Abstract
Background:
As a common pathophysiological condition worldwide, metabolic syndrome (MetS) is a clustering of multiple risk factors implicating in the development of many chronic disorders. Of note, obesity-induced chronic, low-grade inflammation is a major cause of insulin resistance and MetS. In the present study, we evaluated the association of rs3091244 variant of the C-reactive protein (CRP) gene, a well-recognized systemic inflammatory marker, with MetS in Iranian children and adolescents.
Materials and Methods:
Genotyping was performed by mismatched polymerase chain reaction-restriction fragment length polymorphism in 100 MetS and 100 normal individuals aged 9–19 years recruited in the central part of Iran in 2011. A t-test or one-way ANOVA with post-hoc multiple comparisons were used to analyze the differences between groups. Statistical significance was defined as P ≤ 0.05. Logistic regression used to evaluate the association between alleles of the CRP rs3091244 and increased MetS risk.
Results:
There were no differences in the genotype frequencies or allele distribution for −286C>A>T CRP polymorphism between MetS and control groups. Logistic regression showed that only the T allele of the CRP rs3091244 and not any of the genotypes confers a borderline significant (P = 0.059) increased MetS risk compared to A allele with the odds ratio of 1.70 (0.98–2.96).
Conclusions:
This study suggests that in Iranian children and adolescents, −286C>A>T CRP polymorphism is not associated with the increased risk for MetS.
Keywords: Insulin resistance, metabolic syndrome, polymerase chain reaction, restriction fragment length polymorphism, risk factors
INTRODUCTION
As a common pathophysiological condition, metabolic syndrome (MetS) is characterized by abdominal obesity, dysglycemia, dyslipidemia, hypertension, and prothrombotic and proinflammatory states.[1,2] Studies have shown that MetS is associated with the morbidity and mortality from cardiovascular diseases and type 2 diabetes.[3,4] The MetS's prevalence is higher in older age,[5] and is becoming one of the most important public health challenges worldwide. Because of the obesity epidemic in childhood, which is one of the major risk factors of MetS, recent studies have focused on studying the MetS in children and adolescents. Early identification of children at risk will help to design preventive programs as early as possible.[1,6,7] In Iranian children and adolescents, the prevalence of MetS is around 1–2%, much higher than that reported for other countries.[8,9,10]
Dysregulated adipose tissue is associated with the pathogenesis of MetS in part due to the enlargement of adipose cells and infiltration of macrophages followed by overexpression of inflammatory cytokines.[11,12] These cytokines can cause insulin resistance in adipose tissue, skeletal muscle and liver by inhibiting insulin signal transduction.[12]
Due to the important role of inflammation in MetS, investigating the association between inflammatory marker gene polymorphisms, and the risk of MetS is of great value. C-reactive protein (CRP), a well-recognized systemic inflammatory marker, characterizes a pro-inflammatory state when it is increased.[13] Several studies demonstrate that CRP is elevated in major components of MetS, including hypertension, dyslipidemia, obesity, and glucose intolerance.[13,14]
In 2006, Crawford et al. re-sequenced the CRP gene and found 40 single nucleotide polymorphisms (SNPs) within this gene confirming the polymorphic nature of the gene. Among these identified SNPs in CRP, one unique variant is the high-frequency triallelic SNP rs3091244. They reported that all three alleles of rs3091244, A, C, and T, occur with considerable frequency in all population samples examined.[15] Apart from environmental factors and patient behaviors and traits, which can alter the blood CRP levels, newer evidences showed the importance of genetic components as well.[16] Until now, several groups have investigated the link between CRP polymorphisms and baseline blood CRP and it is now evident that these polymorphisms are associated with differences in CRP blood level.[16] Among the CRP polymorphisms, only triallelic SNP rs3091244 (−286C/T/A) has been proven to be functional, that is, to directly contribute to differences in baseline CRP blood levels among individuals.[17]
The association between the CRP rs3091244 and MetS in adult populations has been investigated in a number of studies,[18,19] however, little is known about the effect of CRP −286C/T/A polymorphism on the risk of developing MetS in pediatric age groups. In the present study, we, for the 1st time, evaluated the association of rs3091244 variants of the CRP gene with MetS in Iranian children and adolescents.
MATERIALS AND METHODS
Study population
A total of 100 subjects with MetS (46 boys and 54 girls, mean age: 12.86 ± 0.217 years, range: 9–18 years) and 100 matched healthy, normal weight individuals (48 boys and 52 girls, mean age: 13.36 ± 0.266 years, range: 9–19 years) with no history of systemic inflammation, infection disease, MetS, and type 2 diabetes were included in the present study. They were all recruited from Isfahan (a central province in Iran) university clinics between years 2010 and 2013. MetS subjects were selected according to the modified ATPIII definition.[8] Blood samples were collected in sterile EDTA-treated tubes and centrifuged immediately. The specimens then were stored at –20°C for further analyses. The experimental design reconciled with the Code of Ethics of the World Medical Association (Declaration of Helsinki) and was approved by the Ethics Committees of Isfahan University of Medical Sciences. Oral assent was obtained from participants and written informed consent from their parents.
Laboratory analyses
After an overnight fast, blood samples were collected from all individuals, centrifuged immediately, and plasmas were stored at − 80°C. Lipid profiles and fasting blood sugar (FBS) concentration were measured enzymatically using a Hitachi 7070 analyzer (Diamond Diagnostics, USA) with reagents from Pars Azmoon (Pars Azmoon, Iran). Fasting insulin concentration was determined by a chemiluminescent assay (DiaSorin, Italy) on the LIAISON® analyzer (DiaSorin, Italy).
Extraction of genomic DNA and genotyping of C-reactive protein rs3091244 polymorphism
Genomic DNA was extracted from whole blood using Diatome kit (Isogen Laboratory, Russia) exploiting routine salting-out method. DNA quality and quantity were assessed by agarose gel electrophoresis and spectrophotometry. Mismatched polymerase chain reaction (PCR) – restriction fragment length polymorphism was employed to genotype C>A>T polymorphism in the CRP gene using the following primers:[18] Forward: 5’-ATTTCCCAGTCTGTAAATAAGCAAA-3’ and reverse: 5’-AATGGGAAATGGTAACATATTAATC-3’. The 174 bp amplified fragment possessed two cleavage sites for TaqI and BfaI restriction endonucleases. The TaqI endonuclease cleavage site was created by modifying the reverse primer (bold underlined) to detect the C allele. The differentiation between the A and T alleles was performed by digestion of the same PCR products with BfaI restriction enzyme. Thermal cycling conditions for amplifying the CRP amplicon were as follows: 95°C for 5 min for the initial denaturation followed by 35 cycles consisting of denaturation at 95°C for 40 s; annealing at 55°C for 40 s; extension at 72°C for 40 s and a final extension at 72°C for 10 min. Ten microliter of the amplified product was separately digested with 5 U of TaqI and BfaI restriction endonucleases and analyzed by polyacrylamide gel electrophoresis.
Statistical analyses
SPSS software version 20.0 (SPSS Inc., Chicago, IL, USA) was utilized for statistical analyses. All quantitative values are presented as mean ± SEM. To compare the genotypes and allele frequencies, a Chi-square statistic was calculated. Regarding the levels of biochemical factors and distribution of different genotypes, one-way ANOVA and post-hoc analysis with a least significant difference was performed. Simple and multivariable adjusted odds ratios and 95% confidence intervals were computed using the binary logistic regression. P < 0.05 were considered significant.
RESULTS
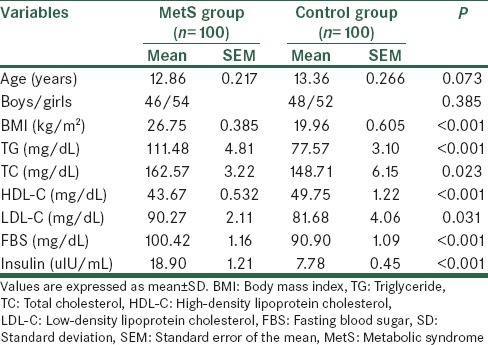
Table 1 presents the clinical and biochemical characteristics of the individuals with and without the MetS. There was a significant difference between the groups in the main risk factors for MetS including triglyceride (TG), total cholesterol (TC), high-density lipoprotein cholesterol (HDL-C), low-density lipoprotein cholesterol (LDL-C), FBS, and insulin levels (P < 0.05).
Table 1.
Clinical and biochemical data in case and control groups
Genotype and allele frequencies of CRP gene − 286C>A>T polymorphism in MetS and control groups are presented in Table 2. The frequencies of the six genotypes between the MetS and control groups were not significantly different (χ2 = 5.673, P = 0.339). In addition, no significant differences were found between the MetS and control groups in allele frequencies (χ2 = 3.760, P = 0.153).
Table 2.
Comparison of genotype and allele frequencies for rs3091244 between MetS and control groups

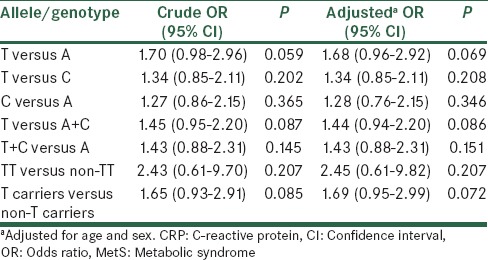
Different allelic and genotypic models were considered then to find the best model, which would fit the effect of CRP gene − 286C>A>T polymorphism [Table 3]. Except for the T allele of the CRP rs3091244 which showed a borderline significant trend to increase MetS risk compared to A allele, no other significant associations were observed between CRP rs3091244 polymorphism and the risk of MetS in any of allelic and genotypic models before and after adjustment.
Table 3.
Logistic regression analyses of association between CRP rs3091244 and risk of MetS
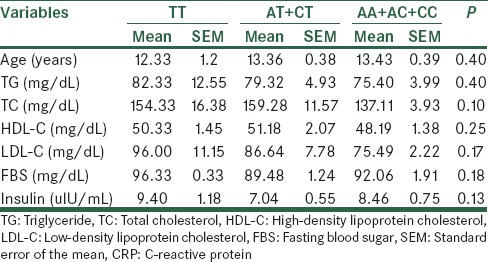
On the basis of the presence of either none, 1, or 2 T alleles, we classified the subjects into three groups: AA + AC + CC genotype group, AT + CT group, and the homozygous TT group. There was no significant difference in body mass index (BMI), TG, TC, HDL-C, LDL-C, FBS or insulin between the three genotype groups in the MetS and control groups based on ANOVA tests [Tables 4 and 5]. However, post-hoc analysis showed a significant difference between FBS levels in MetS subjects with AA + AC + CC genotypes compared to TT genotype (P = 0.028). Control individuals with AT + CT genotypes had also significantly increased TC levels compared to AA + AC + CC genotypes’ carriers (P = 0.038)
Table 4.
The CRP rs3091244 genotypes and their correlation with clinical and biochemical parameters in the MetS group
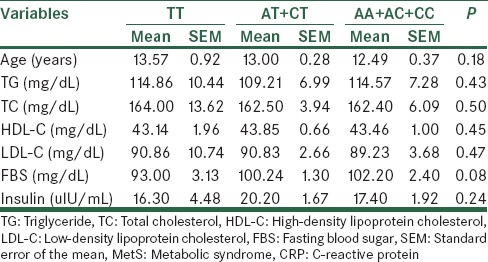
Table 5.
The CRP rs3091244 genotypes and their correlation with clinical and biochemical parameters in the control group
DISCUSSION
The present study is the first investigated the rs3091244 polymorphism in the promoter region of an inflammatory marker gene, CRP, for association with MetS and its different components in a group of pediatric subjects. In our study, A allele observed frequency was 0.245 for control individuals which is similar to what reported (0.261) by 1000 Genomes project for this allele as the second most frequent allele of rs3091244.[20]
By logistic regression using different allelic and genotypic models, we only found a borderline statistically significant association between the T allele of CRP rs8066560 polymorphism and the increased risk for MetS. After genotype stratification, we found that FBS levels were significantly distinct between MetS individuals with no T allele and TT carriers. Furthermore, control children with one T allele had increased TC levels compared to individuals with no T allele.
In 2010, Hsu et al.[18] investigated the effect of rs3091244 on the risk of developing Mets in adults. This study demonstrated that the non-CC genotypes are slightly significantly associated with an increased risk of MetS after adjustment for age, sex, smoking, and BMI. However, after adding the high-sensitivity CRP levels to their model, the statistical significance of the relationship between the rs3091244 non-CC genotype and MetS were lost. Similarly, in another study conducted by Komurcu-Bayrak et al.[19] who explored the effect of different CRP haplotypes including −286C>T>A polymorphism on MetS risk, no association was observed for MetS or its components. In this regard, our data are in accord with above-mentioned studies,[18,19] which showed no association between rs3091244 genotypes and MetS risk. In summary, our study is the first exploring the association between CRP −286C>T>A variant and MetS risk in pediatric subjects. Our results showed that the rs3091244 may not be a major risk factor for the MetS in Iranian children and adolescents. However, our preliminary results obtained from a small population sample should be interpreted with caution and will require confirmation in larger populations. At the current, is not clear why this CRP functional variant has no association with MetS and/or its phenotypes, although as a multi-factorial disorder, a predisposition to MetS will surely involve the interaction of multiple genes and environmental factors.[19]
Footnotes
Source of Support: The study was supported by a research grant from Isfahan University of Medical Sciences, Isfahan
Conflict of Interest: None declared.
REFERENCES
- 1.Alberti KG, Zimmet P, Shaw J. Metabolic syndrome – A new world-wide definition. A consensus statement from the International Diabetes Federation. Diabet Med. 2006;23:469–80. doi: 10.1111/j.1464-5491.2006.01858.x. [DOI] [PubMed] [Google Scholar]
- 2.Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults. Executive Summary of the Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) JAMA. 2001;285:2486–97. doi: 10.1001/jama.285.19.2486. [DOI] [PubMed] [Google Scholar]
- 3.Isomaa B, Almgren P, Tuomi T, Forsén B, Lahti K, Nissén M, et al. Cardiovascular morbidity and mortality associated with the metabolic syndrome. Diabetes Care. 2001;24:683–9. doi: 10.2337/diacare.24.4.683. [DOI] [PubMed] [Google Scholar]
- 4.Aschner P. Metabolic syndrome as a risk factor for diabetes. Expert Rev Cardiovasc Ther. 2010;8:407–12. doi: 10.1586/erc.10.13. [DOI] [PubMed] [Google Scholar]
- 5.Razzouk L, Muntner P. Ethnic, gender, and age-related differences in patients with the metabolic syndrome. Curr Hypertens Rep. 2009;11:127–32. doi: 10.1007/s11906-009-0023-8. [DOI] [PubMed] [Google Scholar]
- 6.Cruz ML, Goran MI. The metabolic syndrome in children and adolescents. Curr Diab Rep. 2004;4:53–62. doi: 10.1007/s11892-004-0012-x. [DOI] [PubMed] [Google Scholar]
- 7.Weiss R, Dziura J, Burgert TS, Tamborlane WV, Taksali SE, Yeckel CW, et al. Obesity and the metabolic syndrome in children and adolescents. N Engl J Med. 2004;350:2362–74. doi: 10.1056/NEJMoa031049. [DOI] [PubMed] [Google Scholar]
- 8.Kelishadi R, Ardalan G, Gheiratmand R, Adeli K, Delavari A, Majdzadeh R, et al. Paediatric metabolic syndrome and associated anthropometric indices: The CASPIAN Study. Acta Paediatr. 2006;95:1625–34. doi: 10.1080/08035250600750072. [DOI] [PubMed] [Google Scholar]
- 9.Schwandt P, Kelishadi R, Haas GM. Ethnic disparities of the metabolic syndrome in population-based samples of german and Iranian adolescents. Metab Syndr Relat Disord. 2010;8:189–92. doi: 10.1089/met.2009.0054. [DOI] [PubMed] [Google Scholar]
- 10.Schwandt P, Kelishadi R, Ribeiro RQ, Haas GM, Poursafa P. A three-country study on the components of the metabolic syndrome in youths: The BIG Study. Int J Pediatr Obes. 2010;5:334–41. doi: 10.3109/17477160903497043. [DOI] [PubMed] [Google Scholar]
- 11.Calabro P, Yeh ET. Intra-abdominal adiposity, inflammation, and cardiovascular risk: New insight into global cardiometabolic risk. Curr Hypertens Rep. 2008;10:32–8. doi: 10.1007/s11906-008-0008-z. [DOI] [PubMed] [Google Scholar]
- 12.de Luca C, Olefsky JM. Inflammation and insulin resistance. FEBS Lett. 2008;582:97–105. doi: 10.1016/j.febslet.2007.11.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ridker PM. Clinical application of C-reactive protein for cardiovascular disease detection and prevention. Circulation. 2003;107:363–9. doi: 10.1161/01.cir.0000053730.47739.3c. [DOI] [PubMed] [Google Scholar]
- 14.Festa A, D’Agostino R, Jr, Howard G, Mykkänen L, Tracy RP, Haffner SM. Chronic subclinical inflammation as part of the insulin resistance syndrome: The Insulin Resistance Atherosclerosis Study (IRAS) Circulation. 2000;102:42–7. doi: 10.1161/01.cir.102.1.42. [DOI] [PubMed] [Google Scholar]
- 15.Crawford DC, Yi Q, Smith JD, Shephard C, Wong M, Witrak L, et al. Allelic spectrum of the natural variation in CRP. Hum Genet. 2006;119:496–504. doi: 10.1007/s00439-006-0160-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hage FG, Szalai AJ. C-reactive protein gene polymorphisms, C-reactive protein blood levels, and cardiovascular disease risk. J Am Coll Cardiol. 2007;50:1115–22. doi: 10.1016/j.jacc.2007.06.012. [DOI] [PubMed] [Google Scholar]
- 17.Szalai AJ, Wu J, Lange EM, McCrory MA, Langefeld CD, Williams A, et al. Single-nucleotide polymorphisms in the C-reactive protein (CRP) gene promoter that affect transcription factor binding, alter transcriptional activity, and associate with differences in baseline serum CRP level. J Mol Med (Berl) 2005;83:440–7. doi: 10.1007/s00109-005-0658-0. [DOI] [PubMed] [Google Scholar]
- 18.Hsu LA, Chang CJ, Wu S, Teng MS, Chou HH, Chang HH, et al. Association between functional variants of the ICAM1 and CRP genes and metabolic syndrome in Taiwanese subjects. Metabolism. 2010;59:1710–6. doi: 10.1016/j.metabol.2010.04.004. [DOI] [PubMed] [Google Scholar]
- 19.Komurcu-Bayrak E, Erginel-Unaltuna N, Onat A, Ozsait B, Eklund C, Hurme M, et al. Association of C-reactive protein (CRP) gene allelic variants with serum CRP levels and hypertension in Turkish adults. Atherosclerosis. 2009;206:474–9. doi: 10.1016/j.atherosclerosis.2009.03.030. [DOI] [PubMed] [Google Scholar]
- 20.1000 Genomes Project Consortium. Abecasis GR, Altshuler D, Auton A, Brooks LD, Durbin RM, et al. A map of human genome variation from population-scale sequencing. Nature. 2010;467:1061–73. doi: 10.1038/nature09534. [DOI] [PMC free article] [PubMed] [Google Scholar]


