Abstract
Background:
Stroke is the second cause of death among elderly people. Oxidative stress plays an important role in brain damage after stroke. Currently, Vitamin D has been shown as an antioxidant. The aim of this study was to evaluate the status of Vitamin D, antioxidant enzymes, and the relation between them in ischemic stroke patients.
Materials and Methods:
This case–control study was carried out on 36 patients with ischemic stroke patients and 36 matched subjects as controls. Intake of fruits and vegetables, exposure of sunlight, serum lipid profile, concentrations of serum 25-dihydroxy Vitamin D (25(OH) D), activities of serum superoxide dismutase, and glutathione peroxidase enzymes were determined.
Results:
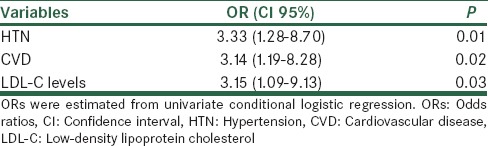
Severe Vitamin D deficiency was seen in 30% of the patients versus 11% of the controls (P < 0.05). Consumption of fruits and vegetables was lower in patients than that of controls (P < 0.05). Activities of antioxidant enzymes and intake of fruits were positively correlated in stroke patients (P = 0.02). The most potent predictors of stroke risk were hypertension, high levels of low-density lipoprotein cholesterol (LDL-C) and history of cardiovascular disease (CVD) (odds ratios: 3.33, 3.15, and 3.14, respectively, P < 0.05 for all). There was no association between 25(OH) D levels with activities of serum antioxidant enzymes and lipid profile in the two groups.
Conclusion:
Ischemic stroke patients have higher prevalence of severe Vitamin D deficiency and lower intakes of fruits and vegetables. Intake of fruits was positive correlated to higher antioxidant enzymes levels. High levels of blood pressure, history of CVD, and high LDL-C levels are the strongest predictors of ischemic stroke.
Keywords: Antioxidant enzymes, fruits and vegetables, Ischemic stroke, Vitamin D
INTRODUCTION
Stroke is the second leading cause of death and third leading contributor to disability-adjusted life-years in the world.[1] Without intervention, the global number of deaths from stroke is expected to rise to 6.5 million by 2015 and 7.8 million by 2030.[2] Cardiovascular diseases (CVDs) including stroke are the main cause of death in Iran.[3] The results of one study provide evidence that the incidence of stroke in Iran is considerably greater than in most Western countries.[4] Eighty-seven percent of stroke patients experience an ischemic stroke, which is caused by occlusion of a blood vessels,[5] that leading to initiation of immune response and oxidative damage.[6] One group of defense mechanisms to cope with oxidative stress is antioxidant enzymes. The most important antioxidant enzymes include superoxide dismutase (SOD) and glutathione peroxidase (GPX).[7] The role of Vitamin D as an antioxidant has not been clearly studied. One of the antioxidant mechanisms of Vitamin D is exerted through its effect on the antioxidant enzymes.[7] Studies from Iran indicated that Vitamin D deficiency has increased during 1999–2010 in all cities including Ahwaz, and 40–60% of people have been reported as Vitamin D deficient.[8] Ischemic strokes can be the consequence of different etiological processes, which could, in turn, be associated with dietary patterns.[9] The possiblepreventive effects of fruits and vegetables in oxidative condition may act through activation of antioxidant enzymes defense systems.[10]
To the best of our knowledge, no study has investigated the relationship between Vitamin D status, fruits and vegetables intake, and the antioxidant enzymes at the same time in stroke patients. This study aimed at determining the association of Vitamin D status and intake of fruits and vegetables as the main dietary antioxidant sources with serum antioxidant enzymes in ischemic stroke.
MATERIALS AND METHODS
Subjects
In this case–control study-which was conducted in Golestan University Hospital and Oil Company Hospital, at city of Ahvaz, South-West of Iran, in autumn 2013-36 stroke cases and 36 controls were selected with convenience nonrandom sampling method. Subjects were 63–65 years old. Those hospitalized in neurology ward and during 24–72 h after onset of acute stroke were chosen as cases and controls chosen from individuals referred to the same hospitals laboratory for a checkup with no history of the cerebrovascular accident. All subjects were informed about the purposes, procedures and signed written informed consent forms. The study protocol was approved by the Medical Ethics Committee of Jundishapur University of Medical Sciences under the registrations number B-9221. The subjects were interviewed by a trained interviewer (LA) to complete all questionnaires.
Assessment of stroke
For all patients, the incidence of ischemic stroke was confirmed by an attendant neurologist using brain computed tomography. One episode of the focal neurologic deficit with acute onset due to a vascular cause and lasting more than 24 h was defined as ischemic stroke.[11]
Assessment of other variables
Anthropometric data such as sex, age, weight, height, and body mass index (BMI) were measured and medical history including CVD, hypertension (HTN), diabetes mellitus (DM), and smoking habits were collected. Patients with a history of previous cerebral infarction, liver and renal disease, cancer and those using Vitamin D and other antioxidants supplements were excluded. Another questionnaire about the duration of exposure to sunlight in the previous month (< 30; 30–60; 60–120 and >120 min/day) completed.
Assessment of dietary intakes
Usual fruits and vegetables intakes of the participants were assessed using a validated 50-item semi-quantitative food frequency questionnaire (FFQ). Validity and reproducibility of the semi-FFQ had previously been assessed before.[12,13] The frequency of fruits and vegetables consumption was asked as daily, weekly, and monthly for each item. In case of aphasia, the patient's spouse answered the questions.
Blood sample collection
After an overnight fast, 5 mL whole blood sample was taken from all participants and transferred to cold boxes for referral to the laboratory. Serums kept frozen at −80°C until further analyses.
Laboratory measurements
Serum lipid profile was measured using auto analyzer at University Central Laboratory. Serum levels of Vitamin D and antioxidant enzymes, SOD and GPX were determined using an enzyme-linked immunosorbent assay by Euroimmun and Eastbiopharm kits, respectively. Serum 25-dihydroxy Vitamin D (25(OH) D) levels less than 4.9 ng/mL (12.25 nmol/L), between 5 and 9.99 ng/mL (12.25–25 nmol/L) and between 10 and 14 ng/mL (25–35 nmol/L), were considered as severe, moderate, and mild Vitamin D deficiency, respectively.[14]
Statistical methods
Based on prior studies, a sample size of 36 was obtained for each group (considering a confidence interval [CI] of 95% and power of 90%).[15] Categorical variables were compared using Chi-square test and continuous variables were compared using an independent t-test. Logistic regression model was used to determine the independent effects on Vitamin D status and also the odds ratios (ORs). To prevent confounding effects, the covariates of sex, history of CVD and HTN were included in the models. Correlations were determined using Spearman Rank test. P < 0.05 was considered statistically significant. Statistical analysis was performed by SPSS® version 16 (SPSS Inc., version 16, Chicago, IL, USA).
RESULTS
Baseline demographic and clinical characteristics of the subjects are shown in Table 1. Age, BMI, history of smoking, and DM were similar between stroke patients and their controls. Biochemical indices are also shown in Table 1. Lipid profile showed no significant difference between the two groups except for low-density lipoprotein cholesterol (LDL-C) concentrations that were significantly higher in stroke patients than those of controls (P = 0.027). In addition, serum concentrations of 25(OH) D and activity of serum antioxidant enzymes (GPX and SOD) showed no significant difference between the two groups [Table 1]. However, the intake of vegetables and fruits and duration of sun exposure were different between the groups (P < 0.05). Furthermore, 30% of patient versus 11% of controls showed severe Vitamin D deficiency (P = 0.04). Serum 25(OH) D levels showed no significant association with sunlight exposure [Table 2]. There were no significant associations between the concentrations of serum 25(OH) D and activities of serum antioxidant enzymes and also lipid profile after adjusting for confounding factors [Table 3]. In post-hoc analysis with Tukey's test, we found a significant positive association between the levels of serum antioxidant enzymes and intake of fruits in stroke patients (P = 0.02). Moreover, there was a significant positive association between intake of vegetables and SOD enzyme activity in the controls [P < 0.04; Table 4]. To evaluate the effect of main factors for predicting the risk of stroke, logistic regression models were used. Accordingly, the most effective predictors of ischemic stroke were HTN, high LDL-C levels, and CVD history (OR [95% CI]: 3.33 [1.28–8.70], 3.15 [1.09–9.13], and 3.14 [1.19–8.28], respectively) [Table 5].
Table 1.
Baseline characteristics and antioxidant levels of stroke patients and their controls
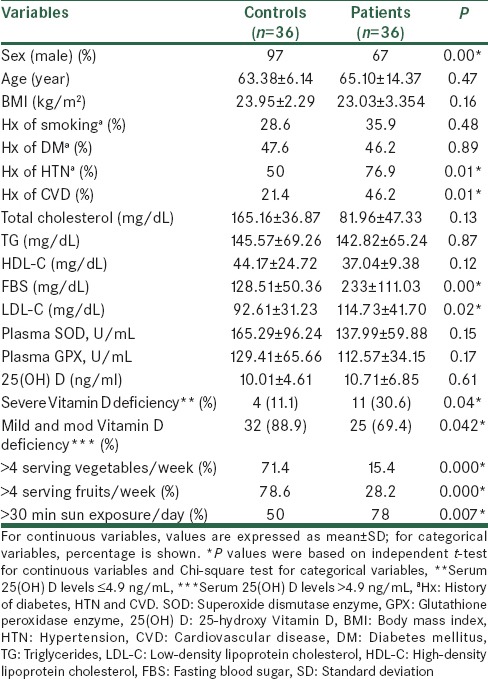
Table 2.
Comparison of Vitamin D levels based on exposure of sunlight between the groups
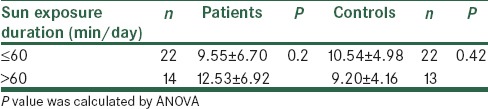
Table 3.
Association between serum levels of vitamin 25(OH) D and activities of antioxidant enzymes (SOD and GPX) and lipid profile in stroke patient and healthy subjects*
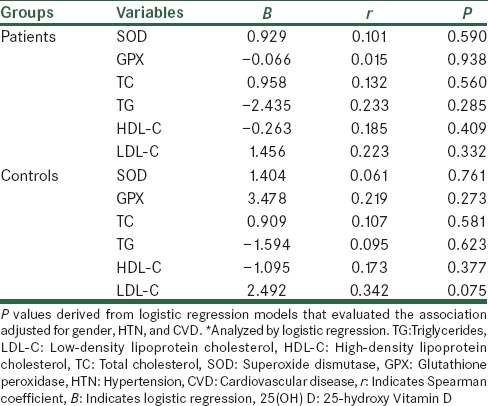
Table 4.
Comparison of fruits and vegetables intake based on activities of SOD and GPX enzymes in stroke patients and controls
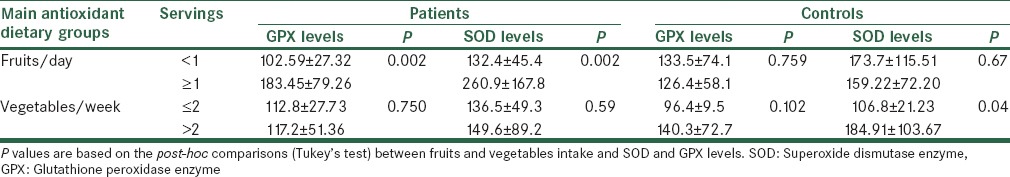
Table 5.
ORs (95% CI) for predicting the effects of the contributing factors of ischemic stroke
DISCUSSION
The main objective of this study was to investigate the association between Vitamin D levels and serum antioxidant enzymes activity in ischemic stroke patients compared with those of matched subjects. We have also evaluated the association between these enzymes and fruits and vegetables intake.
Prevalence of severe Vitamin D deficiency in stroke patients and their controls were 30% and 11%, respectively, indicating that the majority of participants had low levels of Vitamin D. As shown in Table 1, although there is significant difference in duration of sun exposure between the groups, but serum 25(OH) D levels showed no significant association with sunlight exposure in the two groups. Other studies indicated that low serum 25(OH) D levels in Iranian populations are prevalent in all regions, despite plenty of sunshine. In this regard, Rahnavard et al.[16] and Hashemipour et al.[14] showed no association between exposure to sunlight and serum Vitamin D levels confirming our results. Vitamin D deficiency is well-documented in long-term stroke survivors due to reduced sun exposure and is associated with poststroke hip fractures. Less is known regarding its levels in acute stroke.[17] Although, prospective population-based studies have shown that low 25(OH) D levels are predictive of future stroke,[18,19,20,21] we have restricted our comparisons to other cross-sectional studies.[17,22,23]
We also found no significant difference of serum 25(OH) D levels between the cases and their controls [Table 1]. These results are in accordance with other studies carried out on stroke patients[22,23] but are in contrast to Poole et al. study.[17] Iranmanesh and Gadari in a cross-sectional study in Iran, compared the serum 25(OH) D levels of 44 patients suffering from first-ever ischemic stroke with those of 44 healthy ambulant elderly subjects and reported no differences in serum Vitamin D in patients with acute stroke and controls.[22] Furthermore, Gupta et al. in a cross-sectional study in India, measured serum 25(OH) D levels in 73 patients of ischemic stroke, within 7 days of onset of stroke and compared them with 70 matched controls. There was no significant difference in the prevalence of Vitamin D deficiency and mean 25(OH) D levels between the cases and controls.[23] However, Poole et al. found that 77% patients with stroke have lower serum 25(OH) D levels than healthy elderly subjects.[17] In the latter study, the serum samples of 25(OH) D were collected up to 30 days of a first-ever stroke, whereas we measured 25(OH) D status within 1–3 days poststroke.
Regarding lipid profile findings, Boshtam et al. reported that there was no statistical association between serum Vitamin D levels and lipid profile in Iranian female carpet weavers. They aimed to determine the relationship between serum Vitamin D levels and coronary artery disease risk factors such as hyperlipidemia.[24] In our study, although LDL-C levels in stroke patients were higher than the controls (P = 0.027), however, there was no significant association between Vitamin D and LDL-C levels in both groups. Our results are similar to other studies in Iranians with CVD and diabetes[24,25] but were in contrast to another study by Hirschler et al. in Argentine.[26]
Cherubini et al. concluded that activity of SOD and GPX, as antioxidant enzymes in plasma of stroke patients are reduced immediately after an acute ischemic stroke, as a consequence of increased oxidative stress, and revealed an increment over time.[15] In Fang et al. study,[27] antioxidant activity of SOD was significantly decreased after onset of stroke in gerbils. Chen et al. investigated Vitamins C and E influence on the antioxidant enzymes in stroke-prone rats. After 6 weeks of treatment, they found an increased activation of SOD.[28] Taking all results together, it is conceivable to elucidate that cerebral ischemia may result in an enhanced oxidative stress.
To the best of our knowledge, the present study is the first attempt to confirm the hypothesis that Vitamin D is an effective factor that could improve antioxidant defense against oxidative stress imposed by ischemic stroke. However, no significant correlation was found between serum Vitamin D levels and activity of SOD and GPX. One of the reasons pertaining to this result could be due to the high prevalence of Vitamin D deficiency in our subjects. As another reason, we also took the blood samples during 24–72 h after onset of acute stroke, possibly if this time was longer, complications of ischemic stroke related oxidative stress could have been more extended and then positive correlation between Vitamin D levels and antioxidant enzymes could have been detected. Javanbakht et al.[7] found that Vitamin D supplementation could increase erythrocyte SOD and catalase activities in patients with atopic dermatitis. Moreover, in Taheri et al. study on the diabetic subject,[29] there was a positive correlation between Vitamin D concentrations and SOD and GPX enzymes activity.
Epidemiological studies have revealed that the consumption of fruits and vegetables is associated with protective effects against age-related pathologies.[30] The possibility that the complex mixture of phytochemicals in fruits and vegetables may contribute to their preventive effects through activation of endogenous antioxidant defense systems has also been raised.[31]
In our study, consumption of more than four serving of fruits and vegetables per week in stroke patients was 15% and 28%, respectively, indicating both were lower than those of controls. Moreover, there was a significant positive correlation between the activity of SOD and GPX enzymes and fruits consumption in stroke patients. In parallel with this finding, Mahe et al. compared the dietary pattern of ischemic stroke patients with control subjects using a validated 14-item FFQ. Ischemic stroke patients had a lower scores of fruits and vegetables intakes (2.9 ± 1.7 vs. 3.8 ± 1.6; P =0.005).[9] In a meta-analysis on association between fruits and vegetables consumption and the risk of stroke, Hu et al. found an inverse association of total fruits and vegetables consumption with the risk of stroke.[32] In Kim et al. study,[10] the impact of a supplement with nutritional doses of antioxidant nutrients and phytochemicals on maintenance of the antioxidant enzyme activities in subjects with a habitually low intake of fruits and vegetables was assessed. None of the antioxidant enzyme activities were significantly affected by supplementation. However, increasing resistance to DNA damage and LDL oxidation was shown.
Regarding stroke predicting factors, prospective studies have revealed different risk factors for stroke subtypes. The risk factors for ischemic stroke include aging, HTN, diabetes, smoking, history of CVD, atrial fibrillation, and left ventricular hypertrophy.[33] In our study, patients’ history of HTN, high serum levels of LDL-C and CVD history were significantly higher than those of controls (77 vs. 50%, 114.7 vs. 92.6 mg/dL and 46 vs. 21%, respectively). To evaluate the effect of predicting factors of stroke risk, logistic regression models were used. ORs for these predictors were 3.33 for having HTN, 3.15 for high serum LDL-C levels and 3.14 for the history of CVD [Table 5]. These findings are in agreement with other studies in Iran and elsewhere[34,35] emphasizing on common risk factors. Accordingly, we could define the order of the most powerful predictors of ischemic stroke in our patients.
A another study performed on Iranian stroke patients showed that the prevalence of stroke risk factors including HTN, DM, hypercholesterolemia, smoking, valvular heart disease were 53%, 13.5%, 8%, 15%, and 17.7%, respectively.[34] A cross-sectional multihospital-based study performed on patients with ischemic stroke found HTN as the risk factor of greatest impact on ischemic stroke (64%) followed by DM (36%), ischemic heart disease (34%), hypercholesterolemia (32%), and smoking (20%).[35]
As a strength point of our study, patients were recruited within 1–3 days of onset of stroke, so did not have a past history of stroke (because Vitamin D deficiency is common in poststroke patients due to reduced sun exposure). This study also revealed high levels of Vitamin D deficiency in the elderly subject despite plenty of sunshine in the region emphasizing a preventive strategy to be considered. At the end, it should be mentioned that our study had limitations as being a cross-sectional study and limited ability to determine a temporal association between Vitamin D deficiency and ischemic stroke. In addition, serum levels of total antioxidant capacity and antioxidant enzymes in RBCs were not measured which could be an area for future works.
CONCLUSION
Our results showed that ischemic stroke patients have a higher prevalence of severe Vitamin D deficiency and lower intakes of fruits and vegetables. Furthermore, fruits have a positive correlation with antioxidant enzymes levels. Elevated blood pressure and serum LDL-C levels and also the history of CVD are the strongest predictors of ischemic stroke.
Other studies with a higher number of subjects and measuring more specific antioxidant biomarkers are suggested to explore the possible causal effects of specific antioxidants and Vitamin D levels on ischemic patients.
Financial support and sponsorship
Supported by a grant of Vice-chancellor of Research Affairs of Jundishapur University under the Registration No. B-9221.
Conflicts of interest
There are no conflicts of interest.
Acknowledgments
This study was a part of Laleh Afshari's Master of Science thesis supported by a grant of Vice-chancellor of Research Affairs of Jundishapur University and also Arvand branch of AJUMS under the registration no. B-9221. We wish to thank the Educational Deputy of Ahvaz Oil Company (Naft) Hospital for their kind assistance.
REFERENCES
- 1.Hankey GJ. Secondary stroke prevention. Lancet Neurol. 2014;13:178–94. doi: 10.1016/S1474-4422(13)70255-2. [DOI] [PubMed] [Google Scholar]
- 2.Tikhonoff V, Zhang H, Richart T, Staessen JA. Blood pressure as a prognostic factor after acute stroke. Lancet Neurol. 2009;8:938–48. doi: 10.1016/S1474-4422(09)70184-X. [DOI] [PubMed] [Google Scholar]
- 3.Karami M, Soori H, Monfared AB. Estimating the contribution of selected risk factors in attributable burden to stroke in Iran. Iran J Public Health. 2012;41:91–6. [PMC free article] [PubMed] [Google Scholar]
- 4.Azarpazhooh MR, Etemadi MM, Donnan GA, Mokhber N, Majdi MR, Ghayour-Mobarhan M, et al. Excessive incidence of stroke in Iran: Evidence from the Mashhad Stroke Incidence Study (MSIS), a population-based study of stroke in the Middle East. Stroke. 2010;41:e3–10. doi: 10.1161/STROKEAHA.109.559708. [DOI] [PubMed] [Google Scholar]
- 5.Chen H, Yoshioka H, Kim GS, Jung JE, Okami N, Sakata H, et al. Oxidative stress in ischemic brain damage: Mechanisms of cell death and potential molecular targets for neuroprotection. Antioxid Redox Signal. 2011;14:1505–17. doi: 10.1089/ars.2010.3576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chan CP, Jiang HL, Leung LY, Wan WM, Cheng NM, Ip WS, et al. Multiple atherosclerosis-related biomarkers associated with short-and long-term mortality after stroke. Clin Biochem. 2012;45:1308–15. doi: 10.1016/j.clinbiochem.2012.06.014. [DOI] [PubMed] [Google Scholar]
- 7.Javanbakht M, Keshavarz S, Mirshafiey A, Djalali M, Siassi F, Eshraghian M, et al. The effects of vitamins E and D supplementation on erythrocyte superoxide dismutase and catalase in atopic dermatitis. Iran J Public Health. 2010;39:57–63. [PMC free article] [PubMed] [Google Scholar]
- 8.Saidinia A, Larijani B, Jalalinia SH, Farzadfar F, Keshtkar AA, Rezai E. Prevalence of vitamin D deficiency in Iran population 1999-2010. Iran J Diabetes Metab. 2012;12:574–84. [Google Scholar]
- 9.Mahe G, Ronziere T, Laviolle B, Golfier V, Cochery T, De Bray JM, et al. An unfavorable dietary pattern is associated with symptomatic ischemic stroke and carotid atherosclerosis. J Vasc Surg. 2010;52:62–8. doi: 10.1016/j.jvs.2010.02.258. [DOI] [PubMed] [Google Scholar]
- 10.Kim YJ, Ahn YH, Lim Y, Kim JY, Kim J, Kwon O. Daily nutritional dose supplementation with antioxidant nutrients and phytochemicals improves DNA and LDL stability: A double-blind, randomized, and placebo-controlled trial. Nutrients. 2013;5:5218–32. doi: 10.3390/nu5125218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Khosravi-Boroujeni H, Saadatnia M, Shakeri F, Keshteli AH, Esmaillzadeh A. A case-control study on potato consumption and risk of stroke in central Iran. Arch Iran Med. 2013;16:172–6. [PubMed] [Google Scholar]
- 12.Mirmiran P, Esfahani FH, Mehrabi Y, Hedayati M, Azizi F. Reliability and relative validity of an FFQ for nutrients in the Tehran lipid and glucose study. Public Health Nutr. 2010;13:654–62. doi: 10.1017/S1368980009991698. [DOI] [PubMed] [Google Scholar]
- 13.Prohan M, Amani R, Nematpour S, Jomehzadeh N, Haghighizadeh MH. Total antioxidant capacity of diet and serum, dietary antioxidant vitamins intake, and serum hs-CRP levels in relation to depression scales in university male students. Redox Rep. 2014;19:133–9. doi: 10.1179/1351000214Y.0000000085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hashemipour S, Larijani B, Adibi H, Javadi E, Sedaghat M, Pajouhi M, et al. Vitamin D deficiency and causative factors in the population of Tehran. BMC Public Health. 2004;4:38. doi: 10.1186/1471-2458-4-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cherubini A, Polidori MC, Bregnocchi M, Pezzuto S, Cecchetti R, Ingegni T, et al. Antioxidant profile and early outcome in stroke patients. Stroke. 2000;31:2295–300. doi: 10.1161/01.str.31.10.2295. [DOI] [PubMed] [Google Scholar]
- 16.Rahnavard Z, Eybpoosh S, Homami MR, Meybodi HA, Azemati B, Heshmat R, et al. Vitamin D deficiency in healthy male population: Results of the Iranian multi-center osteoporosis study. Iran J Public Health. 2010;39:45–52. [PMC free article] [PubMed] [Google Scholar]
- 17.Poole KE, Loveridge N, Barker PJ, Halsall DJ, Rose C, Reeve J, et al. Reduced vitamin D in acute stroke. Stroke. 2006;37:243–5. doi: 10.1161/01.STR.0000195184.24297.c1. [DOI] [PubMed] [Google Scholar]
- 18.Sun Q, Pan A, Hu FB, Manson JE, Rexrode KM. 25-Hydroxyvitamin D levels and the risk of stroke: A prospective study and meta-analysis. Stroke. 2012;43:1470–7. doi: 10.1161/STROKEAHA.111.636910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kojima G, Bell C, Abbott RD, Launer L, Chen R, Motonaga H, et al. Low dietary vitamin D predicts 34-year incident stroke: The Honolulu Heart Program. Stroke. 2012;43:2163–7. doi: 10.1161/STROKEAHA.112.651752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Daubail B, Jacquin A, Guilland JC, Khoumri C, Aboa-Eboulé C, Giroud M, et al. Association between serum concentration of vitamin D and 1-year mortality in stroke patients. Cerebrovasc Dis. 2014;37:364–7. doi: 10.1159/000362534. [DOI] [PubMed] [Google Scholar]
- 21.Chaudhuri JR, Mridula KR, Alladi S, Anamika A, Umamahesh M, Balaraju B, et al. Serum 25-hydroxyvitamin d deficiency in ischemic stroke and subtypes in Indian patients. J Stroke. 2014;16:44–50. doi: 10.5853/jos.2014.16.1.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Iranmanesh F, Gadari F. Vitamin D and ischemic stroke. J Hormozgan Med Sci. 2011;15:178–83. [Google Scholar]
- 23.Gupta A, Prabhakar S, Modi M, Bhadada SK, Lal V, Khurana D. Vitamin D status and risk of ischemic stroke in North Indian patients. Indian J Endocrinol Metab. 2014;18:721–5. doi: 10.4103/2230-8210.139241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Boshtam M, Rafiei M, Sarrafzadegan N, Mostafavi S, Naderi G. The relationship between serum vitamin D level, coronary artery disease and the associated risk factors in female carpet weavers. J Qazvin Univ Med Sci. 2005;35:44–52. [Google Scholar]
- 25.Bonakdaran S, Varasteh A, Khajeh Dalouie M. Serum 25 hydroxy vitamin D3 and laboratory risk markers of cardiovascular diseases in type 2 diabetic patients. Iran J Endocrinol Metab. 2010;11:504–9. [PubMed] [Google Scholar]
- 26.Hirschler V, Maccallini G, Molinari C, Inés U, Castano LA, et al. San Antonio de los Cobres Study Group. Association between vitamin D and Apo B concentrations in Argentinean Indian children. Clin Chim Acta. 2014;429:147–51. doi: 10.1016/j.cca.2013.12.012. [DOI] [PubMed] [Google Scholar]
- 27.Fang KM, Cheng FC, Huang YL, Chung SY, Jian ZY, Lin MC. Trace element, antioxidant activity, and lipid peroxidation levels in brain cortex of gerbils after cerebral ischemic injury. Biol Trace Elem Res. 2013;152:66–74. doi: 10.1007/s12011-012-9596-1. [DOI] [PubMed] [Google Scholar]
- 28.Chen X, Touyz RM, Park JB, Schiffrin EL. Antioxidant effects of vitamins C and E are associated with altered activation of vascular NADPH oxidase and superoxide dismutase in stroke-prone SHR. Hypertension. 2001;38:606–11. doi: 10.1161/hy09t1.094005. [DOI] [PubMed] [Google Scholar]
- 29.Taheri E, Jalali M, Saedi A, Jazayeri A, Rahimi A. Association between 25(OH) D and antioxidant marker in type 2 diabetes mellitus with healthy subjects. Iran J Diabetes Metab. 2010;10:502–13. [Google Scholar]
- 30.Dauchet L, Amouyel P, Hercberg S, Dallongeville J. Fruit and vegetable consumption and risk of coronary heart disease: A meta-analysis of cohort studies. J Nutr. 2006;136:2588–93. doi: 10.1093/jn/136.10.2588. [DOI] [PubMed] [Google Scholar]
- 31.Liu RH. Health benefits of fruit and vegetables are from additive and synergistic combinations of phytochemicals. Am J Clin Nutr. 2003;78:517S–20. doi: 10.1093/ajcn/78.3.517S. [DOI] [PubMed] [Google Scholar]
- 32.Hu D, Huang J, Wang Y, Zhang D, Qu Y. Fruits and vegetables consumption and risk of stroke: A meta-analysis of prospective cohort studies. Stroke. 2014;45:1613–9. doi: 10.1161/STROKEAHA.114.004836. [DOI] [PubMed] [Google Scholar]
- 33.Fahimfar N, Khalili D, Mohebi R, Azizi F, Hadaegh F. Risk factors for ischemic stroke; results from 9 years of follow-up in a population based cohort of Iran. BMC Neurol. 2012;12:117. doi: 10.1186/1471-2377-12-117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ghandehari K, Izadi-Mood Z. Khorasan stroke registry: Analysis of 1392 stroke patients. Arch Iran Med. 2007;10:327–34. [PubMed] [Google Scholar]
- 35.Delbari A, Salman Roghani R, Tabatabaei SS, Lökk J. A stroke study of an urban area of Iran: Risk factors, length of stay, case fatality, and discharge destination. J Stroke Cerebrovasc Dis. 2010;19:104–9. doi: 10.1016/j.jstrokecerebrovasdis.2009.06.003. [DOI] [PubMed] [Google Scholar]


