Abstract
Purpose
Our previous investigations showed that involuntary treadmill exercise is neuroprotective in a light-induced retinal degeneration mouse model, and it may act through activation of tropomyosin-related kinase B (TrkB) receptors. This study investigated whether voluntary running wheel exercise can be neuroprotective in an inheritable model of the retinal degenerative disease retinitis pigmentosa (RP), rd10 mice.
Methods
Breeding pairs of rd10 and C57BL/6J mice were given free-spinning (active) or locked (inactive) running wheels. Pups were weaned into separate cages with their parents' respective wheel types, and visual function was tested with ERG and a virtual optokinetic system at 4, 5, and 6 weeks of age. Offspring were killed at 6 weeks of age and retinal cross-sections were prepared for photoreceptor nuclei counting. Additionally, separate cohorts of active and inactive rd10 pups were injected daily for 14 days after eye opening with a selective TrkB receptor antagonist (ANA-12) or vehicle solution and assessed as described above.
Results
Mice in the rd10 active group exhibited significant preservation of visual acuity, cone nuclei, and total photoreceptor nuclei number. Injection with ANA-12 precluded the preservation of visual acuity and photoreceptor nuclei number in rd10 mice.
Conclusions
Voluntary running partially protected against the retinal degeneration and vision loss that otherwise occurs in the rd10 mouse model of RP. This protection was prevented by injection of ANA-12, suggesting that TrkB activation mediates exercise's preservation of the retina. Exercise may serve as an effective, clinically translational intervention against retinal degeneration.
Keywords: exercise, retinal degeneration, brain-derived neurotrophic factor
Voluntary running wheel exercise significantly decreases the rate of photoreceptor degeneration and visual acuity loss associated with the rd10 mouse model. Furthermore, systemic administration of a TrkB antagonist, ANA-12, blocks the protective effects on structure and function.
The protective role of exercise in the nervous system is extensive. Documented in both the central nervous system and peripheral nervous system, exercise preserves neuronal structure and promotes rehabilitation of motor and cognitive function in rodents and humans with neurodegeneration.1–3 Rodent models given an array of neurotoxins in both the hippocampus and brainstem while undergoing treadmill exercise retained spatial memory and motor coordination.4 In humans, both aging individuals and Alzheimer's patients exhibited improvements in cognitive function when prescribed regular exercise regimens.5,6 Additionally, exercise has been shown to improve motor skill acquisition after stroke.7
Retinal neurons are also responsive to neuroprotection through exercise. Mice subjected to treadmill exercise training before and after light-induced retinal degeneration (LIRD) had significantly preserved retinal function and structure compared to mice that were exposed to LIRD but were placed only on stationary treadmills.8 Similarly, with a swimming exercise regimen, rodents subjected to acute retinal injury via intraocular pressure elevation exhibited not only significant preservation of retinal physiology, but also suppression of astrocytic gliosis and macrophage activation.9
While the mechanism of such protection with regular exercise remains to be comprehensively understood, brain-derived neurotrophic factor (BDNF), a ligand of the tropomyosin-related kinase B (TrkB) receptor, may play a key role.10–12 Brain-derived neurotrophic factor signaling via the TrkB receptor promotes neuron growth and survival.13,14 TrkB agonists, like N-[2-(-indol-3-yl)ethyl]-2-oxopiperideine-3-carboximide, have been shown to mitigate retinal cell death as a result of LIRD.15 Additionally, 7,8-dihydroxyflavone, another TrkB agonist, is protective against retinal apoptotic events induced by kainic acid or stroke and is also protective against excitotoxic and oxidative stress in primary cultures of rat retinal ganglion cells.16,17 Local11 and systemic10 BDNF levels have been documented to increase post exercise, suggesting a relationship between neuroprotection and physical activity. In support of this hypothesis, Lawson et al.8 found that retinal, hippocampal, and serum BDNF levels were upregulated immediately after nonvoluntary treadmill exercise. When injected with a TrkB receptor antagonist, these mice that were exercised and exposed to toxic light exhibited significantly less protection of retinal structure and function than their vehicle-injected counterparts.8 Similarly, systemic injection of K252a, a tyrosine kinase inhibitor whose use can disrupt BDNF signaling, eliminates the protective effects of exercise in a rodent model of Parkinson's disease, further supporting the hypothesis that BDNF signaling may mediate exercise-induced neuroprotection.12
Previous studies showing protective effects of exercise on the retina have been limited to acute injury models such as LIRD and intraocular pressure elevation and “forced” exercise paradigms such as treadmill running or swimming.8,9 In this study, we examined the benefits of voluntary running wheel exercise to retinal structure and function in an inherited mouse model of RP, the Pde6brd10 (rd10) strain. The rd10 mice have an autosomal recessive mutation in 3′,5′-cyclic phosphodiesterase in rod photoreceptors, causing rods to degenerate significantly by postnatal day 18 (P18).18,19 A selective TrkB receptor antagonist, ANA-12, was then used to preclude the protective effects of exercise and to explore the subsequent consequences on outer nuclear layer (ONL) cell survival in the rd10 strain. C57BL/6J mice were used as a wild-type control. This voluntary exercise scheme enabled us to explore a more clinically applicable intervention for a model of an as yet incurable human neurodegenerative disease.
Methods
Animals
All animal procedures were approved by the Atlanta VA Institutional Animal Care and Use Committee and conform to the ARVO Statement for the Use of Animals in Ophthalmic and Vision Research. C57BL/6J (C57) and Pde6brd10 (rd10) mice were purchased from Jackson Laboratories (Bar Harbor, ME, USA), bred in-house, and raised under a 12-hour light/12-hour dark cycle with ad libitum standard mouse chow and water.
Wheel Exercise Regimen
We randomly assigned adult rd10 breeding pairs into two treatment groups, with either free-spinning (active) or locked (inactive) low-profile running wheels (Med-Associates, Inc., St. Albans, VT, USA). Pups (active, n = 20; inactive, n = 22) were assumed to benefit from their mothers having continuous access to their respective wheels through birth, rearing, and to weaning. All pups from these breeders were weaned at P21 into individual cages with a free-spinning or locked wheel corresponding to their parents' designated treatment group to provide a consistent intervention across age. A separate cohort of C57BL/6J mice was subjected to the experimental paradigm described above to serve as control groups.
Optokinetic Tracking (OKT)
At P27, P34, and P41, we assessed visual acuity of all mice by recording OKT behavior using a virtual optomotor system (OptoMotry; Cerebral Mechanics, Inc., Lethbridge, Alberta, Canada) under photopic conditions.20–22 Mice were placed on a platform within an enclosed testing chamber and monitored remotely via a camera attached to the chamber ceiling. Computer monitors on each of the chamber's four walls projected vertical bars moving laterally at varying spatial frequencies at 100% contrast. We noted reflexive optokinetic head tracking synonymous with the direction of pattern rotation as a positive recognition, and we utilized a staircase pattern of changing spatial frequencies to find the spatial frequency threshold at which a tracking reflex could be elicited. Both clockwise and counterclockwise rotations were used to selectively attain thresholds for left and right eyes, respectively.23 For analysis, we averaged spatial frequency thresholds for left and right eyes of animals within each treatment group and compared these values across time points.
Electroretinography (ERG)
At P28, P35, and P42, we measured retinal function using ERG as previously detailed.24 Briefly, we dark-adapted mice overnight and anesthetized (ketamine [80 mg/kg]/xylazine [16 mg/kg]) the mice under dim red light. After anesthetizing the corneas (1% tetracaine) and dilating the pupils (1% tropicamide, 1% cyclopentolate), we placed the mice on a heating pad to maintain the body temperature at 37°C (ATC 1000; World Precision Instruments, Inc., Sarasota, FL, USA). The ERG stimuli consisted of a five-step, full-field flash stimuli presented by a Ganzfeld dome under scotopic conditions (−3.0 to 2.1 log cd s/m2). Interflash interval increased with flash stimulus from 2 to 70 seconds. We recorded the electrical response of the retina using a gold wire contacting the cornea through a layer of 1% methylcellulose. We referenced and grounded the responses to 1-cm platinum needle electrodes inserted subcutaneously in the cheek and tail, respectively. We stored acquired responses on a commercial ERG system (UTAS 3000; LKC Technologies, Inc., Gaithersburg, MD, USA). After testing, we administered yohimbine (2.1 mg/kg) to the mice to reverse effects of xylazine and prevent corneal ulcers.25
We measured the a-wave, which is the response of the photoreceptors,26,27 from the baseline to the trough of the first negative wave. and the b-wave, which originates from the depolarizing bipolar cells,28 from the trough of the a-wave to the peak of the waveform, or when the a-wave was not present, from the baseline to the peak of the waveform.
BDNF TrkB Antagonist Experiments
We used a low-molecular-weight selective TrkB receptor antagonist, ANA-12 (Sigma-Aldrich Corp., St. Louis, MO, USA) to block TrkB activation. In addition to being specifically designed to cross the blood–brain barrier, ANA-12 selectively binds to TrkB and interrupts TrkB signal transduction while leaving TrkA and TrkC functions unaltered.29
We randomly divided a separate cohort of rd10 mice into four treatment groups: active + ANA-12 (n = 12), inactive + ANA-12 (n = 6), active + vehicle (n = 6), inactive + vehicle (n = 5). We administered either ANA-12 or vehicle (1% DMSO, 16.5% CremophorEL; 16.5% ethanol, 66% Dulbecco's PBS, pH 7.4) by intraperitoneal injection using a dose of 0.2 mg/kg body weight. As before, mice were provided either free-spinning or locked running wheels based on their treatment designation. Starting from eye opening at P14, we injected ANA-12 or vehicle daily 1 hour prior to the commencement of the 12-hour dark period of the daily light cycle (dosing based upon previous characterization of ANA-12 pharmacokinetics29) in order to inhibit TrkB receptor activation as a result of exercise during the period of highest wheel activity. Outcome measures consisted of OKT recordings and photoreceptor nuclei counts (detailed below) at P41 and P44, respectively.
Histology
At P44, we euthanized mice and marked right eyes superiorly for orientation. We then enucleated and fixed eyes in 4% paraformaldehyde for 30 minutes. After rinsing with 0.1 M phosphate buffer, we dissected eyes and removed the lens, dehydrated the posterior eyecups through a graded alcohol series and embedded the eyecups in plastic resin (Embed 812/DER 736; Electron Microscopy Science, Inc., Hatfield, PA, USA). We sectioned the posterior hemispheres in the superior-to-inferior plane into 0.5-μm-thick plastic sections bisecting the optic disc using an ultramicrotome (Reichert Ultracut; Leica, Inc., Buffalo Grove, IL, USA) with a histo diamond knife. We stained retinal sections with 1% aqueous toluidine blue (Sigma-Aldrich Corp.) and imaged the slides using a phase-contrast microscope (Leica DM LB; Leica, Inc.) at 40× power.
We quantified photoreceptor nuclei using an image analysis program (Image-Pro Plus 5.0; Media Cybernetics, Inc., Rockville, MD, USA) in four regions of each retinal section. Nuclei in the ONL were counted superiorly and inferiorly, starting 0.1 mm from the optic nerve, using adjacent micrographs that spanned 0.2 mm. We averaged the photoreceptor nuclei counts taken at each of these four locations across three sections of each eye. Average counts at each location for each eye were then compared between experimental groups. Additionally, the average nuclei counts at each location were summed for each eye and then averaged within experimental groups. Additionally, cone photoreceptors were also quantified in the same manner in rd10 mice. Cones were identified in plastic sections according to characteristic cellular chromatin staining patterns noted by Carter Dawson and LaVail.30 The ONL nuclei in which heterochromatin was noted to form two or more clumps were counted as cones.
Statistical Analyses
We performed one-way and two-way repeated measures ANOVAs and Student's t-tests using commercial statistical analysis software (SigmaStat 3.5; Systat Software, Inc., Chicago, IL, USA). We set significance at P < 0.05 for all analyses, and values are expressed as mean plus or minus SEM. We performed post hoc multiple comparisons using the Holm-Sidak method. The reported n is the total number of animals examined per group.
Results
Exercise Preserved Visual Function in rd10 Mice
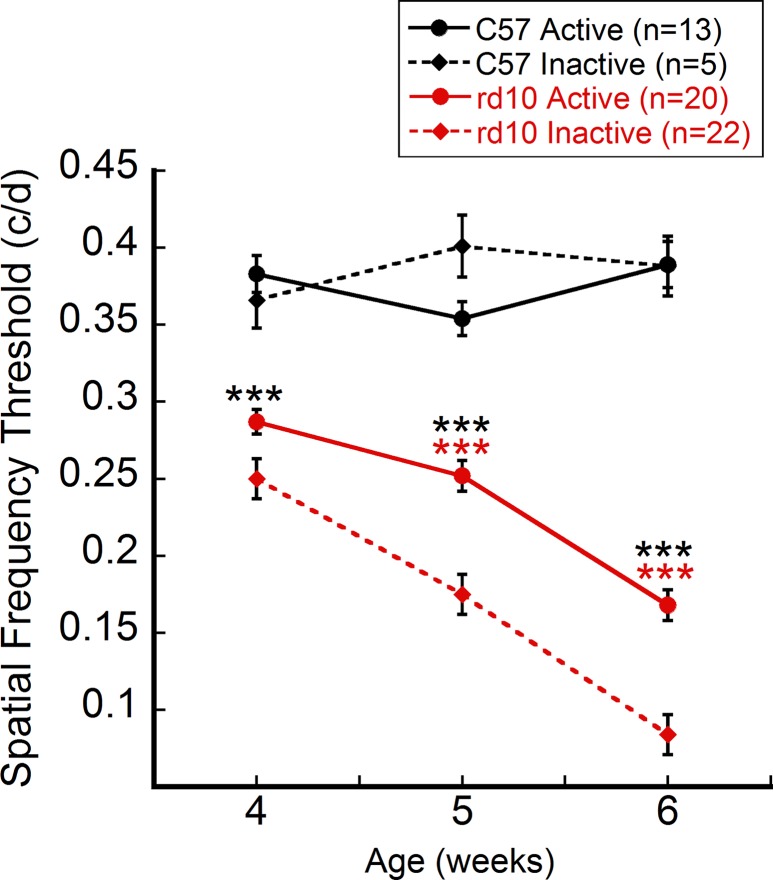
Neither active nor inactive groups of C57 control mice exhibited significant differences in visual acuity during the study (Fig. 1). However, both C57 groups yielded significantly greater spatial frequency thresholds than did both groups of rd10 mice at all ages (two-way ANOVA, F[6,151] = 22,45, P < 0.001; post hoc comparisons P < 0.001 between C57 and rd10 mice).
Figure 1.
Exercise preserved visual acuity in rd10 mice. Active and inactive groups were assessed for visual acuity using OKT at P27, P34, and P41. Active and inactive C57 mice did not exhibit significantly different spatial frequency thresholds, though both were significantly different from both rd10 groups (two-way ANOVA, F[6, 151] = 22.45, P < 0.001; Holm-Sidak post hoc comparisons indicated by black asterisks). Active rd10 mice exhibited significantly greater spatial frequency thresholds at 5 and 6 weeks of age compared to inactive rd10 mice (Holm-Sidak post hoc comparisons indicated by red asterisks). ***P < 0.001.
From 5 weeks of age onward, active rd10 mice had significantly greater visual acuity thresholds than inactive rd10 mice (Holm-Sidak post hoc comparisons, P < 0.001; Fig. 1). From P28 to P42, the progressive retinal degeneration that characterizes the rd10 strain was observed in decreased spatial frequency thresholds at an average of 66.4% in inactive mice, while active mice only declined an average of 41.5%. Dark-adapted ERG a- and b-wave amplitudes were not significantly different between rd10 strains. See Supplementary Figure S1.
Exercise Preserved Photoreceptor Numbers
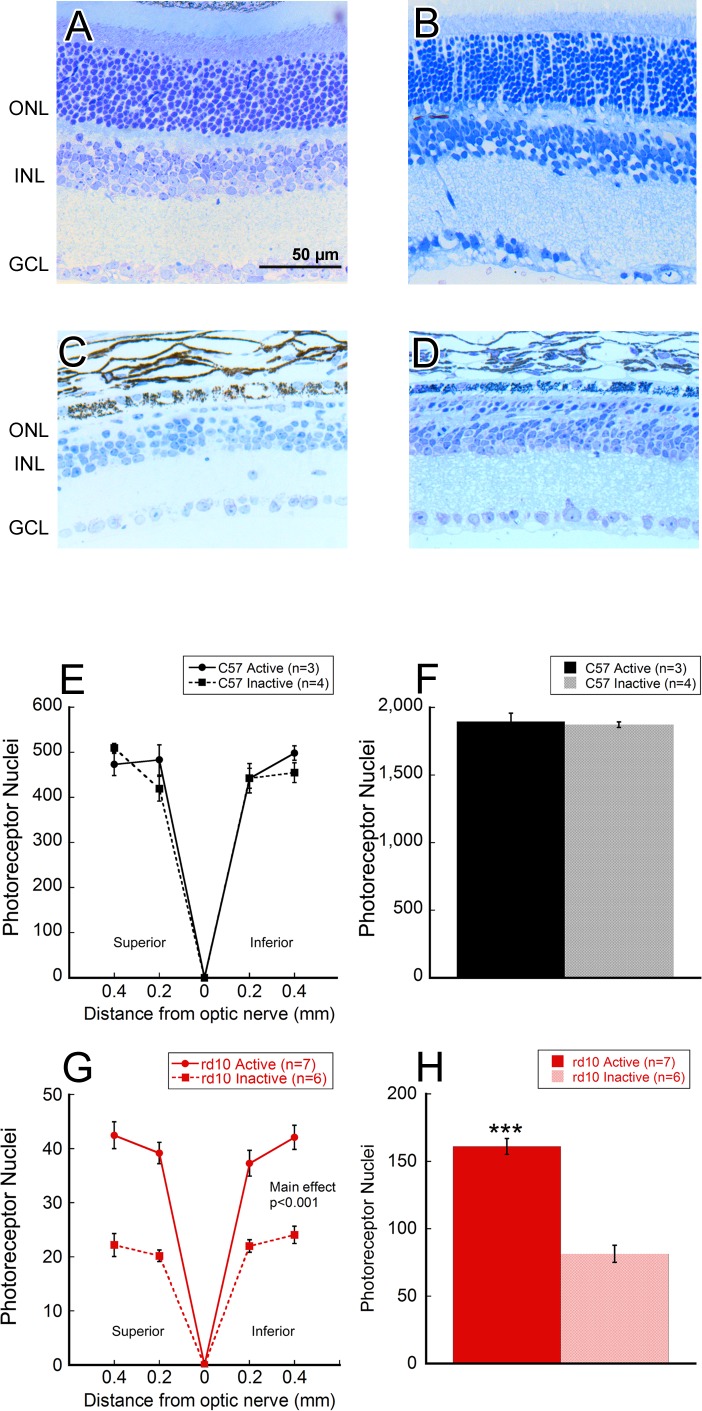
We counted total ONL nuclei in retinal cross-sections of active and inactive mouse eyes embedded in plastic resin. As with visual function, C57 active and inactive groups did not exhibit significant differences in photoreceptor nuclei counts (Figs. 2A, 2B, 2E, 2F). However, morphologic differences between rd10 active and inactive groups were apparent. Though photoreceptor loss was evident in both experimental rd10 groups, active rd10 mice had consistently thicker outer nuclear layers when compared to inactive rd10 mice (Fig. 2C, 2D). Quantitatively, active rd10 mice also had significant preservation of photoreceptor nuclei compared to inactive rd10 mice at different retinal locations (Fig. 2G; two-way ANOVA main effect of treatment, F[1, 57] = 92.4, P < 0.001). Counts between these groups revealed roughly twice the number of photoreceptor nuclei at all locations in active rd10 mice. Furthermore, total photoreceptor counts across the 0.8-mm span of retina were found to be 97.9% more numerous in active rd10 mice (Fig. 2H; active mean ± SD: 161.1 ± 15.6; inactive mean ± SD: 81.4 ± 15.5; Student's t-test, P < 0.001). There were no significant differences detected between superior and inferior retinal locations for either group, nor in the number of inner nuclear layer (INL) nuclei (data not shown).
Figure 2.
Exercise preserved ONL composition. Representative retinal cross-section at P44 of (A) an inactive C57 mouse, (B) an active C57 mouse, (C) an inactive rd10 mouse, and (D) an active rd10 mouse. (E, F) C57 mice that were given either free-spinning or locked wheels did not exhibit any significant differences in quantities of photoreceptors measured at any distance from the optic nerve measured. (G) The number of photoreceptors counted at all locations, relative to the optic nerve head, was significantly greater in active rd10 mice (two-way repeated measures ANOVA, main effect of treatment, F[1, 57] = 92.4, P < 0.001). (H) Active rd10 mice had a significantly greater number of total photoreceptor nuclei compared to inactive rd10 mice (Student's t-test, P < 0.001). ***P < 0.001.
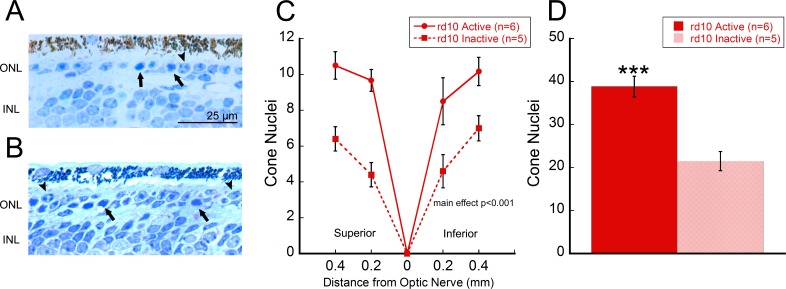
From the same active and inactive rd10 retinal cross-sections, we also counted cone nuclei. At every location analyzed, active rd10 mice possessed significantly higher cone nuclei numbers than inactive rd10 mice (Figs. 3A, 3B, 3C; two-way repeated measures ANOVA, main effect of treatment, F[1, 54] = 25.3, P < 0.001). Summed cone counts across all locations showed similar results, with active rd10 mice showing significantly greater quantities of cone nuclei than inactive rd10 mice (active: 38.8 ± 5.9, inactive: 21.5 ± 5.0; Student's t-test, P = 0.001).
Figure 3.
Exercise preserved cones in rd10 mice. Representative cross-section of (A) an inactive or (B) an active rd10 mouse retina at 100× magnification. Cones (indicated by arrowheads) were identified based on heterochromatin staining patterns displaying characteristic “clumping” into two or more spots within the nucleus, while rods (indicated by arrows) displayed a single, unified region of staining within the nucleus.30 (C) At all locations examined relative to the optic nerve, active rd10 mouse retinas exhibited significantly greater cone counts than inactive rd10 mouse retinas (two-way repeated measures ANOVA, main effect of treatment, F[1, 54] = 25.3, P < 0.001). (D) Summed cone nuclei acquired from all locations yielded similar results with total cone counts. Active rd10 mice had significantly greater cone counts than inactive rd10 mice (Student's t-test, P = 0.001). ***P < 0.001.
TrkB Antagonist Blocked the Protective Effects of Exercise
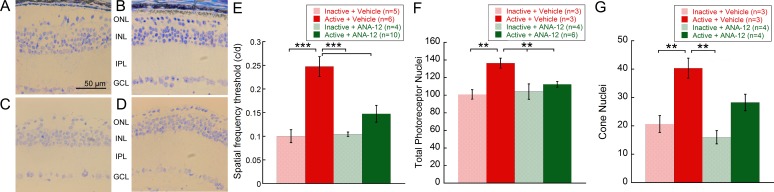
Daily injections of the TrkB antagonist, ANA-12, starting at P14 significantly reduced the protective effects of exercise on visual acuity and photoreceptor numbers in rd10 mice (Fig. 4). At P41, the spatial frequency threshold of inactive rd10 mice injected daily with either ANA-12 or vehicle were indistinguishable (Fig. 4E; inactive + ANA-12: 0.104 ± 0.011; inactive + vehicle: 0.100 ± 0.030). In contrast, active rd10 mice injected with vehicle demonstrated significantly higher spatial frequency thresholds than all other treatment groups (active + vehicle: 0.248 ± 0.051; one-way ANOVA, F[3, 28] = 11.51, P < 0.001). Active rd10 mice treated with ANA-12 showed no visual acuity preservation, with values that matched those of the inactive rd10 groups (active + ANA-12: 0.148 ± 0.062). Delivery of ANA-12 to inactive rd10 mice did not further accelerate degeneration of retinal morphology or function.
Figure 4.
ANA-12 treatment negated neuroprotective effects of exercise in rd10 mice. Retinal cross-sections of active + vehicle (A) rd10 mice showed visibly greater preservation of ONL morphology than those of inactive + vehicle (B), active + ANA-12 (C), and inactive + ANA-12 (D) rd10 mice. (E) The OKT spatial frequency thresholds measured at P41 showed active + vehicle mice had significantly greater spatial frequency thresholds than all other groups (one-way ANOVA, F[3, 28] = 11.51, P < 0.001). Importantly, ANA+12 treatment completely blocked the protective effects of exercise in the active + ANA-12 mice, such that they were statistically indistinguishable from the inactive treatment groups. (F) Summed photoreceptor nuclei across all locations revealed that active + vehicle rd10 mice had statistically more photoreceptors than all other experimental groups (one-way ANOVA, F(3, 15) = 5.94, P = 0.01], which were statistically indistinguishable from each other. (G) Active vehicle rd10 mice possessed significantly greater summed cone nuclei than inactive vehicle and inactive ANA-12 mice (one-way ANOVA, F[3, 13] = 10.73, P < 0.01). Significant differences were not found between active vehicle and active ANA-12 groups. **P < 0.01; ***P < 0.001.
Treatment with ANA-12 also negated preservation of retinal photoreceptor structure in active rd10 mice (Fig. 4A–D). Comparison of total area nuclei counts for all experimental groups matched the pattern of findings observed in the OKT data. The average sum of photoreceptor nuclei across all retinal locations examined in active + vehicle mice was significantly greater than all other experimental groups (Fig. 4F; active + vehicle: 136.4 ± 9.9, P = 0.01; one-way ANOVA F[3, 15] = 5.94, P = 0.01). Furthermore, average sums of photoreceptor nuclei for all other groups were statistically indistinguishable from one another (active + ANA-12: 112.4 ± 8.1; inactive + ANA-12: 104.1 ± 17.5; inactive + vehicle: 100.9 ± 9.3). Average sums of cone nuclei measured between groups revealed significantly greater quantities of cones in active + vehicle mice than in inactive + ANA-12 and inactive + vehicle mice (active + vehicle: 40.3 ± 6.1; inactive + ANA-12: 16.0 ± 4.7; inactive + vehicle: 20.7 ± 5.1; one-way ANOVA, F[3, 13] = 10.73, P < 0.01). However, only a trend toward statistical significant differences in cone nuclei was noted between active + vehicle and active + ANA-12 groups (post hoc comparison, P < 0.06; active + ANA-12: 28.3 ± 7.2).
Discussion
Voluntary Exercise Preserves Retinal Structure and Visual Function in rd10 Mice
We assessed the efficacy of voluntary exercise in slowing the advancement of inheritable retinal degeneration. We found that mice born to active rd10 breeders who were granted continuous access to free-spinning wheels after weaning maintained higher levels of visual acuity and overall photoreceptor cell density than those given locked wheels (Figs. 1, 2). Importantly, exercise also significantly preserved cone photoreceptors in active rd10 mice compared to inactive rd10 mice (Fig. 3). As the preservation of overall photoreceptor cell density exceeds that of observed cone photoreceptor protection, and with cone photoreceptors comprising approximately 25% of the total number of photoreceptors in the rd10 mice at P44, it would appear that voluntary exercise benefited both rod and cone structure.
The OKT measurements were performed under photopic conditions that would primarily test cone pathway function. However, there have been reports that rod pathways may also contribute to photopic OKT in mice.31,32 Our attempts to measure rod function through dark-adapted ERGs with dim stimuli did not reveal any significant effects, nor did dark-adapted ERGs with bright stimuli, which elicit a mixed rod/cone response (Supplementary Fig. S1). Since the ERG is a full-field potential from all the regions of the retina, the ERG may not have the sensitivity to measure the response from the few remaining photoreceptors in the rd10 retina, while a reliable OKT response can be obtained from a small number of photoreceptors. Thus, voluntary exercise preserved cone, and potentially rod, function in the rd10 mice. Further examination of light-adapted ERGs, scotopic OKT, and other behavioral assessment would be beneficial to determine what retinal cell types and pathways benefit from exercise.
Voluntary Exercise Is More Similar to Human Activity
Though previous studies had established a link between forced exercise and its capacity to preserve retinal physiology and structure, a voluntary exercise regimen had yet to be explored. Our use of running wheels to investigate the protective effect of exercise on the degenerating retina is both novel and more clinically applicable than forced exercise. Treadmills were used to exercise mice exposed to toxic light damage,8 and swimming had been used in mice subjected to intraocular pressure elevation, both of which were forced paradigms of exercise that yielded significant protection.9 While such exercise paradigms are neuroprotective, there are several advantages to adopting a voluntary scheme. Although it is still unclear which method yields greater protection, voluntary, habitual running is more similar to what would be prescribed in the clinic. Additionally, the electric shock impetus given by rodent treadmills and forced swimming sessions without the option of rest may introduce levels of stress that may impact, and most likely diminish, the protective outcomes. For example, rats subjected to regular foot shock stress exhibited impaired hippocampal neurogenesis and expression of BDNF.33,34 Our implementation of a voluntary, shock-free running scheme eliminated the possibility of confounding effects of forced-exercise stress.
Inheritable Retinal Degeneration More Closely Resembles Human Disease
We also used an inheritable model of retinal degeneration in lieu of acute injury. Though models like LIRD simulate the phenotype of retinal degeneration, the rd10 mouse strain provides a model of photoreceptor death that more closely resembles human autosomal RP. The rd10 mice display a progressive loss of photoreceptors due to a missense mutation in exon 13 of the Pde6b gene, which encodes the β subunit of rod photoreceptor cGMP phosphodiesterase type 6. Defects in the Pde6b gene have been observed in some forms of human autosomal recessive RP.35 Therefore, the rd10 mutation is a viable model through which we may study the onset and prevention of a currently untreatable human disease. Though other Pde6b mutant rodent strains exist, the pace of the rd10 degeneration is preferable to more aggressive rodent models of RP such as the rd1 mouse, in which photoreceptor synaptogenesis is already arrested before eye opening at P14.36 In the rd10 mice, photoreceptors begin to degenerate around P16, with significant loss by P18 and nearly complete loss by P60.18 This slower rate of degeneration provides a greater window to intervene and optimize the treatment regimen.
Mechanism of Neuroprotection With Exercise
As an initial exploration of a potential mechanism for the observed protective effects in voluntarily exercised rd10 mice, we examined whether TrkB activation is requisite for exercise-induced retinal and visual protection. Tropomyosin-related kinase B activation and signal transduction has been implicated in the protective effects of exercise on memory through upregulation in the hippocampus10–12 and has been shown to be involved in the protective effects of treadmill exercise on retinal degeneration.8 In the rd10 mice, we found daily injections of a TrkB receptor antagonist, starting at P14, effectively suppressed the previously observed retinal protection in active rd10 mice (Fig. 4). Visual acuity thresholds and total photoreceptor nuclei counts in active + ANA-12 rd10 mice were blocked to the levels of inactive mice, while cone nuclei counts had a strong trend toward the same.
Exactly how TrkB activation provides benefit to the photoreceptors and retina is still not well understood, though there is evidence that BDNF may play a role in this effect. The expression of retinal BDNF, a ligand of TrkB, is widespread in the retina and is upregulated during exercise.10,11 In rodents, BDNF has been localized to retinal ganglion cells, amacrine cells, cone outer segments, and Muller cells.37–40 In teleost fish used in aging research, BDNF mRNA was detectable in all retinal layers.41 Receptors for BDNF and TrkB have been colocalized to the retinal pigmented epithelium, retinal ganglion cell layer, inner plexiform layer, INL, outer plexiform layer, and Muller cells in rats subjected to toxic light conditions.42,43 Furthermore, Di Polo et al.38 reported colocalization of BDNF and TrkB receptor proteins in red-green–sensitive cone outer segments in rat retinas. However, whether BDNF could have a direct effect on photoreceptors remains unclear as TrkB signaling is not solely governed by BDNF. Other ligands of TrkB include neurotrophin-3, neurotrophin-4 (NF4), and nerve growth factor (NGF), all of which influence survival and development of neurons.44 Of these, NF4 and NGF have also been observed to be upregulated in the brain after exercise.45,46 Additionally, downstream targets of BDNF upregulation and increased TrkB receptor activation may activate mechanistic targets such as rapamycin complex 1.47 While these factors have not been observed to be upregulated in the retina after exercise, our data do not rule out their potential contributions to the protective effects of exercise through TrkB activation. Future experiments should pursue quantification of these factors in the retina post exercise to complement the observations made in this study.
Limitations and Future Aims
While this study illustrated the benefit of daily exercise, it is still unclear whether exercise during specific developmental stages of life is more effective than during others. Interestingly, active rd10 mice possessed significantly higher visual acuity at the earliest (P27) testing point, suggesting that exercise by the parents prior to weaning may have had positive effects on the pups. Both maternal and paternal exercise habits have been correlated with neurologic benefits in their offspring. Pups of female Wistar rats that exercised in swimming sessions before and during pregnancy were found to have higher hippocampal neurogenesis and neurotrophin levels 7 days after birth.48 Additionally, hippocampal BDNF, spatial learning, and memory in male offspring whose fathers exercised for 6 weeks prior to mating were found to be greater than those with sedentary fathers.49 Future experimentation should investigate differences in structural and functional protection in response to exercise regimen implemented at different developmental stages—natal, weaning, and postweaning periods.
Our interpretation is further limited by the absence of data to quantify the distance run by each animal. Obtaining information on distances run by experimental animals and correlating these data with their functional test results could enable us to determine an optimal distance that must be run by an animal to positively impact retinal health. Although we lack sufficient data to describe the volume of exercise required for maximum protection, our data suggest that an overall lifestyle of frequent exercise can benefit neuronal health. Running is a relatively cheap and accessible means of activity that can be promoted in the clinic and pursued in the patients' choice of time and place. Within the bounds of these experiments, exercise is an effective intervention in progressive retinal degeneration, which may translate to the human condition.
Supplementary Material
Acknowledgments
Supported by National Institutes of Health (Bethesda, MD, USA) Grants NIH P30 EY006360 (JHB/MTP) and NIH R01 EY014026 (JHB), and by a grant from the Abraham J. & Phyllis Katz Foundation (JHB; Atlanta, GA, USA). This work was also supported by a Veterans Affairs Research Career Scientist Award (MTP; Washington, DC, USA), by the Atlanta VA Center of Excellence in Vision and Neurocognitive Rehabilitation Pilot Award (MTP/JHB; Atlanta, GA, USA), and by a departmental award from Research to Prevent Blindness (New York, NY, USA).
Disclosure: A.M. Hanif, None; E.C. Lawson, None; M. Prunty, None; M. Gogniat, None; M.H. Aung, None; R. Chakraborty, None; J.H. Boatright, None; M.T. Pardue, None
References
- 1. Austin MW,, Ploughman M,, Glynn L,, Corbett D. Aerobic exercise effects on neuroprotection and brain repair following stroke: a systematic review and perspective. [published online ahead of print July 2 2014]. Neuroscience Res. doi: http://dx.doi.org/10.1016/j.neures.2014.06.007. [DOI] [PubMed]
- 2. Huang T,, Larsen KT,, Ried-Larsen M,, Moller NC,, Andersen LB. The effects of physical activity and exercise on brain-derived neurotrophic factor in healthy humans: a review. Scand J Med Sci Sports. 2014; 24: 1–10. [DOI] [PubMed] [Google Scholar]
- 3. Wood K,, Wilhelm JC,, Sabatier MJ,, Liu K,, Gu J,, English AW. Sex differences in the effectiveness of treadmill training in enhancing axon regeneration in injured peripheral nerves. Dev Neurobiol. 2012; 72: 688–698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Carro E,, Trejo JL,, Busiguina S,, Torres-Aleman I. Circulating insulin-like growth factor I mediates the protective effects of physical exercise against brain insults of different etiology and anatomy. J Neurosci. 2001; 21: 5678–5684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Intlekofer KA,, Cotman CW. Exercise counteracts declining hippocampal function in aging and Alzheimer's disease. Neurobiol Dis. 2013; 57: 47–55. [DOI] [PubMed] [Google Scholar]
- 6. Erickson KI,, Gildengers AG,, Butters MA. Physical activity and brain plasticity in late adulthood. Dialogues Clin Neurosci. 2013; 15: 99–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Mang CS,, Campbell KL,, Ross CJ,, Boyd LA. Promoting neuroplasticity for motor rehabilitation after stroke: considering the effects of aerobic exercise and genetic variation on brain-derived neurotrophic factor. Phys Ther. 2013; 93: 1707–1716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Lawson EC,, Han MK,, Sellers JT,, et al. Aerobic exercise protects retinal function and structure from light-induced retinal degeneration. J Neurosci. 2014; 34: 2406–2412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Chrysostomou V,, Kezic JM,, Trounce IA,, Crowston JG. Forced exercise protects the aged optic nerve against intraocular pressure injury. Neurobiol Aging. 2014; 35: 1722–1725. [DOI] [PubMed] [Google Scholar]
- 10. Ploughman M,, Granter-Button S,, Chernenko G,, et al. Exercise intensity influences the temporal profile of growth factors involved in neuronal plasticity following focal ischemia. Brain Res. 2007; 1150: 207–216. [DOI] [PubMed] [Google Scholar]
- 11. Griffin EW,, Mullally S,, Foley C,, Warmington SA,, O'Mara SM,, Kelly AM. Aerobic exercise improves hippocampal function and increases BDNF in the serum of young adult males. Physiol Behav. 2011; 104: 934–941. [DOI] [PubMed] [Google Scholar]
- 12. Real CC,, Ferreira AF,, Chaves-Kirsten GP,, Torrao AS,, Pires RS,, Britto LR. BDNF receptor blockade hinders the beneficial effects of exercise in a rat model of Parkinson's disease. Neuroscience. 2013; 237: 118–129. [DOI] [PubMed] [Google Scholar]
- 13. Hofer MM,, Barde YA. Brain-derived neurotrophic factor prevents neuronal death in vivo. Nature. 1988; 331: 261–262. [DOI] [PubMed] [Google Scholar]
- 14. Soppet D,, Escandon E,, Maragos J,, et al. The neurotrophic factors brain-derived neurotrophic factor and neurotrophin-3 are ligands for the trkB tyrosine kinase receptor. Cell. 1991; 65: 895–903. [DOI] [PubMed] [Google Scholar]
- 15. Iuvone PM,, Boatright JH,, Tosini G,, Ye K. N-acetylserotonin: circadian activation of the BDNF receptor and neuroprotection in the retina and brain. Adv Exp Med Biol. 2014; 801: 765–771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Jang SW,, Liu X,, Chan CB,, et al. Amitriptyline is a TrkA and TrkB receptor agonist that promotes TrkA/TrkB heterodimerization and has potent neurotrophic activity. Chem Biol. 2009; 16: 644–656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Gupta VK,, You Y,, Li JC,, Klistorner A,, Graham SL. Protective effects of 7,8-dihydroxyflavone on retinal ganglion and RGC-5 cells against excitotoxic and oxidative stress. J Mol Neurosci. 2013; 49: 96–104. [DOI] [PubMed] [Google Scholar]
- 18. Chang B,, Hawes NL,, Pardue MT,, et al. Two mouse retinal degenerations caused by missense mutations in the beta-subunit of rod cGMP phosphodiesterase gene. Vision Res. 2007; 47: 624–633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Gargini C,, Terzibasi E,, Mazzoni F,, Strettoi E. Retinal organization in the retinal degeneration 10 (rd10) mutant mouse: a morphological and ERG study. J Comp Neurol. 2007; 500: 222–238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Prusky GT,, Alam NM,, Beekman S,, Douglas RM. Rapid quantification of adult and developing mouse spatial vision using a virtual optomotor system. Invest Ophthal Vis Sci. 2004; 45: 4611–4616. [DOI] [PubMed] [Google Scholar]
- 21. Aung MH,, Kim MK,, Olson DE,, Thule PM,, Pardue MT. Early visual deficits in streptozotocin-induced diabetic long evans rats. Invest Ophthal Vis Sci. 2013; 54: 1370–1377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Aung MH,, Park HN,, Han MK,, et al. Dopamine deficiency contributes to early visual dysfunction in a rodent model of type 1 diabetes. J Neurosci. 2014; 34: 726–736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Douglas RM,, Alam NM,, Silver BD,, McGill TJ,, Tschetter WW,, Prusky GT. Independent visual threshold measurements in the two eyes of freely moving rats and mice using a virtual-reality optokinetic system. Vis Neurosci. 2005; 22: 677–684. [DOI] [PubMed] [Google Scholar]
- 24. Mocko JA,, Kim M,, Faulkner AE,, Cao Y,, Ciavatta VT,, Pardue MT. Effects of subretinal electrical stimulation in mer-KO mice. Invest Ophthalmol Vis Sci. 2011; 52: 4223–4230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Turner PV,, Albassam MA. Susceptibility of rats to corneal lesions after injectable anesthesia. Comp Med. 2005; 55: 175–182. [PubMed] [Google Scholar]
- 26. Penn RD,, Hagins WA. Signal transmission along retinal rods and the origin of the electroretinographic a-wave. Nature. 1969; 223: 201–204. [DOI] [PubMed] [Google Scholar]
- 27. Hood DC,, Birch DG. The A-wave of the human electroretinogram and rod receptor function. Invest Ophthal Vis Sci. 1990; 31: 2070–2081. [PubMed] [Google Scholar]
- 28. Robson JG,, Frishman LJ. Response linearity and kinetics of the cat retina: the bipolar cell component of the dark-adapted electroretinogram. Vis Neurosci. 1995; 12: 837–850. [DOI] [PubMed] [Google Scholar]
- 29. Cazorla M,, Premont J,, Mann A,, Girard N,, Kellendonk C,, Rognan D. Identification of a low-molecular weight TrkB antagonist with anxiolytic and antidepressant activity in mice. J Clin Invest. 2011; 121: 1846–1857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Carter-Dawson LD,, LaVail MM. Rods and cones in the mouse retina. I. Structural analysis using light and electron microscopy. J Comp Neurol. 1979; 188: 245–262. [DOI] [PubMed] [Google Scholar]
- 31. Schmucker C,, Seeliger M,, Humphries P,, Biel M,, Schaeffel F. Grating acuity at different luminances in wild-type mice and in mice lacking rod or cone function. Invest Ophthal Vis Sci. 2005; 46: 398–407. [DOI] [PubMed] [Google Scholar]
- 32. Alam NM,, Altimus CM,, Douglas RM,, Hattar S,, Prusky GT. Photoreceptor regulation of spatial visual behavior. Invest Ophthal Vis Sci. 2014; 56: 1842–1849. [DOI] [PubMed] [Google Scholar]
- 33. Dagyte G,, Van der Zee EA,, Postema F,, et al. Chronic but not acute foot-shock stress leads to temporary suppression of cell proliferation in rat hippocampus. Neuroscience. 2009; 162: 904–913. [DOI] [PubMed] [Google Scholar]
- 34. Rasmusson AM,, Shi L,, Duman R. Downregulation of BDNF mRNA in the hippocampal dentate gyrus after re-exposure to cues previously associated with footshock. Neuropsychopharmacology. 2002; 27: 133–142. [DOI] [PubMed] [Google Scholar]
- 35. Hartong DT,, Berson EL,, Dryja TP. Retinitis pigmentosa. Lancet. 2006; 368: 1795–1809. [DOI] [PubMed] [Google Scholar]
- 36. Blanks JC,, Adinolfi AM,, Lolley RN. Photoreceptor degeneration and synaptogenesis in retinal-degenerative (rd) mice. J Comp Neurol. 1974; 156: 95–106. [DOI] [PubMed] [Google Scholar]
- 37. Avwenagha O,, Bird MM,, Lieberman AR,, Yan Q,, Campbell G. Patterns of expression of brain-derived neurotrophic factor and tyrosine kinase B mRNAs and distribution and ultrastructural localization of their proteins in the visual pathway of the adult rat. Neuroscience. 2006; 140: 913–928. [DOI] [PubMed] [Google Scholar]
- 38. Di Polo A,, Cheng L,, Bray GM,, Aguayo AJ. Colocalization of TrkB and brain-derived neurotrophic factor proteins in green-red-sensitive cone outer segments. Invest Ophthal Vis Sci. 2000; 41: 4014–4021. [PubMed] [Google Scholar]
- 39. Fujieda H,, Sasaki H. Expression of brain-derived neurotrophic factor in cholinergic and dopaminergic amacrine cells in the rat retina and the effects of constant light rearing. Exp Eye Res. 2008; 86: 335–343. [DOI] [PubMed] [Google Scholar]
- 40. Seki M,, Nawa H,, Fukuchi T,, Abe H,, Takei N. BDNF is upregulated by postnatal development and visual experience: quantitative and immunohistochemical analyses of BDNF in the rat retina. Invest Ophthal Vis Sci. 2003; 44: 3211–3218. [DOI] [PubMed] [Google Scholar]
- 41. Gatta C,, Castaldo L,, Cellerino A,, de Girolamo P,, Lucini C,, D'Angelo L. Brain derived neurotrophic factor in the retina of the teleost N. furzeri. Ann Anat. 2014; 196: 192–196. [DOI] [PubMed] [Google Scholar]
- 42. Asai N,, Abe T,, Saito T,, Sato H,, Ishiguro S,, Nishida K. Temporal and spatial differences in expression of TrkB isoforms in rat retina during constant light exposure. Exp Eye Res. 2007; 85: 346–355. [DOI] [PubMed] [Google Scholar]
- 43. Saito T,, Abe T,, Wakusawa R,, et al. TrkB-T1 receptors on Muller cells play critical role in brain-derived neurotrophic factor-mediated photoreceptor protection against phototoxicity. Curr Eye Res. 2009; 34: 580–588. [DOI] [PubMed] [Google Scholar]
- 44. Reichardt LF. Neurotrophin-regulated signalling pathways. Philos Trans R Soc Lond B Biol Sci. 2006; 361: 1545–1564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Hong YP,, Lee HC,, Kim HT. Treadmill exercise after social isolation increases the levels of NGF BDNF, and synapsin I to induce survival of neurons in the hippocampus, and improves depression-like behavior. J Exerc Nutrition Biochem. 2015; 19: 11–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Chung JY,, Kim MW,, Bang MS,, Kim M. Increased expression of neurotrophin 4 following focal cerebral ischemia in adult rat brain with treadmill exercise. PLoS One. 2013; 8: e52461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Watson K,, Baar K. mTOR and the health benefits of exercise. Semin Cell Dev Biol. 2014; 36: 130–139. [DOI] [PubMed] [Google Scholar]
- 48. Marcelino TB,, Longoni A,, Kudo KY,, et al. Evidences that maternal swimming exercise improves antioxidant defenses and induces mitochondrial biogenesis in the brain of young Wistar rats. Neuroscience. 2013; 246: 28–39. [DOI] [PubMed] [Google Scholar]
- 49. Yin MM,, Wang W,, Sun J,, et al. Paternal treadmill exercise enhances spatial learning and memory related to hippocampus among male offspring. Behav Brain Res. 2013; 253: 297–304. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.