Abstract
Background
Spinal cord injury (SCI) is a neurological condition which paralyses the patient below the level of injury and could occur due to damage, infection and tumors. Presently, there is no cure for SCI. The treatment options used for SCI include corticosteroid (methylprednisolone sodium succinate), surgical interventions, and physiotherapy and lowering of body temperature. The research on treatment options for SCI has been shifted to cell-based therapies. Use of human embryonic stem cells (hESCs) have been explored in animal models in which these cells have been found to hold a potential to repair and regenerate.
Purpose
We wanted to assess the safety and efficacy of hESCs in the treatment of patients with spinal cord injury.
Methods
Five patients who were either paraplegic or quadriplegic were treated with hESC therapy.
Results
Following the treatment, all patients showed significant improvement in their sitting balance, control and sensation of bowel and bladder, power and movement of limbs (lower limbs and upper limbs). No adverse events were reported.
Conclusion
In conclusion, hESC is safe and effective therapy for SCI.
Keywords: Quadriplegia, Paraplegia, hESC, Stem cell therapy
 Introduction
Introduction
Spinal cord injury (SCI) is a neurological condition that occurs due to injury, infection and tumors.1,2 A systemic review estimated the incidence rate of traumatic spinal cord injury (TSCI) in Asia to range from 12.06 to 61.6 per million in persons with the average age ranging from 26.8 to 56.6 years. Motor vehicle collisions and falls were recognized to be the main causes of TSCI(3). Today, the research on treatment options for SCI aims at regaining the lost functions of spinal cord by promoting remyelination with oligodendrocytes, and formation of neurons.4,5 Stem cell therapy has already been explored in vitro and in vivo as a source of cell replacement therapy in the treatment of SCI.5,8 We report the use of hESC in the treatment of five patients who were either paraplegic or quadriplegic. All the patients were scored on the basis of a scale developed by the American Spinal Injury Association (ASIA), both before and after the treatment.9
Methods
hESCs (NTECH-2000 n/nn) were cultured and maintained as per our proprietary in-house patented technology in a Good Manufacturing Practices, Good Laboratory Practices and Good Tissue Practices certified laboratory at Nutech Mediworld (Patent-WO 2007/141657A PCT/1B 2007 Published 13 Dec 2007). The evidence for the use of hESCs at Nutech Mediworld has also been submitted in written and accepted at House of Lords, Regenerative Medicine, Science and Technology Committee.10 The cell lines are free of animal product and are chromosomally stable.
The treatment strategy was divided into phases. In first phase, T1 (8-Week for paraplegics and 12-Week for quadriplegics), 0.25 ml (<4 million cells) hESCs were administered through intramuscular (i.m) route twice daily to “prime” the body and allow for the recipient immune system not to reject the stem cells, 1 ml hESCs (<16 million cells) were administered every 10 days through intravenous (i.v) route to “home in” to the required area and 1 to 5 ml hESCs were administered every 7 days by any of the supplemental routes including brachial plexus block, intrathecal, caudal, epidural, popliteal block and/or deep spinal muscle and epidural catheter, in order to introduce the stem cells as near the injured site as possible (local action). After a gap period of 4–8 months, the successive phases like T2 (4 to 6 weeks) and T3 (4 to 6 weeks) also used the same dosage regime as T1. The treatment was repeated annually, if needed. This treatment protocol was developed on the basis of a pilot study conducted on 72 patients, which found that the extension of the treatment period more than 8 weeks in paraplegics and more than 12 weeks in quadriplegics do not lead to any better results. A gap of 4 months between the subsequent treatment phases was decided to allow the injected hESCs to develop into mature cells and regenerate the affected part. The treatment periods T2 and T3 were incorporated to add more cells into the body, thus, allowing more repair and regeneration.
The patient provided written informed consent prior to start of the treatment. The condition of patient was videographed before, during and after the treatment periods. The biochemical and radiological investigations were done before the start of the treatment and then at regular intervals. The characteristics of all the patients are described in Table 1. In-house physicians and nurses carefully observed all the patients for antigenic or paraphylactic responses.
Table 1: Characteristics of Patients.
| Patients | Age (Yr) | Injury date | Admission Date (Visits) | Discharge Date | ASIA Score | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 1 | 2 | 3 | 4 | 5 | 6 | Before | After | |||
| 1 | 32 | 17 Apr 94 | 8 Mar 08 | 1 Aug 10 | 28 Mar 11 | 24 Oct 13 | 2 Jun 08 | 10 Sep 10 | 11 May 11 | 8 Nov 13 | A | C | ||||
| 2 | 20 | 31 Oct 10 | 8 Oct 11 | 3 Oct 13 | 10 Dec 11 | 30 Oct 13 | A | B | ||||||||
| 3 | 26 | 30 Apr 11 | 26 Sep 11 | 15 Nov 12 | 2 Dec 13 | 23 Nov 11 | 13 Dec 12 | 26 Dec 13 | A | A | ||||||
| 4 | 48 | 18 Apr 92 | 16 Aug 13 | 7 Apr 14 | 26 Dec 13 | 8 May 14 | A | A | ||||||||
| 5 | 37 | 31 Aug 07 | 1 Feb 10 | 1 Sep 10 | 23 Feb 11 | 24 Feb 12 | 30 Oct 12 | 15 Nov 13 | 28Apr 10 | 18 Oct 10 | 29 Mar 11 | 1 Apr 12 | 12 Dec 12 | 23 Dec 13 | A | B |
The data for all the patients was validated by Moody’s International (Document number NH-heSC-10–1), GVK Biosciences (NM-Hesc-10–1, 18 November 2010) and Quality of Austria Central Asia Pvt. Ltd. Accreditation Company (Document number QACA/OCT/2013/26). These companies were allowed to examine the medical and statistical data present at the institute and were also able to meet the patients.
Results
Patient 1
An Australian patient with quadriplegia since last 14 years was admitted to Nutech Mediworld on 8 March 2008. Patient history revealed that he suffered a major trauma to the neck while playing rugby in April 1994, which resulted in injury to the spine at C1 level that later receded to C2 level. He was on ventilator support via tracheostomy since his injury. His ASIA score as assessed by his investigator was A.
At the time of admission, the patient was unable to move his upper limbs (ULs) as well as lower limbs (LLs) and had complete loss of sensation except on his face. On examination, patient was on ventilator support with tracheostomy at 17 breaths per minute, speech was co-incident with the ventilator. He had no sitting balance and the plantar reflex and the abdominal reflex were absent with an exaggerated ankle jerk. His LL had clonus. There was no deep sensation. He had no bladder and bowel control or sensation. He needed three full time care-takers at all times. He could not eat more than one meal a day. Magnetic resonance imaging (MRI) tractography showed visualization of nerve fibers/tracts in the upper cervical cord from cervicomedullary junction caudally up to C2 level; the cord fibers were not discerned upto D1 level (Figure 1a).
Fig. 1:
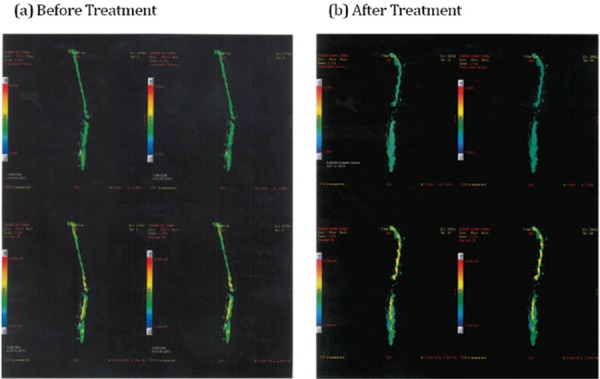
Before treatment and after treatment Tractrography Images (Patient 1).
The patient underwent four sessions of hESC therapy at Nutech Mediworld (Table 1) and was last followed up on 8 November 2013.
Following the treatment with hESC, the patient weaned off his ventilator and was able to remain away from ventilator for up to 12 hours. He was able to move his neck freely, shrug shoulders and showed movement of his arms and hands. His sitting balance also improved significantly. He could stand with chest and Hip Knee Ankle Foot Orthosis (HKAFO) and with support. His deep sensation was increased up to the abdomen. His ASIA score after the treatment was C (Figure 1b).
Patient 2
A 28-year male from India was admitted to Nutech Mediworld on 8 October 2011 with complaints of loss of bowel and bladder control, left (Lt) LL paralyzed with intact sensation and right (Rt) LL movements present with no sensation.
Patient’s history revealed that he met with an accident in October 2010 sustaining D11-D12 fracture and right ulnar fracture. Patient underwent surgical procedure with instrumentation at D9-L1 level and had regular session of physiotherapy and occupational therapy post surgery which helped him in improving motor function in Rt LL and sensation in Lt LL. He was even able to lift and hold his Rt LL, could hop on crutches but couldn’t walk. His ASIA score was A.
The MRI scan showed fracture of D11 vertebral body with anterior subluxation of D10 over D11, compression fracture of D12 vertebral body, decreased spinal size at D11 due to fracture subluxation with indentation of the cord at D10-11, minimal extradural hematoma at D10–11 level. Cord contusion was noted at D10–11 level and D11 laminar fracture was seen on Rt side Figure 2(b).
Fig. 2:
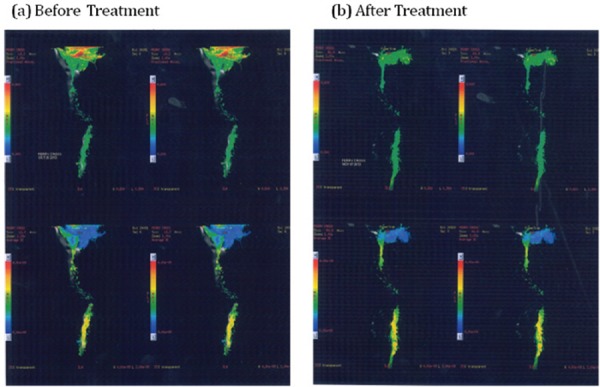
Before treatment and after treatment Tractrography Images (Patient 2).
Patient underwent two sessions of hESC therapy. Following the first session of the therapy, patient showed improvement in bladder and bowel sensation with partial control, improvement in sensation in Lt LL and Rt LL power (as he could bend his knees and hold it). There was decrease in clonus and jerks in LL and calf pain. The patient was able to walk with Ankle Foot Orthosis (AFO) calipers and walkers and could climb stairs with little help.
On 3 October 2013, patient came back for the second session of hESC therapy. The gap between the two sessions of therapy was more than 2 years. Following the second session of the therapy, patient showed improved LL strength, improved Lt LL sensation at foot-planar and dorsal aspect and calf and gait improved with quadripod stick in bars. The Rt thigh and toes movements were stronger than earlier. Patient was last followed up on 30 October 2013.
During the therapy he was also given supplements including calcium supplement (Calcitriol -500 mg × OD), vitamin B capsules (B-complex-15 units), iron supplements (Ferrous Fumarate and Folic Acid - OD), Vitamin D3 (Cholecalciferol sachets – one sachet a week) and nectra powder.
The patient had an improved power and sensation in Lt leg and improved power in Rt leg thigh and toes. The patient had improved bowel and bladder control with full voiding and evacuation sensation. His gait improved with quadripod sticks. He was able to walk with elbow crutches and climb stairs with little support. His ASIA score was B following the treatment (Figure 1b).
Patient 3
A 26-year old male from the US was admitted to Nutech Mediworld on 26 September 2011 with complaints of no movements of LL, no sensation below umbilicus and no bladder and bowel control or sensation.
The patient was apparently well until April 2011 when he met with a car accident, sustaining T11-T12 fracture with SCI along with other injuries including T11 rib fracture and Lt pneumothorax. After the injury he had a complete loss of sensation below the umbilicus and control and sensation over bowel/bladder. He was hospitalized and surgical correction for the compression fracture with reduction and posterior instrumentation of T9-L1 was done. Patient was wheelchair bound since then with no movement of B/L LL. He had regular physiotherapy sessions after the surgery and was able to take a few steps with Knee Ankle Foot Orthosis (KAFO) calipers and elbow crutches. He had some bladder sensation on catheterization but did not have any filling or voiding sensation. His limbs were atrophic and flaccid. His score, on ASIA impairment scale was A. He had undergone stabilization and physiotherapy in the U.S.
On examination, the patient had one bed sore at coccyx region and B/L LL edema. Investigation with MRI revealed D10-11 which reduced with significant spinal canal encroachment by post protruded intervening disc or marginal osteophytes which compressed the thecal sac and post-contusion residual myelomalacic changes in spinal canal at D10-11. Electromyography (EMG) showed severely impaired post column conduction from B/L LL with evidence of active de-nervation on EMG.
Patient was given hESC therapy. At the end of first session of therapy, the patient showed marked improvement in his health. He had mild sensation in knee, muscle sensation in quads and calf better, deep knee sensation, partial voiding sensation and had a feeling of pain in back. He could crawl and walk with KAFO and crutches and had a flicker movement in B/L ankle and toes. The patient had plantar reflex in Lt foot and excessive sweating at the sole of the feet.
After 11 months, the patient came for second session of the therapy on 15 November 2012. The patient did not follow the protocol. Before the second session of hESC therapy, MRI tractography was done which showed marked artifactual distortion and non-visualization of the tracts in dorsal cord at the level of injury and pedicle screw fixation. No definitive improvement was observed on the integrity of white matter fibers at the site of cord injury. Following the second sensation of hESC therapy, the patient developed sensation upto B/L thighs and Rt knee.
On 2 December 2013, the patient visited our facility for the third session of therapy after a gap of 11 months. He again underwent investigation with MR tractography which showed no definitive improvement on the integrity of white matter fibers at the site of cord injury. There was anterior wedging of D11 vertebral body with break in height reduction of D11-D12 disc. There was a mild thecal sac indentation from mild posterior bulge of the disc. Cystic myelomalacia was noted caudal to D9-D10 disc level with marked thinning of the cord caudal to D10. We, however, observed that the tracts seem to have increased in size and diameter.
Clinically, patient had a remarkable improvement as he was able to walk on the treadmill for almost half an hour at a stretch just with an ankle support. Overall, patient showed improvement in the knee movement, kneeling strength (ability to shift in squatting position) and had flicker movements in B/L ankle and toes. The patient developed partial sensation and control over bowel and bladder. He had improvement in LL muscle contraction (quads and hamstrings), his core strength improved and he had increased his muscle mass of the body. The patient was able to move with KAFO and elbow crutches. His ASIA score continued at A.
Patient 4
A 48-year male from India was admitted to Nutech Mediworld on 16 August 2013 with complaints of tingling sensation at the level of injury (D10), decreased sensation and power in B/L LL, impaired bowel and bladder sensation and function with jerky movements in both LL with spasticity.
Patient was apparently well until 18 April 1992, when he had multiple gun shots at T9-T10 level of spine. He noticed sudden loss of sensation and motor power below the T10 thoracic vertebral level with no bowel/bladder control and sensation. Patient underwent surgical procedures for removal of bullets. His ASIA impairment score as assessed by his investigator was A.
He was wheelchair bound since then with poor sitting balance, severe spasticity and tightness in B/L LL and on Foley’s catheter. He had no bowel sensation and control.
Patient’s medical history revealed that he had undergone laproscopic removal of foreign bodies from the spine in April 1992, with anastomosis in July 1992 and poor healing of closure site in 1995. On examination, patient had exaggerated reflexes (L>R), clonus in knees, bed sore at sacral area and Rt iliac area and the absence of plantar and ankle reflexes. On investigation, MR tractography showed complete interruption of nerve fibers at T9 level, other radiographic and blood investigations were non-significant except for raised TLC (13, 300/L) and he was found to have urine infection with E. coli and was treated with Norfloxacin (400 mg twice daily × 5 days). The patient underwent first session of hESC therapy for 8 weeks and showed improvement in his ability to feel the bowel/bladder fullness, tingling sensation in B/L LL, slight movement in his LL inwards and outwards. The patient could feel weight on his heels and knees when on calipers, had good sitting balance, felt reduction in spasticity and tightness and was able to stand and take steps with KAFO calipers and minimum external support.
Patient underwent skin grafting of sacral area sore in September 2013 with complete healing and his Rt iliac sore was also completely healed.
After a gap of 3 months, the patient came for the second session of hESC therapy on 7 April 2014. During second session of the therapy, the patient had fever and sore throat with elevated TLC (18, 200/L), which was treated with injectable Cefpodoxime (2gm OD × 3 days). The patient showed further improvements in power of LL and also in the sensation of temperature, touch and pain. The patient was able to feel complete bladder fullness, partial bowel fullness and could feel weight at the heels and the knees on standing with calipers. He could also feel the needle prick in his spinal procedures. The patient was last followed up on 8 May 2014.
Overall, the patient showed improvement in standing capability with calipers, a mild flicker in B/L knee extensors, had sensation to temperature, touch and pain, bowel and bladder sensation on fullness. He showed improved standing balance and posture with good step taking, had tingling sensation all over LL and could move LL inwards and outwards in supine position. His ASIA score after the treatment remained at A.
Patient 5
A 36-year female from the U.S. was admitted to Nutech Mediworld on 1 February, 2010 with complaints of no movement of B/L LL, slight movement in B/L UL, absence of sensation below sternum, no bowel bladder sensation and control, pain from waist to toe, inability to grasp any object, poor sitting balance, could not walk, and felt dizzy even on sitting up. Her ASIA score was A.
The patient was apparently healthy till 2007 when she met with an accident sustaining injury at C6-C7 level. She underwent surgical fixation of C5-T1 with rods. She was wheel chair bound on a quad support since then with poor sitting control, unable to walk and felt neuralgic pain below waist. The patient’s history revealed that spiral fracture of Rt humerus was surgically fixed with titanium plates and screw.
On examination, she had claw hand, thumb movement were absent in Rt hand and weak in Lt hand, no hand functions, weak hand grip, was dependent for activities of daily living and had poor pelvic control in quadruped position. The patient was paralysed in all the four limbs and had no bowel/bladder sensation and control.
The patient was treated with hESC along with extensive physiotherapy and occupational therapy. The patient underwent six sessions of hESC therapy, with a gap phase between the sessions varying from 4 to 11 months. She last followed up on 23 December 2013. Following the treatment, the patient showed improvement in UL muscle strength, contraction of B/L hips and knees, muscle wasting and improvement in posture with good sitting balance. The patient had developed good standing balance with calipers, good grasping with Rt hand, good thumb movement in both hands and good pinch and release. She was able to write, make a near normal fist, developed sensation till B/L knees and at sole of foot, appreciable extension in both knees; could stand for long duration and even take steps with KAFO caliper and binder. The patient also showed improvement in bladder sensation (can hold the speed of voiding) and bowel sensation (pushes while passing motions). She was able to crawl independently and also able to stand from a sitting position while holding onto bars. Her ASIA score after treatment was B.
Discussion
Several factors contribute to inability of neural regeneration and slow functional recovery after SCI. The injury leads to degeneration of the axons.11 It also causes death of multiple cell types including neurons and oligodendrocytes. Death of oligodendrocytes results in demyelination of axons which further disrupts the action potential1 contributing to impaired locomotion.5 Presently, there is no cure for SCI. The treatment options used for SCI include corticosteroid (methylprednisolone sodium succinate), surgical interventions, physiotherapy and lowering of body temperature.12,13 Recently, research on the use of cell-based therapies has gained momentum. Cells having capability of self renewal and differentiation into multiple cell types could be used as a novel therapy for the treatment of SCI.5,14 Stem cells have been recognized to possess the potential to differentiate into multiple cell types.15 Use of ESCs is an attractive therapeutic option because of their pluripotent nature.16 Transplanted cells are known to function by replacing the lost cells thus, promoting the recovery.5
Keirstead et al were the first to show that remyelination using hESCs could be an option for the restoration of lost function after SCI. They observed enhanced remyelination and improved locomotion in adult rats with SCIs, following the injection of hESC-derived oligodendrocyte progenitor cells (OPCs).5 Nistor and colleagues presented a method for the generation of oligodendrocyte from hESCs. They injected oligodendroglial cells into the shiverer model of dysmyelination, where they differentiated into oligodendrocytes and showed myelin formation.17 Kakinohana et al reported that the injection of hESC-derived neural cell precursors (hNPCs) into ischemia-injured lumbar spinal cord of rats or lumbar spinal cord of naive immunosuppressed minipigs resulted in successful engraftment of hNPCs and their maturation into neurons.18 However, till date only one human trial (the Geron trial) on the use of hESC-derived OPCs in patients with acute SCI has been conducted but was stopped in between due to financial concerns.19
In the present study, in-house cultured hESCs were used to treat quadriplegic and paraplegic patients. The cell line has been created from a single fertilized ovum 24–48 hr after fertilization when the conceptus is assumed to have reached the 4–16 cell stage. Media used in culturing the cell lines are free from animal contaminants and cells of animal origin. The hESCs were administered through i.m, i.v as well as through other supplemental routes (brachial plexus block, intrathecal, epidural, caudal, deep spinal muscle and/or popliteal block). Treatment protocol consisted of different phases such as T1, T2 and T3 with a gap phase of 4–6 months between the successive phases so as to allow hESCs to grow, repair and regenerate. The duration of gap phase was decided based on a concept of time taken for organogenesis (14–16 weeks) in human fetus.20
Previous studies have shown that stem cells communicate with damaged cells, and home at the site of injury. Homing signals including cytokines, chemokines and growth factors released at the injured site attracts systemically or locally administered stem cells to the site. These signals function by inducing upregulation of selectins and activation of integrins, present on the stem cells. Mesenchymal stem cells (MSCs) have been shown to follow the same homing pattern.21,22
Thus, we could assume that hESCs in our study might have followed the same pattern of homing at the injured site and regenerating the affected region. We cannot rule out the possibility that in our study patients, the hESCs might have differentiated into neurons, help in the tissue repair at the site of injury in spinal cord and regenerate cells for improvement in functioning of the spinal cord.
Dramatic improvement in the health of all the patients was observed following the treatment with hESC in our study. ASIA score of two patients improved from A to B and for one patient, it improved from A to C. Although, the score of other two patients remained A after the treatment but they showed various signs of improvement that cannot be assessed with ASIA score such as fullness of bowel and bladder and control over bowel and bladder. None of the patient developed teratomas and were not given any immunosuppressant.
In conclusion, hESCs could be an effective and safe therapeutic option for treatment of patients with SCI. However, there is a need to conduct clinical trials on large number of patients with SCI to confirm the safety and efficacy of hESC-based therapy.
Acknowledgements
The authors acknowledge all the doctors, a staff and patients of the Nutech Mediworld.
Authorship Contribution
Geeta Shrauff: Involved in the conception, design and conducted the study, reviewed each draft of the manuscript, Rakesh Gupta: Involved in the clinical examination of some patients
Abbreviations
- hESC
human embryonic stem cell
- TSCI
Traumatic spinal cord injury
- SCI
Spinal cord injury
- MSCs
Mesenchymal stem cells
- ASIA
American Spinal Injury Association
- UP:
Upper Limb
- LL
Lower limb
- OPCs
Oligodendrocyte progenitor cells
- KAFO
Knee Ankle Foot Orthosis
- MRI
Magnetic resonance imaging
| Serial no. | Comment | Response |
|---|---|---|
| Review Round 1 | ||
| Reviewer Message: “The world community should not lose out on the first human data on embryonic stem cell therapy in spinal injuries.” | ||
| Caveat: All regulatory/monitoring permissions have been claimed to be taken by the investigator. | ||
| 1 | That patients were not charged of any fees. | Patients were charged as a package which included the physical therapy, occupational therapy, food and lodging. |
| The statement for the same has also been added in the letter drafted for the comment no. 8 | ||
| 2 | Please mention if pregnant or lactating patients received any therapies. | The data for all the patients was validated by GVK Biosciences (NM-Hesc-10-1, 18 November 2010). We are providing the validation report from GVK for your reference. As per exclusion criteria, pregnant or lactating patients were not included in the study |
| 3 | The choice of patients in this study is widely disparate, ranging from 5 months to 21 years after injury, making interpretation of results difficult. | All the five patients in our study had a chronic injury. They came after taking others treatments that did not result in any benefits. All patients were injured since long duration (Patient 1: 14 yrs, Patient no. 2: 1 yr, Patient no. 4: 21 yrs, Patient no. 5: 3 yrs) except the Patient no. 3, who suffered recently (5 months). |
| 4 | Authors’ Nutech Mediworld’s hESC has no validated and published study on cell line or animal model for understanding the utility and mechanism of this patent on the CNS | The paper on cell utilities is under process. |
| We have recently published two studies on the use of hESC therapy in patients with CP (http://www.ncbi.nlm.nih.gov/pubmed/25496119 and CVI (Accepted in Cell Sciences and Therapy, not yet published). Other papers have been submitted and are under review. | ||
| 5 | There is no investigation to prove that these hESCs homed into the spinal cord of the patient | Homing of stem cells in the patients with spinal cord injury and other medical conditions has been investigated by several studies. We are providing some references in support of the same. |
| A review by Kang et al stated that chemokines, cytokines, and growth factors released upon injury provide migratory cues for systemically or locally administered stem cells. The cues induce upregulation of selectins and activation of integrins on the stem cell surface, enabling cells to interact with the endothelium. Stem cells subsequently adhere and transmigrate across the endothelial layer into tissues. Regarding the homing capability of MSCs, numerous studies have confirmed that systemically infused | ||
| MSCs can migrate to injured, inflamed tissues and exert therapeutic effects. (Journey of mesenchymal stem cells for homing: strategies to enhance efficacy and safety of stem cell therapy. Stem Cells Int. 2012; 2012:342968. Epub 2012/07/04). | ||
| A study by Chapel et al found that MSCs homed into numerous tissues following a severe multi-organ injury in primates. (Mesenchymal stem cells home to injured tissues when co-infused with hematopoietic cells to treat a radiation-induced multi-organ failure syndrome.J Gene Med. 2003 Dec;5(12):1028-38.) | ||
| 6 | The authors need to mention what was the “supplemental route” taken for injecting the stem cells | The following supplemental routes were used to inject the hESCs in our study patients: Brachial plexus block, intrathecal, caudal, epidural, popliteal block and/or deep spinal muscle and epidural cathetar |
| 7 | The mentioned videos should be given as supplementary data | We are providing the links where you can find the videos uploaded by the patients themselves. (http://pcsrf.org.au/) and (http://www.shannondavisjourney.com/) |
| 8 | The details of the patients’ clinical status before and after treatment can be shortened into ASIA grade. The details given in the text about the neurological grade and outcome can be given as supplemental data | We have shortened before and after clinical status of the patients in the manuscript. |
| Patient no. 1: ASIA score before treatment: A | ||
| SIA score after treatment: C | ||
| Patient no. 2: ASIA score before treatment: A | ||
| ASIA score after treatment: B | ||
| Patient no. 3: ASIA score before treatment: A | ||
| ASIA score after treatment: A | ||
| Patient no. 4: ASIA score before treatment: A | ||
| ASIA score after treatment: A | ||
| Patient no. 5: ASIA score before treatment: A | ||
| ASIA score after treatment: A | ||
| 9 | Their data has only been presented at the House of Lords which has not commented on its usefulness/validity | We have recently published two studies on the use of hESC therapy in patients with CP (http://www.ncbi.nlm.nih.gov/pubmed/25496119 and CVI (Accepted in Cell Sciences and Therapy, not yet published). Other papers have been submitted and are under review. |
| The data has also been presented at the 13 different scientific events. | ||
| 10 | The Geron trial was not stopped only on financial grounds but there were litigations based on ethicality | Tom Okarma, CEO of Geron made a public statement that the decision to halt the clinical trial on hESC was completely based on financial concerns. By stopping its stem cell programme, the company will cut its workforce by more than a third and save millions of dollars. (http://www.bbc.com/news/health-15740133) |
| (Money and Morals: Ending Clinical Trials for Financial Reasons. Eaton ML, Kwon BK, Scott CT. Curr Top Behav Neurosci. 2014 Jul 26. [Epub ahead of print]) | ||
| We are not aware if the study stopped based on ethical litigations. | ||
| 11 | What were the necessary regulatory permis-sions obtained for the 72 patients’ pilot study? | The approval for the pilot study on 72 patients was taken from the independent ethics committee of the institute. |
| 12 | What were the necessary regulatory permis-sions obtained for the 5 patients in this study? | The approval for this study was taken from independent ethics committee. |
| 13 | If this was a trial, then the necessary permission from DCGI and other authorities would be required and if this was a therapy, this is in direct contravention to ICMR-DBT Guidelines for Stem Cell Research which were already in place by 2012. | We followed the 2012 guidelines of Indian Council of Medical Research (http://icmr.nic.in/stem_cell/stem_cell_guidelines.pdf). Please see page no. 14 of the guideline. |
| Review Round 2 | ||
| 14 | There is reference to pilot study for 72 patients conducted before the current study. Authors are requested to provide Ethical approval for the pilot study of 72 patients. | The study for 72 patients was a retrospective study. All patients had given consent to any study being carried out prior to the human embryonic stem cell transplantation. |
| 15 | It is difficult to interpret data from such a disparate patients | We agree that no analysis can be done from this patient population. That is why we have presented the results as such. Please note that this was the initial study and was not done to analyze anything. All these patients had irreversible conditions and no therapy had benefitted them. |
| Our primary objective of giving hESC therapy was to collect the safety data. We also noted the efficacy of these cells as a secondary objective. | ||
| Through this publication, we just want to convey to the audience that hESC cultured in our laboratory that are free of any animal products do not cause any adverse events as teratomas or immunogenicity and are clinically effective. | ||
| Now, it’s been 13 years and we have data for more than 1300 patients with irreversible conditions who have benefitted from this therapy. Till date, none of our patient had any adverse event during or after the therapy. | ||
| Everybody knows the potential of hESCs in curing irreversible conditions but the fear of adverse events is mainly because of the animal products that are used in culture and the immunogenic response. We use a unique technology. That’s why it has been awarded patent in over 126 countries including US. | ||
| 16 | It is not clear if author has published any animal or cell study. | Here is the list of our studies conducted in humans that have been published: Shroff G, Gupta A, Barthakur J. Therapeutic potential of human embryonic stem cell transplantation in patients with cerebral palsy. J Transl Med 2014; 12: 318. |
| Shroff G, Das L. Human Embryonic Stem Cell Therapy in Cerebral Palsy Children with Cortical Visual Impairment: A Case Series of 40 Patients. Journal of Cell Science and Therapy 2015. | ||
| Shroff G. A novel approach of human embryonic stem cells therapy in treatment of Friedrich’s Ataxia. Inter-national Journal of Case Reports and Images (IJCRI) Forthcoming 2015. | ||
| Treatment of Lyme Disease with Human Embryonic Stem Cells: A Case Series. In Press | ||
| Human Embryonic Stem Cells in the Treatment of Spinocerebellar Ataxia: A Case Series. In Press | ||
| 17 | Author was suggested to validate the homing of stem cells in the spinal cord of patients. Providing references does not mean that homing is done in current study presented by author | We have done tractography in our patients before and after the therapy that showed axonal reconnections. This could have been possible only if the injected hESCs migrated to the injury site. |
| Validating homing of the stem cells is beyond the scope of this study. The references quoted have given proof that once injected any kind of stem cells have the tendency and capability of homing at the injured site. | ||
| 18 | Authors are requested mention all supple-mental routes in the text of paper. | We have added the supplemental routes as suggested. |
| 19 | The ASIA scores of patient 2 and 5 are different here as compared to Table. 1. Please incorporate whichever is the correct one. | Correction made |
| 20 | The reply is not related to que-ry(Comment-Their data has only been pre-sented at the House of Lords which has not commented on its usefulness/validity. Re-sponse- We have recently published two studies on the use of hESC therapy in patients with CP (http://www.ncbi.nlm.nih.gov/pubmed/25496119 and CVI (Accepted in Cell Sciences and Therapy, not yet published). Other papers have been submitted and are under review. | Please note that House of Lords does not accept evidences from one and all. |
| Nutech Mediworld was invited by House of Lords to present evidence on hESC therapy. They publish the evidences only after screening them. We were the single entry from India to be accepted as written evidence. | ||
| They found the data beneficial to the patients and interesting and published it. | ||
| 21 | 12 These are not regulatory.(Comment - What were the necessary regulatory permissions obtained for the 72 patients’ pilot study? Response- The approval for the pilot study on 72 patients was taken from the independent ethics committee of the institute Comment -What were the necessary regulatory permissions obtained for the 5 patients in this study? Response- The approval for this study was taken from independent ethics committee | At the time when this therapy was started, the only guidelines available were Guidelines on Biomedical Research on Human participants (2000 version). We complied fully to these guidelines. The first National guidelines on stem cells were framed in the year 2005 and evolved yearly after that. Nutech Mediworld also adapted itself to the evolving guidelines in principle. As such, there is no law for regulatory oversight. |
| 22 | Author have mentioned that they have followed the 2012 ICMR guidelines. However reviewer interpretation is just the converse. There was no oversight/monitoring/regulatory mechanism in place which is entirely different from an Institutional Ethics Clearance. | As mentioned previously, ICMR and DBT were fully informed about the cases taken up and their progress or detailed after therapy report was also sent to time on a regular basis. |
Footnotes
This article complies with International Committee of Medical Journal editor’s uniform requirements for manuscript.
Conflict of interest: None; Funding: None.
References
- 1.Lukovic D, Moreno Manzano V et al. Concise review: human pluripotent stem cells in the treatment of spinal cord injury. Stem Cells. 2012;30(9):1787–92. doi: 10.1002/stem.1159. [DOI] [PubMed] [Google Scholar]
- 2.Sekhon LH, Fehlings MG. Epidemiology, demographics, and pathophysiology of acute spinal cord injury. Spine (Phila Pa 1976) 2001;26(24 Suppl):S2–12. doi: 10.1097/00007632-200112151-00002. [DOI] [PubMed] [Google Scholar]
- 3.Ning GZ, Wu Q, Li YL et al. Epidemiology of traumatic spinal cord injury in Asia: a systematic review. J Spinal Cord Med. 2012;35(4):229–39. doi: 10.1179/2045772312Y.0000000021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Erceg S, Ronaghi M, Oria M et al. Transplanted oligodendrocytes and motoneuron progenitors generated from human embryonic stem cells promote locomotor recovery after spinal cord transection. Stem Cells. 2010;28(9):1541–9. doi: 10.1002/stem.489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Keirstead HS, Nistor G, Bernal G et al. Human embryonic stem cell-derived oligodendrocyte progenitor cell transplants remyelinate and restore locomotion after spinal cord injury. J Neurosci. 2005;25(19):4694–705. doi: 10.1523/JNEUROSCI.0311-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lee H, Shamy GA, Elkabetz Y et al. Directed differentiation and transplantation of human embryonic stem cell-derived motoneurons. Stem Cells. 2007;25(8):1931–9. doi: 10.1634/stemcells.2007-0097. [DOI] [PubMed] [Google Scholar]
- 7.Park HC, Shim YS, Ha Y et al. Treatment of complete spinal cord injury patients by autologous bone marrow cell transplantation and administration of granulocyte-macrophage colony stimulating factor. Tissue Eng. 2005;11(5–6):913–22. doi: 10.1089/ten.2005.11.913. [DOI] [PubMed] [Google Scholar]
- 8.Lima C, Escada P, Pratas-Vital J et al. Olfactory mucosal autografts and rehabilitation for chronic traumatic spinal cord injury. Neurorehabil Neural Repair. 2010;24(1):10–22. doi: 10.1177/1545968309347685. [DOI] [PubMed] [Google Scholar]
- 9.American Spinal Injury Association/International Medical Society of Paraplegia (ASIA/IMSOP) International Standards for Neurological and Functional Classification of Spinal Cord Injury patients (Revised).. Chicago, IL. American Spinal Injury Association; 1996. [Google Scholar]
- 10.Available from http://www.parliament.uk/documents/lords-committees/science-technology/RegenerativeMedicine/RegenMed.pdf ; House of Lords SATSC. [accessed on Sep 26;2014 ].
- 11.Fawcett JW. Overcoming inhibition in the damaged spinal cord. J Neurotrauma. 2006;23(3–4):371–83. doi: 10.1089/neu.2006.23.371. [DOI] [PubMed] [Google Scholar]
- 12.Baptiste DC, Fehlings MG. Update on the treatment of spinal cord injury. Prog Brain Res. 2007;161:217–33. doi: 10.1016/S0079-6123(06)61015-7. [DOI] [PubMed] [Google Scholar]
- 13.Azmoon S, Demarest C, Pucillo AL et al. Neurologic and cardiac benefits of therapeutic hypothermia. Cardiol Rev. 2011;19(3):108–14. doi: 10.1097/CRD.0b013e31820828af. [DOI] [PubMed] [Google Scholar]
- 14.Ware CB, Nelson AM, Mecham B et al. Derivation of naive human embryonic stem cells. Proc Natl Acad Sci U S A. 2014;111(12):4484–9. doi: 10.1073/pnas.1319738111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Barnabe-Heider F, Frisen J. Stem cells for spinal cord repair. Epub 2008/07/03. Cell Stem Cell. 2008;3(1):16–24. doi: 10.1016/j.stem.2008.06.011. [DOI] [PubMed] [Google Scholar]
- 16.Martin GR. Isolation of a pluripotent cell line from early mouse embryos cultured in medium conditioned by teratocarcinoma stem cells. Epub 1981/12/01. Proc Natl Acad Sci U S A. 1981;78(12):7634–8. doi: 10.1073/pnas.78.12.7634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nistor GI, Totoiu MO, Haque N et al. Human embryonic stem cells differentiate into oligodendrocytes in high purity and myelinate after spinal cord transplantation. Epub 2004/11/13. Glia. 2005;49(3):385–96. doi: 10.1002/glia.20127. [DOI] [PubMed] [Google Scholar]
- 18.Kakinohana O, Juhasova J, Juhas S et al. Survival and differentiation of human embryonic stem cell-derived neural precursors grafted spinally in spinal ischemia-injured rats or in naive immunosuppressed minipigs: a qualitative and quantitative study. Cell Transplant. 2012;21(12):2603–19. doi: 10.3727/096368912X653200. [DOI] [PubMed] [Google Scholar]
- 19.Lukovic D, Stojkovic M, Moreno-Manzano V et al. Perspectives and future directions of human pluripotent stem cell-based therapies: lessons from Geron’s clinical trial for spinal cord injury. Stem Cells Dev. 2014;23(1):1–4. doi: 10.1089/scd.2013.0266. [DOI] [PubMed] [Google Scholar]
- 20.Available at; http://www.nlm.nih.gov/medlineplus/ency/article/002398.htm . MedlinePlus. [Accessed Aug 29;2014 ].
- 21.Kang SK, Shin IS, Ko MS et al. Journey of mesenchymal stem cells for homing: strategies to enhance efficacy and safety of stem cell therapy. Stem Cells Int. 2012;2012:342968. doi: 10.1155/2012/342968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Borlongan CV, Glover LE, Tajiri N et al. The great migration of bone marrow-derived stem cells toward the ischemic brain: therapeutic implications for stroke and other neurological disorders. Prog Neurobiol. 2011;95(2):213–28. doi: 10.1016/j.pneurobio.2011.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]


