Abstract
Background:
This study was aimed to evaluate the prevalence of obstructive sleep apnea (OSA) and restless legs syndrome (RLS) in patients with end-stage renal disease (ESRD) after kidney transplantation.
Materials and Methods:
Two hundred kidney transplant recipients were enrolled in this cross-sectional study. Data on age, gender, etiology of ESRD, history of previous kidney transplantation, serum creatinine, and the presence or absence of OSA and RLS were collected. Symptoms of RLS were identified using the RLS questionnaire which was completed by the patients. The Berlin questionnaire and polysomnography were used for diagnosing OSA.
Results:
The mean age of the studied patients was 45.86 ± 10.24 years. The prevalence of OSA was 26% (52 of 200 studied patients) and of RLS was 51.5% (103 of 200 studied patients). Majority of the patients with high-risk OSA were male and significantly older than the patients with low-risk OSA (P < 0.05). The prevalence of RLS was higher in patients with high-risk OSA and a higher level of creatinine compared to that in those with a low risk of OSA (P < 0.0001). Level of creatinine in patients with positive RLS was significantly higher than in those with negative RLS (P < 0.0001). OSA was observed in almost 42% of patients with positive RLS, compared to 9% of patients with negative RLS (P < 0.0001).
Conclusion:
In summary, our results indicate that the prevalence of OSA and RLS in kidney transplant recipients was higher than in the general population. Also, there was a significant association between OSA and RLS in these patients.
Keywords: Chronic kidney disease, end-stage renal disease, kidney transplant, obstructive sleep apnea, restless legs syndrome
INTRODUCTION
Chronic kidney disease (CKD) and end-stage renal disease (ESRD) are highly prevalent in both industrialized and developing countries of the world and have become a major public health problem.[1,2,3] CKD is associated with substantial morbidity, including poor health outcome due to cardiovascular diseases and end-stage kidney disease which affect 10–13% of the general population.[4,5] The epidemiology of CKD and ESRD has changed remarkably in the last decade, particularly in the developed countries, with diabetic nephropathy now assuming epidemic proportions.[6,7] Over the past 10 years, the prevalence of CKD has noticeably increased and this alarming trend is likely to continue.[5] Around the world, the incidence of ESRD that is treated by kidney transplantation varies. In 2010, globally, there were approximately 75,000 kidney transplants; but today, this level would rise to 350,000 or four to five times.[8,9]
Breathing abnormalities induced by sleep-disordered breathing are now recognized as a prevalent condition with serious adverse consequences.[10] The most common form of sleep-disordered breathing is obstructive sleep apnea (OSA). It is a highly prevalent disease affecting 2–4% of the general population (found in 4% of men and 2% of women), and is strongly linked to impaired quality of life and the current obesity epidemic.[11,12,13] Previous studies showed a high prevalence of OSA (27–54%) in patients with CKD.[14,15,16] The prevalence of the disorder in CKD patients not requiring kidney replacement was 54% and the apnea–hypopnea index was correlated with the renal function.[17]
Although dialysis does not reduce the prevalence of sleep disorders in ESRD patients,[18] instances of reversal of sleep-disordered breathing after kidney transplantation have been described in previous case reports.[19,20] The prevalence of sleep-disordered breathing in transplant patients shows that it does not differ from that of the general population;[21] also, discrepant results have been reported in some case series studies.[22,23] A few case reports have reported that following successful kidney transplantation, OSA is corrected.[19,20]
Restless legs syndrome (RLS) is characterized by distressing deep sensations in the limbs, particularly legs; it is a sensorimotor disturbance that is associated with an urge to move often at bedtime and may cause profound sleep disorders.[24] Prevalence of RLS shows variation from country to country and has been described to affect between 5 and 10% of the population.[25] RLS has been reported to be particularly frequent in association with some clinical conditions such as peripheral neuropathy, type 2 diabetes, uremia, multiple sclerosis, iron deficiency, hypothyroidism or hyperthyroidism, acute intermittent porphyria, and pregnancy.[26,27,28,29] A common cause of sleep disturbance is RLS, which is frequently experienced by hemodialysis patients compared with the normal population (6.6–21.5% vs 1–15%, respectively).[13] Also, in hemodialysis patients, RLS has been reported in association with insomnia, poor quality of sleep, and poor quality of life.[29,30] Among uremic patients under hemodialysis therapy, hyperphosphatemia, anxiety, and stress have been found to be independently related to the presence of RLS; therefore, improvement in renal function after kidney transplantation improves the symptoms and reduces the occurrence of RLS.[31]
Studies that have systematically evaluated the changes in OSA and RLS following kidney transplantation are limited. The present study tested the prevalence of OSA and RLS in patients with ESRD after kidney transplantation.
MATERIALS AND METHODS
This cross-sectional study was investigated and approved by the ethics committee of Isfahan University of Medical Sciences. Two hundred kidney transplant recipients who were regularly followed at Transplant Clinic of the Department of Transplantation and Surgery at Isfahan, Iran were enrolled in this study. Patients of both sexes, age older than 18 years, and who had received their kidney transplant between 2007 and 2013 were eligible, if they had no clinical evidence of pulmonary disease, radiological and functional alterations, symptomatic heart failure, autoimmune disease, or cancer. Also, supplemental oxygen use, asthma and unwillingness to participate in the study were the study exclusion criteria. Written informed consent was obtained from the patients, after which they were explained and informed of the purpose of the study.
Demographic data and details of medical history including age, gender, etiology of ESRD, history of previous kidney transplantation, serum creatinine, and the presence or absence of OSA and RLS were collected. A morning urine sample was analyzed for clear creatinine concentrations at a single laboratory. Symptoms of RLS were identified by using the RLS questionnaire that was completed by the patients. The questionnaire had been validated as a screening instrument for RLS in the sleep disorders clinic; it has been shown to be a reliable screening instrument for RLS and was used in a recent epidemiologic survey. The patients were asked to complete the first part of the questionnaire that included eight diagnostic questions and RLS was identified only if the patient met all the diagnostic criteria.[32,33] OSA was assessed in patients using the Berlin questionnaire. The Berlin questionnaire includes information about age, gender, height, weight, and neck size, and has five items on snoring (items 1–5, category 1), three items on daytime somnolence (items 6–8, category 2), and one item on the history of hypertension (item 9, category 3). If the scores were positive in two or more of the three categories, patients were classified as being at high risk of having OSA and if they were positive in less than two categories, patients were identified as being at a low risk of having OSA.[34]
Polysomnography is a precise method for diagnosing OSA, but this method has its disadvantages such as expensiveness, inaccessibility, and difficulty to perform. Thus, to better ensure the diagnostic validity of apnea–hypopnea index, some cases (22 patients who were willing) defined as OSA patients were examined with polysomnography and the polysomnography results of all 22 cases were found to be positive. The polysomnography was done in the sleep lab using the Somnoscreen system (SSC; Somnomedics, Germany). The polysomnography consisted of 14-channel, continuous polygraphic recording from surface leads for electroencephalography, electrooculography, chin electromyography, electrocardiography, sensors for nasal airflow, tracheal sounds, thoracic and abdominal respiratory effort, finger pulse oximeter, leg movements, body position, and light sleep. An apnea–hypopnea index of >5 was considered for the diagnosis of OSA.[10]
The present survey was carried out in a sample of 233 patients, during 6 years (2007–2013). SPSS software for Windows, version 20 (SPSS Inc.; Chicago, IL, USA), was used for statistical analyses, and descriptive data are presented as mean ± SD, median [IQR], and number (percent). Significant differences between the study subgroups were determined by using independent samples t-test for continuous variables and Chi-square test for categorical variables. Correlation analysis was performed using Spearman correlation analysis and Cramer's V test. The level of significance is considered to be P < 0.05.
RESULTS
The study period was between March and September 2013. Among the 233 kidney transplant recipients screened for potential participation, 33 patients were excluded (14 patients did not meet the inclusion criteria and 19 patients refused to participate).
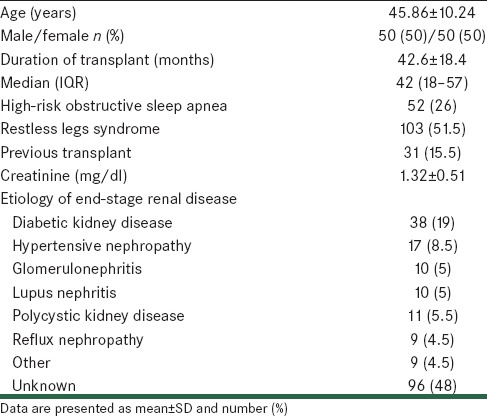
Characteristics and clinical parameters of the studied patients are shown in Table 1. The mean age of the studied patients was 45.86 ± 10.24 years. One hundred patients (50%) were male and the remaining 100 patients (50%) were female. The prevalence of high-risk OSA was 26% (52 of 200 studied patients) and the prevalence of RLS was 51.5% (103 of 200 studied patients). Etiology of ESRD in most the studied patients was unknown (48%), and diabetic kidney disease was found to be another main cause of ESRD (19%).
Table 1.
Characteristics of the study patients
Results obtained on comparing age, sex, duration of transplant, etiology of ESRD, history of previous kidney transplantation, serum creatinine, and RLS in the studied patients with regard to the presence or absence of OSA are presented in Table 2. It was observed that patients with high-risk OSA were significantly older than the patients with low-risk OSA (P = 0.029). A higher percentage of patients with high-risk OSA were male, as compared to those with low-risk OSA (P = 0.004). The prevalence of RLS was higher (82%) in patients with high-risk OSA, as compared to 40% in patients with low-risk OSA (P < 0.0001). Also, patients with high-risk OSA had significantly higher level of creatinine than those with low-risk OSA (P < 0.0001). Etiology of ESRD and history of previous kidney transplantation were similar in patients with high-risk or low-risk OSA (P > 0.05).
Table 2.
Clinical and demographic characteristics of OSA patients and those without OSA
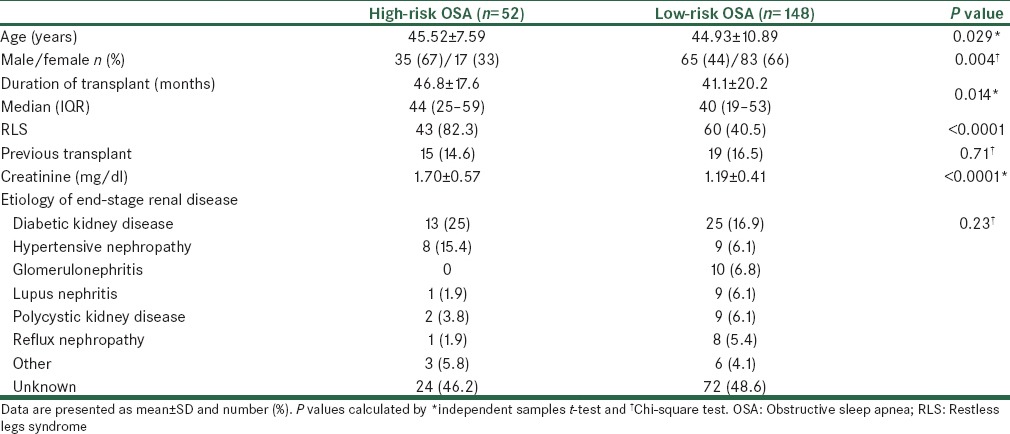
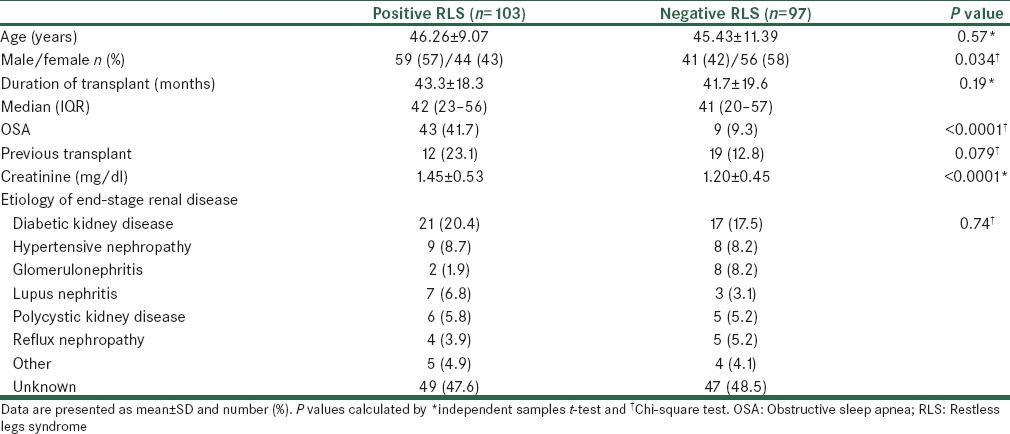
Table 3 shows the results obtained on comparing age, sex, duration of transplant, etiology of ESRD, history of previous kidney transplantation, serum creatinine, and OSA in the studied patients with regard to the presence or absence of RLS. Age, duration of transplant, history of previous kidney transplantation, and etiology of ESRD were similar in patients with positive RLS, as compared to those with negative RLS (P > 0.05). Sex distribution, the prevalence of OSA, and the level of creatinine were significantly different between patients with positive and negative RLS. Level of creatinine in patients with positive RLS was significantly higher than in patients with negative RLS (P < 0.0001). OSA was observed in 42% of patients with positive RLS, as compared to 9% in patients with negative RLS (P < 0.0001).
Table 3.
Clinical and demographic characteristics of RLS patients and those without RLS
DISCUSSION
In the present study, the association between kidney transplantation with OSA and RLS in patients with ESRD was assessed and the results showed that a higher percentage of patients with high-risk OSA were male and were older than the patients with negative OSA. The prevalence of RLS was higher (82%) in patients with OSA, as compared to 40% in patients without OSA. Also, patients with high-risk OSA had significantly higher level of creatinine than those with low-risk OSA. Etiology of ESRD and history of previous kidney transplantation were similar in patients with positive or low-risk OSA. Level of creatinine in patients with positive RLS was significantly higher than in those with negative RLS. High-risk OSA was observed in 42% of patients with positive RLS, as compared to 9% in patients with negative RLS.
Disorders of sleep and wakefulness are widely prevalent nowadays and have a significant impact on different aspects of health in patients receiving maintenance dialysis and also in CKD patients in the predialysis stage.[35,36,37,38] There is only very limited data available regarding the prevalence of sleep disorders in the kidney-transplanted population, although several studies describe the high prevalence of sleep disorders in patients on maintenance dialysis[39,40,41] and OSA is reported to be remarkably common among individuals with ESRD (an estimated 50% of patients).[39] In 1993, the first case study was reported presenting two patients with sleep apnea (one patient with obstructive apnea and the other one with central apnea) in whom the syndrome disappeared after kidney transplantation.[19] A similar study reported a case in 1999.[20] In another study, the prevalence of high-risk OSA was reported as 27% in the transplanted group;[42] in this study, patients were asked to complete the Berlin Sleep Apnea Questionnaire and the Epworth Sleepiness Scale to assess their risk status for OSA and daytime sleepiness, respectively. The authors reported worse renal function, male gender, and older age as independent predictors for high risk of OSA.[42] In the present study, we found a similar prevalence of OSA (26%) in kidney transplant recipients, male gender and older age with OSA as compared to those without OSA. Our findings were similar to those of others, but these studies were limited by small size, lack of controls and adjustment for potential confounders, and use of a selected population with poor generalizability. These results may create the impression that kidney transplantation corrects OSA in all patients with ESRD.
The prevalence of RLS has been found to be between 5 and 15% in the general population.[12] The prevalence of RLS in dialysis patients was reported to be 6.6–21.5% in recent studies.[39,43] Data on RLS in kidney-transplanted patients are limited and only two studies have been published on RLS in kidney-transplanted patients. In one study by Molnar and colleagues published in 2007,[42] the presence of RLS in kidney-transplanted patients was assessed using the Restless Legs Syndrome Questionnaire and the prevalence of RLS was found to be 4.8%. In another study by Winkelmann and colleagues,[44] 11 patients with uremic RLS who had undergone successful kidney transplantation were followed, and within a month, the symptoms of RLS disappeared after transplantation in all patients; but they reappeared after several years in some of them and in most of the patients in whom the transplanted kidney was failing. Molnar and colleagues[45] showed that the presence of RLS was associated with mortality in kidney transplant recipients and concluded that at the time of the initial assessment, patients with RLS had twice as high a chance to die within 4 years than the patients without RLS. The prevalence of RLS in kidney transplantation patients was found to be 51% of the studied patients in the present study, which was higher than that in the general population and Molnar et al.'s[45] findings but similar to Winkelmann et al.'s[44] findings. The small sample size and the studied population could be the reasons for the differences observed.
The study also has some limitations. First, we cannot comment on causality because of the cross-sectional nature of the study. In addition, while the measurements preceded the sleep measurements by 7 years, we have no information regarding the presence or absence of RLS and OSA at baseline; therefore, our study cannot address causality.
In summary, our results indicate that the prevalence of high-risk OSA and RLS in kidney transplant recipients was 26% and 51%, respectively, which was higher than that in the general population. Also, there was a significant association between OSA and RLS in these patients; however, we cannot address causality due to the cross-sectional design of the study.
Financial support and sponsorship
Isfahan University of Medical Sciences.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Hanly PJ, Pierratos A. Improvement of sleep apnea in patients with chronic renal failure who undergo nocturnal hemodialysis. N Engl J Med. 2001;344:102–7. doi: 10.1056/NEJM200101113440204. [DOI] [PubMed] [Google Scholar]
- 2.Stepanski E, Faber M, Zorick F, Basner R, Roth T. Sleep disorders in patients on continuous ambulatory peritoneal dialysis. J Am Soc Nephrol. 1995;6:192–7. doi: 10.1681/ASN.V62192. [DOI] [PubMed] [Google Scholar]
- 3.Unruh ML, Sanders MH, Redline S, Piraino BM, Umans JG, Hammond TC, et al. Sleep apnea in patients on conventional thrice-weekly hemodialysis: Comparison with matched controls from the Sleep Heart Health Study. J Am Soc Nephrol. 2006;17:3503–9. doi: 10.1681/ASN.2006060659. [DOI] [PubMed] [Google Scholar]
- 4.Wen CP, Cheng TY, Tsai MK, Chang YC, Chan HT, Tsai SP, et al. All-cause mortality attributable to chronic kidney disease: A prospective cohort study based on 462293 adults in Taiwan. Lancet. 2008;371:2173–82. doi: 10.1016/S0140-6736(08)60952-6. [DOI] [PubMed] [Google Scholar]
- 5.Coresh J, Selvin E, Stevens LA, Manzi J, Kusek JW, Eggers P, et al. Prevalence of chronic kidney disease in the United States. JAMA. 2007;298:2038–47. doi: 10.1001/jama.298.17.2038. [DOI] [PubMed] [Google Scholar]
- 6.Fleischmann G, Fillafer G, Matterer H, Skrabal F, Kotanko P. Prevalence of chronic kidney disease in patients with suspected sleep apnoea. Nephrol Dial Transplant. 2010;25:181–6. doi: 10.1093/ndt/gfp403. [DOI] [PubMed] [Google Scholar]
- 7.Gooneratne IK, Ranaweera AK, Liyanarachchi NP, Gunawardane N, Lanerolle RD. Epidemiology of chronic kidney disease in a Sri Lankan population. Int J Diabetes Dev Ctries. 2008;28:60–4. doi: 10.4103/0973-3930.43101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chapman JR. What are the key challenges we face in kidney transplantation today? Transplant Res. 2013;2:S1. doi: 10.1186/2047-1440-2-S1-S1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Welberry Smith MP, Baker RJ. Assessment and management of a patient with a renal transplant. Br J Hosp Med. 2007;68:656–62. doi: 10.12968/hmed.2007.68.12.656. [DOI] [PubMed] [Google Scholar]
- 10.Sleep-related breathing disorders in adults: Recommendations for syndrome definition and measurement techniques in clinical research. The report of an American Academy of Sleep Medicine Task force. Sleep. 1999;22:667–89. [PubMed] [Google Scholar]
- 11.Mirrakhimov AE, Sooronbaev T, Mirrakhimov EM. Prevalence of obstructive sleep apnea in Asian adults: A systematic review of the literature. BMC Pulm Med. 2013;13:10. doi: 10.1186/1471-2466-13-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Unruh ML, Levey AS, D’Ambrosio C, Fink NE, Powe NR, Meyer KB. Restless legs symptoms among incident dialysis patients: Association with lower quality of life and shorter survival. Am J Kidney Dis. 2004;43:900–9. doi: 10.1053/j.ajkd.2004.01.013. [DOI] [PubMed] [Google Scholar]
- 13.Molnar MZ, Novak M, Ambrus C, Szeifert L, Kovacs A, Pap J, et al. Restless legs syndrome in patients after renal transplantation. Am J Kidney Dis. 2005;45:388–96. doi: 10.1053/j.ajkd.2004.10.007. [DOI] [PubMed] [Google Scholar]
- 14.Canales MT, Lui LY, Taylor BC, Ishani A, Mehra R, Stone KL, et al. Renal function and sleep-disordered breathing in older men. Nephrol Dial Transplant. 2008;23:3908–14. doi: 10.1093/ndt/gfn364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Roumelioti ME, Buysse DJ, Sanders MH, Strollo P, Newman AB, Unruh ML. Sleep-disordered breathing and excessive daytime sleepiness in chronic kidney disease and hemodialysis. Clin J Am Soc Nephrol. 2011;6:986–94. doi: 10.2215/CJN.05720710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nicholl DD, Ahmed SB, Loewen AH, Hemmelgarn BR, Sola DY, Beecroft JM, et al. Declining kidney function increases the prevalence of sleep apnea and nocturnal hypoxia. Chest. 2012;141:1422–30. doi: 10.1378/chest.11-1809. [DOI] [PubMed] [Google Scholar]
- 17.Markou N, Kanakaki M, Myrianthefs P, Hadjiyanakos D, Vlassopoulos D, Damianos A, et al. Sleep-disordered breathing in nondialyzed patients with chronic renal failure. Lung. 2006;184:43–9. doi: 10.1007/s00408-005-2563-2. [DOI] [PubMed] [Google Scholar]
- 18.Mendelson WB, Wadhwa NK, Greenberg HE, Gujavarty K, Bergofsky E. Effects of hemodialysis on sleep apnea syndrome in end-stage renal disease. Clin Nephrol. 1990;33:247–51. [PubMed] [Google Scholar]
- 19.Langevin B, Fouque D, Léger P, Robert D. Sleep apnea syndrome and end-stage renal disease. Cure after renal transplantation. Chest. 1993;103:1330–5. doi: 10.1378/chest.103.5.1330. [DOI] [PubMed] [Google Scholar]
- 20.Auckley DH, Schmidt-Nowara W, Brown LK. Reversal of sleep apnea hypopnea syndrome in end-stage renal disease after kidney transplantation. Am J Kidney Dis. 1999;34:739–44. doi: 10.1016/S0272-6386(99)70401-4. [DOI] [PubMed] [Google Scholar]
- 21.Mallamaci F, Leonardis D, Tripepi R, Parlongo G, Catalano C, Tripepi G, et al. Sleep disordered breathing in renal transplant patients. Am J Transplant. 2009;9:1373–81. doi: 10.1111/j.1600-6143.2009.02653.x. [DOI] [PubMed] [Google Scholar]
- 22.Jurado-Gámez B, Martin-Malo A, Rodriguez-Benot A, Muñoz-Cabrera L, Cosano Povedano A, Aljama P. Kidney transplantation improves sleep-related breathing in hemodialysis patients. Blood Purif. 2008;26:485–90. doi: 10.1159/000157373. [DOI] [PubMed] [Google Scholar]
- 23.Beecroft JM, Zaltzman J, Prasad R, Meliton G, Hanly PJ. Impact of kidney transplantation on sleep apnea in patients with end-stage renal disease. Nephrol Dial Transplant. 2007;22:3028–33. doi: 10.1093/ndt/gfm309. [DOI] [PubMed] [Google Scholar]
- 24.Berger K, Luedemann J, Trenkwalder C, Ulrich J, Kessler C. Sex and the risk of restless legs syndrome in the general population. Arch Intern Med. 2004;164:196–202. doi: 10.1001/archinte.164.2.196. [DOI] [PubMed] [Google Scholar]
- 25.Ulfberg J, Nystrom B, Carter N, Edling C. Restless legs syndrome among working-aged women. Eur Neurol. 2001;46:17–9. doi: 10.1159/000050750. [DOI] [PubMed] [Google Scholar]
- 26.Walters AS. The International Restless Legs Syndrome study group. Toward a better definition of the restless legs syndrome. Mov Disord. 1995;10:634–42. doi: 10.1002/mds.870100517. [DOI] [PubMed] [Google Scholar]
- 27.Allen RP, Picchietti D, Hening WA, Trenkwalder C, Walters AS, Montplaisi J. Restless legs syndrome: Diagnostic criteria, special considerations, and epidemiology. A report from the restless legs syndrome diagnosis and epidemiology workshop at the National Institutes of Health. Sleep Med. 2003;4:101–19. doi: 10.1016/s1389-9457(03)00010-8. [DOI] [PubMed] [Google Scholar]
- 28.Ulfberg J, Nystrom B, Carter N, Edling C. Prevalence of restless legs syndrome among men aged 18 to 64 years: An association with somatic disease and neuropsychiatric symptoms. Mov Disord. 2001;16:1159–63. doi: 10.1002/mds.1209. [DOI] [PubMed] [Google Scholar]
- 29.Tachibana N, Tanigawa T. Prevalance and clinical characteristics of restless legs syndrome among Japanese industrial workers. Neurology. 2003;60:38. [Google Scholar]
- 30.Tan EK, Seah A, See SJ, Lim E, Wong MC, Koh KK. Restless legs syndrome in an Asian population: A study in Singapore. Mov Disord. 2001;16:577–8. doi: 10.1002/mds.1102. [DOI] [PubMed] [Google Scholar]
- 31.Azar SA, Hatefi R, Talebi M. Evaluation of effect of renal transplantation in treatment of restless legs syndrome. Transplant Proc. 2007;39:1132–3. doi: 10.1016/j.transproceed.2007.03.097. [DOI] [PubMed] [Google Scholar]
- 32.Nichols DA, Allen RP, Grauke JH, Brown JB, Rice ML, Hyde PR, et al. Restless legs syndrome symptoms in primary care: A prevalence study. Arch Intern Med. 2003;163:2323–9. doi: 10.1001/archinte.163.19.2323. [DOI] [PubMed] [Google Scholar]
- 33.Allen RP, Earley CJ. Validation of a diagnostic questionnaire for the restless legs syndrome (RLS) Neurology. 2001;56:4. [Google Scholar]
- 34.Netzer NC, Stoohs RA, Netzer CM, Clark K, Strohl KP. Using the Berlin Questionnaire to identify patients at risk for the sleep apnea syndrome. Ann Intern Med. 1999;131:485–91. doi: 10.7326/0003-4819-131-7-199910050-00002. [DOI] [PubMed] [Google Scholar]
- 35.Gusbeth-Tatomir P, Boisteanu D, Seica A, Buga C, Covic A. Sleep disorders: A systematic review of an emerging major clinical issue in renal patients. Int Urol Nephrol. 2007;39:1217–26. doi: 10.1007/s11255-007-9262-2. [DOI] [PubMed] [Google Scholar]
- 36.Iliescu EA, Coo H, McMurray MH, Meers CL, Quinn MM, Singer MA, et al. Quality of sleep and health-related quality of life in haemodialysis patients. Nephrol Dial Transplant. 2003;18:126–32. doi: 10.1093/ndt/18.1.126. [DOI] [PubMed] [Google Scholar]
- 37.Iliescu EA, Yeates KE, Holland DC. Quality of sleep in patients with chronic kidney disease. Nephrol Dial Transplant. 2004;19:95–9. doi: 10.1093/ndt/gfg423. [DOI] [PubMed] [Google Scholar]
- 38.Parker KP, Kutner NG, Bliwise DL, Bailey JL, Rye DB. Nocturnal sleep, daytime sleepiness, and quality of life in stable patients on hemodialysis. Health Qual Life Outcomes. 2003;1:68. doi: 10.1186/1477-7525-1-68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mucsi I, Molnar MZ, Rethelyi J, Vamos E, Csepanyi G, Tompa G, et al. Sleep disorders and illness intrusiveness in patients on chronic dialysis. Nephrol Dial Transplant. 2004;19:1815–22. doi: 10.1093/ndt/gfh130. [DOI] [PubMed] [Google Scholar]
- 40.Eryavuz N, Yuksel S, Acarturk G, Uslan I, Demir S, Demir M, et al. Comparison of sleep quality between hemodialysis and peritonealdialysis patients. Int Urol Nephrol. 2008;40:785–91. doi: 10.1007/s11255-008-9359-2. [DOI] [PubMed] [Google Scholar]
- 41.Mucsi I, Molnar MZ, Ambrus C, Szeifert L, Kovacs AZ, Zoller R, et al. Restless legs syndrome, insomnia and quality of life in patients on maintenance dialysis. Nephrol Dial Transplant. 2005;20:571–7. doi: 10.1093/ndt/gfh654. [DOI] [PubMed] [Google Scholar]
- 42.Molnar MZ, Szentkiralyi A, Lindner A, Czira ME, Szabo A, Mucsi I, et al. High prevalence of patients with a high risk for obstructive sleep apnoea syndrome after kidney transplantation association with declining renal function. Nephrol Dial Transplant. 2007;22:2686–92. doi: 10.1093/ndt/gfm246. [DOI] [PubMed] [Google Scholar]
- 43.Gigli GL, Adorati M, Dolso P, Piani A, Valente M, Brotini S, et al. Restless legs syndrome in end-stage renal disease. Sleep Med. 2004;5:309–15. doi: 10.1016/j.sleep.2004.01.014. [DOI] [PubMed] [Google Scholar]
- 44.Winkelmann J, Stautner A, Samtleben W, Trenkwalder C. Long-term course of restless legs syndrome in dialysis patients after kidney transplantation. Mov Disord. 2002;17:1072–6. doi: 10.1002/mds.10231. [DOI] [PubMed] [Google Scholar]
- 45.Molnar MZ, Novak M, Mucsi I. Sleep disorders and quality of life in renal transplant recipients. Int Urol Nephrol. 2009;41:373–82. doi: 10.1007/s11255-009-9527-z. [DOI] [PubMed] [Google Scholar]


