lost (range: 79–128 deaths/1000 py) remained much higher than in patients in care (range: 24–37 deaths/1000 py) across all definitions of loss.
DISCUSSION
Understanding the timing of mortality of patients in care is critical to taking action to improve outcomes. Investigations of long-term retention in ART care have documented high rates of LTF, with considerable variation between countries.13,28,29 A recent review of 31 South African ART cohorts found the proportion of patients retained 12 months after ART initiation was 83% overall, falling to 77% after 24 months, and 62% after 60 months, with 40% of attrition because of deaths and 60% because of LTF.30 Although some patients who leave care will seek treatment elsewhere, mortality remains high among patients considered LTF,16,17 and our finding that 36% of deaths off ART occur within 6 months of loss is consistent with other studies.31
Our findings confirm that although MRs in patients lost are much higher than in care, most ART-related mortality occurs while patients are still in care. The high early mortality observed suggests that death is more likely to lead to loss from care than the reverse, yet what happens in the short window around loss remains unknown. It is possible that critically ill patients, sensing that death is near, simply leave care to return home or are unable to make it to the clinic. Interviews with family or other reliable informants close to the patient could help shed light on this issue.
As may be expected, using longer definitions of loss results in a greater proportion of deaths being classified as deaths in care, resulting in a decreasing hazard of death among patients lost. With loss defined as ≥3 months late for a scheduled visit, we found nearly 19% of patients lost died, less than half of the combined mortality reported in a systematic review,31 suggesting patients classified as lost in our cohort may be more likely to transfer into care at other clinics or are healthier when leaving care. However, without a universal definition for classifying patients as LTF, it is difficult to compare programs performance, including rates of loss and subsequent mortality, between facilities or cohorts. Efforts to standardize definitions of lost have found that defining loss as ≥180 days because the last clinic encounter minimizes the misclassification of loss,23 although this definition may not be ideal for patient management or the guiding of tracing activities. Our analysis shows relatively stable estimates of loss and subsequent MRs when the definition of loss is varied between 2 and 6 months late for a scheduled visit. To improve patient management and recall efforts, it may therefore be preferable to use a shorter definition of loss such as ≥2 months late for a scheduled visit, although other authors have stressed the importance of choosing a definition based on outcomes of interest, available visit data, and visit schedules.25
Limitations of this study likely include differential misclassification of exposure status and nondifferential misclassification of death. Active tracing programs have found that nearly a fifth of patients suspected of being lost are in fact alive and in care (self-transfers),27 and it is likely that some patients classified as lost in our cohort also remain in care. Likewise, unrecorded early deaths may lead to patients being misclassified as lost. These two phenomena are likely to bias our HR estimates toward the null. However, because ascertainment of death is achieved through linkage with the NPR, which is highly sensitive, only a small proportion of deaths may not be recorded. Although patients without valid national IDs were not included in our study, they have been identified as more likely to drop out of care than patients with valid national IDs,32 making it likely that overall MRs are even higher in this group.
In South Africa, there are signs that patients who started ART more recently may be more likely to be lost than those who initiated in earlier years,9,10,33 although some authors have pointed out that much of this trend may be attributed to bias.34 If genuine, this trend may reflect a decreasing capacity to adequately support patients in the long term as ART treatment programs expand, or it could be that earlier treatment initiation, as recommended in recent treatment guidelines, is associated with greater loss from care. With access to treatment expanding, it could also be that patients are more likely to self-transfer (leading to misclassification of loss) than was true in earlier years. These questions stress the importance of continued careful monitoring of the relationship between mortality and retention in ART care.
Supplementary Material
ACKNOWLEDGMENTS
The authors thank the directors and staff of Themba Lethu Clinic and to Right to Care, the nongovernmental organization supporting the study site through a partnership with US Agency for International Development (USAID). The authors also thank the Gauteng and National Department of Health for providing for the care of the patients at the Themba Lethu Clinic. Most of all, we thank the patients attending the clinic for their continued trust in the treatment provided. This study was made possible by the generous support of the American people through the USAID. The contents are the responsibility of the authors and do not necessarily reflect the views of USAID or the US Government.
Footnotes
Supported by the South Africa Mission of the US Agency for International Development (USAID) under the terms of Cooperative Agreement 674-A-12-00029 to the Health Economics and Epidemiology Research Office (HE2RO), a Division of the Wits Health Consortium (Pty) Ltd.
Presented at the 20th International AIDS Conference, July 20–25, 2014, Melbourne, Australia. (Poster number THPE068).
The authors have no conflicts of interest to disclose.
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal's Web site (www.jaids.com).
REFERENCES
- 1.WHO, UNAIDS, UNICEF. Global Update on HIV Treatment 2013: Results, Impact and Opportunities. 2013. Available at: www.who.int/hiv/pub/progressreports/update2013/en/. Accessed March 18, 2014. [Google Scholar]
- 2.Reniers G, Slaymaker E, Nakiyingi-Miiro J, et al. Mortality trends in the era of antiretroviral therapy: evidence from the Network for Analysing Longitudinal Population based HIV/AIDS data on Africa (ALPHA). AIDS. 2014;28(suppl 4):S533–S542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nsanzimana S, Remera E, Kanters S, et al. Life expectancy among HIV-positive patients in Rwanda: a retrospective observational cohort study. Lancet Glob Heal. 2015;3:e169–e177. [DOI] [PubMed] [Google Scholar]
- 4.Mills EJ, Bakanda C, Birungi J, et al. Life expectancy of persons receiving combination antiretroviral therapy in low-income countries: a cohort analysis from Uganda. Ann Intern Med. 2011;155:209–216. [DOI] [PubMed] [Google Scholar]
- 5.Johnson LF, Mossong J, Dorrington RE, et al. Life expectancies of South African adults starting antiretroviral treatment: collaborative analysis of cohort studies. PLoS Med. 2013;10:e1001418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bor J, Herbst AJ, Newell ML, et al. Increases in adult life expectancy in rural South Africa: valuing the scale-up of HIV treatment. Science. 2013;339:961–965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Statistics South Africa. Mid-Year Population Estimates 2015. Statistical release P0302; Pretoria, South Africa; 2015. Available at: www.statssa.gov.za/?page_id=1854&PPN=P0302&SCH=6334. Accessed August 20, 2015. [Google Scholar]
- 8.Boulle A, Van Cutsem G, Hilderbrand K, et al. Seven-year experience of a primary care antiretroviral treatment programme in Khayelitsha, South Africa. AIDS. 2010;24:563–572. [DOI] [PubMed] [Google Scholar]
- 9.Fox MP, Shearer K, Maskew M, et al. Treatment outcomes after 7 years of public-sector HIV treatment. AIDS. 2012;26:1823–1828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cornell M, Grimsrud A, Fairall L, et al. Temporal changes in programme outcomes among adult patients initiating antiretroviral therapy across South Africa, 2002-2007. AIDS. 2010;24:2263–2270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Keiser O, Orrell C, Egger M, et al. Public-health and individual approaches to antiretroviral therapy: township South Africa and Switzerland compared. PLoS Med. 2008;5:e148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Boulle A, Schomaker M, May MT, et al. Mortality in patients with HIV-1 infection starting antiretroviral therapy in South Africa, Europe, or North America: a collaborative analysis of Prospective studies. PLoS Med. 2014;11:e1001718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fox M, Rosen S. Retention of adult patients on antiretroviral therapy in low- and middle-income countries: systematic review and meta-analysis 2008-2013. J Acquir Immune Defic Syndr. 2015;69:98–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.The INSIGHT START Study Group. Initiation of antiretroviral therapy in early asymptomatic HIV infection. N Engl J Med. 2015;373:795–807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Statistics South Africa. Mortality and Causes of Death in South Africa: Findings from Death Notification, 2013. Pretoria, South Africa: Statistical release P0309.3; Statistics South Africa; 2014. Available at: www.statssa.gov.za/?page_id=1854&PPN=P0309.3&SCH=5955. Accessed August 20, 2015. [Google Scholar]
- 16.Fox MP, Brennan A, Maskew M, et al. Using vital registration data to update mortality among patients lost to follow-up from ART programmes: evidence from the Themba Lethu Clinic, South Africa. Trop Med Int Health. 2010;15:405–413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Van Cutsem G, Ford N, Hildebrand K, et al. Correcting for mortality among patients lost to follow up on antiretroviral therapy in South Africa: a cohort analysis. PLoS One. 2011;6:e14684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cornell M, Lessells R, Fox MP, et al. Mortality among adults transferred and lost to follow-up from antiretroviral therapy programmes in South Africa: a multicentre cohort study. J Acquir Immune Defic Syndr. 2014;67:e67–75. 10.1097/QAI.0000000000000269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fox MP, Maskew M, MacPhail AP, et al. Cohort profile: the Themba Lethu clinical cohort, Johannesburg, South Africa. Int J Epidemiol. 2013;42:430–439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.National Department of Health. National Antiretroviral Treatment Guidelines. 1st ed Pretoria, South Africa: Jacana, Minuteman Press; 2004. [Google Scholar]
- 21.National Department of Health. The South African Antiretroviral Treatment Guidelines. Pretoria, South Africa: National Department of Health Republic of South Africa; 2010. [Google Scholar]
- 22.National Department of Health. The South African Antiretroviral Treatment Guidelines. Pretoria, South Africa: National Department of Health, Republic of South Africa; 2013. Available at: www.kznhealth.gov.za/medicine/2013_art_guidelines.pdf. Accessed March 17, 2014. [Google Scholar]
- 23.Chi BH, Yiannoutsos CT, Westfall AO, et al. Universal definition of loss to follow-up in HIV treatment programs: a statistical analysis of 111 facilities in Africa, Asia, and Latin America. PLoS Med. 2011;8:e1001111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Grimsrud AT, Cornell M, Egger M, et al. Impact of definitions of loss to follow-up (LTFU) in antiretroviral therapy program evaluation: variation in the definition can have an appreciable impact on estimated proportions of LTFU. J Clin Epidemiol. 2013;66:1006–1013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shepherd BE, Blevins M, Vaz LME, et al. Impact of definitions of loss to follow-up on estimates of retention, disease progression, and mortality: application to an HIV program in Mozambique. Am J Epidemiol. 2013;178:819–828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hennekens C, Buring J. Epidemiology in Medicine. Mayrent S, ed. Philadelphia, PA: Lippincott Williams & Wilkins; 1987. [Google Scholar]
- 27.Wilkinson L, Skordis-Worrall J, Ajose O, et al. Self-transfer and mortality amongst adults lost to follow-up in ART programmes in low- and middle-income countries: systematic review and meta-analysis. Trop Med Int Heal. 2015;20:365–379. [DOI] [PubMed] [Google Scholar]
- 28.Tassie JM, Baijal P, Vitoria MA, et al. Trends in retention on antiretroviral therapy in national programs in low-income and middle-income countries. J Acquir Immune Defic Syndr. 2010;54:437–441. [DOI] [PubMed] [Google Scholar]
- 29.Fox M, Rosen S. Systematic review of retention of pediatric patients on HIV treatment in low and middle-income countries 2008-2013. AIDS. 2015;29:493–502. [DOI] [PubMed] [Google Scholar]
- 30.Rosen S, Fox MP. Retention on Antiretroviral Therapy in South Africa: Evidence from a Systematic Review. Johannesburg, South Africa: HERO Policy Brief Number 8, Health Economics and Epidemiology Research Office; 2014. Available at: www.heroza.org/publications/policy-brief-8-retention-antiretroviral-therapy-south-africa-evidence-systemic-review. Accessed June 16, 2014. [Google Scholar]
- 31.Brinkhof MWG, Pujades-Rodriguez M, Egger M. Mortality of patients lost to follow-up in antiretroviral treatment programmes in resource-limited settings: systematic review and meta-analysis. PLoS One. 2009;4:e5790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shearer K, Maskew M, Long L, et al. Impacts of disaggregating programmatic outcomes by documentation of citizenship in South Africa. Paper presented at: Poster TUPE418, 20th International AIDS Conference; July 20–25, 2014; Melbourne, Australia. [Google Scholar]
- 33.Nglazi MD, Lawn SD, Kaplan R, et al. Changes in programmatic outcomes during 7 years of scale-up at a community-based antiretroviral treatment service in South Africa. J Acquir Immune Defic Syndr. 2011;56:e1–e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Johnson L, Estill J, Keiser O, et al. Do increasing rates of loss to follow-up in antiretroviral treatment programs imply deteriorating patient retention? Am J Epidemiol. 2014;180:1208–1212. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


