Abstract
Introduction
Infliximab, an antibody against tumor necrosis factor alpha, is used to treat inflammatory bowel disease and has well-established efficacy and proven safety. Complications of this treatment are related to immunosuppression and include higher risk of serious infections and malignant neoplasia. Although extremely rare, fulminant liver damage related to infliximab therapy has been reported.
Case presentation
We present the case of a 38-year-old Afro-Brazilian woman with refractory ulcerative colitis who was started on infliximab. She had no previous history of liver disease, alcohol abuse, or infection. After the fifth dose of the medication, drug-induced liver injury was diagnosed. Treatment was discontinued but our patient’s condition was aggravated by severe cholestasis and grade III/IV encephalopathy, requiring liver transplantation.
Conclusion
Drug-induced liver injury is an uncommon complication of infliximab. Current consensus recommends screening for liver dysfunction prior to and during therapy. This case emphasizes the need for vigilance and highlights a rare and potentially lethal complication.
Keywords: Hepatitis, Infliximab, Liver failure, Liver transplantation, Ulcerative colitis
Introduction
Antibodies against tumor necrosis factor alpha, such as infliximab (IFX), are increasingly being used to treat inflammatory bowel disease [1, 2]. They were originally used to manage moderate to severe active Crohn’s disease but have recently been approved for severe ulcerative colitis (UC) [3]. The most worrisome adverse effects of IFX are the increased risks of developing serious infections and malignancies; therefore, close monitoring of patients is required to increase safety. Although uncommon, IFX therapy has been associated with drug-induced liver injury, which frequently occurs 13 weeks after its initiation but may be diagnosed up to 6 months later [4–11]. We report a rare case of severe UC treated with IFX that was complicated by fulminant hepatic failure and required urgent liver transplantation.
Case presentation
A 38-year-old Afro-Brazilian woman with refractory UC was started on IFX (5 mg/kg). Her baseline liver and renal functions were entirely normal. Our patient became asymptomatic after IFX-induction therapy (weeks 0, 2, and 6). After the fifth dose of infliximab, routine laboratory examinations showed an increase in transaminases levels, with aspartate aminotransferase (AST) of 325 U/L and alanine aminotransferase (ALT) of 408 U/L, and the medication was discontinued. Our patient had no history of alcohol abuse or infection. Serology tests for hepatitis B and C viruses, human immunodeficiency virus (HIV), cytomegalovirus, and Epstein–Barr virus were negative. No serum smooth-muscle, anti-nucleic, or antimitochondrial antibodies could be detected. Even after we discontinued therapy, our patient’s condition worsened. She developed severe cholestasis with marked jaundice and fatigue, requiring hospitalization. Laboratory tests were as follows: bilirubin 23.38 mg/dL (normal range <1.2 mg/dL), AST 1,254 U/L (normal range <35 U/L), and ALT 2,177 U/L (normal range <105 U/L). Her platelet count decreased to 54,000 and the plasma internationalized normalized ratio (INR) was elevated to a value of 4.1. A nuclear magnetic resonance scan demonstrated acute liver damage. She developed grade III encephalopathy with marked confusion and incomprehensible speech and had an emergency whole liver transplantation. The surgery was successful. A histopathological analysis of her explanted liver revealed extensive necrosis compatible with fulminant hepatic failure (Fig. 1). After the procedure, results from hepatic tests normalized (Fig. 2). Our patient made a full recovery and is receiving immunosuppressive therapy with tacrolimus. No signs of liver rejection or colitis reactivation have yet been noted.
Fig. 1.
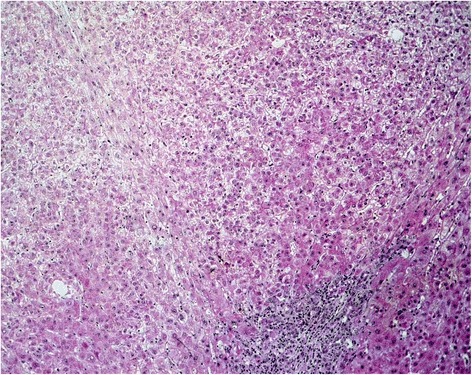
Hepatic necrosis. Hematoxylin and eosin staining, original magnification 10×
Fig. 2.
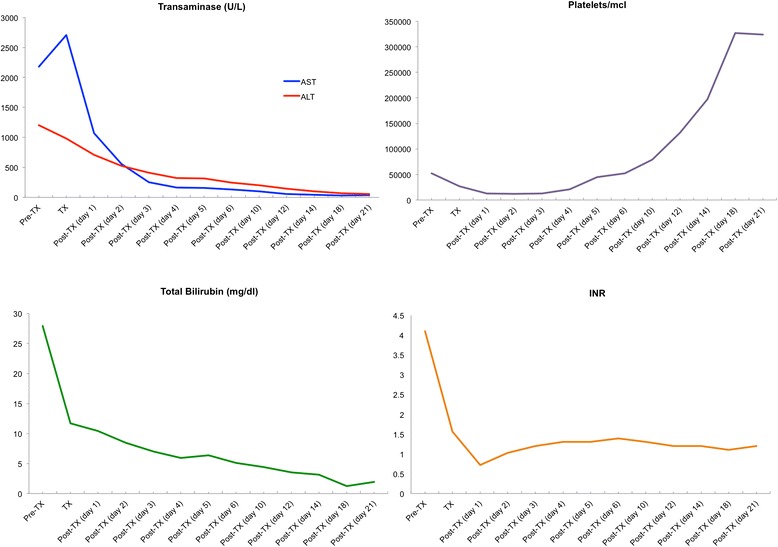
Laboratory examinations before (Pre-TX) and after (Post-TX) the liver transplant, expressed in days. Shown are transaminase levels (aspartate aminotransferase [ALT], shown in blue; alanine aminotransferase [ALT], shown in red), bilirubin levels, plasma internationalized normalized ratio (INR), and platelet count. IFX infliximab, TX transplant
Discussion
The number of patients treated with IFX has rapidly increased worldwide. An estimated 2 million people were exposed to this drug from 1998 to 2014. The colectomy rate in UC significantly decreased after the introduction of this class of drugs [12–15]. Despite this notable success, physicians must be aware of the possible complications, especially those related to immunosuppression such as serious infections and malignant neoplasia.
Elevated liver enzymes (especially ALT) have been reported during IFX treatment but are usually transitory and have no clinical implications. Fulminant liver failure is an extremely rare and serious event that requires a liver transplant, with high morbidity and mortality [16–18]. IFX-induced acute liver failure may be explained in three possible ways: autoimmune hepatitis, cholestatic injury, and direct toxicity [19].
In the present case, our patient had no history of alcohol consumption or concomitant use of any hepatotoxic drugs. Serological tests for infectious hepatitis, HIV, or other viruses were negative. Autoimmune disease was also excluded. Histopathological analysis of the explanted liver evidenced diffuse hepatitis intertwined with areas of necrosis, suggesting direct liver damage. A diagnosis of IFX-induced hepatitis was made considering the temporal relationship with IFX exposure, lack of other possible causes of liver injury, laboratory changes, and clinical deterioration.
Current guidelines support screening for liver dysfunction at 4-month intervals. It is also important to rule out any hepatotoxic risk factor prior to IFX therapy. Discontinuation of IFX is recommended if transaminase levels reach three times the upper normal limits, especially if associated with clinical manifestations [20].
Conclusion
This report calls attention to a rare and potentially lethal adverse effect of IFX. All efforts should be made to rule out any pre-existing liver disease before initiating IFX therapy and vigilance must continue during the maintenance treatment, which must be interrupted if aminotransferases elevate more than three times above the normal levels. Signs of abrupt clinical deterioration should raise suspicion for fulminant liver disease.
Consent
Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the Editor-in-Chief of this journal.
Acknowledgements
The authors have no disclosures to make and have had no source of funding in the preparation of this manuscript. We would like to thank Prof. Ênio David Mente for his technical support.
Abbreviations
- AST
aspartate aminotransferase
- ALT
alanine aminotransferase
- HIV
human immunodeficiency virus
- IFX
infliximab
- INR
internationalized normalized ratio
- UC
ulcerative colitis
Footnotes
Competing interests
The authors declare that they have no competing interests.
Authors’ contributions
RSP interpreted the patient data and was a major contributor to writing the manuscript. MRF and VFM collected and helped interpret patient data, and contributed to writing the manuscript. LNZR analyzed histopathological findings. JJRR and OF designed, reviewed, and approved the final version of the manuscript. All authors read and approved the final manuscript.
Contributor Information
Rogerio Serafim Parra, Email: rogeriosparra@gmail.com.
Marley Ribeiro Feitosa, Email: marleyfeitosa@yahoo.com.br.
Vanessa Foresto Machado, Email: v_anfm@yahoo.com.br.
Leandra Naira Zambelli Ramalho, Email: lramalho@fmrp.usp.br.
Jose Joaquim Ribeiro da Rocha, Email: jjrocha1@bol.com.br.
Omar Feres, Email: omar.feres@hspaulo.com.br.
References
- 1.Magro F, Portela F. Management of inflammatory bowel disease with infliximab and other anti-tumor necrosis factor alpha therapies. BioDrugs. 2010;24(Suppl 1):3–14. doi: 10.2165/11586290-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 2.Singh S, Pardi DS. Update on anti-tumor necrosis factor agents in Crohn disease. Gastroenterol Clin North Am. 2014;43(3):457–78. doi: 10.1016/j.gtc.2014.05.008. [DOI] [PubMed] [Google Scholar]
- 3.Seo GS, Chae SC. Biological therapy for ulcerative colitis: an update. World J Gastroenterol. 2014;20(37):13234–8. doi: 10.3748/wjg.v20.i37.13234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Björnsson ES, Gunnarsson BI, Gröndal G, Jonasson JG, Einarsdottir R, Ludviksson BR, et al. Risk of drug-induced liver injury from tumor necrosis factor antagonists. Clin Gastroenterol Hepatol. 2015;13(3):602–8. doi: 10.1016/j.cgh.2014.07.062. [DOI] [PubMed] [Google Scholar]
- 5.Tobon GJ, Cañas C, Jaller JJ, Restrepo JC, Anaya JM. Serious liver disease induced by infliximab. Clin Rheumatol. 2007;26(4):578–81. doi: 10.1007/s10067-005-0169-y. [DOI] [PubMed] [Google Scholar]
- 6.Menghini VV, Arora AS. Infliximab-associated reversible cholestatic liver disease. Mayo Clin Proc. 2001;76:84. doi: 10.4065/76.1.84. [DOI] [PubMed] [Google Scholar]
- 7.Mancini S, Amorotti E, Vecchio S, Ponz de Leon M, Roncucci L. Infliximab-related hepatitis: discussion of a case and review of the literature. Intern Emerg Med. 2010;5(3):193–200. doi: 10.1007/s11739-009-0342-4. [DOI] [PubMed] [Google Scholar]
- 8.Ierardi E, Valle ND, Nacchiero MC, De Francesco V, Stoppino G, Panella C. Onset of liver damage after a single administration of infliximab in a patient with refractory ulcerative colitis. Clin Drug Investig. 2006;26:673. doi: 10.2165/00044011-200626110-00008. [DOI] [PubMed] [Google Scholar]
- 9.Cheng FK, Bridges EE, Betteridge JD. Drug-induced liver injury from initial dose of infliximab. Mil Med. 2015;180(6):e723-4. [DOI] [PubMed]
- 10.Moum B, Konopski Z, Tufteland KF, Jahnsen J. Occurrence of hepatoxicicty and elevated liver enzymes in a Crohn’s disease patient treated with infliximab. Inflamm Bowel Dis. 2007;13:1584. doi: 10.1002/ibd.20230. [DOI] [PubMed] [Google Scholar]
- 11.Ghabril M, Bonkovsky HL, Kum C, Davern T, Hayashi PH, Kleiner DE, et al. Liver injury from tumor necrosis factor-α antagonists: analysis of thirty-four cases. Clin Gastroenterol Hepatol. 2013;11(5):558–64. doi: 10.1016/j.cgh.2012.12.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sandborn WJ, Rutgeerts P, Feagan BG, Reinisch W, Olson A, Johanns J, et al. Colectomy rate comparison after treatment of ulcerative colitis with placebo or infliximab. Gastroenterology. 2009;137(4):1250–60. doi: 10.1053/j.gastro.2009.06.061. [DOI] [PubMed] [Google Scholar]
- 13.Colombel JF, Rutgeerts P, Reinisch W, Esser D, Wang Y, Lang Y, et al. Early mucosal healing with infliximab is associated with improved long-term clinical outcomes in ulcerative colitis. Gastroenterology. 2011;141(4):1194–201. doi: 10.1053/j.gastro.2011.06.054. [DOI] [PubMed] [Google Scholar]
- 14.Arias MT, Vande Casteele N, Vermeire S, de Buck van Overstraeten A, Billiet T, Baert F, et al. A panel to predict long-term outcome of infliximab therapy for patients with ulcerative colitis. Clin Gastroenterol Hepatol. 2015;13(3):531–8. doi: 10.1016/j.cgh.2014.07.055. [DOI] [PubMed] [Google Scholar]
- 15.Gustavsson A, Järnerot G, Hertervig E, Friis-Liby I, Blomquist L, Karlén P, et al. Clinical trial: colectomy after rescue therapy in ulcerative colitis - 3-year follow-up of the Swedish-Danish controlled infliximab study. Aliment Pharmacol Ther. 2010;32(8):984–9. doi: 10.1111/j.1365-2036.2010.04435.x. [DOI] [PubMed] [Google Scholar]
- 16.Rowe BW, Gala-Lopez B, Tomlinson C, Girgis S, Shapiro JA. Fulminant hepatic failure necessitating transplantation following the initiation of infliximab therapy: a cautionary tale times two. Transpl Int. 2013;26(12):e110–2. doi: 10.1111/tri.12185. [DOI] [PubMed] [Google Scholar]
- 17.Kinnunen U, Färkkilä M, Mäkisalo H. A case report: ulcerative colitis, treatment with an antibody against tumor necrosis factor (infliximab), and subsequent liver necrosis. J Crohns Colitis. 2012;6(6):724–7. doi: 10.1016/j.crohns.2012.02.004. [DOI] [PubMed] [Google Scholar]
- 18.Khokhar OS, Lewis JH. Hepatotoxicity of agents used in the management of inflammatory bowel disease. Dig Dis. 2010;28(3):508–18. doi: 10.1159/000320410. [DOI] [PubMed] [Google Scholar]
- 19.Carvalheiro J, Mendes S, Sofia C. Infliximab induced liver injury in Crohn's disease: a challenging diagnosis. J Crohns Colitis. 2014;8(5):436–7. doi: 10.1016/j.crohns.2013.11.013. [DOI] [PubMed] [Google Scholar]
- 20.Miehsler W, Novacek G, Wenzl H, Vogelsang H, Knoflach P, Kaser A, et al. A decade of infliximab: the Austrian evidence based consensus on the safe use of infliximab in inflammatory bowel disease. J Crohns Colitis. 2010;4(3):221–56. doi: 10.1016/j.crohns.2009.12.001. [DOI] [PubMed] [Google Scholar]


