Abstract
Background
Retinal ischemia is a major cause of visual impairment and blindness worldwide. The available therapeutic strategies have limited potential.
Purpose
In order to understand the pathophysiology and validating therapies for retinal ischemia, establishment of reproducible animal models is necessary.
Methods
In the model discussed in this article, the pterygopalatine artery (PPA) is ligated along with the external carotid artery for 3.5 hours and thereafter allowed to reperfuse. Because PPA supplies the blood to the ophthalmic artery, the ligation of this artery causes retinal ischemia.
Results
This article describes the validation of retinal ischemia-reperfusion model in mouse through PPA ligation and its validation through fluorescein fundus angiography (FFA) and immunofluorescence staining for glial fibrillary acidic protein (GFAP), a glial injury marker.
Conclusions
In conclusion this article describes the creation of mouse model of retinal ischemia-reperfusion injury which can be reproduced in a shorter time duration resulting in reduced mortality.
Keywords: Ischemia-reperfusion, Mouse model, Pterygopalatine artery, External carotid artery
 Introduction
Introduction
Retinal ischemia is a major cause of blindness world-wide and is associated with various disorders such as diabetic retinopathy, glaucoma, stroke, optic neuropathy and other retinopathies.1 It is caused by obstruction in blood supply to retina decreasing the oxygen and glucose supply. If the blood supply is not restored, the damage becomes permanent leading to cell death and consequent vision loss. The damage caused to the retina can be due to various pathways which result in energy failure, excitotoxicity, ion channels imbalance, free-radical generation.2 Reperfusion i.e. the restoration of blood flow to the retina leads to more damage to the retina than the ischemia itself. The re-establishment of blood supply to the ischemic retina increases the infiltration of macrophages and also leads to the generation of free-radicals causing more damage.3
Small animals, such as rats and mice are attractive models because they have vascular and phylogenetic similarities to humans. Besides, the availability, reproducibility and the relevance to humans are other factors that are important for use of mice for pre-clinical investigations.
Many different approaches have been used in establishment of animal models of retinal ischemia. One approach is through the elevation of intraocular pressure (IOP).4 In this method the IOP is increased above the systolic pressure. Selles-Navarro et al. quantified the retinal ganglion cells (RGC) survival at different time points in the high IOP model of retinal ischemia.5 Photocoagulation of blood vessels is another means to cause retinal ischemia.6 Another model for transient retinal ischemia involves temporary ligation of ophthalmic vessels. Lafuente et al. examined the RGC loss in this model of retinal ischemia by ligating the vessels for varying intervals of time and concluded that the degree of RGC loss depends on the severity of injury caused.7,8 In the same model, another study showed a significant decrease in the ERG wave amplitude along with the histological changes represented as decrease in inner retinal layer thickness.9 Another approach includes middle cerebral artery occlusion (MCAO)10 and chronic bilateral common carotid artery occlusion (BCCAO).11 Some of these vascular models are based on the obstruction of blood supply to the ophthalmic artery which also receives the blood supply from the pterygopalatine artery (PPA) and the external carotid artery (ECA) in mice. The ligation of PPA along with the external carotid artery thus reduces the blood supply to the retina.12–14 This information has been successfully exploited to establish mouse model of retinal ischemia.
Thus, this study describes the methodology for creation of mouse model of transient retinal ischemia and its validation through fluorescein fundus angiography and immunofluorescence analysis for glial fibrillary acidic protein (GFAP).
Methods
The experiments were performed on 6 to 8 weeks old C57BL/6J male mice. The mice were anaesthetized using xylazine (50mg/ml) and ketamine (50mg/ml). The right common carotid artery and its branches were exposed after a midline incision (Figure 1). The external carotid artery (ECA) and the pterygopalatine artery (PPA), a branch of internal carotid artery (ICA) were ligated using suture (size 7–0; Ethicon, New Jersey, USA). After 3.5 hours of ligation, the blood supply was restored to allow reperfusion (Supplementary video S1). The changes in the cerebral blood flow were measured using Laser-doppler blood flow-meter (Moor Instruments, UK) by placing the probe in the cortical region on the skull on the ipsilateral side of the brain. The ligation was validated using fluorescein fundus angiography, where sodium fluorescein was injected intravenously after 45 minutes of ligation of PPA and fundus images of right and left eye were captured using Spectralis HRA + OCT (Heidelberg Engineering, Heidelberg, Germany).
Fig. 1:
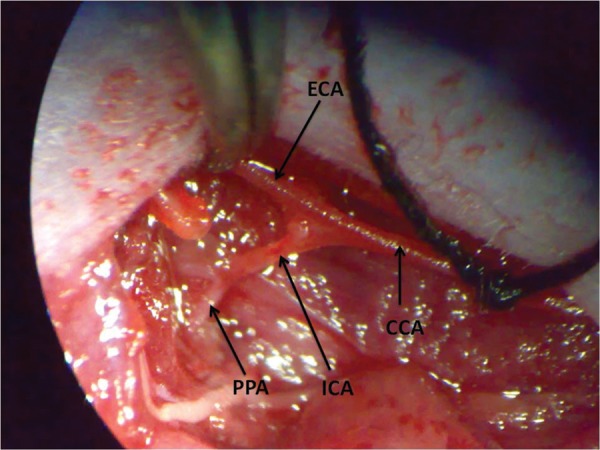
Screen-shot for the mouse arteries. The figure shows the right common carotid artery and its branches, where CCA- common carotid artery; ECA – external carotid artery; ICA – internal carotid artery; PPA – pterygopalatine artery.
After 5 days of reperfusion, the mice were sacrificed and the eyes were enucleated. The cryosections were cut and placed on slides. The control and injured sections were blocked in the serum of animal species in which secondary antibody was raised and incubated with primary antibody for glial fibrillary acidic protein (GFAP) overnight at 4OC. After washing with PBS, the sections were incubated with secondary antibody for 1 hour and counterstained with the nuclear stain, DAPI (1:1000, Life Technologies, USA). The sections were mounted with FluorSave reagent (Calbiochem, USA). The slides were observed under an upright fluorescent microscope (BX51, Olympus Corporation, Japan). The primary antibody used for immunofluorescence was GFAP goat polyclonal IgG antibody (1:100, Santa Cruz Biotechnology Inc., USA) and the secondary antibody was Cy3 conjugated donkey anti-goat antibody (1:200, Jackson ImmunoResearch Laboratories Inc., USA). The images were adjusted for contrast using Adobe Photoshop software.
All procedures were carried out following the GLP norms and according to the Standard Operating Procedures (SOP) established in the research facility. The experiments were approved by the Institutional Animal Ethical Committee (53/IAEC/273).
Results
In this study, the changes in blood flow to the brain were examined using laser doppler (n = 3). The laser doppler trend showed a decrease in blood flow during the ligation and was restored after the reperfusion of blood supply (Figure 2). The injury was further validated through fluorescein fundus angiography (FFA), where the fundus of the mouse after ligation was evaluated for fluorescein circulation (n = 4). The fundus photographs after the ligation of the artery showed thinning of the vessels as compared to the contralateral eye (Figure 3).
Fig. 2:
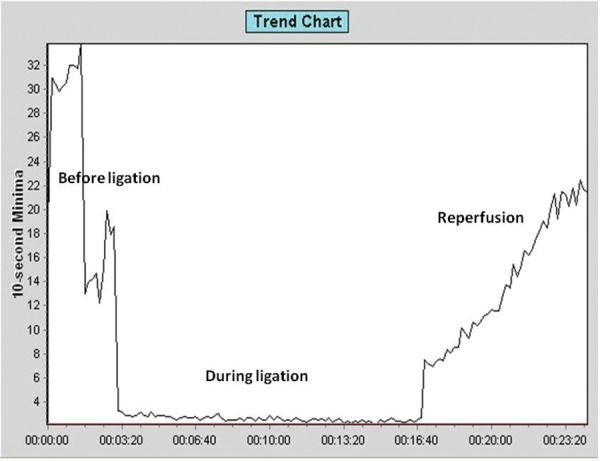
Blood flow measurement using Laser Doppler. The trend chart from laser doppler blood-flow meter demonstrates the changes in the cerebral blood flow before ligation, during the ECA and PPA ligation and after the ligation is removed for reperfusion.
Fig. 3:
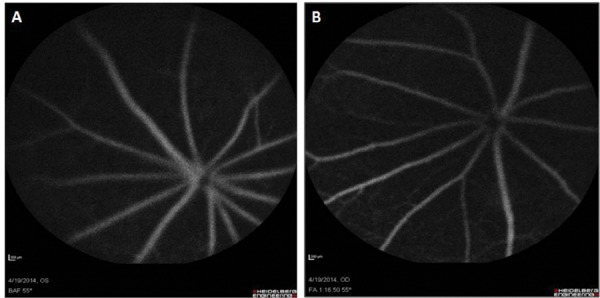
Fluorescein fundus angiography (FFA) of mouse eye. The figure shows the fundus photographs of mouse eye after PPA ligation. A. Left eye is without PPA ligation; B. Right eye is after PPA ligation – which shows thinning of the vessels.
The mice were sacrificed after 5 days of reperfusion and the validation of retinal ischemia model was done through histological and immunohistochemical analysis of retinal cryo-sections for injury markers such as glial fibrillary acidic protein (GFAP; n = 3). In this model of retinal ischemia, 3.5 hrs of ligation and 5 days of reperfusion was shown to have an increased expression of GFAP (Figure 4).
Fig. 4:
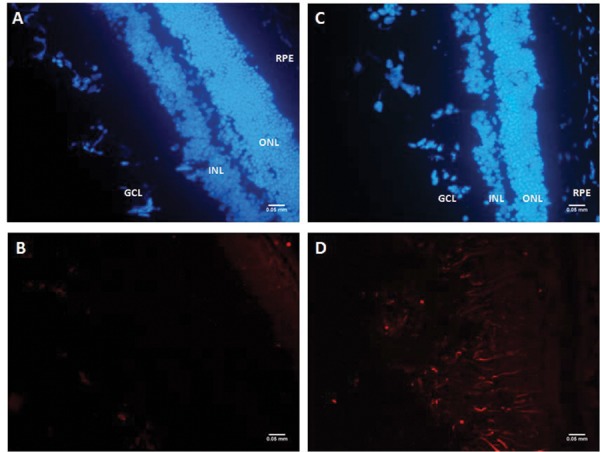
Immunofluorescence of GFAP expression after PPA ligation. Immunohistochemical staining for glial fibrillary acidic protein (GFAP) in cryosections for control (A-B) and experimental mice (C-D); GFAP is stained with Cy3; blue is DAPI which stains the nuclei (Scale bar – 50 microns).
Discussion
Retinal ischemia can be validated through histological and immunohistochemical analysis of retinal cryosections, quantitation of mRNA expression of injury markers in retina homogenate, blood flow measurements using laser doppler blood flow meter, electroretinography for electrophysiological changes and fluorescein fundus angiography.
The glial fibriallry acidic protein (GFAP) being a glial injury marker is known to be upregulated in response to neuronal injury and/or photoreceptor degeneration.15–17 Osborne et al. have shown that the decrease in blood supply to retina caused by clamping of both carotid arteries led to increased GFAP expression which could be used as an index of retinal degeneration.18 GFAP over expression has been reported in rat model of chronic BCCAO19 as well as in the optic nerve crush model.20 Eisenfield et al. reported elevated GFAP expression in RCS rats with genetic retinal degeneration.21
The mouse model of retinal ischemia reported in this article involves the ligation of both the external carotid artery and the pterygopalatine artery for the stipulated time-period. This leads to retinal damage by transient ischemia-reperfusion injury. This model has advantages over the other models that are being used to study retinal ischemia. The model established by elevation of intraocular pressure causes damage to retina through both ischemia and high pressure22,23 unlike the PPA ligation model which is more specific and well defined. The photocoagulation model, on the contrary, cannot be used to study transient ischemia as the damage caused is permanent. The middle cerebral artery occlusion model leads to both cerebral and retinal ischemia thus enhancing the mortality rate in the experimental animals.24
This model can be created in short period of time and has low mortality rate. The few confounders that need to be taken care of include the body temperature, infection, feed and housing conditions. The model can also be modified for the degree of retinal injury caused by changing the duration of ligation as well as reperfusion and can be further used to test new drugs and other therapeutic strategies for retinal ischemia.
Authorship contributions
Gillipsie Minhas: Performed the surgery Ryuichi Morishita, Munehisa Shimamura: Provided training, Reema Bansal: Supervised the FFA, Akshay Anand: Designed, supervised and provided resources for the study.
Acknowledgements
The authors acknowledge the Department of Science and Technology (F.No. SR/SO/HS-0001/2010), Council of Scientific and Industrial Research, India and Disease Models and Mechanisms Fellowship, London UK.
Footnotes
This article complies with International Committee of Medical Journal editor’s uniform requirements for manuscript.
Conflict of interest: None; Funding: None.
Competing Interests: The authors declare no competing interests.
References
- 1.Osborne NN, Casson RJ, Wood JP et al. Retinal ischemia: mechanisms of damage and potential therapeutic strategies. Progress in Retinal and Eye Research. 2004;23(1):91–147. doi: 10.1016/j.preteyeres.2003.12.001. [DOI] [PubMed] [Google Scholar]
- 2.Bonne C, Muller A, Villain M. Free radicals in retinal ischemia. General Pharmacology. 1998;30(3):275–80. doi: 10.1016/s0306-3623(97)00357-1. [DOI] [PubMed] [Google Scholar]
- 3.Jennings RB, Sommers HM, Smyth GA. Myocardial necrosis induced by temporary occlusion of a coronary artery in the dog. Archives of Pathology. 1960;70:68–78. [PubMed] [Google Scholar]
- 4.Buchi ER, Suivaizdis I, Fu J. Pressure-induced retinal ischemia in rats: An experimental model for quantitative study. Ophthalmologica. 1991;203(3):138–47. doi: 10.1159/000310240. [DOI] [PubMed] [Google Scholar]
- 5.Selles-Navarro I, Villegas-Perez MP, Salvador-Silva M et al. Retinal ganglion cell death after different transient periods of pressure-induced ischemia and survival intervals. A quantitative in vivo study. Investigative Ophthalmology & Visual Science. 1996;37(10):2002–14. [PubMed] [Google Scholar]
- 6.Mosinger JL, Olney JW. Photothrombosis-induced ischemic neuronal degeneration in the rat retina. Experimental Neurology. 1989;105(1):110–3. doi: 10.1016/0014-4886(89)90178-7. [DOI] [PubMed] [Google Scholar]
- 7.Lafuente Lopez-Herrera MP, Mayor-Torroglosa S, Miralles de Imperial J et al. Transient ischemia of the retina results in altered retrograde axoplasmic transport: Neuroprotection with brimonidine. Experimental Neurology. 2002;178(2):243–58. doi: 10.1006/exnr.2002.8043. [DOI] [PubMed] [Google Scholar]
- 8.Lafuente MP, Villegas-Perez MP, Selles-Navarro I et al. Retinal ganglion cell death after acute retinal ischemia is an ongoing process whose severity and duration depends on the duration of the insult. Neuroscience. 2002;109(1):157–68. doi: 10.1016/s0306-4522(01)00458-4. [DOI] [PubMed] [Google Scholar]
- 9.Mayor-Torroglosa S, De la Villa P, Rodriguez ME et al. Ischemia results 3 months later in altered ERG, degeneration of inner layers, and deafferented tectum: Neuroprotection with brimonidine. Investigative Ophthalmology & Visual Science. 2005;46(10):3825–35. doi: 10.1167/iovs.05-0392. [DOI] [PubMed] [Google Scholar]
- 10.Steele EC, Jr, Guo Q, Namura S. Filamentous middle cerebral artery occlusion causes ischemic damage to the retina in mice. Stroke. 2008;39(7):2099–104. doi: 10.1161/STROKEAHA.107.504357. [DOI] [PubMed] [Google Scholar]
- 11.Block F, Schwarz M, Sontag KH. Retinal ischemia induced by occlusion of both common carotid arteries in rats as demonstrated by electroretinography. Neuroscience Letters. 1992;144(1–2):124–6. doi: 10.1016/0304-3940(92)90731-l. [DOI] [PubMed] [Google Scholar]
- 12.Lelong DC, Bieche I, Perez E. Novel mouse model of monocular amaurosis fugax. Stroke. 2007;38(12):3237–44. doi: 10.1161/STROKEAHA.107.499319. [DOI] [PubMed] [Google Scholar]
- 13.Ogishima H, Nakamura S, Nakanishi T et al. Ligation of the pterygopalatine and external carotid arteries induces ischemic damage in the murine retina. Investigative Ophthalmology & Visual Science. 2011;52(13):9710–20. doi: 10.1167/iovs.11-8160. [DOI] [PubMed] [Google Scholar]
- 14.Ishizuka F, Shimazawa M, Umigai N et al. Crocetin, a carotenoid derivative, inhibits retinal ischemic damage in mice. European Journal of Pharmacology. 2013;703(1–3):1–10. doi: 10.1016/j.ejphar.2013.02.007. [DOI] [PubMed] [Google Scholar]
- 15.Ekstrom P, Sanyal S, Narfstrom K et al. Accumulation of glial fibrillary acidic protein in Muller radial glia during retinal degeneration. Investigative Ophthalmology & Visual Science. 1988;29(9):1363–71. [PubMed] [Google Scholar]
- 16.Xue LP, Lu J, Cao Q et al. Muller glial cells express nestin coupled with glial fibrillary acidic protein in experimentally induced glaucoma in the rat retina. Neuroscience. 2006;139(2):723–32. doi: 10.1016/j.neuroscience.2005.12.032. [DOI] [PubMed] [Google Scholar]
- 17.Xue L, Ding P, Xiao L et al. Nestin is induced by hypoxia and is attenuated by hyperoxia in Muller glial cells in the adult rat retina. International Journal of Experimental Pathology. 2011;92(6):377–81. doi: 10.1111/j.1365-2613.2011.00786.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Osborne NN, Safa R, Nash MS. Photoreceptors are preferentially affected in the rat retina following permanent occlusion of the carotid arteries. Vision Research. 1999;39(24):3995–4002. doi: 10.1016/s0042-6989(99)00127-3. [DOI] [PubMed] [Google Scholar]
- 19.Barnett NL, Osborne NN. Prolonged bilateral carotid artery occlusion induces electrophysiological and immunohistochemical changes to the rat retina without causing histological damage. Experimental Eye Research. 1995;61(1):83–90. doi: 10.1016/s0014-4835(95)80061-1. [DOI] [PubMed] [Google Scholar]
- 20.Bignami A, Dahl D. The radial glia of Muller in the rat retina and their response to injury. An immunofluorescence study with antibodies to the glial fibrillary acidic (GFA) protein. Experimental Eye Research. 1979;28(1):63–9. doi: 10.1016/0014-4835(79)90106-4. [DOI] [PubMed] [Google Scholar]
- 21.Eisenfeld AJ, Bunt-Milam AH, Sarthy PV. Muller cell expression of glial fibrillary acidic protein after genetic and experimental photoreceptor degeneration in the rat retina. Investigative Ophthalmology & Visual Science. 1984;25(11):1321–8. [PubMed] [Google Scholar]
- 22.Peachey NS, Green DJ, Ripps H. Ocular ischemia and the effects of allopurinol on functional recovery in the retina of the arterially perfused cat eye. Investigative Ophthalmology & Visual Science. 1993;34(1):58–65. [PubMed] [Google Scholar]
- 23.Marmor MF, Dalal R. Irregular retinal and RPE damage after pressure-induced ischemia in the rabbit. Investigative Ophthalmology & Visual Science. 1993;34(8):2570–5. [PubMed] [Google Scholar]
- 24.Minhas G, Morishita R, Anand A. Preclinical models to investigate retinal ischemia: advances and drawbacks. Frontiers in Neurology. 2012;3:75. doi: 10.3389/fneur.2012.00075. [DOI] [PMC free article] [PubMed] [Google Scholar]


