Abstract
LSD1 is a histone lysine demethylase, which is highly expressed in multiple types of human cancer. Although its roles in transcriptional regulation have been well-studied, functional regulation of LSD1 by post-translational modifications still remains unknown. Here, we demonstrate that the histone lysine methyltransferase SUV39H2 trimethylated LSD1 on lysine 322. Knockdown of SUV39H2 resulted in a decrease of LSD1 protein even though the mRNA levels were unchanged. SUV39H2-induced LSD1 methylation suppresses LSD1 polyubiquitination and subsequent degradation. In addition, we also observed indirect effect of SUV39H2 overexpression on LSD1-target genes. Our results reveal the regulatory mechanism of LSD1 protein through its lysine methylation by SUV39H2 in human cancer cells.
Keywords: SUV39H2, carcinogenesis, LSD1, non-histone protein methylation
INTRODUCTION
LSD1 (also known as KDM1A, AOF2, and BHC110), the first-identified histone lysine-specific demethylase [1], is an amine oxidase that catalyzes lysine demethylation in a flavin adenine dinucleotide (FAD)-dependent oxidative reaction. LSD1 is composed of three domains; a SWIRM domain that is a conserved motif shared by many chromatin regulatory complexes, an amine oxidase (AO) domain and a Tower domain [2-4]. LSD1 is involved in both transcriptional gene repression and activation through its demethylation of H3K4 and H3K9 [1, 5]. LSD1 can only demethylate mono- and dimethyl lysine residues, and not trimethyl lysine residues, because it requires a lone pair of electrons only present on mono- and dimethylated lysine residues [6]. It was also reported that LSD1 demethylates lysine residues at non-histone proteins including p53, MYPT1, DNMT1 and E2F1 [7-10]. LSD1 demethylates p53 at lysine 370 and represses p53-mediated transcriptional upregulation including induction of apoptosis [8, 11, 12]. Subsequently LSD1 was found as a member of multiple complexes including a CoREST complex and a NuRD complex, both of which function in transcription repression [13-15]. In addition, overexpression of LSD1 was detected in various types of human cancer, and high levels of LSD1 were correlated with poor outcome of cancer patients [16-18]. Concordantly, depletion of LSD1 inhibits cancer cell proliferation [16]. These results suggested that LSD1 could be a promising therapeutic target for development of drugs to treat cancers. Indeed, clinical trials of LSD1 inhibitors have just started for small cell lung carcinoma and acute myeloid leukemia [6]. With regard to the post-translational modifications of LSD1, Nam et al. reported that protein kinase Cα (PKCα) phosphorylates LSD1 and plays a critical role in rhythmicity and phase resetting of the circadian clock [19]. However, other modifications influencing functions of LSD1 have not been elucidated, so far.
Suppressor of Variegation 3-9 Homologue 2 (SUV39H2, also known as KMT1B) is a histone lysine-specific methyltransferase that was firstly reported to methylate lysine 9 of histone H3 (H3K9) [20]. In general, histone H3K9 methylation is involved in heterochromatin formation and transcriptional repression. Genetic ablation in the SUV39H1 and SUV39H2 genes result in severe chromosomal instabilities, such as abnormally-long telomeres with reduced binding of chromobox proteins Cbx1, Cbx3 and Cbx5 [21, 22]. We recently reported that SUV39H2 methylates histone H2AX and regulates the DNA repair pathway through regulation of γ-H2AX activity in human cancer [23]. Since the expression of SUV39H2 is restricted in testis in adult tissues and is significantly elevated in various cancer types such as non-small cell lung cancer, bladder cancer and prostate cancer [23], SUV39H2 appears to be an ideal target for development of anti-cancer treatment.
In the present study, we demonstrate that SUV39H2 trimethylates LSD1 on lysine 322. SUV39H2-mediated LSD1 methylation inhibits polyubiquitination, which leads to stabilization of the LSD1 protein. Our studies unveil a novel mechanism of SUV39H2 in human cancer through the lysine methylation of LSD1.
RESULTS
SUV39H2 methylates lysine 322 on LSD1 both in vitro and in vivo
We performed an in vitro methyltransferase assay using recombinant LSD1 protein with a variety of recombinant histone methyltransferases to identify an enzyme(s) that would possibly methylate LSD1 and found that the histone methyltransferase SUV39H2 could methylate LSD1 in a dose-dependent manner (Figure 1A, 1B and Supplementary Figure S1). Subsequently, we applied liquid chromatography-tandem mass spectrometry (LC-MS/MS) and identified that lysine 322 on LSD1 was trimethylated by SUV39H2 (Figure 1C and 1D). To further confirm this methylation site, we synthesized the wild-type peptide covering amino acids 313-330 of LSD1 (WT) and the Lys 322-substituted LSD1 peptide (K322R), and performed an in vitro methyltransferase assay. Consequently, we detected a strong methylation signal only in the wild-type LSD1 peptide but not the substituted LSD1 peptide (K322R) (Figure 2A). In addition, lysine 322 of LSD1, the methylation site, is highly conserved across species (Figure 2B), supporting that this lysine methylation might have a critical role in the function of LSD1. Furthermore, we validated the methylation of LSD1 by SUV39H2 in 293T cells that were transfected with a FLAG-LSD1 wild-type (WT) vector or a FLAG-LSD1-K322R vector together with an HA-SUV39H2. In vivo labeling experiments revealed a strong signal corresponding to methylated LSD1 in FLAG-LSD1-WT-transfected cells, but the specific signal was significantly diminished in FLAG-LSD1-K322R-tranfected cells (Figure 2C). Taken together, these results imply that SUV39H2 methylates lysine 322 on LSD1 both in vitro and in vivo.
Figure 1. SUV39H2 methylates LSD1.
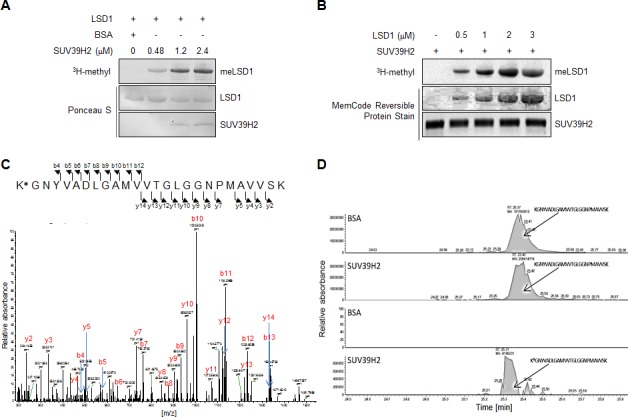
A. Recombinant LSD1 protein was methylated by SUV39H2 in a dose-dependent manner. An in vitro methyltransferase assay was performed by using purified His-tagged LSD1 and different amount of SUV39H2 recombinant proteins. Methylated LSD1 was detected by fluorography. Amounts of loading proteins were evaluated by staining with Ponceau S. B. Confirmation of the in vitro methyltransferase assay. Different amount of LSD1 protein was mixed with SUV39H2 in the presence of S-adenosyl-L-[methyl-3H]-methionine. Methylated LSD1 was detected by fluorography. Amounts of loading proteins were evaluated by staining with MemCodeTM Reversible Protein Stain (Thermo Fisher Scientific). C. The MS-MS spectrum corresponding to the trimethylated LSD1 322–347 peptide. The 42 Da increase of the Lys 322 residue was observed. D. MS chromatograms of unmodified and trimethylated LSD1 322–347 peptides.
Figure 2. Lys 322 on LSD1 methylation by SUV39H2 both in vitro and in vivo.
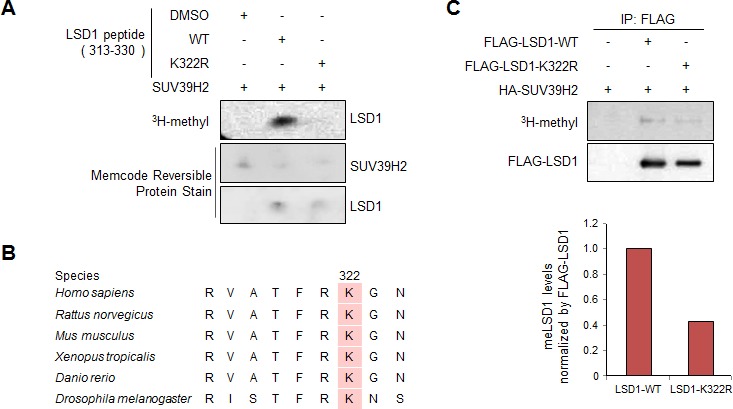
A. In vitro methyltransferase assay indicated that LSD1 peptide (amino acid residues 313-330) was methylated by SUV39H2 but not Lys 322-substituted LSD1 peptide (K322R). Amounts of loading proteins were evaluated by staining the MemCodeTM Reversible Protein Stain (Thermo Fisher Scientific). B. Amino acid sequence indicated that the methylation site Lys 322 was highly conserved across species. C. Methylation of LSD1 in human cells was confirmed by in vivo labeling experiment. 293T cells were transfected with FLAG-LSD1-WT or FLAG-LSD1-K322R in the presence of HA-SUV39H2 and treated with methionine-free medium, including cycloheximide and chloramphenicol. They were then labeled with L-[methyl-3H] methionine for 3 hours. Cell lysates were immunoprecipitated with FLAG-M2 agarose, and methylated LSD1 was visualized by fluorography. The membrane was immunoblotted with an anti-FLAG (an internal control) antibody.
SUV39H2 stabilizes LSD1 via inhibiting polyubiquitination
We previously reported that biological effects of lysine methylation are categorized into 5 classes, and one of them is to regulate the stability of substrate protein [6]. To examine whether SUV39H2-mediated methylation affects protein stability of LSD1, we co-expressed FLAG-tagged LSD1 with Mock vector or HA-tagged SUV39H2 in 293T cells. The cells were treated with cycloheximide (CHX) to block new protein synthesis. We found that LSD1 protein degraded much more rapidly in the cells co-transfected with a control mock vector, compared to those co-transfected with HA-tagged SUV39H2 (Figure 3A), indicating that LSD1 proteins were degraded much faster probably due to the lack of its methylation by SUV39H2. Consistently, we detected a remarkable reduction of LSD1 protein levels when knocking down endogenous expression of SUV39H2 in A549 cells even though mRNA levels of LSD1 were unchanged (Figure 3B). In addition, when LSD1 was immunoprecipitated with an anti-LSD1 antibody from whole cell extracts of A549 or SBC5 cells treated with siEGFP, siLSD1 or siSUV39H2, a significant decrease of LSD1 protein was also observed in the immunoprecipitants after treatment with siSUV39H2 as well as siLSD1 (Figure 3C). Hence, these results indicate that SUV39H2 appears to stabilize LSD1 protein via methylation.
Figure 3. SUV39H2 stabilizes LSD1 protein.
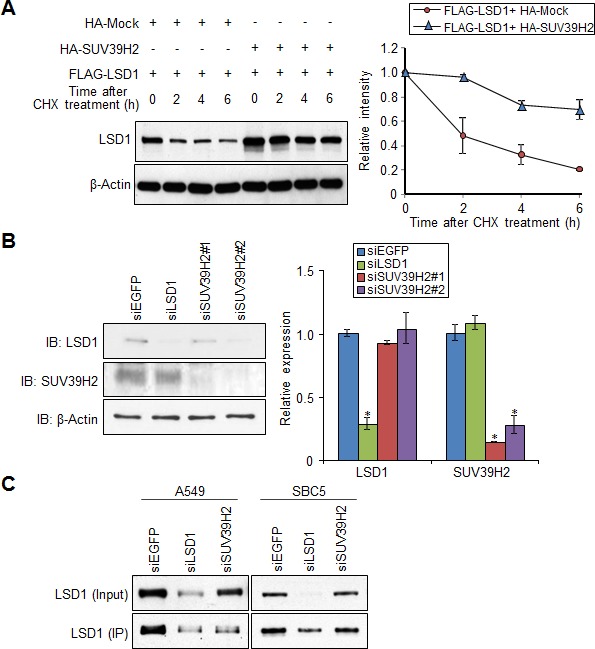
A. FLAG-LSD1 was co-expressed with HA-Mock or HA-SUV39H2 into 293T cells. After treating cells with cycloheximide (CHX) (100 μg/ml) for indicated time intervals, expression of LSD1 was examined (left panel). The intensity of LSD1 protein for each time point was quantified by densitometry and plotted (right panel). Results are the mean ± SD of three independent experiments. B. A549 cells were transfected with control EGFP, LSD1 and two different SUV39H2 siRNAs. Expression of LSD1, SUV30H2 and β-Actin (internal control) was examined by western blot (left panel). The mRNA levels of LSD1 and SUV39H2 were quantified by real-time PCR. All error bars indicate SEM of two independent experiments. P-values were calculated using Student's t-test (*P < 0.05). C. Lysates from A549 and SBC5 cells transfected with control EGFP, LSD1 and SUV39H2 siRNA were immunoprecipitated with an anti-LSD1 antibody. LSD1 protein levels were examined by western blot analysis.
Protein polyubiquitination is known as a signal for protein degradation. We hypothesized that SUV39H2 could stabilize LSD1 protein through inhibiting polyubiquitination of LSD1, and co-expressed FLAG-LSD1 and HA-Ubiquitin with HA-Mock or HA-SUV39H2 in 293T cells. Ubiquitinated LSD1 was observed in the FLAG-LSD1 immunoprecipitants purified from the cells treated with MG115 and MG132. We observed remarkable reduction of ubiquitinated LSD1 in the LSD1 immunoprecipitants purified from the cells co-expressing with SUV39H2, indicating that SUV39H2 seems to reduce polyubiquitination levels of LSD1 protein (Figure 4A). Furthermore, endogenous ubiquitination levels of LSD1 protein were examined by knocking down the expression of SUV39H2. Consistently, attenuation of SUV39H2 expression resulted in an increase of polyubiquitination levels on LSD1 in A549 and SBC5 cancer cells (Figure 4B). These results imply that SUV39H2 stabilizes LSD1 through inhibiting polyubiquitination levels of LSD1.
Figure 4. SUV39H2-dependent LSD1 methylation inhibits LSD1 protein degradation mediated by polyubiquitination.
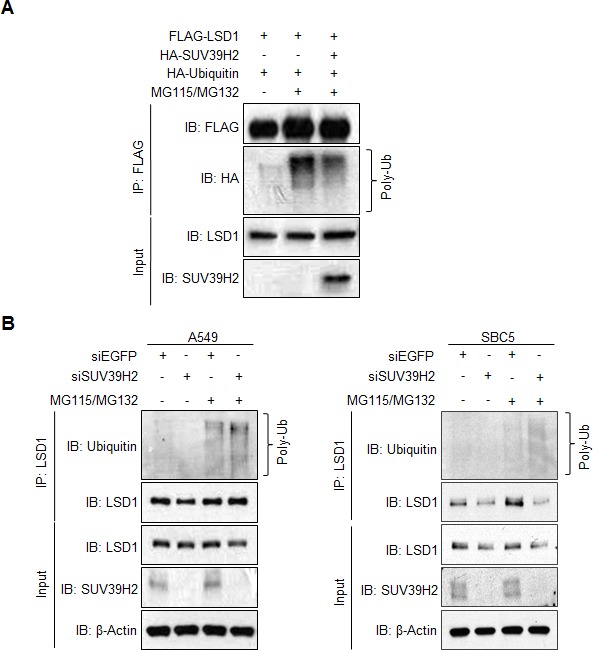
A. FLAG-LSD1 and HA-Ubiquitin were co-expressed in 293T cells with or without HA-SUV39H2. Cells were incubated with 5 μM MG115 and 10 μM MG132 for 6 hours before lysis. LSD1 protein was immunoprecipitated with anti-FLAG M2 agarose beads, and polyubiquitinated LSD1 proteins were detected by anti-HA antibody. B. After incubation with siEGFP or siSUV39H2 for 72 hours, A549 (left panel) or SBC5 (right panel) cells were treated with 5 μM MG115 and 10 μM MG132 for 6 hours. Cell lysates were immunoprecipitated with anti-LSD1 antibody. Polyubiquitinated LSD1 protein was detected using an anti-Ubiquitin antibody.
SUV39H2-mediated lysine 322 methylation regulates LSD1-downstream genes
According to our previous report, lysine methylation also regulates protein-protein interactions [6]. LSD1 is a part of the CoREST complex, and lysine 322, the methylation site by SUV39H2, is close to the portion of LSD1 that was reported to be critical for the interaction between LSD1 and CoREST based on the structural analysis [24]. Therefore, we investigated whether SUV39H2-dependent LSD1 methylation on lysine 322 affects the interaction between LSD1 and CoREST. As shown in Figure 5A, we examined binding affinities of wild-type LSD1 (LSD1-WT) and K322R mutant-type LSD1 (LSD1-K332R) to CoREST, and interestingly, LSD1-K332R showed a significant reduction of binding affinities to CoREST compared to LSD1-WT. Moreover, K322R mutant of LSD1 caused lower binding affinities to H3K4me1, H3K4me2 and total histone H3 (Figure 5A). Taken together, SUV39H2-mediated methylation of LSD1 at lysine 322 was important for its binding affinities to CoREST.
Figure 5. Methylation of LSD1 at lysine 322 is critical for its binding to CoREST, and regulating targeted genes.
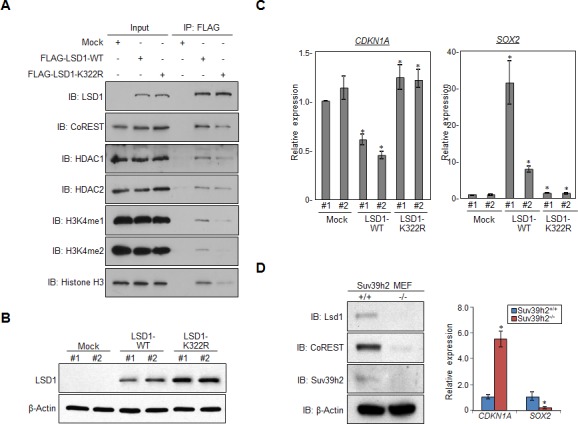
A. 293T cells were individually transfected with FLAG-Mock, FLAG-LSD1 or FLAG-LSD1mutant (K322R) vectors, and cell extracts were immunoprecipitated with anti-FLAG M2 agarose beads. B. Expression levels of LSD1 proteins were examined in stably expressing wild-type LSD1 or mutant LSD1 (K322R) HeLa cells. C. The mRNA levels of CDKN1A and SOX2 were quantified by real-time PCR. All error bars indicate SEM of four independent experiments. P-values were calculated using Student's t-test (*P < 0.05). D. Expression levels of Lsd1 and CoREST proteins from Suv39h2 wild-type (Suv39h2+/+) and Suv39h2 null (Suv39h2−/−) MEF cells were examined by western blot (left panel). The mRNA levels of CDKN1A and SOX2 were quantified by real-time PCR. All error bars indicate SEM of two independent experiments. P-values were calculated using Student's t-test (*P < 0.05).
LSD1 is an essential epigenetic regulator, and critical in regulating expression of several downstream target genes [25]. To examine whether SUV39H2-medidated methylation has an influence on transcriptional regulation of LSD1-target genes, we established stable HeLa cell clones that constitutively overexpress LSD1-WT or LSD1-K322R (Figure 5B). We then examined expression levels of CDKN1A (p21/CIP1) and SOX2, which are LSD1-target genes [26, 27], and found that LSD1-WT overexpressing cells showed lower CDKN1A expression and higher SOX2 expression compared to control Mock cells. However, we couldn't observe expression changes of these genes in LSD1-K322R overexpressing cells (Figure 5C). Consistent with these results, LSD1-K322R overexpressing cells showed lower growth rate than LSD1-WT overexpressing cells (Supplementary Figure S2). Next, we examined expression of Cdkn1a and Sox2 in Suv39h2-null (Suv39h2−/−) mouse embryonic fibroblast (MEF) cells established from Suv39h2 knockout mouse [21]. Compared to wild-type MEFs, we observed diminished protein expressions of Lsd1 and CoREST in Suv39h2−/− MEFs (Figure 5D). Consistently, we found increased Cdkn1a and reduced Sox2 mRNA levels in Suv39h2−/− MEFs compared to wild-type MEFs (Figure 5D). Similar results were also observed in the cells treated with chaetocin, an inhibitor of the SU(VAR)3-9 [28, 29] (Figure 6A and 6B).
Figure 6. Methylation of LSD1 at lysine 322 affects LSD1 regulation of targeted genes.
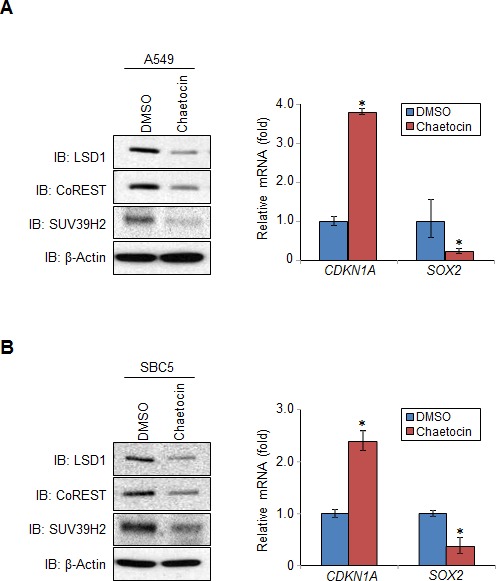
Chaetocin, an inhibitor of the SU(VAR)3-9 was treated into A549 cells A. and SBC5 cells B. at the dose of 0.5 μM for 24 hours. The expression levels of LSD1 and CoREST proteins were examined by western blot (left panel). The mRNA levels of CDKN1A and SOX2 were quantified by real-time PCR (right panel). All error bars indicate SEM of two independent experiments. P-values were calculated using Student's t-test (*P < 0.05).
DISCUSSION
In the present study, we identified that LSD1 was trimethylated by SUV39H2 at lysine 322, and that SUV39H2-mediated LSD1 methylation stabilized the protein levels of LSD1 through inhibition of its polyubiquitination. Overexpression of SUV39H2 attenuated LSD1 polyubiquitination, whereas depletion of SUV39H2 enhanced LSD1 polyubiquitination and enhanced degradation of LSD1, indicating that SUV39H2-induced LSD1 methylation impedes LSD1 polyubiquitination. In addition, the methylation of lysine 322 on LSD1 by SUV39H2, was critical for the interaction of LSD1 with CoREST. Moreover, since this SUV39H2-mediated LSD1 methylation enhanced the binding affinity to histone H3, we hypothesized that methylation could possibly influence transcriptional regulation of LSD1-target genes. Indeed, we confirmed that LSD1 methylation at lysine 322 played critical roles in the regulation of LSD1-target genes, CDKN1A and SOX2, whose dysregulations are involved in human tumorigenesis [30, 31]. Among biological functions of protein lysine methylation in human cancer we previously categorized [6], this study implies that SUV39H2-mediated LSD1 methylation may increase the stability of LSD1 proteins by suppressing polyubiquitination, and may also alter LSD1-CoREST interaction, which causes aberrant transcriptional regulation of LSD1-downstream genes.
Many reports have indicated that dysregulation of protein lysine methyltransferases and protein lysine demethylases has important roles in human tumorigenesis [32-37]. LSD1 is highly expressed in multiple types of cancer, including bladder cancer, oestrogen-receptor-negative breast cancer, colorectal cancer, lung cancer and prostate cancer [16, 17, 38]. Previous data have also indicated that LSD1 functioned as an essential regulator of leukemia stem cell (LSC) potential [39], and its inhibition could reactivate the all-trans-retinoic acid differentiation pathway in acute myeloid leukemia [40]. As mentioned above, we previously reported that overexpression of SUV39H2 in various cancer types such as non-small cell lung cancer, bladder cancer and prostate cancer [23]. These accumulated data indicate that both SUV39H2 and LSD1 could be promising candidates for anti-cancer drug development. Several results have shown that LSD1 inhibitor could suppress different types of cancer [41, 42]. In particular, a Phase I clinical trial of GSK2879552, an LSD1-specific inhibitor, has been initiated for patients with relapsed/refractory small cell lung cancer, and a Phase I clinical trial of the novel LSD1 inhibitor ORY-1001 has begun for acute myeloid leukaemia [6]. Combination therapy involving LSD1 inhibitors could also be a viable approach, such as HDACs inhibitors and ATRA inhibitors. In fact, inhibition of LSD1 could sensitize cancer cells to HDAC inhibitors [42, 43]. Our study indicated a high correlation between SUV39H2 and LSD1, which imply a new viable approach combining LSD1 and SUV39H2 inhibitors in cancer therapy.
MATERIALS AND METHODS
Cell culture
293T, HeLa and A549 cells were from American Type Culture Collection (ATCC) in 2001 and 2003, and tested and authenticated by DNA profiling for polymorphic short tandem repeat (STR) markers (Supplementary Table S1). SBC-5 cells were from the Japanese Collection of Research Bioresources in 2001, and were tested and authenticated by DNA profiling for polymorphic short tandem-repeat markers (Supplementary Table S1). Both cell lines were grown in monolayers in appropriate media: Dulbecco's modified Eagle's medium (D-MEM) for 293T cells; RPMI1640 medium for A549; Eagle's Minimum Essential Medium (E-MEM) for HeLa and SBC-5 cells supplemented with 10% fetal bovine serum and 1% antibiotic/antimycotic solution (Sigma-Aldrich, St. Louis, MO). We also generated stable HeLa cell lines constitutively expressing LSD1. The pCAGGS-LSD1-3xFLAG or empty pCAGGS-3xFLAG mock vector was transfected into HeLa cells by FuGENE6 (Roche Applied Science, Penzberg, Germany) according to the manufacturer's protocol [16, 33], and the antibiotics-resistant clones were selected with the culture media containing 0.5 mg/ml Geneticin®. A549 and SBC5 cells were transfected with SUV39H2-specific siRNA duplex, LSD1-specific siRNA duplex or siEGFP siRNA duplex as a negative control, respectively, by using Lipofectamin RNAiMAX (Life Technologies, Carlsbad, CA) according to the manufacturer's recommendations. The siRNA sequences are described in Supplementary Table S2.
Mouse Suv39h2 wild-type and Suv39h2−/− MEF cells were established by Dr. Thomas Jenuwein group [21, 23]. Cells were cultured with Dulbecco's modified Eagle's medium (D-MEM) supplemented with 10% fetal bovine serum, 1% antibiotic/antimycotic solution (Sigma-Aldrich, St. Louis, MO), 2 mM L-glutamine, 0.1 mM β-mercaptoethanol, 1x non-essential amino acid solution (Life Technologies) and sodium pyruvate.
Mass spectrometry
The reaction mixture of in vitro methyltransferase assay was subjected to SDS-PAGE, and the bands on the gel were visualized by SimplyBlueTM SafeStain (Life Technologies, Carlsbad, CA). The bands corresponding to LSD1 were excised from the gel, and digested with sequencing grade TPCK-trypsin (Worthington Biochemical, Lakewood, NJ) in 30 μL of digestion buffer (10 mM Tris-HCl, 0.05% decyl glucoside, pH 8.0) at 37°C for 12 h. The digest mixture was separated using a nanoflow LC (Easy nLC, Thermo Fisher Scientific, Waltham, MA) on an NTCC analytical column (C18, Φ0.075 × 100 mm, 3 μm, Nikkyo Technos, Tokyo, Japan) with a linear gradient of 35% buffer B (100% acetonitrile and 0.1% formic acid) at a flow rate of 300 nL/min over 10 min, and subjected on-line to a Q-Exactive mass spectrometer (Thermo Fisher Scientific) with a nanospray ion source using data dependent TOP10 method. The MS/MS spectra were searched against the in-house database using local MASCOT server (version 2.3; Matrix Sciences, London, United Kingdom). The quantitative analysis using Qual Browser (version2.2; Thermo Fisher Scientific) was performed as described previously [7].
RNA extraction, real-time PCR
Total RNA is purified from the cells by using RNeasy Mini Kits (QIAGEN, Venlo, Netherlands) according to manufacturer's recommendations. Synthesis of cDNA is performed with SuperScriptTM III First-Strand Synthesis System for RT-PCR kit (Life Technologies). Detailed information for the primers is available in Supplementary Table S3.
In vitro methyltransferase assay
In vitro methyltransferase assays were performed as described previously [7, 44-50]. Briefly, 1 μg of His-LSD1 protein was incubated with 1 μg of His-SUV39H2 in 50 mM Tris-HCl (pH 8.8), 1.0 μCi/ml S-adenosyl-L-[methyl-3H]-methionine (Perkin Elmer, Waltham, MA) and Milli-Q water for 1 hour at 30°C. After boiling in sample buffer, the samples were subjected to SDS-PAGE, and visualized by fluorography [44].
Immunoprecipitation and antibodies
293T cells were seeded at a density of 40% on a 100-mm dish. After cell attachment, the cells were transfected with expression vectors using FuGENE6, and after 48 h, transfected 293T cells were washed with PBS and lysed in RIPA buffer (50 mM Tris-HCl (pH 7.4), 150 mM NaCl, 0.5% sodium deoxycholate, 0.1% SDS, 1% Nonidet-P40, 0.1 mM PMSF) with complete protease inhibitor cocktail (Roche Applied Science). Cell extracts were incubated with anti-FLAG M2 agarose (Sigma-Aldrich) for 2 h at 4°C. After the beads were washed 3 times with 1 ml of TBS buffer (pH 7.6), the FLAG-tagged proteins bound to the beads were eluted by boiling in Lane Marker Sample Buffer (Thermo Fisher Scientific). Samples were then subjected to SDS-PAGE, and detected by western blot. The following antibodies were used: anti-LSD1 (ab17721, Abcam, Cambridge, UK), anti-CoREST (ab32631, Abcam), anti-HDAC1 (sc7872, Santa Cruz Biotechnology, Dallas, TX), anti-HDAC2 (sc7899, Santa Cruz Biotechnology), anti-Ubiquitin (sc8017, Santa Cruz Biotechnology), anti-H3K4me1 (ab8895, Abcam), anti-H3K4me2 (ab32356, Abcam) and anti-histone H3 (ab1791, Abcam).
In vivo labeling
In vivo labeling was performed as described previously [51]. 293T cells were starved for 0.5 hour in methionine-free medium, including cycloheximide (100 μg/ml) and chloramphenicol (40 μg/ml). They were then labeled with L-[methyl-3H] methionine (10 μCi/ml, Perkin Elmer) for 3 hours. FLAG-LSD1-WT or FLAG-LSD1-K322R in the presence of HA-SUV39H2 was immunoprecipitated with FLAG-M2 agarose and methylated LSD1 was visualized by fluorography.
SUPPLEMENTARY MATERIAL FIGURES AND TABLES
ACKNOWLEDGMENTS AND FUNDING
We thank Drs. Gouji Toyokawa and Yataro Daigo in the University of Tokyo for technical support, and Drs. Makoto Nakakido and Kenbun Sone in the University of Chicago for helpful discussion. We are also grateful to Drs. Thomas Jenuwein and Nicholas Shukeir for providing Suv39h2−/−MEF cells.
Footnotes
CONFLICTS OF INTEREST
Y. Nakamura is a stock holder and a scientific advisor of Oncotherapy Science, Inc. No potential conflicts of interest were disclosed by the other authors.
REFERENCES
- 1.Shi Y, Lan F, Matson C, Mulligan P, Whetstine JR, Cole PA, Casero RA. Histone demethylation mediated by the nuclear amine oxidase homolog LSD1. Cell. 2004;119:941–953. doi: 10.1016/j.cell.2004.12.012. [DOI] [PubMed] [Google Scholar]
- 2.Shi Y, Whetstine JR. Dynamic regulation of histone lysine methylation by demethylases. Molecular cell. 2007;25:1–14. doi: 10.1016/j.molcel.2006.12.010. [DOI] [PubMed] [Google Scholar]
- 3.Mimasu S, Sengoku T, Fukuzawa S, Umehara T, Yokoyama S. Crystal structure of histone demethylase LSD1 and tranylcypromine at 2. 25 A. Biochemical and biophysical research communications. 2008;366:15–22. doi: 10.1016/j.bbrc.2007.11.066. [DOI] [PubMed] [Google Scholar]
- 4.Yang M, Culhane JC, Szewczuk LM, Gocke CB, Brautigam CA, Tomchick DR, Machius M, Cole PA, Yu H. Structural basis of histone demethylation by LSD1 revealed by suicide inactivation. Nature Structural Molecular Biology. 2007;14:535–539. doi: 10.1038/nsmb1255. [DOI] [PubMed] [Google Scholar]
- 5.Metzger E, Wissmann M, Yin N, Muller JM, Schneider R, Peters AH, Gunther T, Buettner R, Schule R. LSD1 demethylates repressive histone marks to promote androgen-receptor-dependent transcription. Nature. 2005;437:436–439. doi: 10.1038/nature04020. [DOI] [PubMed] [Google Scholar]
- 6.Hamamoto R, Saloura V, Nakamura Y. Critical roles of non-histone protein lysine methylation in human tumorigenesis. National Review Cancer. 2015;15:110–124. doi: 10.1038/nrc3884. [DOI] [PubMed] [Google Scholar]
- 7.Cho HS, Suzuki T, Dohmae N, Hayami S, Unoki M, Yoshimatsu M, Toyokawa G, Takawa M, Chen T, Kurash JK, Field HI, Ponder BA, Nakamura Y, Hamamoto R. Demethylation of RB regulator MYPT1 by histone demethylase LSD1 promotes cell cycle progression in cancer cells. Cancer research. 2011;71:1–6. doi: 10.1158/0008-5472.CAN-10-2446. [DOI] [PubMed] [Google Scholar]
- 8.Huang J, Sengupta R, Espejo AB, Lee MG, Dorsey JA, Richter M, Opravil S, Shiekhattar R, Bedford MT, Jenuwein T, Berger SL. p53 is regulated by the lysine demethylase LSD1. Nature. 2007;449:105–108. doi: 10.1038/nature06092. [DOI] [PubMed] [Google Scholar]
- 9.Kontaki H, Talianidis I. Lysine methylation regulates E2F1-induced cell death. Molecular cell. 2010;39:152–160. doi: 10.1016/j.molcel.2010.06.006. [DOI] [PubMed] [Google Scholar]
- 10.Wang J, Hevi S, Kurash JK, Lei H, Gay F, Bajko J, Su H, Sun W, Chang H, Xu G, Gaudet F, Li E, Chen T. The lysine demethylase LSD1 (KDM1) is required for maintenance of global DNA methylation. Nature genetics. 2009;41:125–129. doi: 10.1038/ng.268. [DOI] [PubMed] [Google Scholar]
- 11.Scoumanne A, Chen X. The lysine-specific demethylase 1 is required for cell proliferation in both p53-dependent and -independent manners. The Journal of biological chemistry. 2007;282:15471–15475. doi: 10.1074/jbc.M701023200. [DOI] [PubMed] [Google Scholar]
- 12.Tsai WW, Nguyen TT, Shi Y, Barton MC. p53-targeted LSD1 functions in repression of chromatin structure and transcription in vivo. Molecular and cellular biology. 2008;28:5139–5146. doi: 10.1128/MCB.00287-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lee MG, Wynder C, Cooch N, Shiekhattar R. An essential role for CoREST in nucleosomal histone 3 lysine 4 demethylation. Nature. 2005;437:432–435. doi: 10.1038/nature04021. [DOI] [PubMed] [Google Scholar]
- 14.Shi YJ, Matson C, Lan F, Iwase S, Baba T, Shi Y. Regulation of LSD1 histone demethylase activity by its associated factors. Molecular cell. 2005;19:857–864. doi: 10.1016/j.molcel.2005.08.027. [DOI] [PubMed] [Google Scholar]
- 15.Wang Y, Zhang H, Chen Y, Sun Y, Yang F, Yu W, Liang J, Sun L, Yang X, Shi L, Li R, Li Y, Zhang Y, Li Q, Yi X, Shang Y. LSD1 is a subunit of the NuRD complex and targets the metastasis programs in breast cancer. Cell. 2009;138:660–672. doi: 10.1016/j.cell.2009.05.050. [DOI] [PubMed] [Google Scholar]
- 16.Hayami S, Kelly JD, Cho HS, Yoshimatsu M, Unoki M, Tsunoda T, Field HI, Neal DE, Yamaue H, Ponder BA, Nakamura Y, Hamamoto R. Overexpression of LSD1 contributes to human carcinogenesis through chromatin regulation in various cancers. International journal of cancer Journal international du cancer. 2011;128:574–586. doi: 10.1002/ijc.25349. [DOI] [PubMed] [Google Scholar]
- 17.Kahl P, Gullotti L, Heukamp LC, Wolf S, Friedrichs N, Vorreuther R, Solleder G, Bastian PJ, Ellinger J, Metzger E, Schule R, Buettner R. Androgen receptor coactivators lysine-specific histone demethylase 1 and four and a half LIM domain protein 2 predict risk of prostate cancer recurrence. Cancer research. 2006;66:11341–11347. doi: 10.1158/0008-5472.CAN-06-1570. [DOI] [PubMed] [Google Scholar]
- 18.Schulte JH, Lim S, Schramm A, Friedrichs N, Koster J, Versteeg R, Ora I, Pajtler K, Klein-Hitpass L, Kuhfittig-Kulle S, Metzger E, Schule R, Eggert A, Buettner R, Kirfel J. Lysine-specific demethylase 1 is strongly expressed in poorly differentiated neuroblastoma: implications for therapy. Cancer research. 2009;69:2065–2071. doi: 10.1158/0008-5472.CAN-08-1735. [DOI] [PubMed] [Google Scholar]
- 19.Nam HJ, Boo K, Kim D, Han DH, Choe HK, Kim CR, Sun W, Kim H, Kim K, Lee H, Metzger E, Schuele R, Yoo SH, Takahashi JS, Cho S, Son GH, et al. Phosphorylation of LSD1 by PKCalpha is crucial for circadian rhythmicity and phase resetting. Molecular cell. 2014;53:791–805. doi: 10.1016/j.molcel.2014.01.028. [DOI] [PubMed] [Google Scholar]
- 20.O'Carroll D, Scherthan H, Peters AH, Opravil S, Haynes AR, Laible G, Rea S, Schmid M, Lebersorger A, Jerratsch M, Sattler L, Mattei MG, Denny P, Brown SD, Schweizer D, Jenuwein T. Isolation and characterization of Suv39h2, a second histone H3 methyltransferase gene that displays testis-specific expression. Molecular and cellular biology. 2000;20:9423–9433. doi: 10.1128/mcb.20.24.9423-9433.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Peters AH, O‘Carroll D, Scherthan H, Mechtler K, Sauer S, Schofer C, Weipoltshammer K, Pagani M, Lachner M, Kohlmaier A, Opravil S, Doyle M, Sibilia M, Jenuwein T. Loss of the Suv39h histone methyltransferases impairs mammalian heterochromatin and genome stability. Cell. 2001;107:323–337. doi: 10.1016/s0092-8674(01)00542-6. [DOI] [PubMed] [Google Scholar]
- 22.Garcia-Cao M, O'Sullivan R, Peters AH, Jenuwein T, Blasco MA. Epigenetic regulation of telomere length in mammalian cells by the Suv39h1 and Suv39h2 histone methyltransferases. Nature genetics. 2004;36:94–99. doi: 10.1038/ng1278. [DOI] [PubMed] [Google Scholar]
- 23.Sone K, Piao L, Nakakido M, Ueda K, Daigo Y, Jenuwein T, Nakamura Y, Hamamoto R. Critical role of lysine 134 methylation on histone H2AX for gamma-H2AX production and DNA repair. Nature communications. 2014;5:5691. doi: 10.1038/ncomms6691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yang M, Gocke CB, Luo X, Borek D, Tomchick DR, Machius M, Otwinowski Z, Yu H. Structural basis for CoREST-dependent demethylation of nucleosomes by the human LSD1 histone demethylase. Molecular cell. 2006;23:377–387. doi: 10.1016/j.molcel.2006.07.012. [DOI] [PubMed] [Google Scholar]
- 25.Lim S, Janzer A, Becker A, Zimmer A, Schule R, Buettner R, Kirfel J. Lysine-specific demethylase 1 (LSD1) is highly expressed in ER-negative breast cancers and a biomarker predicting aggressive biology. Carcinogenesis. 2010;31:512–520. doi: 10.1093/carcin/bgp324. [DOI] [PubMed] [Google Scholar]
- 26.Adamo A, Sese B, Boue S, Castano J, Paramonov I, Barrero MJ, Izpisua Belmonte JC. LSD1 regulates the balance between self-renewal and differentiation in human embryonic stem cells. Nature cell biology. 2011;13:652–659. doi: 10.1038/ncb2246. [DOI] [PubMed] [Google Scholar]
- 27.Zhang X, Lu F, Wang J, Yin F, Xu Z, Qi D, Wu X, Cao Y, Liang W, Liu Y, Sun H, Ye T, Zhang H. Pluripotent stem cell protein Sox2 confers sensitivity to LSD1 inhibition in cancer cells. Cell reports. 2013;5:445–457. doi: 10.1016/j.celrep.2013.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Greiner D, Bonaldi T, Eskeland R, Roemer E, Imhof A. Identification of a specific inhibitor of the histone methyltransferase SU(VAR)3-9. Nature Chemical Biology. 2005;1:143–145. doi: 10.1038/nchembio721. [DOI] [PubMed] [Google Scholar]
- 29.Chiba T, Saito T, Yuki K, Zen Y, Koide S, Kanogawa N, Motoyama T, Ogasawara S, Suzuki E, Ooka Y, Tawada A, Otsuka M, Miyazaki M, Iwama A, Yokosuka O. Histone lysine methyltransferase SUV39H1 is a potent target for epigenetic therapy of hepatocellular carcinoma. International journal of cancer Journal international du cancer. 2015;136:289–298. doi: 10.1002/ijc.28985. [DOI] [PubMed] [Google Scholar]
- 30.Liu K, Lin B, Zhao M, Yang X, Chen M, Gao A, Liu F, Que J, Lan X. The multiple roles for Sox2 in stem cell maintenance and tumorigenesis. Cellular signalling. 2013;25:1264–1271. doi: 10.1016/j.cellsig.2013.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Warfel NA, El-Deiry WS. p21WAF1 and tumourigenesis: 20 years after. Current opinion in oncology. 2013;25:52–58. doi: 10.1097/CCO.0b013e32835b639e. [DOI] [PubMed] [Google Scholar]
- 32.Cho HS, Kelly JD, Hayami S, Toyokawa G, Takawa M, Yoshimatsu M, Tsunoda T, Field HI, Neal DE, Ponder BA, Nakamura Y, Hamamoto R. Enhanced expression of EHMT2 is involved in the proliferation of cancer cells through negative regulation of SIAH1. Neoplasia. 2011;13:676–684. doi: 10.1593/neo.11512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hamamoto R, Furukawa Y, Morita M, Iimura Y, Silva FP, Li M, Yagyu R, Nakamura Y. SMYD3 encodes a histone methyltransferase involved in the proliferation of cancer cells. Nature cell biology. 2004;6:731–740. doi: 10.1038/ncb1151. [DOI] [PubMed] [Google Scholar]
- 34.Hamamoto R, Silva FP, Tsuge M, Nishidate T, Katagiri T, Nakamura Y, Furukawa Y. Enhanced SMYD3 expression is essential for the growth of breast cancer cells. Cancer Science. 2006;97:113–118. doi: 10.1111/j.1349-7006.2006.00146.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hayami S, Yoshimatsu M, Veerakumarasivam A, Unoki M, Iwai Y, Tsunoda T, Field HI, Kelly JD, Neal DE, Yamaue H, Ponder BA, Nakamura Y, Hamamoto R. Overexpression of the JmjC histone demethylase KDM5B in human carcinogenesis: involvement in the proliferation of cancer cells through the E2F/RB pathway. Molecular Cancer. 2010;9:59. doi: 10.1186/1476-4598-9-59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Saloura V, Cho HS, Kyiotani K, Alachkar H, Zuo Z, Nakakido M, Tsunoda T, Seiwert T, Lingen M, Licht J, Nakamura Y, Hamamoto R. WHSC1 Promotes Oncogenesis through Regulation of NIMA-related-kinase-7 in Squamous Cell Carcinoma of the Head and Neck. Molecular Cancer Research. 2015;13:293–304. doi: 10.1158/1541-7786.MCR-14-0292-T. [DOI] [PubMed] [Google Scholar]
- 37.Tsuge M, Hamamoto R, Silva FP, Ohnishi Y, Chayama K, Kamatani N, Furukawa Y, Nakamura Y. A variable number of tandem repeats polymorphism in an E2F-1 binding element in the 5′ flanking region of SMYD3 is a risk factor for human cancers. Nature genetics. 2005;37:1104–1107. doi: 10.1038/ng1638. [DOI] [PubMed] [Google Scholar]
- 38.Kauffman EC, Robinson BD, Downes MJ, Powell LG, Lee MM, Scherr DS, Gudas LJ, Mongan NP. Role of androgen receptor and associated lysine-demethylase coregulators, LSD1 and JMJD2A, in localized and advanced human bladder cancer. Molecular Carcinogenesis. 2011;50:931–944. doi: 10.1002/mc.20758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Harris WJ, Huang X, Lynch JT, Spencer GJ, Hitchin JR, Li Y, Ciceri F, Blaser JG, Greystoke BF, Jordan AM, Miller CJ, Ogilvie DJ, Somervaille TC. The histone demethylase KDM1A sustains the oncogenic potential of MLL-AF9 leukemia stem cells. Cancer Cell. 2012;21:473–487. doi: 10.1016/j.ccr.2012.03.014. [DOI] [PubMed] [Google Scholar]
- 40.Schenk T, Chen WC, Gollner S, Howell L, Jin L, Hebestreit K, Klein HU, Popescu AC, Burnett A, Mills K, Casero RA, Jr, Marton L, Woster P, Minden MD, Dugas M, Wang JC, et al. Inhibition of the LSD1 (KDM1A) demethylase reactivates the all-trans-retinoic acid differentiation pathway in acute myeloid leukemia. Nature Medicine. 2012;18:605–611. doi: 10.1038/nm.2661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Etani T, Suzuki T, Naiki T, Naiki-Ito A, Ando R, Iida K, Kawai N, Tozawa K, Miyata N, Kohri K, Takahashi S. NCL1, a highly selective lysine-specific demethylase 1 inhibitor, suppresses prostate cancer without adverse effect. Oncotarget. 2015;6:2865–78. doi: 10.18632/oncotarget.3067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Fiskus W, Sharma S, Shah B, Portier BP, Devaraj SG, Liu K, Iyer SP, Bearss D, Bhalla KN. Highly effective combination of LSD1 (KDM1A) antagonist and pan-histone deacetylase inhibitor against human AML cells. Leukemia. 2014;28:2155–2164. doi: 10.1038/leu.2014.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Singh MM, Manton CA, Bhat KP, Tsai WW, Aldape K, Barton MC, Chandra J. Inhibition of LSD1 sensitizes glioblastoma cells to histone deacetylase inhibitors. Neuro-oncology. 2011;13:894–903. doi: 10.1093/neuonc/nor049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cho HS, Shimazu T, Toyokawa G, Daigo Y, Maehara Y, Hayami S, Ito A, Masuda K, Ikawa N, Field HI, Tsuchiya E, Ohnuma S, Ponder BA, Yoshida M, Nakamura Y, Hamamoto R. Enhanced HSP70 lysine methylation promotes proliferation of cancer cells through activation of Aurora kinase B. Nature communications. 2012;3:1072. doi: 10.1038/ncomms2074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Cho HS, Hayami S, Toyokawa G, Maejima K, Yamane Y, Suzuki T, Dohmae N, Kogure M, Kang D, Neal DE, Ponder BA, Yamaue H, Nakamura Y, Hamamoto R. RB1 Methylation by SMYD2 Enhances Cell Cycle Progression through an Increase of RB1 Phosphorylation. Neoplasia. 2012;14:476–486. doi: 10.1593/neo.12656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hamamoto R, Toyokawa G, Nakakido M, Ueda K, Nakamura Y. SMYD2-dependent HSP90 methylation promotes cancer cell proliferation by regulating the chaperone complex formation. Cancer letters. 2014;351:126–133. doi: 10.1016/j.canlet.2014.05.014. [DOI] [PubMed] [Google Scholar]
- 47.Kogure M, Takawa M, Saloura V, Sone K, Piao L, Ueda K, Ibrahim R, Tsunoda T, Sugiyama M, Atomi Y, Nakamura Y, Hamamoto R. The oncogenic polycomb histone methyltransferase EZH2 methylates lysine 120 on histone H2B and competes ubiquitination. Neoplasia. 2013;15:1251–1261. doi: 10.1593/neo.131436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kunizaki M, Hamamoto R, Silva FP, Yamaguchi K, Nagayasu T, Shibuya M, Nakamura Y, Furukawa Y. The lysine 831 of vascular endothelial growth factor receptor 1 is a novel target of methylation by SMYD3. Cancer research. 2007;67:10759–10765. doi: 10.1158/0008-5472.CAN-07-1132. [DOI] [PubMed] [Google Scholar]
- 49.Silva FP, Hamamoto R, Kunizaki M, Tsuge M, Nakamura Y, Furukawa Y. Enhanced methyltransferase activity of SMYD3 by the cleavage of its N-terminal region in human cancer cells. Oncogene. 2008;27:2686–2692. doi: 10.1038/sj.onc.1210929. [DOI] [PubMed] [Google Scholar]
- 50.Takawa M, Cho HS, Hayami S, Toyokawa G, Kogure M, Yamane Y, Iwai Y, Maejima K, Ueda K, Masuda A, Dohmae N, Field HI, Tsunoda T, Kobayashi T, Akasu T, Sugiyama M, et al. Histone Lysine Methyltransferase SETD8 Promotes Carcinogenesis by Deregulating PCNA Expression. Cancer research. 2012;72:3217–3227. doi: 10.1158/0008-5472.CAN-11-3701. [DOI] [PubMed] [Google Scholar]
- 51.Cho HS, Suzuki T, Dohmae N, Hayami S, Unoki M, Yoshimatsu M, Toyokawa G, Takawa M, Chen T, Kurash JK, Field H, Ponder BA, Nakamura Y, Hamamoto R. Demethylation of RB regulator MYPT1 by histone demethylase LSD1 promotes cell cycle progression in cancer cells. Cancer Research. 2011;71:655–60. doi: 10.1158/0008-5472.CAN-10-2446. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


