Abstract
Background
The aim of this study was to investigate and interpret the expression level and potential function of TCEA3 in gastric cancer.
Material/Methods
qRT-PCR was used to determine the expression level of TCEA3 in gastric cancer tissues. Pearson χ2 test was performed to clarify the correlation between TCEA3 expression and patients’ clinicopathologic characteristics. Biological function of TCEA3 was tested by proliferation assay and colony formation assay. Flow cytometry was used to study the potential function of TCEA3 in apoptosis induction.
Results
TCEA3 expression was significantly downregulated in gastric cancer tissues compared with paired normal tissues. Poor prognoses were observed in the low TCEA3 expression group of patients in contrast to the high TCEA3 expression group. Functionally, upregulation of TCEA3 inhibited gastric cancer cell proliferation and colony formation. We also found that TCEA3 may attenuate cell growth through apoptosis induction.
Conclusions
Our findings suggest that TCEA3 attenuates the proliferation and induces apoptosis of gastric cancer cells.
MeSH Keywords: Apoptosis, Cell Proliferation, Stomach Neoplasms
Background
Gastric cancer (GC) is one of the most common cancers and is the second leading cause of cancer deaths worldwide [1–3]. Despite great achievements in treatment of gastric cancer, the long-term survival rate for advanced gastric cancer patients is still quite low and the absolute number of gastric cancer patients and deaths is still a large burden[2,4,5]. Uncontrolled growth of cancer cells leads to most of the mortalities and play a critical role in the poor prognosis [6,7]. However, the underlying molecular mechanisms are still poorly understood. Therefore, a better understanding of the molecular mechanisms related to the carcinogenesis of gastric cancer is essential and urgent to develop novel avenues for gastric cancer therapy.
TCEA is one of most important transcription elongation factors; it can directly bind RNA polymerase II, allowing it to read through various transcription arrest sites [8–10]. TCEA has 3 distinct isoforms. TCEA1 is ubiquitously expressed, TCEA2 is a testis-specific isoform [11,12], and TCEA3 is another isoform of TCEA about which little is known. In previous studies, Tcea3 was reported to control cell fate of mESCs via regulating the Lefty1-Nodal-Smad2 pathway and that its appropriate level is essential for the balanced pluripotency of mESCs [13]. Moreover, TCEA3 was identified to bind with TGF-beta receptor I and induces Smad-independent, JNK-dependent apoptosis in ovarian cancer cells [8]. However, the potential role of TCEA3 in gastric cancer is still unclear.
The present study demonstrated that TCEA3 expression was significantly downregulated in gastric cancer tissues compared with paired normal tissues. Worse prognoses were observed in patients in the low TCEA3 expression group compared to those in the high TCEA3 expression group. Functionally, upregulation of TCEA3 inhibited gastric cancer cell proliferation and colony formation. We also found that TCEA3 can attenuate cell growth through apoptosis induction.
Material and Methods
Tissue specimens and cell culture
We collected 34 tissue specimens from patients diagnosed with gastric cancer at the First Affiliated Hospital of Wenzhou Medical University. Tissues were harvested freshly after sample dissection and preserved at –80°C. Immortalized normal gastric mucosal epithelial cell line GES-1, gastric cancer cell lines MKN-45 and SGC-7901 were purchased from the American Type Culture Collection (ATCC, Manassas, VA, USA) and maintained in RPMI 1640 medium containing 10% FBS (GIBCO, Carlsbad, CA, USA) and cultured at 37°C with 5% CO2.
RNA extraction and quantitative real-time PCR (qRT-PCR)
Total RNA was extracted from tissues or cells by using Trizol reagent (Invitrogen, Carlsbad, CA, USA). qRT-PCR was performed using a standard protocol from the SYBR Green PCR kit (Toyobo, Osaka, Japan). ΔCt values were normalized to GAPDH levels. Each sample was analyzed in triplicate. Primers for TCEA3 were: 5′-AAGAGCACGGACATGAAGTACC-3′ (forward) 5′-CTCTGCCGTCATCTTGGCTA-3′ (reverse); for GAPDH were: 5′-GGACCTGACCTGCCGTCTAG-3′ (forward) and 5′-GTAGCCCAGGATGCCCTTGA-3′ (reverse).
Western blot
CDK6 and β-actin protein expression was performed as previously described [14]. The primary antibody for TCEA3 was purchased from ProteinTech (ProteinTech Group, Wuhan, China). GAPDH primary antibody was purchased from Abcam (Abcam, USA).
Plasmid and transfection
TCEA3 full-length cDNA was cloned from GES-1 cDNA and then inserted into the Lenti-virus vector PCDH. One day before transfection, MKN-45 and SGC-7901 cells were plated onto 6-well plates. After 24 h, when the cell confluence was about 70%, transfection was performed with Lipofectamine 2000 (Invitrogen) following the manufacturer’s instructions.
Cell proliferation assay
Cell proliferation rates were calculated at 0, 24, 48, and 72 h after seeding in 96-well plates. Cells were cultured in 10% CCK-8 (DOJINDO) diluted in RPMI 1640 medium at 37°C until visual color conversion appeared. Quantification was carried out with a microtiter plate reader according to the manufacturer’s instructions.
Colony formation
Cells were suspended in RPMI-1640 and seeded in 6-well culture plates at a density of 1×103 cells per dish. The plates were incubated for 2 weeks and the number of colonies was counted after staining with 0.1% crystal violet solution. Colonies of more than 50 cells were manually counted. The experiments were independently triplicated.
Flow cytometric analysis for apoptosis
Cells transfected with TCEA3 or negative control (NC) were plated in 6-well plates. After 48-h incubation, the cells were harvested and flow cytometry was performed with the AnnexinV/PI double-staining kit (BD Biosciences, USA) according to the manufacturer’s instructions. All experiments were repeated 3 times.
Statistics
IBM SPSS 19.0 statistical software was used to analyze the data, which are expressed as mean ± standard deviation (SD). The differences between groups were analyzed using the t test or one-way ANOVA, as appropriate. Differences were considered statistically significant at P<0.05.
Results
TCEA3 was downregulated and was associated with poor prognosis in gastric cancer
In 34 gastric cancer patients, TCEA3 expression was investigated by qPCR. We found the expression level of TCEA3 in cancer tissues was significantly lower than in paired normal tissues (P<0.05) (Figure 1). To investigate the correlation between TCEA3 expression and the clinicopathologic characteristics of gastric cancer, we analyzed the expression of TCEA3 combined with clinicopathologic data. As shown in Table 1, while the expression of TCEA3 was not related to sex (P=0.6), we found significant association of age (P=0.66), tumor location (P=0.56), lymph node metastasis (P=0.19), or differentiation degree (P=0.23), TCEA3 with TNM stage (P=0.009) and depth of invasion (P=0.03).
Figure 1.
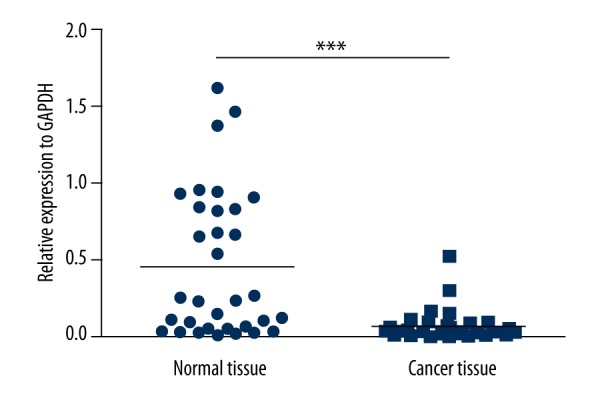
TCEA3 mRNA expression in 34 gastric cancer tissues and paired adjacent non-tumor tissues examined by qRT-PCR and normalized to GAPDH.
Table 1.
Association between TCEA3 expression and clinicopathologic characteristics of 34 gastric cancer patients.
| Clinicopathologic characteristics | Low expression (n=26) | High expression (n=8) | P value |
|---|---|---|---|
| Gender | 0.6 | ||
| Male | 17 | 5 | |
| Female | 9 | 3 | |
| Age | 0.66 | ||
| <60 | 11 | 2 | |
| ≥60 | 15 | 6 | |
| Location | 0.56 | ||
| Middle proximal | 10 | 3 | |
| Distal | 16 | 5 | |
| Depth of invasion | 0.03 | ||
| T1, T2 | 5 | 5 | |
| T3, T4 | 21 | 3 | |
| Lymph node metastasis | 0.19 | ||
| Yes | 20 | 4 | |
| No | 6 | 4 | |
| TNM stage | 0.009 | ||
| I, II | 3 | 5 | |
| III, IV | 23 | 3 | |
| Differentiation | 0.23 | ||
| High, middle | 9 | 4 | |
| Moderate, low | 17 | 4 |
TCEA3 inhibited the growth of gastric cancer cells
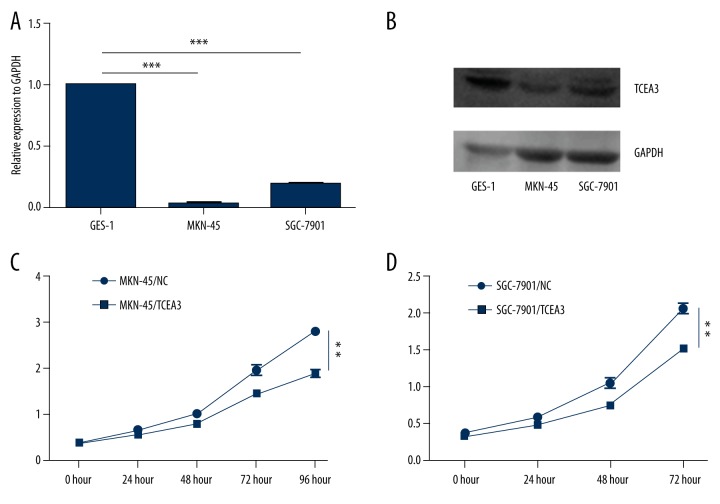
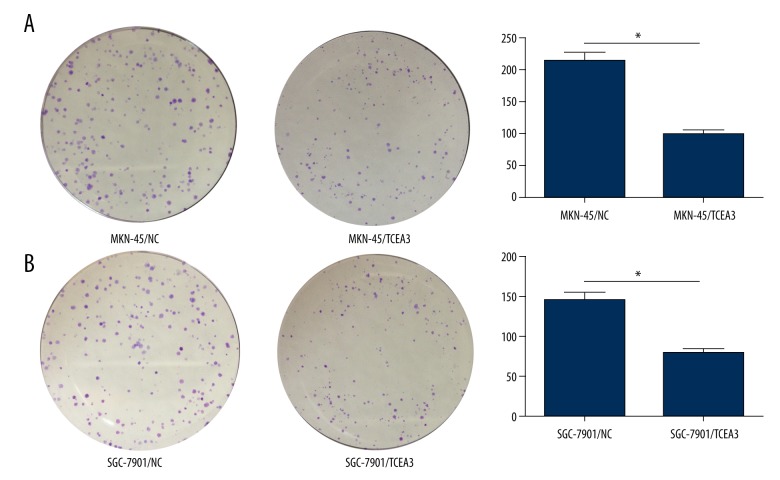
In gastric cancer cell lines MKN-45 and SGC-7901, TCEA3 also showed relatively lower expression than in immortalized normal gastric mucosal epithelial cell line (GES-1) (Figure 2A, 2B). To explore the potential role of TCEA3 in gastric cancer, we transfected MKN-45 and SGC-7901 cells with exogenous TCEA3 or negative control (NC). Then, we used proliferation and colony formation assays to evaluate the effect of TCEA3 on cell proliferative ability. As indicated in Figure 2C and 2D, the TCEA3 had a significantly slower function in proliferation for both MKN-45 and SGC-7901 cells. Consistent with the results of the proliferation assay, TCEA3 also remarkably inhibited colony formation (Figure 3A, 2B). Our results show that TCEA3 can suppress the proliferation of gastric cancer cells.
Figure 2.
TCEA3 attenuated gastric cancer cell proliferation. (A, B) TCEA3 mRNA/protein expression in immortalized normal gastric mucosal epithelial cell line GES-1 and gastric cancer cell lines MKN-45 and SGC-7901. (C, D) Up regulation of TCEA3 inhibited MKN-45 and SGC-7901 proliferation.
Figure 3.
TCEA3 inhibited the colony formation of gastric cancer cell. (A, B) Up-regulation of TCEA3 inhibited MKN-45 and SGC-7901 colony formation.
TCEA3 induced gastric cancer cell apoptosis
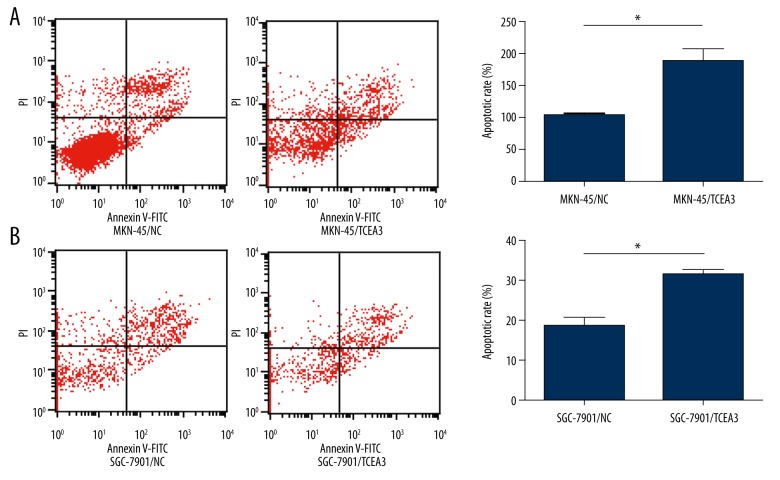
To determine if the attenuation effect in cell growth by TCEA3 was due to the induction of apoptosis, flow cytometry was performed to evaluate the apoptotic cell rate. As shown in Figure 4A and 4B, the number of apoptotic cells significantly increased in MKN-45 and SGC-7901 transfected with TCEA3 compared to the NC group.
Figure 4.
TCEA3 induced gastric cancer cell apoptosis. (A, B) Up-regulation of TCEA3 promoted MKN-45 and SGC-7901 apoptosis as measured by flow cytometry.colony formation.
Discussion
TCEA3 was reported to bind with TGF-beta receptor I and induces Smad-independent, JNK-dependent apoptosis in ovarian cancer cells [8]. In the present study, we found that TCEA3 expression was significantly downregulated in gastric cancer tissues compared with paired normal tissues. Poor prognoses, especially the advanced TNM stage, were observed in the low TCEA3 expression group of patients in contrast to the high TCEA3 expression group, which indicates the potential correlation between TCEA3 and gastric cancer growth. Functionally, up-regulation of TCEA3 inhibited gastric cancer cell proliferation and colony formation. Interestingly, we also found that TCEA3 may attenuate cell growth through apoptosis induction.
Numerous suppressive genes have been demonstrated to be downregulated in gastric cancer and associated with the prognosis, like KIAA1324, MiR-361-5p, and EGR3 [15–17]. Apoptosis induction is a critical function by which suppressive genes inhibit tumor growth. TCEA3 was reported to promote apoptosis in ovarian cancer through the TGF-beta pathway [8]. We hypothesized that TCEA3 inhibited tumor growth through apoptosis induction in gastric cancer. Then, we performed the flow cytometry and determined that the involvement of apoptosis in TCEA3 induced tumor restriction.
Conclusions
TCEA3 was significantly downregulated in gastric cancer and can cause attenuation of cell growth via induction of cell apoptosis, which might provide a new strategy for gastric cancer treatment.
Footnotes
Source of support: Departmental sources
References
- 1.Yang L. Incidence and mortality of gastric cancer in China. World J Gastroenterol. 2006;12:17–20. doi: 10.3748/wjg.v12.i1.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jemal A, Center MM, DeSantis C, et al. Global patterns of cancer incidence and mortality rates and trends. Cancer Epidem Biomar. 2010;19:1893–907. doi: 10.1158/1055-9965.EPI-10-0437. [DOI] [PubMed] [Google Scholar]
- 3.Xu Y, Zhao F, Wang Z, et al. MicroRNA-335 acts as a metastasis suppressor in gastric cancer by targeting Bcl-w and specificity protein 1. Oncogene. 2012;31:1398–407. doi: 10.1038/onc.2011.340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Xu Y, Sun J, Xu J, et al. miR-21 Is a promising novel biomarker for lymph node metastasis in patients with gastric cancer. Gastroent Res Pract. 2012;2012:640168. doi: 10.1155/2012/640168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Thiel A, Ristimaki A. Gastric cancer: basic aspects. Helicobacter. 2012;17:26–29. doi: 10.1111/j.1523-5378.2012.00979.x. [DOI] [PubMed] [Google Scholar]
- 6.Ko H, Kim JM, Kim SJ, et al. Induction of apoptosis by genipin inhibits cell proliferation in AGS human gastric cancer cells via Egr1/p21 signaling pathway. Bioorg Med Chem Lett. 2015;25(19):4191–96. doi: 10.1016/j.bmcl.2015.08.005. [DOI] [PubMed] [Google Scholar]
- 7.Xia T, Chen S, Jiang Z, et al. Long noncoding RNA FER1L4 suppresses cancer cell growth by acting as a competing endogenous RNA and regulating PTEN expression. Sci Rep. 2015;5:13445. doi: 10.1038/srep13445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cha Y, Kim DK, Hyun J, et al. TCEA3 binds to TGF-beta receptor I and induces Smad-independent, JNK-dependent apoptosis in ovarian cancer cells. Cell Signal. 2013;25:1245–51. doi: 10.1016/j.cellsig.2013.01.016. [DOI] [PubMed] [Google Scholar]
- 9.Reines D, Conaway JW, Conaway RC. The RNA polymerase II general elongation factors. Trends Biochem Sci. 1996;21:351–55. [PMC free article] [PubMed] [Google Scholar]
- 10.Wind M, Reines D. Transcription elongation factor SII. BioEssays. 2000;22:327–36. doi: 10.1002/(SICI)1521-1878(200004)22:4<327::AID-BIES3>3.0.CO;2-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hubbard K, Catalano J, Puri RK, et al. Knockdown of TFIIS by RNA silencing inhibits cancer cell proliferation and induces apoptosis. BMC Cancer. 2008;8:133. doi: 10.1186/1471-2407-8-133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Weaver ZA, Kane CM. Genomic characterization of a testis-specific TFIIS (TCEA2) gene. Genomics. 1997;46:516–19. doi: 10.1006/geno.1997.5073. [DOI] [PubMed] [Google Scholar]
- 13.Park KS, Cha Y, Kim CH, et al. Transcription elongation factor Tcea3 regulates the pluripotent differentiation potential of mouse embryonic stem cells via the Lefty1-Nodal-Smad2 pathway. Stem Cells. 2013;31:282–92. doi: 10.1002/stem.1284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhang J, Yang Y, Lei L, et al. Rhizoma paridis saponins induces cell cycle arrest and apoptosis in non-small cell lung carcinoma A549 cells. Med Sci Monit. 2015;21:2535–41. doi: 10.12659/MSM.895084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Liao F, Ji MY, Shen L, et al. Decreased EGR3 expression is related to poor prognosis in patients with gastric cancer. J Mol Histol. 2013;44:463–68. doi: 10.1007/s10735-013-9493-8. [DOI] [PubMed] [Google Scholar]
- 16.Ma F, Song H, Guo B, et al. MiR-361-5p inhibits colorectal and gastric cancer growth and metastasis by targeting staphylococcal nuclease domain containing-1. Oncotarget. 2015;6:17404–16. doi: 10.18632/oncotarget.3744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kang JM, Park S, Kim SJ, et al. KIAA1324 suppresses gastric cancer progression by inhibiting the oncoprotein GRP78. Cancer Res. 2015;75:3087–97. doi: 10.1158/0008-5472.CAN-14-3751. [DOI] [PubMed] [Google Scholar]