Abstract
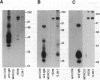
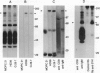
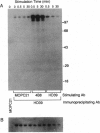
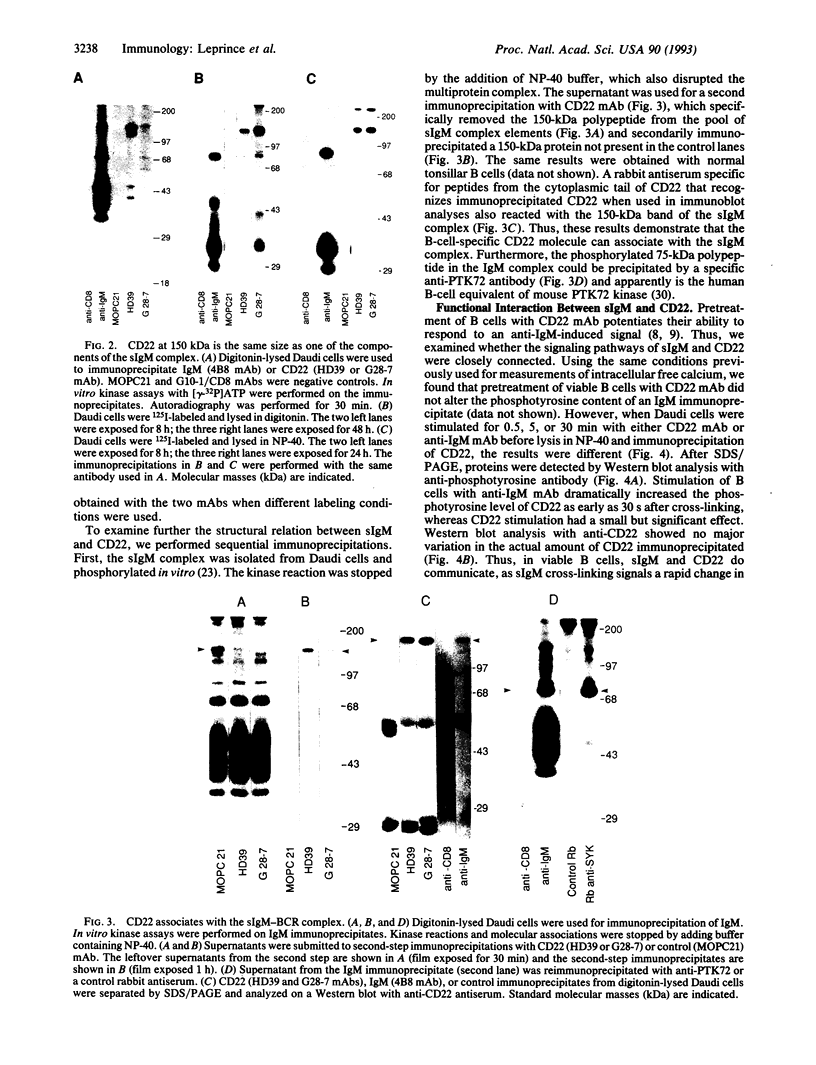
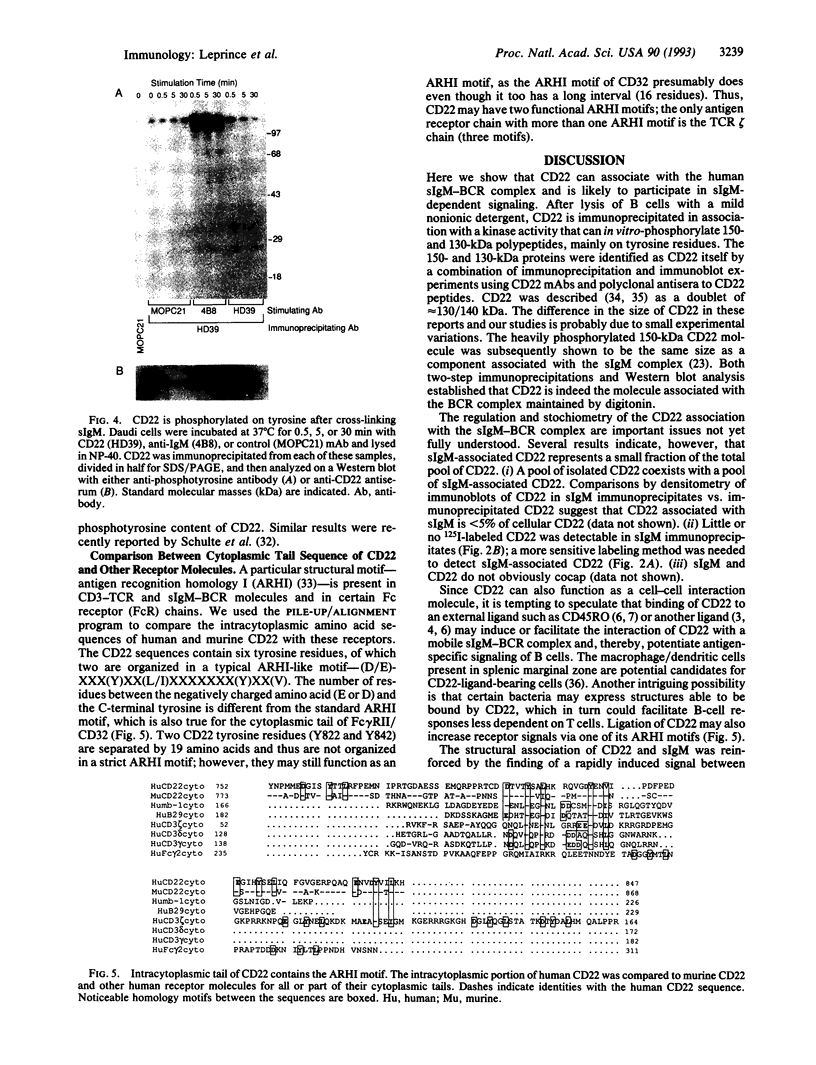
The B-cell surface molecule CD22, when cross-linked, modulates signaling through the surface IgM (sIgM)-B-cell receptor (BCR) complex. Here we analyzed the basis of this interaction between CD22 and the human sIgM complex. After lysis of B cells or B-cell lines in digitonin, CD22 coimmunoprecipitated a kinase activity that in vitro-phosphorylated two polypeptides of 150 and 130 kDa on tyrosine residues. By immunoblot analysis with a rabbit anti-serum specific for a synthetic peptide of CD22, we found these proteins to be CD22 itself. Furthermore, the phosphorylated 150-kDa CD22 was found in the sIgM-BCR complex maintained by digitonin, along with Ig alpha/mb-1, Ig beta/B29, and a 75-kDa polypeptide precipitated by an antiserum specific to protein-tyrosine kinase PTK72. CD22 is likely to be an important signaling partner in the sIgM-BCR complex since it is very rapidly and strikingly phosphorylated after sIgM is cross-linked and since it contains the antigen recognition homology I (ARHI) motif, present in other antigen receptor molecules.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Armitage R. J., Rowe D. J., Beverly P. C. A new antigen identified by the monoclonal antibody UCHB 1 delivers a costimulatory signal to a subset of human B cells. Eur J Immunol. 1988 Jan;18(1):67–76. doi: 10.1002/eji.1830180111. [DOI] [PubMed] [Google Scholar]
- Aruffo A., Kanner S. B., Sgroi D., Ledbetter J. A., Stamenkovic I. CD22-mediated stimulation of T cells regulates T-cell receptor/CD3-induced signaling. Proc Natl Acad Sci U S A. 1992 Nov 1;89(21):10242–10246. doi: 10.1073/pnas.89.21.10242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boué D. R., Lebien T. W. Structural characterization of the human B lymphocyte-restricted differentiation antigen CD22. Comparison with CD21 (complement receptor type 2/Epstein-Barr virus receptor). J Immunol. 1988 Jan 1;140(1):192–199. [PubMed] [Google Scholar]
- Burkhardt A. L., Brunswick M., Bolen J. B., Mond J. J. Anti-immunoglobulin stimulation of B lymphocytes activates src-related protein-tyrosine kinases. Proc Natl Acad Sci U S A. 1991 Aug 15;88(16):7410–7414. doi: 10.1073/pnas.88.16.7410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cambier J. C. Signal transduction by T- and B-cell antigen receptors: converging structures and concepts. Curr Opin Immunol. 1992 Jun;4(3):257–264. doi: 10.1016/0952-7915(92)90074-o. [DOI] [PubMed] [Google Scholar]
- Campana D., Janossy G., Bofill M., Trejdosiewicz L. K., Ma D., Hoffbrand A. V., Mason D. Y., Lebacq A. M., Forster H. K. Human B cell development. I. Phenotypic differences of B lymphocytes in the bone marrow and peripheral lymphoid tissue. J Immunol. 1985 Mar;134(3):1524–1530. [PubMed] [Google Scholar]
- Campbell K. S., Cambier J. C. B lymphocyte antigen receptors (mIg) are non-covalently associated with a disulfide linked, inducibly phosphorylated glycoprotein complex. EMBO J. 1990 Feb;9(2):441–448. doi: 10.1002/j.1460-2075.1990.tb08129.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell K. S., Hager E. J., Friedrich R. J., Cambier J. C. IgM antigen receptor complex contains phosphoprotein products of B29 and mb-1 genes. Proc Natl Acad Sci U S A. 1991 May 1;88(9):3982–3986. doi: 10.1073/pnas.88.9.3982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell M. A., Sefton B. M. Association between B-lymphocyte membrane immunoglobulin and multiple members of the Src family of protein tyrosine kinases. Mol Cell Biol. 1992 May;12(5):2315–2321. doi: 10.1128/mcb.12.5.2315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan A. C., Irving B. A., Fraser J. D., Weiss A. The zeta chain is associated with a tyrosine kinase and upon T-cell antigen receptor stimulation associates with ZAP-70, a 70-kDa tyrosine phosphoprotein. Proc Natl Acad Sci U S A. 1991 Oct 15;88(20):9166–9170. doi: 10.1073/pnas.88.20.9166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan A. C., Iwashima M., Turck C. W., Weiss A. ZAP-70: a 70 kd protein-tyrosine kinase that associates with the TCR zeta chain. Cell. 1992 Nov 13;71(4):649–662. doi: 10.1016/0092-8674(92)90598-7. [DOI] [PubMed] [Google Scholar]
- Chen J. Z., Stall A. M., Herzenberg L. A., Herzenberg L. A. Differences in glycoprotein complexes associated with IgM and IgD on normal murine B cells potentially enable transduction of different signals. EMBO J. 1990 Jul;9(7):2117–2124. doi: 10.1002/j.1460-2075.1990.tb07380.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark E. A., Lane P. J. Regulation of human B-cell activation and adhesion. Annu Rev Immunol. 1991;9:97–127. doi: 10.1146/annurev.iy.09.040191.000525. [DOI] [PubMed] [Google Scholar]
- Clark E. A., Shu G. Activation of human B cell proliferation through surface Bp35 (CD20) polypeptides or immunoglobulin receptors. J Immunol. 1987 Feb 1;138(3):720–725. [PubMed] [Google Scholar]
- Clark M. R., Campbell K. S., Kazlauskas A., Johnson S. A., Hertz M., Potter T. A., Pleiman C., Cambier J. C. The B cell antigen receptor complex: association of Ig-alpha and Ig-beta with distinct cytoplasmic effectors. Science. 1992 Oct 2;258(5079):123–126. doi: 10.1126/science.1439759. [DOI] [PubMed] [Google Scholar]
- Gold M. R., Matsuuchi L., Kelly R. B., DeFranco A. L. Tyrosine phosphorylation of components of the B-cell antigen receptors following receptor crosslinking. Proc Natl Acad Sci U S A. 1991 Apr 15;88(8):3436–3440. doi: 10.1073/pnas.88.8.3436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hermanson G. G., Eisenberg D., Kincade P. W., Wall R. B29: a member of the immunoglobulin gene superfamily exclusively expressed on beta-lineage cells. Proc Natl Acad Sci U S A. 1988 Sep;85(18):6890–6894. doi: 10.1073/pnas.85.18.6890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hombach J., Lottspeich F., Reth M. Identification of the genes encoding the IgM-alpha and Ig-beta components of the IgM antigen receptor complex by amino-terminal sequencing. Eur J Immunol. 1990 Dec;20(12):2795–2799. doi: 10.1002/eji.1830201239. [DOI] [PubMed] [Google Scholar]
- Hombach J., Tsubata T., Leclercq L., Stappert H., Reth M. Molecular components of the B-cell antigen receptor complex of the IgM class. Nature. 1990 Feb 22;343(6260):760–762. doi: 10.1038/343760a0. [DOI] [PubMed] [Google Scholar]
- Hutchcroft J. E., Harrison M. L., Geahlen R. L. Association of the 72-kDa protein-tyrosine kinase PTK72 with the B cell antigen receptor. J Biol Chem. 1992 Apr 25;267(12):8613–8619. [PubMed] [Google Scholar]
- Kamps M. P., Sefton B. M. Acid and base hydrolysis of phosphoproteins bound to immobilon facilitates analysis of phosphoamino acids in gel-fractionated proteins. Anal Biochem. 1989 Jan;176(1):22–27. doi: 10.1016/0003-2697(89)90266-2. [DOI] [PubMed] [Google Scholar]
- Kappes D. J., Tonegawa S. Surface expression of alternative forms of the TCR/CD3 complex. Proc Natl Acad Sci U S A. 1991 Dec 1;88(23):10619–10623. doi: 10.1073/pnas.88.23.10619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lane P. J., Ledbetter J. A., McConnell F. M., Draves K., Deans J., Schieven G. L., Clark E. A. The role of tyrosine phosphorylation in signal transduction through surface Ig in human B cells. Inhibition of tyrosine phosphorylation prevents intracellular calcium release. J Immunol. 1991 Jan 15;146(2):715–722. [PubMed] [Google Scholar]
- Leprince C., Draves K. E., Ledbetter J. A., Torres R. M., Clark E. A. Characterization of molecular components associated with surface immunoglobulin M in human B lymphocytes: presence of tyrosine and serine/threonine protein kinases. Eur J Immunol. 1992 Aug;22(8):2093–2099. doi: 10.1002/eji.1830220820. [DOI] [PubMed] [Google Scholar]
- Pezzutto A., Dörken B., Moldenhauer G., Clark E. A. Amplification of human B cell activation by a monoclonal antibody to the B cell-specific antigen CD22, Bp 130/140. J Immunol. 1987 Jan 1;138(1):98–103. [PubMed] [Google Scholar]
- Pezzutto A., Dörken B., Rabinovitch P. S., Ledbetter J. A., Moldenhauer G., Clark E. A. CD19 monoclonal antibody HD37 inhibits anti-immunoglobulin-induced B cell activation and proliferation. J Immunol. 1987 May 1;138(9):2793–2799. [PubMed] [Google Scholar]
- Pezzutto A., Rabinovitch P. S., Dörken B., Moldenhauer G., Clark E. A. Role of the CD22 human B cell antigen in B cell triggering by anti-immunoglobulin. J Immunol. 1988 Mar 15;140(6):1791–1795. [PubMed] [Google Scholar]
- Reth M. Antigen receptor tail clue. Nature. 1989 Mar 30;338(6214):383–384. doi: 10.1038/338383b0. [DOI] [PubMed] [Google Scholar]
- Sakaguchi N., Kashiwamura S., Kimoto M., Thalmann P., Melchers F. B lymphocyte lineage-restricted expression of mb-1, a gene with CD3-like structural properties. EMBO J. 1988 Nov;7(11):3457–3464. doi: 10.1002/j.1460-2075.1988.tb03220.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulte R. J., Campbell M. A., Fischer W. H., Sefton B. M. Tyrosine phosphorylation of CD22 during B cell activation. Science. 1992 Nov 6;258(5084):1001–1004. doi: 10.1126/science.1279802. [DOI] [PubMed] [Google Scholar]
- Schwartz-Albiez R., Dörken B., Monner D. A., Moldenhauer G. CD22 antigen: biosynthesis, glycosylation and surface expression of a B lymphocyte protein involved in B cell activation and adhesion. Int Immunol. 1991 Jul;3(7):623–633. doi: 10.1093/intimm/3.7.623. [DOI] [PubMed] [Google Scholar]
- Stamenkovic I., Seed B. The B-cell antigen CD22 mediates monocyte and erythrocyte adhesion. Nature. 1990 May 3;345(6270):74–77. doi: 10.1038/345074a0. [DOI] [PubMed] [Google Scholar]
- Stamenkovic I., Sgroi D., Aruffo A., Sy M. S., Anderson T. The B lymphocyte adhesion molecule CD22 interacts with leukocyte common antigen CD45RO on T cells and alpha 2-6 sialyltransferase, CD75, on B cells. Cell. 1991 Sep 20;66(6):1133–1144. doi: 10.1016/0092-8674(91)90036-x. [DOI] [PubMed] [Google Scholar]
- Taniguchi T., Kobayashi T., Kondo J., Takahashi K., Nakamura H., Suzuki J., Nagai K., Yamada T., Nakamura S., Yamamura H. Molecular cloning of a porcine gene syk that encodes a 72-kDa protein-tyrosine kinase showing high susceptibility to proteolysis. J Biol Chem. 1991 Aug 25;266(24):15790–15796. [PubMed] [Google Scholar]
- Torres R. M., Law C. L., Santos-Argumedo L., Kirkham P. A., Grabstein K., Parkhouse R. M., Clark E. A. Identification and characterization of the murine homologue of CD22, a B lymphocyte-restricted adhesion molecule. J Immunol. 1992 Oct 15;149(8):2641–2649. [PubMed] [Google Scholar]
- Van Noesel C. J., Borst J., De Vries E. F., Van Lier R. A. Identification of two distinct phosphoproteins as components of the human B cell antigen receptor complex. Eur J Immunol. 1990 Dec;20(12):2789–2793. doi: 10.1002/eji.1830201238. [DOI] [PubMed] [Google Scholar]
- Venkitaraman A. R., Williams G. T., Dariavach P., Neuberger M. S. The B-cell antigen receptor of the five immunoglobulin classes. Nature. 1991 Aug 29;352(6338):777–781. doi: 10.1038/352777a0. [DOI] [PubMed] [Google Scholar]
- Wange R. L., Kong A. N., Samelson L. E. A tyrosine-phosphorylated 70-kDa protein binds a photoaffinity analogue of ATP and associates with both the zeta chain and CD3 components of the activated T cell antigen receptor. J Biol Chem. 1992 Jun 15;267(17):11685–11688. [PubMed] [Google Scholar]
- Wegener A. M., Letourneur F., Hoeveler A., Brocker T., Luton F., Malissen B. The T cell receptor/CD3 complex is composed of at least two autonomous transduction modules. Cell. 1992 Jan 10;68(1):83–95. doi: 10.1016/0092-8674(92)90208-t. [DOI] [PubMed] [Google Scholar]
- Wilson G. L., Fox C. H., Fauci A. S., Kehrl J. H. cDNA cloning of the B cell membrane protein CD22: a mediator of B-B cell interactions. J Exp Med. 1991 Jan 1;173(1):137–146. doi: 10.1084/jem.173.1.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamanashi Y., Kakiuchi T., Mizuguchi J., Yamamoto T., Toyoshima K. Association of B cell antigen receptor with protein tyrosine kinase Lyn. Science. 1991 Jan 11;251(4990):192–194. doi: 10.1126/science.1702903. [DOI] [PubMed] [Google Scholar]
- Zioncheck T. F., Harrison M. L., Isaacson C. C., Geahlen R. L. Generation of an active protein-tyrosine kinase from lymphocytes by proteolysis. J Biol Chem. 1988 Dec 15;263(35):19195–19202. [PubMed] [Google Scholar]
- van Noesel C. J., van Lier R. A., Cordell J. L., Tse A. G., van Schijndel G. M., de Vries E. F., Mason D. Y., Borst J. The membrane IgM-associated heterodimer on human B cells is a newly defined B cell antigen that contains the protein product of the mb-1 gene. J Immunol. 1991 Jun 1;146(11):3881–3888. [PubMed] [Google Scholar]