Abstract
Background:
Schizophrenia severely influences function and quality of life. The benefit of newer antipsychotics in improving the quality of life in schizophrenia still remains controversial. The aim of the present study is to evaluate the effect of memantine on global function and quality of life in patients with schizophrenia.
Materials and Methods:
This was a randomized controlled trial on inpatient cases of schizophrenia in Noor University Hospital, Isfahan, Iran. A number of 64 patients were selected through sequential sampling; patients were randomly allocated in intervention and placebo groups. The intervention group was treated with memantine plus previously administered, stabled-dose, atypical antipsychotic, while the control group received placebo plus previously administered, stabled-dose, atypical antipsychotic. Memantine administration was initiated at 5 mg daily; the dosage was increased at weekly intervals by 5 mg and finally up-titrated to 20 mg daily within 4 weeks. All patients were assessed by means of Global Assessment of Functioning (GAF) and quality of life scale (QLS) initially and every four weeks to the end of the 12th week.
Results:
Analysis of baseline GAF and QLS scores showed no significant differences between the two groups (P = 0.081 and P = 0.225, respectively). GAF and QLS scores increased in both groups; but it was higher in the intervention group. The difference between the two groups was statistically significant. (P < 0.001 and P < 0.001, respectively) memantine was well tolerated, with no significant side effects.
Conclusion:
Add-on memantine was significantly effective in improving the global function of patients as well as their quality of life.
Keywords: Function, global assessment of functioning, memantine, quality of life, quality of life scale, schizophrenia
INTRODUCTION
Schizophrenia is a chronic debilitating disorder, leading to chronic disability, impaired function and diminished quality of life.[1,2] It is among the top 10 medical disorders causing disability.[3] Cognitive deficits, negative and positive symptoms and medication side effects can adversely affect the outcome of the disorder.[4]
With the beginning of deinstitutionalization process, there has been a growing interest toward the quality of life in patients with schizophrenia.[5] Quality of life can be viewed as a personal sense of well-being and satisfaction with life, access to opportunities and resources, and health.[6] In fact, social function and outcome are key elements for long-term evaluation and management in patients with schizophrenia.[5] From a clinical point of view, quality of life is considered as both intermediate and distal outcome in schizophrenia.[7]
The concept of quality of life has been the subject of interest for decades. Yet there are various definitions; some researchers focus on narrow issues such as absence of disease and health-related symptoms, whilst others consider quality of life as an overall assessment of one's whole experience of life. This may explain the controversial views regarding subjectivity or objectivity in the quality of life. Although the correlation of psychopathologic features of schizophrenia and quality of life has already been identified, the degree of functional impairment and defects of quality of life cannot be explained merely through the apparent psychopathologic symptoms.[5,6]
Although the advent of newer antipsychotic agents held great promise in terms of better efficacy and tolerability, their benefit in improving the quality of life in schizophrenia still remains controversial.[1,7]
Although the pathophysiological process of schizophrenia is ambiguous, glutamatergic signaling through N-methyl-D-aspartate (NMDA) receptor has been proposed to play pivotal role in positive and negative symptoms of schizophrenia.[3,8] Experimental studies show that persistent blockade of NMDA receptor recreates the clinical pathological features of schizophrenia.[9] On the other hand, NMDA-modulating agents, including D-serine, D-alanine, D-cycloserine and sarcosine, have been the focus of several trials for treatment of schizophrenia and other major psychiatric conditions.[10,11,12,13,14] Memantine, an uncompetitive NMDA blocking agent, has been presented to treat severe residual negative symptoms of schizophrenia in previous case studies.[15] Several reports suggest the neuroprotective role of memantine in schizophrenia. However, it is not known if this property results in improvement of function and quality of life.[8]
The aim of the present study is to evaluate the effect of memantine on global function and quality of life in patients with schizophrenia.
MATERIALS AND METHODS
This was a randomized controlled trial on inpatient cases of schizophrenia in Noor Hospital, an Educational Hospital affiliated to Isfahan University of Medical Sciences, in downtown Isfahan. The diagnosis was based on clinical interview and Diagnostic and Statistical Manual of Mental Disorders-IV-TR criteria. A number of 64 patients were selected through sequential sampling among hospitalized patients with schizophrenia [Figure 1]. Inclusion criteria were age 18–65 years, normal intellectual ability, not being pregnant, not breast feeding, not receiving electro convulsive therapy in the current 2 weeks, being diagnosed with schizophrenia for the past 2 years, being treated with atypical antipsychotic in the past 3 months, and initial Brief Psychiatric Rating Scale score >26. Exclusion criteria were sensitivity to memantine, drug or substance abuse and dependence, comorbidity of other psychiatric disorders or neurological conditions. Patients were assessed through the following scales:
Figure 1.
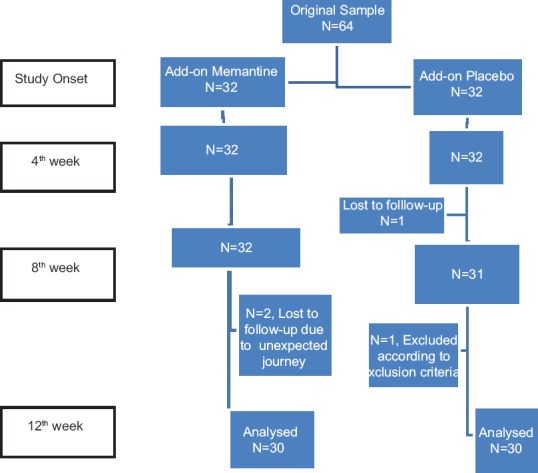
Flowchart of study steps
Global assessment of functioning
Global Assessment of Functioning (GAF) is a global functioning assessment, widely used numeric scale, for evaluation of psychological, social and occupational functioning in psychiatric outpatients and inpatients. It evaluates three domains: Social function, psychological function and personal function. It is rated from 1 to 100, and the score is often presented as a range. The higher the score is, the better the individual functions. GAF has proven to be valid and reliable. It covers the range from mental health to deep psychopathology in generic global measures.[16]
Quality of life scale
Quality of life scale (QLS) is a scale with 21 items in four categories, including interpersonal relation (8 items), instrumental role (4 items), intra-psychic foundation (7 items) and common objects and activities (2 items). The range of score in interpersonal relations is 0–48, in instrumental role 0–24, in intra-psychic foundation 0–42, and in common objects and activities 0–12. It is based on a semi-structured interview designed to assess quality of life in patients with schizophrenia. The score may vary from 0 to 126. Higher scores present better quality. Face and content validity is acceptable; reliability was measured through Cronbach's alpha (84–97%).[5]
Before the onset of trial, families and guardians were informed about the study process, probable side effects, positive and negative aspects of participation; and their questions were answered by the clinicians who conducted the study. Written informed consents were then obtained from family or guardian. Patients were allocated in random blocks according to their sequence. Each block included two patients, one allocated to intervention group and the other allocated to the control. The intervention group was treated with memantine plus previously administered stabled-dose atypical antipsychotic, while the control group received placebo plus atypical antipsychotic. Memantine administration was initiated at 5 mg daily; the dosage was increased at weekly intervals by 5 mg and finally up-titrated to 20 mg daily within 4 weeks. The other group received add-on placebo. Both intervention and control groups were on a stable dosage of atypical antipsychotic regimen in the past 3 months and also during the trial. The previous routine treatment was continued during the study.
All patients were assessed by means of GAF and QLS initially and every 4 weeks to the end of the 12th week. Patients, families or guardians, and the clinicians who evaluated the patients were unaware of the group allocation. Memantine and placebo were packed identically and labeled alphabetically by the pharmaceutical laboratory to ensure double-blinding. Data were analyzed using repeated measure ANOVA in SPSS 17.0. (SPSS Statistics for Windows, Chicago: SPSS Inc). Age, duration of schizophrenia, and number of prior psychiatric admission were compared through t-tests, while the differences between other demographic features were measured by means of Chi-square; results were presented as mean (standard deviation).
RESULTS
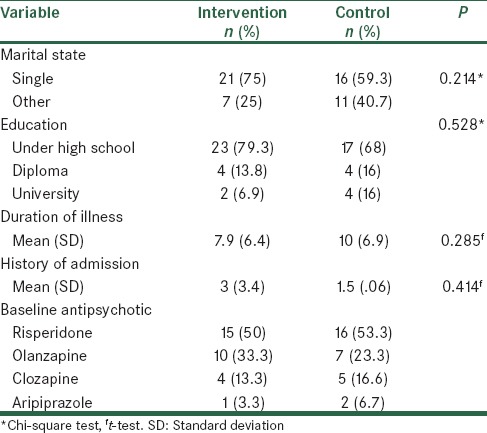
A total of 64 patients were included, however, four patients dropped out during the study [Figure 1]. Analysis of baseline demographic information, including age, marital status, education, duration of illness and hospitalization history, revealed no significant differences between the two groups [Table 1]. The intervention group included 18 males and 12 females, while the control group included 14 males and 16 females (P = 0.301). The mean ages of intervention and control groups were 32.3 (9.9) and 34.2 (10.6) respectively (P = 0.471). Among patients in the intervention group, 10 were living with parents, seven with one parent, two with spouse, and two alone, while, among control patients, nine were living with parents, six with one parent, five with spouse, and one alone. Moreover, there were 18 missing responses for social status, five for marital status, and six for education level.
Table 1.
Demographic and baseline clinical profile
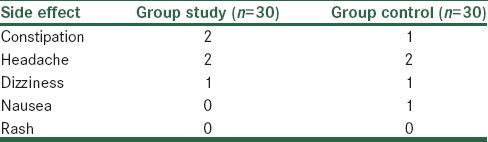
Analysis of baseline GAF and QLS scores showed no significant differences between the two groups [Table 2]. During the study, GAF and QLS scores increased in both groups; however, the increase was higher in the intervention group [Figure 2]. The difference between the two groups was statistically significant (P < 0.001) [Table 3]. Memantine was well tolerated, and side effects were not significant [Table 4].
Table 2.
Baseline comparison of GAF and QLS scores
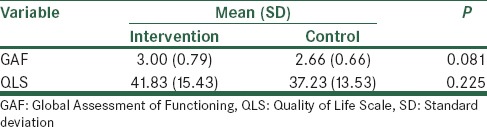
Figure 2.
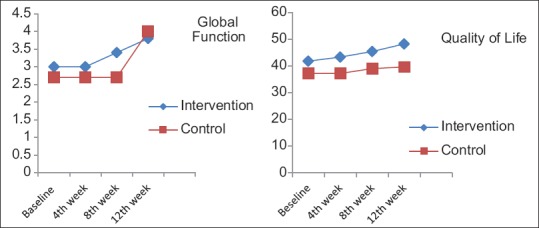
The effect of memantine and placebo on global function and quality of life
Table 3.
The effect of memantine and placebo on GAF and QLS
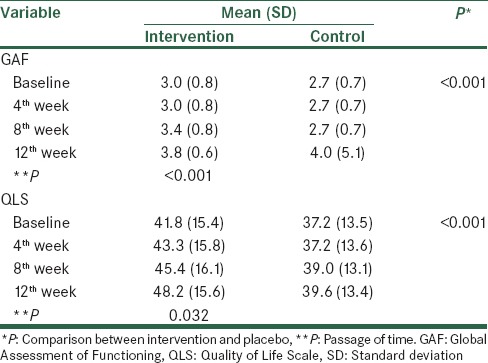
Table 4.
Frequency of reported side effects during the study, in study and control groups
The effect of memantine on negative and positive symptoms were also evaluated which will be depicted in detail, in another paper.
DISCUSSION
This is the first study, to our knowledge, that has assessed the effect of add-on memantine on global function and quality of life in schizophrenia.
In recent decades, new generation antipsychotics offered a more tolerable profile, and therefore, they were expected to yield improvement in quality of life; however, their benefit in promoting the quality of life still remains controversial.[5,6]
Memantine is a low to moderate affinity uncompetitive open channel NMDA receptor antagonist approved for Alzheimer's disease.[17] Memantine can block pathological activation and overstimulation, while preserving normal synaptic function.[18]
Memantine has been tried in a broad range of psychiatric disorders. Ghaleiha et al. showed that memantine add-on significantly improved short-term outcomes in moderate to severe OCD.[19] It has also been discussed that memantine might have antidepressant-like and mood-stabilizing effect. In a study by Lee et al. memantine has been effective in ameliorating metabolic profile of patients with bipolar II disorder; therefore, it is discussed that memantine can reduce binge eating episodes and weight.[20] It is also studied in children with autism and proven to be effective in reducing irritability and stereotypic behaviour.[21] It has also been studied in attention deficit hyperactivity disorder (ADHD), based on the concept of glutamate dysregulation in prefrontal cortex and striatum of untreated ADHD children.[17,22]
Administration of memantine in schizophrenia has also been studied in several trials. Krivoy et al. indicated that adjunctive memantine treatment, in schizophrenia can improve the clinical status, particularly negative symptoms, but not cognitive deficits.[23] Also, in another study by John et al., memantine has proven to be clinically effective in improving positive and negative psychopathology and cognitive domains.[8] However, Lee et al. proposed that adjunctive memantine treatment did not either affect psychopathology or improve cognitive function in chronic schizophrenia;[4] although it was well tolerated and did not exacerbate positive symptoms.[4] Likewise, Fakhari et al. showed that add-on memantinetreatment of refractory schizophrenia was not associated with improvement in negative and positive symptoms in refractory schizophrenia.[24] Moreover, Lieberman et al. found that adjunctive memantine had no efficacy in treatment of schizophrenia with residual psychopathology and was even associated with a higher incidence of adverse effects.[25]
The present study showed add-on memantine was significantly effective in improving the global function of patients as well as their quality of life. This could be explained by the fact that negative and cognitive symptoms are the most influencing factors in the disability of patients with schizophrenia.[5] If memantine ameliorates the cognitive or functional abilities in schizophrenia, it can subsequently improve patient function and quality of life.[8] However, regarding the short duration of treatment in the present study, heterogeneous baseline treatment, further studies are required to confirm the results.
Financial support and sponsorship
Isfahan University of Medical Sciences, Isfahan, Iran.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Palmer BW, Martin AS, Depp CA, Glorioso DK, Jeste DV. Wellness within illness: Happiness in schizophrenia. Schizophr Res. 2014;159:151–6. doi: 10.1016/j.schres.2014.07.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Irmak MK. Schizophrenia or possession? J Relig Health. 2014;53:773–7. doi: 10.1007/s10943-012-9673-y. [DOI] [PubMed] [Google Scholar]
- 3.Bosia M, Pigoni A, Cavallaro R. Genomics and epigenomics in novel schizophrenia drug discovery: Translating animal models to clinical research and back. Expert Opin Drug Discov. 2015;10:125–39. doi: 10.1517/17460441.2015.976552. [DOI] [PubMed] [Google Scholar]
- 4.Lee JG, Lee SW, Lee BJ, Park SW, Kim GM, Kim YH. Adjunctive memantine therapy for cognitive impairment in chronic schizophrenia: A placebo-controlled pilot study. Psychiatry Investig. 2012;9:166–73. doi: 10.4306/pi.2012.9.2.166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Galuppi A, Turola MC, Nanni MG, Mazzoni P, Grassi L. Schizophrenia and quality of life: How important are symptoms and functioning? Int J Ment Health Syst. 2010;4:31. doi: 10.1186/1752-4458-4-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Eack SM, Newhill CE. Psychiatric symptoms and quality of life in schizophrenia: A meta-analysis. Schizophr Bull. 2007;33:1225–37. doi: 10.1093/schbul/sbl071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bobes J, Garcia-Portilla MP, Bascaran MT, Saiz PA, Bousoño M. Quality of life in schizophrenic patients. Dialogues Clin Neurosci. 2007;9:215–26. doi: 10.31887/DCNS.2007.9.2/jbobes. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.John JP, Lukose A, Manjunath S. Off-label use of memantine as adjunctive treatment in schizophrenia: A retrospective case series study. Pharmacopsychiatry. 2014;47:202–9. doi: 10.1055/s-0034-1385931. [DOI] [PubMed] [Google Scholar]
- 9.Coyle JT. NMDA receptor and schizophrenia: A brief history. Schizophr Bull. 2012;38:920–6. doi: 10.1093/schbul/sbs076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hashimoto K. Targeting of NMDA receptors in new treatments for schizophrenia. Expert Opin Ther Targets. 2014;18:1049–63. doi: 10.1517/14728222.2014.934225. [DOI] [PubMed] [Google Scholar]
- 11.Attari A, Rajabi F, Maracy MR. D-cycloserine for treatment of numbing and avoidance in chronic post traumatic stress disorder: A randomized, double blind, clinical trial. J Res Med Sci. 2014;19:592–8. [PMC free article] [PubMed] [Google Scholar]
- 12.Omranifard V, Shirzadi E, Samandari S, Afshar H, Maracy MR. Memantine add on to citalopram in elderly patients with depression: A double-blind placebo-controlled study. J Res Med Sci. 2014;19:525–30. [PMC free article] [PubMed] [Google Scholar]
- 13.Choi KH, Wykes T, Kurtz MM. Adjunctive pharmacotherapy for cognitive deficits in schizophrenia: Meta-analytical investigation of efficacy. Br J Psychiatry. 2013;203:172–8. doi: 10.1192/bjp.bp.111.107359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lane HY, Lin CH, Huang YJ, Liao CH, Chang YC, Tsai GE. A randomized, double-blind, placebo-controlled comparison study of sarcosine (N-methylglycine) and D-serine add-on treatment for schizophrenia. Int J Neuropsychopharmacol. 2010;13:451–60. doi: 10.1017/S1461145709990939. [DOI] [PubMed] [Google Scholar]
- 15.Paraschakis A. Tackling negative symptoms of schizophrenia with memantine. Case Rep Psychiatry. 2014;2014:384783. doi: 10.1155/2014/384783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Aas IH. Global Assessment of Functioning (GAF): Properties and frontier of current knowledge. Ann Gen Psychiatry. 2010;9:20. doi: 10.1186/1744-859X-9-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hosenbocus S, Chahal R. Memantine: A review of possible uses in child and adolescent psychiatry. J Can Acad Child Adolesc Psychiatry. 2013;22:166–71. [PMC free article] [PubMed] [Google Scholar]
- 18.Sani G, Serra G, Kotzalidis GD, Romano S, Tamorri SM, Manfredi G, et al. The role of memantine in the treatment of psychiatric disorders other than the dementias: A review of current preclinical and clinical evidence. CNS Drugs. 2012;26:663–90. doi: 10.2165/11634390-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 19.Ghaleiha A, Entezari N, Modabbernia A, Najand B, Askari N, Tabrizi M, et al. Memantine add-on in moderate to severe obsessive-compulsive disorder: Randomized double-blind placebo-controlled study. J Psychiatr Res. 2013;47:175–80. doi: 10.1016/j.jpsychires.2012.09.015. [DOI] [PubMed] [Google Scholar]
- 20.Lee SY, Chen SL, Chang YH, Chen PS, Huang SY, Tzeng NS, et al. Add-on memantine to valproate treatment increased HDL-C in bipolar II disorder. J Psychiatr Res. 2013;47:1343–8. doi: 10.1016/j.jpsychires.2013.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ghaleiha A, Asadabadi M, Mohammadi MR, Shahei M, Tabrizi M, Hajiaghaee R, et al. Memantine as adjunctive treatment to risperidone in children with autistic disorder: A randomized, double-blind, placebo-controlled trial. Int J Neuropsychopharmacol. 2013;16:783–9. doi: 10.1017/S1461145712000880. [DOI] [PubMed] [Google Scholar]
- 22.Kavirajan H. Memantine: A comprehensive review of safety and efficacy. Expert Opin Drug Saf. 2009;8:89–109. doi: 10.1517/14740330802528420. [DOI] [PubMed] [Google Scholar]
- 23.Krivoy A, Weizman A, Laor L, Hellinger N, Zemishlany Z, Fischel T. Addition of memantine to antipsychotic treatment in schizophrenia inpatients with residual symptoms: A preliminary study. Eur Neuropsychopharmacol. 2008;18:117–21. doi: 10.1016/j.euroneuro.2007.07.008. [DOI] [PubMed] [Google Scholar]
- 24.Fakhari A, Herizchi S, Goldust M, Yousefi-Jafarabadi A. The efficacy of complementary use of memantine in treatment of schizophrenia with chronic course. J Am Sci. 2013;9:71–4. [Google Scholar]
- 25.Lieberman JA, Papadakis K, Csernansky J, Litman R, Volavka J, Jia XD, et al. A randomized, placebo-controlled study of memantine as adjunctive treatment in patients with schizophrenia. Neuropsychopharmacology. 2009;34:1322–9. doi: 10.1038/npp.2008.200. [DOI] [PubMed] [Google Scholar]


