Abstract
The increasing use of highly potent strains of cannabis prompted this new evaluation of human toxicology and subjective effects following passive exposure to cannabis smoke. The study was designed to produce extreme cannabis smoke exposure conditions tolerable to drug-free nonsmokers. Six experienced cannabis users smoked cannabis cigarettes [5.3% Δ9-tetrahydrocannabinol (THC) in Session 1 and 11.3% THC in Sessions 2 and 3] in a closed chamber. Six nonsmokers were seated alternately with smokers during exposure sessions of 1 h duration. Sessions 1 and 2 were conducted with no ventilation and ventilation was employed in Session 3. Oral fluid, whole blood and subjective effect measures were obtained before and at multiple time points after each session. Oral fluid was analyzed by ELISA (4 ng/mL cutoff concentration) and by LC–MS-MS (limit of quantitation) for THC (1 ng/mL) and total THCCOOH (0.02 ng/mL). Blood was analyzed by LC–MS-MS (0.5 ng/mL) for THC, 11-OH-THC and free THCCOOH. Positive tests for THC in oral fluid and blood were obtained for nonsmokers up to 3 h following exposure. Ratings of subjective effects correlated with the degree of exposure. Subjective effect measures and amounts of THC absorbed by nonsmokers (relative to smokers) indicated that extreme secondhand cannabis smoke exposure mimicked, though to a lesser extent, active cannabis smoking.
Introduction
Cannabis continues to be the most widely used illicit drug globally. In the USA, there were 19.8 million past month users in 2013 (7.5% of those aged 12 or older), representing an increase in reported rates from 2002 to 2009 (range 5.8–7.0%) (1). Δ9-tetrahydrocannabinol (THC), the primary euphoriant of cannabis, is present in cannabis plant material as THC and as carboxylic acid precursor molecular forms [precursor acids are referred to as Δ9-tetrahydrocannabinolic acid A (THCA-A) and Δ9-tetrahydrocannabinolic acid B (THCA-B)]. Decarboxylation of the precursor THC acids, THCA-A and THCA-B, to THC in cannabis occurs during storage, upon heating (e.g., smoking) or under alkaline conditions.
Smoking is the most commonly used route of cannabis self-administration. The dynamics of smoking are complex, and several factors influence bio-delivery of THC including potency of the plant material, amount of THC in inhaled smoke, amount of THC destroyed by pyrolysis, amount of THC released in sidestream smoke and the pattern of smoking (e.g., smoking duration, number of puffs, puff duration and depth of inhalation). Davis et al. (2) reported that the burning temperature of cannabis when smoked by human subjects was ∼800°C and, that upon initial lighting of a cannabis cigarette, there is heavy pyrolytic destruction of THC in material near the burning tip. THC that is not destroyed by pyrolysis migrates away from the heat to concentrate in the cooler part of the cigarette. Drawing upon the cigarette delivers THC in the mainstream smoke to the user. As smoking progresses, mainstream smoke delivers greater amounts of THC per unit weight of cannabis burned as the cigarette is consumed.
The amount of THC delivered to the smoker in mainstream smoke from standardized cannabis cigarettes is estimated to be in the range of 20–37% of the cigarette content and 23–30% is destroyed by pyrolysis (3). The remainder, 33–57% of the original THC, is presumed to be discharged to the environment as smoke and aerosol particles due to sidestream smoke, and additional air contamination occurs from mainstream smoke after it is exhaled. “Secondhand” smoke is a combination of these sources.
Exposure to secondhand smoke from combusted cannabis poses similar health risks as exposure to secondhand tobacco smoke because of the mutagenic and carcinogenic properties of constituents in smoke formed during organic combustion (4, 5). In addition, there is concern that exposure to secondhand cannabis smoke might produce a positive drug test in oral fluid, similar to concerns expressed for urine (6), in nonsmokers. Cannabinoid-related analytes that have been identified in oral fluid after cannabis use include THC, 9-carboxy-11-nor-Δ9-tetrahydrocannabinol (THCCOOH) and its conjugate, cannabidiol (CBD) and cannabinol (CBN) (7–14). The 2004 US Department of Health and Human Services (DHHS) proposed revisions to mandatory guidelines for federal workplace drug testing programs (Mandatory Guidelines) with oral fluid called for initial testing for THC parent drug and a metabolite at a 4-ng/mL cutoff concentration (oral fluid) and a confirmatory test at 2 ng/mL for THC (15). Although final guidelines for oral fluid testing have not been published, there is considerable private sector testing of oral fluid at similar cutoff concentrations as those proposed in the Mandatory Guidelines.
The concern that positive oral fluid tests might result from exposure to secondhand cannabis smoke has been addressed in only a limited number of studies. Niedbala et al. (16–18) performed a series of studies to evaluate the risk of positive oral fluid tests from passive exposure to cannabis smoke. In initial studies, nonsmokers seated next to cannabis smokers in small enclosed spaces produced specimens that screened and confirmed positive for THC following exposure to dense secondhand cannabis smoke (17). However, the authors recognized that specimen contamination had occurred when oral fluid collection was performed inside the exposure chamber (18). This discovery called into question results from earlier studies in which specimen contamination could have occurred when oral fluid was not collected in a clean environment (17). When the passive exposure study was repeated with collections conducted in a clean environment, participants uniformly tested negative at the screening/confirmation cutoff concentrations (3.0/1.5 ng/mL) for 8 h after passive exposure.
In another study, Moore et al. (19) measured THC in oral fluid in a clean environment during 3 h exposure to secondhand cannabis smoke in a Dutch coffee house and for up to 22 h after exposure. Peak concentrations of THC during exposure for each of the 10 subjects were as follows: 4.3 (2 h), 6.8 (2 h), 5.8 (2 h), 3.8 (2 h), 1.5 (3 h), 5.1 (3 h), 2.3 (3 h), 17 (3 h), 12 (3 h) and 1.3 (3 h) ng/mL. Testing oral fluid at 12–22 h following exposure indicated that residual THC concentrations were detectable for two subjects (1.0 and 1.2 ng/mL), whereas the remaining subjects were negative.
Considering the methodological problems with specimen contamination and the substantial increase in the potency of cannabis over the last decade (20), a comprehensive evaluation of exposure to secondhand cannabis smoke was needed. The current study was designed to evaluate cannabinoid concentrations and subjective effects associated with extreme secondhand cannabis smoke exposure. Specifically, the current study was designed to assess the effects of cannabis potency and room ventilation on resulting cannabinoid concentrations in oral fluid, urine and whole blood, corresponding subjective ratings of intoxication, and performance on a brief battery of cognitive performance measures. Urine test results and the effect of room ventilation on pharmacodynamic outcomes have been reported in detail previously (6, 21). This report provides detailed screening and confirmatory data for oral fluid specimens (collected in a clean environment) and whole blood specimens collected at the same nominal times from subjects exposed to smoked or secondhand cannabis smoke. Data from these paired set of specimens allowed examination of the possible relationship of THC oral fluid concentrations with blood concentrations. Participant ratings of self-reported drug effects are also provided to highlight differences in the functional consequences of the observed biological cannabinoid levels across study conditions.
Experimental methods
Participants and study design
Three secondhand cannabis smoke exposure sessions were conducted at the Johns Hopkins University Behavioral Pharmacology Research Unit, Baltimore, MD. The study setting was a controlled environmental laboratory containing a specially constructed exposure chamber. A complete description of the chamber, study conditions, participant demographics and procedures has been published (6). Briefly, six drug-free nonsmokers and six experienced cannabis smokers participated in each session. Smokers were recruited who self-reported the use of cannabis at least two times per week during the prior 90 days. Nonsmokers were recruited who had a history of lifetime cannabis exposure, but had not used cannabis or other illicit substances within the previous 6 months. The first session involved exposure to smoked cannabis containing 5.3% THC in an unventilated environment, the second session involved exposure to smoked cannabis containing 11.3% THC in an unventilated environment and the third session involved exposure to smoked cannabis containing 11.3% THC in a ventilated environment. Each session was conducted in a specially constructed Plexiglas smoke exposure chamber [10 ft. × 13 ft. (3.05 m × 3.96 m) with a 7 ft. (2.13 m) ceiling]. The chamber had an adjustable ventilation/exhaust system. Smokers and nonsmokers sat around a table in alternating seats. The three sessions were conducted at weekly or greater intervals. Nonsmokers were unique across sessions, but smokers participated in multiple sessions. The duration of each exposure session was 1 h, during which smokers consumed cannabis ad libitum in the presence of nonsmokers inside the closed chamber.
Cannabis for research purposes was obtained through the US Federal Drug Supply Program. Moderate potency cannabis cigarettes were machine rolled, were 85 mm in length × 25 mm circumference and weighed a mean weight (SD) of 0.92 (0.06) g/cigarette; the cigarettes had an assayed mean content of 5.3% (0.48%) total THC. High-potency cigarettes were hand-rolled, were 70 mm in length (24.5 mm) and had a mean weight (SD) of 1.0 (0.04) g/cigarette; the cigarettes had an assayed mean content of 11.3% (0.29%) total THC.
Written informed consent was obtained prior to study participation. The study was approved by the Johns Hopkins Medicine Institutional Review Board and conducted in accordance with the ethical standards of the Helsinki Declaration. All subjects were compensated for their participation.
To reduce contamination issues associated with specimen collection, all participants donned disposable paper clothing including booties over their own clothing before entering the experimental chamber for each session. Following cannabis exposure, participants exited the chamber and immediately discarded their disposable clothing, and washed their hands and face with soap and water. After drying, they proceeded to a cannabis-free room (investigative area) for participation in specimen collections and subjective and physiological assessments.
Participants were supplied with goggles for use as needed for reduction of eye irritation from the smoke. During each session, participants remained in their assigned seats and played games, conversed or engaged in other activities (e.g., listened to music and used cell phone). Smokers were allowed to drink from bottles of water (supplied at the start of the session). Nonsmokers were not allowed to eat or drink during the session or after the session until after the first oral fluid specimen was collected. As a safety measure, pulse oximeter readings were collected pre-session and at 15-min intervals during each session to ensure that an adequate oxygen supply was maintained within the chamber.
Specimen collection
Oral fluid was collected in a clean environment by expectoration for a period of up to 5 min into 8 mL glass screw cap culture tubes (Thermo Fisher Scientific, Waltham, MA, USA, 16 × 100 mm, #14-959-35AA). Prior to collection, the inner surface of the collection tubes was silanized with Sylon-CT™ (Sigma-Aldrich, St Louis, MO, USA, #33065-U) and rinsed with methanol and dried. Caps for the tubes contained a PTFE liner (Thermo Fisher Scientific, #4506615). Specimens were collected from nonsmokers immediately prior to each session and following the 1-h exposure session nominally at 0.25, 0.5, 1, 1.5, 2, 3, 4, 5, 6, 8, 10, 12, 22, 26, 30 and 34 h after the end of the exposure session (designated time 0). Smokers' oral fluid specimens were collected at the same nominal times through 8 h; smokers were discharged from the study after 8 h. No food or drink was allowed for a period of 10 min prior to or during each scheduled oral fluid collection. Each specimen was sealed with a plastic screw cap, wrapped with parafilm and stored refrigerated until shipped overnight in refrigerated containers to the laboratory within 3 days after study completion.
Whole blood collections were made via an indwelling intravenous catheter inserted prior to each session. Ten milliliters of blood were collected into vacutainer tubes (gray top). Specimens were collected from nonsmokers immediately prior to each session and following the 1-h exposure session nominally at 0.25, 0.5, 1, 1.5, 2, 3, 4, 5, 6, 8, 12, 22, 26, 30 and 34 h after the end of each exposure session. Smokers' blood specimens were collected at the same time through 8 h. Each specimen was divided in half and transferred to two plastic cryotubes, and stored frozen at −60°C until shipped frozen to the laboratory for analysis.
Because of the logistics involved in collecting multiple types of measures from 12 participants in each session, the exact timing of early specimen collections was somewhat variable; consequently, all specimen times should be considered as nominal values (i.e., ±10 min).
Analytical methods
Oral fluid and whole blood specimens were analyzed by Immunalysis Corporation (Pomona, CA, USA). Oral fluid was tested according to the manufacturer's procedure with the Immunalysis Saliva/Oral Fluids Cannabinoids ELISA kit at a cutoff concentration of 4 ng/mL. Oral fluid and blood specimen analyses were initiated upon receipt at the laboratory. Cross-reactivities for this assay as listed in the manufacturer's brochure were as follows: THC (100%), Δ8-THC (66.7%), CBN (4%) and CBD (50%).
Oral fluid and whole blood analyses were conducted by LC–MS-MS at Immunalysis Corporation according to published methods (22, 23). Oral fluid was hydrolyzed per the published method with sodium hydroxide solution (1 N; 0.2 mL) prior to extraction. Consequently, THCCOOH concentrations in oral fluid are reported as ‘total’ THCCOOH. The limit of quantitation (LOQ) and upper limit of linearity (ULOL), respectively, for analyses of oral fluid were—THC: 1, 100 ng/mL and THCCOOH: 0.02, 0.1 ng/mL. Blood specimens were not hydrolyzed prior to analyses. Consequently, blood concentrations of THCCOOH are reported as ‘free’ THCCOOH concentrations. The LOQ and ULOL, respectively, for analyses of blood were—THC: 0.5, 100 ng/mL; THCCOOH: 0.5, 100 ng/mL and 11-OH-THC: 0.5, 100 ng/mL. Control samples were prepared from Cerillant (Round Rock, TX, USA) solutions. Oral fluid control samples were prepared at target concentrations of 8 and 500 ng/mL for THC and 0.1 and 5 ng/mL for THCCOOH. Whole blood control samples were prepared at target concentrations of 1 ng/mL for THC and 11-OH-THC, and 1, 5, 10 and 20 ng/mL for THCCOOH. Control samples were analyzed with each batch of oral fluid and whole blood specimens. The ranges of percent deviation from the target concentration of control samples prepared for oral fluid analyses were—THC: 8 ng/mL (n = 5), −15.0 to −6.3%; THC: 500 ng/mL (n = 7), −7.0 to 1.4%; THCCOOH: 0.1 ng/mL (n = 5), 8.0–14.0% and THCCOOH: 5 ng/mL (n = 6), −8.4 to 9.7%. The inter-run precisions for oral fluid control samples were—THC: 8 ng/mL (n = 5), 4.2%; THC: 500 ng/mL (n = 7), 3.0%; THCCOOH: 0.1 ng/mL (n = 5), 2.7% and THCCOOH: 5 ng/mL (n = 6), 6.5%. The ranges of percent deviation from the target concentration of control samples prepared for whole blood analyses were—THC: 1 ng/mL (n = 12), 0.98–1.00%; 11-OH-THC: 1 ng/mL (n = 12), 1.0–1.2%; THCCOOH: 1 ng/mL (n = 12), −20.0 to 10.0%; THCCOOH: 5 ng/mL (n = 12), 2.0–20.0%; THCCOOH: 10 ng/mL, 10.0–20% and THCCOOH: 20 ng/mL (n = 12), 0–15.0%. The inter-run precisions for oral fluid control samples were—THC: 1 ng/mL (n = 12), 2.6%; 11-OH-THC: 1 ng/mL (n = 12), 8.6%; THCCOOH: 1 ng/mL (n = 12), 9.4%; THCCOOH: 5 ng/mL (n = 11), 4.8% and THCCOOH: 10 ng/mL (n = 12), 3.8%.
Self-report of drug effects
Nonsmokers and smokers completed a 15-item Drug Effect Questionnaire (DEQ) to assess subjective ratings of pharmacodynamic drug effects. Individual items on the DEQ included three ratings of drug effect (‘do you feel a drug effect?’, ‘do you feel a pleasant drug effect?’, ‘do you feel an unpleasant drug effect?’). These ratings are hereafter referred to as ‘drug effect’, ‘pleasant drug effect’ and ‘unpleasant drug effect’. Participants rated each item using a 100-mm visual analog scale (VAS) anchored with ‘not at all’ on one end and ‘extremely’ on the other. The DEQ was administered at baseline and at 0, 0.5, 1, 1.5, 2, 3, 4, 5, 6 and 8 h post-exposure.
Sensitivity, specificity and agreement
The sensitivity, specificity and agreement of the immunoassays (IAs) for detection of cannabinoids in oral fluid were calculated by comparison of the qualitative IA response with the quantitative LC–MS-MS result for THC. The initial test for THC parent drug and metabolite (≥4 ng/mL) and confirmatory test cutoff concentration (≥2 ng/mL) for THC, as proposed in the 2004 Mandatory Guidelines (15), were used to determine if a specimen was positive. True-positive (TP; IA response ≥ cutoff concentration and LC–MS-MS positive), true-negative (TN; IA response < cutoff concentration and LC–MS-MS negative), false-positive (FP; IA response ≥ cutoff concentration and LC–MS-MS negative) and false-negative (FN; IA response < cutoff concentration and LC–MS-MS positive) were calculated versus LC–MS-MS at the 2-ng/mL cutoff concentration. Sensitivity, specificity and agreement were calculated, respectively, as follows: 100 × [TP/(TP + FN)], 100 × [TN/(TN + FP)] and 100 × [(TP + TN)/(TP + TN + FP + FN)].
Correlations of THC and THCCOOH within and between matrices
Paired oral fluid and whole blood THC and THCCOOH concentrations determined by LC–MS-MS were compared to evaluate the degree of linear dependence between the two matrices using linear regression and determination of Pearson's product–moment correlation coefficients (r). The significance of the relationship (two-tailed) was calculated utilizing r and the degrees of freedom (d.f., defined as n − 2) to indicate nondirectional probability. For each individual nonsmoker, the number of paired specimens with measurable drug concentrations (>LOQ) was insufficient for correlational analysis. However, a composite correlation of all nonsmokers' individually paired specimens could be performed (same analyte). For each smoker, the number of paired specimens was sufficient for correlation on an individual basis. Corresponding analyses were performed for THC and THCCOOH concentrations (same analyte) for each individual smoker per session, and a composite of all smokers' individually paired specimens was also conducted. Similar correlations were conducted comparing THC with THCCOOH (same matrix) for individual smokers.
Estimation of ‘body-load’ (dose) of THC inhaled by nonsmokers compared with smokers
Area under the curve (AUC) calculations of THC concentrations in oral fluid and blood were calculated by the linear trapezoidal rule. Individual AUC0−t calculations were made from time 0 to the time of the last detectable concentration (nonsmokers) or to the last collected specimen (8 h for smokers). For smokers who had baseline concentrations of THC present prior to smoking, concentrations of THC were adjusted by subtraction of estimated residual THC concentrations from the total THC measured at each time point. The equation employed for estimating residual THC concentrations was as follows: log Ct = log C0 − kt/2.303, where Ct = observed concentration, log C0 = residual concentration at baseline, k = first-order elimination rate constant for THC and t = time. The first-order rate constant, k, was estimated for all smokers based on an estimated half-life (T1/2) of 1 h.
The amount of THCCOOH excreted in urine by individual nonsmokers was calculated by multiplication of the concentration of THCCOOH (GC–MS analyses) in each individual urine specimen by the total volume of the specimen. The cumulative total amount of THCCOOH excreted by each nonsmoker was compared with 0.1974 mg of THCCOOH; the amount of THCCOOH reported to be excreted in urine over a 7-day period by smokers who smoked a single 3.55% THC cigarette (24). AUC measures for a ‘pleasant drug effect’ for each individual were calculated in a similar manner as AUCs for oral fluid and blood.
Results
Session conditions and cannabis use
Generally, smokers began smoking immediately at the start of the session, took occasional short breaks and then resumed smoking until the end of the session. The average amounts of cannabis consumed per smoker in Sessions 1, 2 and 3 were 1.7, 2.4 and 2.8 g, respectively. This corresponded to means of 90.1, 271.2 and 316.4 mg of THC per smoker and to a mean total THC per session of 545.9, 1627.2 and 1864.5 mg for all smokers combined. For comparison, the smokers reported prior daily consumption of an average of 1.5 g of cannabis.
During Sessions 1 and 2 (nonventilated conditions), there was rapid accumulation of smoke inside the chamber that persisted throughout the 1-h period, whereas in Session 3 (ventilated condition), visible smoke was present, but at lower levels. The use of goggles helped alleviate eye irritation that occurred in the unventilated sessions.
Oral fluid analyses of nonsmoker specimens
Oral fluid specimens were initially tested for THC by IA (ELISA, 4 ng/mL cutoff concentrations) and by LC–MS-MS for THC (LOQ = 0.5 ng/mL) and THCCOOH (LOQ = 0.02 ng/mL). A tabulation of initial test results and THC concentrations in oral fluid is summarized in Table I for the 18 different nonsmokers who participated in Sessions 1, 2 and 3. THCCOOH was not detected in any nonsmokers' specimens. The tabular values for THC in Table I are listed over time to the last measurable concentration for each session. Thereafter, all specimens tested uniformly negative by IA and mass spectrometry. Prior to each session, all nonsmokers' baseline oral fluid specimens tested negative by IA and LC–MS-MS. Mean (range) detection times to the last positive result by IA were—Session 1: 1.25 (0.25–3) h, Session 2: 1.38 (0.25–3) h and Session 3: 0.38 (0–1.5) h. Maximum THC concentrations (Cmax) by LC–MS-MS in oral fluid occurred in the first collected specimen (Tmax = 0.25 h). Cmax concentrations (range) were—Session 1: 34.0 (4.9–86) ng/mL, Session 2: 81.5 (12–308) ng/mL and Session 3: 16.9 (1.7–75) ng/mL. Thereafter, THC concentrations dropped rapidly over the next 1–3 h. Mean (range) detection times (time to the last measurable amount of THC) by LC–MS-MS (LOQ = 1 ng/mL) were—Session 1: 5.4 (1.5–12) h, Session 2: 10.8 (3–26) h and Session 3: 1.4 (0.25–3) h.
Table I.
Screening and Confirmation of Nonsmokers' Oral Fluid Specimens Following Exposure to Concentrated Secondhand Cannabis Smoke
| Time (h)a | THC ELISA (cutoff = 4 ng/mL) | THC, LC–MS-MS (ng/mL) | THC ELISA (cutoff = 4 ng/mL) | THC, LC–MS-MS (ng/mL) | THC ELISA (cutoff = 4 ng/mL) | THC, LC–MS-MS (ng/mL) | THC ELISA (cutoff = 4 ng/mL) | THC, LC–MS-MS (ng/mL) | THC ELISA (cutoff = 4 ng/mL) | THC, LC–MS-MS (ng/mL) | THC ELISA (cutoff = 4 ng/mL) | THC, LC–MS-MS (ng/mL) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Session 1 | ||||||||||||
| Subject | S7 | S11 | S13 | S14 | S15 | S16 | ||||||
| −1 | NEG | 0 | NEG | 0 | NEG | 0 | NEG | 0 | NEG | 0 | NEG | 0 |
| 0.25 | POS | 20 | POS | 10 | POS | 86 | POS | 67 | POS | 4.9 | POS | 16 |
| 0.5 | POS | 11 | NEG | 2.6 | POS | 21 | POS | 22 | NEG | 2.1 | NEG | 3.7 |
| 1 | POS | 8.5 | POS | 3.2 | POS | 13 | POS | 11 | NEG | 1.5 | NEG | 2 |
| 1.5 | POS | 10 | NEG | 1.9 | POS | 4.2 | POS | 6.3 | NEG | 1.6 | NEG | 1.6 |
| 2 | NEG | 4.2 | NEG | 0 | POS | 7 | NEG | 3.5 | MS | MS | NEG | 1.1 |
| 3 | NEG | 1.7 | NEG | 0 | POS | 1.7 | NEG | 1.3 | NEG | 1 | NEG | 1 |
| 4 | NEG | 1.8 | NEG | 0 | MS | AF | NEG | 1.2 | NEG | 1.1 | NEG | 1.3 |
| 5 | NEG | 1.9 | NEG | 0 | NEG | 0 | NEG | 0 | MS | MS | NEG | 0 |
| 6 | NEG | 0 | NEG | 0 | NEG | 1.1 | NEG | 0 | NEG | 2.7 | NEG | AF |
| 8 | NEG | AF | NEG | 0 | NEG | 0 | NEG | 0 | NEG | 1.6 | NEG | 0 |
| 10 | NEG | 0 | NEG | 0 | NEG | 0 | NEG | 0 | NEG | 1.0 | NEG | 0 |
| 12 | NEG | 0 | NEG | 0 | NEG | 0 | NEG | 0 | NEG | 1.0 | NEG | 0 |
| Session 2 | ||||||||||||
| Subject | S8 | S23 | S37 | S38 | S40 | S41 | ||||||
| −1 | NEG | 0 | NEG | 0 | NEG | 0 | NEG | 0 | NEG | 0 | NEG | 0 |
| 0.25 | POS | 32 | POS | 53 | POS | 60 | POS | 308 | POS | 24 | POS | 12 |
| 0.5 | POS | 10 | POS | 12 | POS | 13 | POS | 11 | POS | 4.1 | NEG | 3.3 |
| 1 | NEG | 2.9 | NEG | 5.0 | NEG | 2.1 | NEG | 3.8 | NEG | 2.8 | NEG | 0 |
| 1.5 | NEG | 2.0 | POS | 6.8 | POS | 4.2 | POS | 10 | NEG | 1.4 | NEG | 2.7 |
| 2 | NEG | 0 | POS | 4.1 | POS | 2.9 | POS | 6.4 | NEG | 0 | NEG | 1.0 |
| 3 | NEG | 1.0 | POS | 2.9 | NEG | 1.1 | NEG | 0 | NEG | 1.0 | NEG | 0 |
| 4 | NEG | 1.7 | NEG | 2.6 | NEG | 1.3 | NEG | 1.7 | NEG | 0 | NEG | 0 |
| 5 | NEG | 1.0 | NEG | 1.5 | NEG | 1.2 | NEG | 1.8 | NEG | 0 | NEG | 0 |
| 6 | NEG | 0 | NEG | 2.3 | NEG | 1.0 | NEG | 1.5 | NEG | 0 | NEG | 0 |
| 8 | NEG | 1.8 | NEG | 2.4 | NEG | 0 | NEG | 0 | NEG | 0 | NEG | 0 |
| 10 | NEG | 0 | NEG | 1.1 | NEG | 0 | NEG | 1.9 | NEG | 0 | NEG | 1.2 |
| 12 | NEG | 0 | NEG | 0 | NEG | 0 | NEG | 2.8 | NEG | 0 | NEG | 0 |
| 22 | NEG | 0 | NEG | 1.0 | NEG | 0 | NEG | 0 | NEG | 0 | NEG | 0 |
| 26 | NEG | 0 | NEG | 1.0 | NEG | 0 | NEG | 0 | NEG | 0 | NEG | 0 |
| Session 3 | ||||||||||||
| Subject | S25 | S26 | S27 | S28 | S29 | S36 | ||||||
| −1 | NEG | 0 | NEG | 0 | NEG | 0 | NEG | 0 | NEG | 0 | NEG | 0 |
| 0.25 | NEG | 1.7 | POS | 15 | POS | 4.8 | NEG | 2.0 | NEG | 2.9 | POS | 75 |
| 0.5 | NEG | 0 | POS | 6.1 | NEG | 1.5 | NEG | 0 | NEG | 1.1 | POS | 13 |
| 1 | NEG | 0 | NEG | 5.0 | NEG | 1.0 | NEG | 0 | NEG | 0 | NEG | 0 |
| 1.5 | NEG | 0 | POS | 2.9 | NEG | 1.3 | NEG | 0 | NEG | 0 | NEG | 0 |
| 2 | NEG | 0 | NEG | 2.5 | NEG | 0 | NEG | 0 | NEG | 0 | NEG | 1.6 |
| 3 | NEG | 0 | NEG | 1.2 | NEG | 0 | NEG | 0 | NEG | 0 | NEG | 1.9 |
MS, missing specimen; AF, analysis failed; NEG, negative; POS, positive.
aData are tabulated over time to the last specimen collection that any subject had measurable drug content at the LC–MS-MS assay's LOQ. Specimens thereafter were uniformly negative.
Application of the Proposed Guidelines (2004) oral fluid cutoff concentrations for the initial IA test (4 ng/mL) and confirmatory test (2 ng/mL) to the nonsmokers' specimens (n = 302, four missed specimens) from the three sessions discussed in Table I indicated a total of 41 specimens (17, 18 and 6 in Sessions 1, 2 and 3, respectively) that met criteria for a confirmed positive result (TP = positive initial test, ≥4 ng/mL/positive confirmatory test, ≥2 ng/mL). Mean (range) detection times to the last TP were—Session 1: 1.3 (0.25–2) h, Session 2: 1.4 (0.25–3) h and Session 3: 0.4 (0–1.5) h. There was 1 FP [Session 1, Subject 13 (S13), collected at 3 h], 23 FNs (7, 12 and 4 in Sessions 1, 2 and 3, respectively) and 237 TNs. The overall sensitivity, specificity and agreement of IA relative to mass spectrometry tests were 64.1, 99.6 and 92.1%, respectively.
Oral fluid analyses of smoker specimens
Four of the smokers participated in all three cannabis smoking sessions, two smokers in two sessions and two additional smokers participated in a single session. Oral fluid specimens were collected over 8 h following each session prior to discharge of the smokers. Baseline oral fluid specimens for smokers tested positive for THC by ELISA with the exception of two participants (S20, Session 1 and S18, Session 2). Following smoking, the majority of specimens from the three sessions (n = 186, 94.9%) tested positive by ELISA. There were eight specimens (12.5%) in Session 1, and one (1.5%) in Session 2 that tested negative after smoking. The times for the negative specimens varied from 2 to 8 h.
Data from the analyses of smokers' oral fluid specimens by LC–MS-MS for THC (LOQ = 1 ng/mL) and THCCOOH (LOQ = 0.02 ng/mL) are presented in Table II. Baseline specimens were generally positive for THC and THCCOOH. The mean (range) concentrations of THC prior to smoking were—Session 1: 12.0 (5.1–23) ng/mL, Session 2: 77.5 (0–316) ng/mL and Session 3: 157.4 (5–506) ng/mL. The mean (range) concentrations of THCCOOH prior to smoking were—Session 1: 0.342 (0.045–1.105) ng/mL, Session 2: 0.878 (0.076–3.460) ng/mL and Session 3: 0.450 (0.079–1.234) ng/mL. Following smoking, Cmax concentrations for THC occurred uniformly across the three sessions and across all subjects in the first collected specimen (0.25 h). The mean (range) Cmax concentrations of THC after smoking were—Session 1: 969.5 (102–3,512) ng/mL, Session 2: 721 (369–1,358) ng/mL and Session 3: 1,089 (168–3,207) ng/mL. Cmax concentrations for THCCOOH were less affected from smoking cannabis and occurred at variable times (range: 0.25–4 h). The mean (range) concentrations of THCCOOH after smoking were—Session 1: 1.248 (0.065–3.349) ng/mL, Session 2: 0.955 (0.105–3.173) ng/mL and Session 3: 0.884 (0.081–3.042) ng/mL.
Table II.
LC–MS-MS Analyses of Smokers' Oral Fluid Specimens Before and After Smoking Cannabis
| Time (h) | THC (ng/mL) | THCCOOH (ng/mL) | THC (ng/mL) | THCCOOH (ng/mL) | THC (ng/mL) | THCCOOH (ng/mL) | THC (ng/mL) | THCCOOH (ng/mL) | THC (ng/mL) | THCCOOH (ng/mL) | THC (ng/mL) | THCCOOH (ng/mL) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Session 1 | ||||||||||||
| Subject | S3 | S5 | S9 | S17 | S18 | S20 | ||||||
| −1 | 23 | 0.6 | 19 | 1.105 | 8.9 | 0.113 | 10 | 0.141 | 5.9 | 0.046 | 5.1 | 0.045 |
| 0.25 | 3512 | 1.381 | 434 | 2.303 | 904 | 0.457 | 220 | 0.065 | 102 | 0.056 | 645 | 0.171 |
| 0.5 | MS | MS | 275 | 3.349 | 243 | 0.458 | 128 | 0.062 | 47 | 0.069 | 226 | 0.13 |
| 1 | 961 | 3.212 | 164 | 3.754 | 44 | 0.213 | 51 | 0.061 | 40 | 0.09 | 77 | 0.2 |
| 1.5 | 655 | 3.323 | 112 | 2.493 | 48 | 0.162 | 34 | 0.065 | 11 | 0.066 | 68 | 0.205 |
| 2 | 135 | 0.601 | 43 | 2.28 | 32 | 0.271 | 16 | 0.061 | 3.2 | 0.043 | 29 | 0.109 |
| 3 | 25 | 0.108 | 6.9 | 1.02 | 7 | 0.121 | 4.3 | 0.054 | 2.4 | 0.032 | 9.5 | 0.084 |
| 4 | 7.8 | 0.141 | 8.7 | 1.556 | 20 | 0.175 | 4.6 | 0.051 | 4.1 | 0.051 | 17 | 0.095 |
| 5 | 35 | 0.452 | 13 | 1.186 | 8.5 | 0.074 | 4.4 | 0.051 | AF | 0.02 | 6.4 | 0.04 |
| 6 | 18 | 0.294 | 13 | 1.121 | 8.3 | 0.123 | 2.9 | 0.048 | 0 | 0.032 | 5 | 0.039 |
| 8 | 17 | 0.137 | 7.6 | 0.676 | 5.4 | 0.16 | 14 | 0.085 | 0 | 0 | 6.4 | 0.037 |
| Session 2 | ||||||||||||
| Subject | S1 | S5 | S9 | S17 | S18 | S20 | ||||||
| −1 | 118 | 1.37 | 316 | 3.46 | 8.3 | 0.115 | 4.5 | 0.16 | 0 | 0.085 | 18 | 0.076 |
| 0.25 | 588 | 3.173 | 466 | 1.598 | 1099 | 0.373 | 1358 | 0.353 | 446 | 0.125 | 369 | 0.093 |
| 0.5 | 287 | 2.88 | 50 | 0.515 | 140 | 0.164 | 225 | 0.212 | 132 | 0.124 | 166 | 0.089 |
| 1 | 24 | 0.595 | 21 | 0.442 | 143 | 0.119 | 44 | 0.115 | 10 | 0.107 | 85 | 0.083 |
| 1.5 | 58 | 1.028 | 18 | 0.602 | 113 | 0.095 | 65 | 0.173 | 11 | 0.067 | 55 | 0.081 |
| 2 | 34 | 0.643 | 16 | 0.635 | 22 | 0.046 | 33 | 0.111 | 10 | 0.078 | 42 | 0.089 |
| 3 | 13 | 0.47 | 33 | 0.893 | 23 | 0.09 | 7.1 | 0.09 | 4.4 | 0.075 | 12 | 0.072 |
| 4 | 30 | 0.997 | 24 | 0.746 | 23 | 0.125 | 19 | 0.195 | 6.9 | 0.154 | 37 | 0.105 |
| 5 | 25 | 0.966 | 10 | 0.918 | 18 | 0.145 | 18 | 0.259 | 2.7 | 0.116 | 18 | 0.085 |
| 6 | 32 | 0.887 | 24 | 1.871 | 8.2 | 0.117 | 25 | 0.206 | 6.1 | 0.104 | 6.5 | 0.067 |
| 8 | 11 | 0.406 | 10 | 1.355 | 6.7 | 0.077 | 5.1 | 0.171 | 3 | 0.113 | 11 | 0.057 |
| Session 3 | ||||||||||||
| Subject | S1 | S5 | S9 | S17 | S18 | S10 | ||||||
| −1 | 69 | 1.234 | 506 | 1.067 | 9.2 | 0.079 | 338 | 0.137 | 17 | 0.098 | 5 | 0.086 |
| 0.25 | 168 | 3.042 | 752 | 1.109 | 1575 | 0.331 | 3207 | 0.135 | 585 | 0.199 | 248 | 0.081 |
| 0.5 | 43 | 1.154 | 544 | 1.086 | 461 | 0.197 | 333 | 0.059 | 293 | 0.194 | 57 | 0.066 |
| 1 | 70 | 0.836 | 51 | 0.372 | 139 | 0.09 | 169 | 0.075 | 65 | 0.095 | 62 | 0.064 |
| 1.5 | 42 | 0.907 | 133 | 0.776 | 185 | 0.128 | 106 | 0.074 | 36 | 0.077 | 33 | 0.066 |
| 2 | 37 | 0.502 | 93 | 0.65 | 70 | 0.092 | 128 | 0.091 | 13 | 0.105 | 19 | 0.056 |
| 3 | 7.6 | 0.358 | 34 | 0.658 | 22 | 0.11 | 24 | 0.041 | 14 | 0.096 | 12 | 0.043 |
| 4 | 19 | 0.618 | 62 | 1.515 | 51 | 0.136 | 101 | 0.135 | 19 | 0.142 | 7.7 | 0.062 |
| 5 | 23 | 0.76 | 47 | 1.424 | 31 | 0.16 | 86 | 0.126 | 21 | 0.087 | 7.9 | 0.071 |
| 6 | 30 | 1.41 | 32 | 1.351 | 23 | 0.108 | 64 | 0.123 | 18 | 0.153 | 6.7 | 0.068 |
| 8 | 3.5 | 0.383 | 11 | 1.135 | 29 | 0.228 | 22 | 0.063 | 17 | 0.106 | 4.2 | 0.062 |
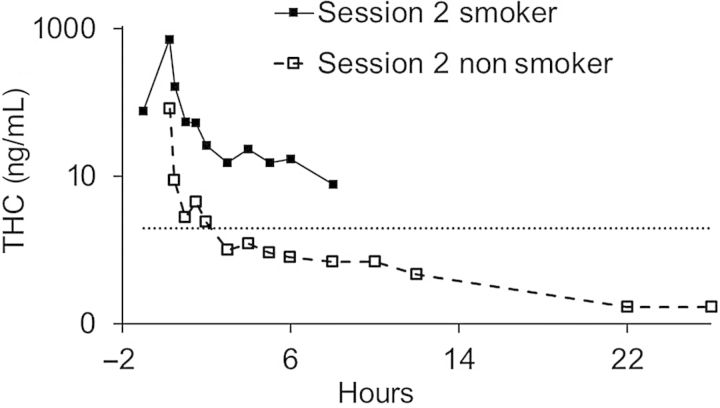
Figure 1 illustrates the concentrations of THC in oral fluid for smokers (baseline included) in comparison with those for nonsmokers (zero baseline; not shown) for Session 2. The data are plotted on a logarithmic scale because of the large difference in concentrations between the two groups.
Figure 1.
Mean THC concentrations in oral fluid of nonsmokers and smokers in Session 2.
Whole blood analyses of nonsmoker specimens
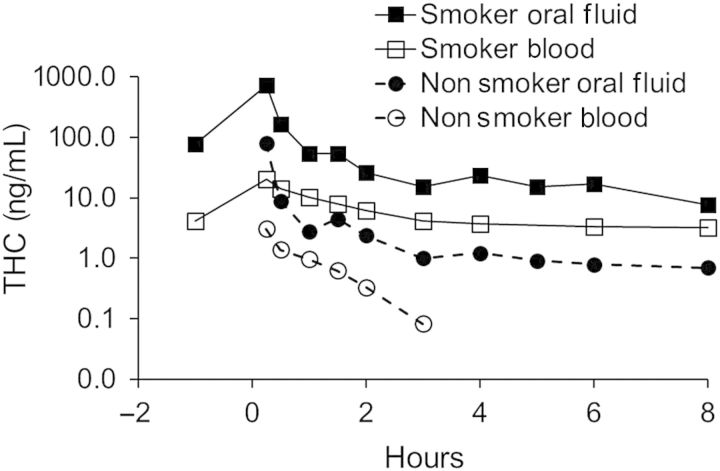
Whole blood specimens were collected from the 18 nonsmokers and analyzed for THC, 11-OH-THC and THCCOOH by LC–MS-MS (LOQ = 0.5 ng/mL). The results are listed in Table III. Figure 2 shows THC concentrations in blood and oral fluid for nonsmokers compared with smokers for Session 2. Quantitative values are listed over time to the last measurable THC or THCCOOH concentration for each session. Thereafter, all specimens tested uniformly negative. Prior to each session, all nonsmokers' baseline specimens tested negative for all analytes by LC–MS-MS. Maximum THC and THCCOOH concentrations (Cmax) by LC–MS-MS in blood generally occurred in the first collected specimen (Tmax = 0.25 h). Mean (range) Cmax concentrations of THC and THCCOOH were—Session 1: THC, 1.4 (0.6–1.8) ng/mL and THCCOOH, 1.2 (0.8–1.7) ng/mL; Session 2: THC, 3.1 (1.2–5.6) ng/mL and THCOOH, 2.5 (0–5.1) ng/mL and Session 3: THC, 0.5 (0–0.9) ng/mL and THCCOOH, 0.2 (0–0.7) ng/mL. 11-OH-THC was only detected in two specimens from a single subject in one session (Session 2, S38, 0.6 ng/mL, collected at 0.25 h, and 0.7 ng/mL, collected at 0.5 h).
Table III.
LC–MS-MS Analyses of Nonsmokers' Whole Blood Specimens Following Exposure to Concentrated Secondhand Cannabis Smoke
| Time (h)a | THC (ng/mL) | THCCOOH (ng/mL) | THC (ng/mL) | THCCOOH (ng/mL) | THC (ng/mL) | THCCOOH (ng/mL) | THC (ng/mL) | THCCOOH (ng/mL) | THC (ng/mL) | THCCOOH (ng/mL) | THC (ng/mL) | THCCOOH (ng/mL) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Session 1 | ||||||||||||
| Subject | S7 | S11 | S13 | S14 | S15 | S16 | ||||||
| −1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 0.25 | 1.6 | 1.7 | 1.7 | 1.5 | 1.8 | 1.3 | 1.1 | 0.8 | 0.6 | 0.8 | 1.5 | 1.1 |
| 0.5 | 0.7 | 1.4 | 1.0 | 1.5 | 0.9 | 1.1 | 0.7 | 0.8 | 0 | 0.7 | 0.8 | 1.3 |
| 1 | 0.6 | 1.4 | 0.7 | 1.2 | 0.7 | 1.2 | 0.5 | 0.8 | 0 | 0.6 | 0.6 | 1.2 |
| 1.5 | 0.5 | 1.2 | 0 | 1.0 | 0.5 | 1.1 | 0 | 0.8 | 0 | 0.5 | 0 | 1.0 |
| 2 | 0 | 1.2 | 0 | 1.0 | 0 | 0.9 | 0 | 0.8 | 0 | 0 | 0 | 1.0 |
| 3 | 0 | 1.0 | 0 | 0.8 | 0 | 0.6 | 0 | 0.8 | 0 | 0 | 0 | 1.0 |
| 4 | 0 | 1.0 | 0 | 0.6 | 0 | 0.5 | 0 | 0.7 | 0 | 0 | 0 | 0.8 |
| 6 | 0 | 0.9 | 0 | 0.6 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 8 | 0 | 1 | 0 | 0.5 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 12 | 0 | 0.7 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Session 2 | ||||||||||||
| Subject | S8 | S23 | S37 | S38 | S40 | S41 | ||||||
| −1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 0.25 | 2.8 | 2.1 | 5.6 | 2.7 | 1.2 | 1.9 | 2.8 | 2.9 | 4.2 | 0 | 1.8 | 1.8 |
| 0.5 | 1.1 | 2.1 | 2.0 | 2.6 | 0.7 | 2.4 | 1.6 | 5.1 | 2.3 | 0 | 0.7 | 2.1 |
| 1 | 0.7 | 1.6 | 1.3 | 2.6 | 0.8 | 2.5 | 0.8 | 4.5 | 1.6 | 0 | 0.6 | 2.4 |
| 1.5 | 0.5 | 1.5 | 1.0 | 2.4 | 0 | 1.6 | 0.8 | 3.6 | 1.0 | 0 | 0.5 | 2.7 |
| 2 | 0 | 1.2 | 0.7 | 2.4 | 0 | 1.6 | 0.6 | 3.3 | 0.7 | 0 | 0 | 2.7 |
| 3 | 0 | 1.0 | 0.5 | 2.0 | 0 | 2.3 | 0 | 3.7 | 0 | 0 | 0 | 1.1 |
| 4 | 0 | 0.9 | 0 | 2.0 | 0 | 1.1 | 0 | 3.6 | 0 | 0 | 0 | 2.0 |
| 6 | 0 | 0.7 | 0 | 1.3 | 0 | 1.3 | 0 | 2.1 | 0 | 0 | 0 | 1.4 |
| 8 | 0 | 0 | 0 | 1.0 | 0 | 1.1 | 0 | 1.8 | 0 | 0 | 0 | 0.8 |
| 12 | 0 | 0 | 0 | 0.9 | 0 | 0.6 | 0 | 1.1 | 0 | 0 | 0 | 1.5 |
| 22 | 0 | 0 | 0 | 0.5 | 0 | 0 | 0 | 0.8 | 0 | 0 | 0 | 1.0 |
| Session 3 | ||||||||||||
| Subject | S25 | S26 | S27 | S28 | S29 | S36 | ||||||
| −1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 0.25 | 0 | 0 | 0.7 | 0 | 0.7 | 0.6 | 0.9 | 0 | 0 | 0 | 0.7 | 0.7 |
| 0.5 | 0 | 0 | 0 | 0 | 0 | 0.5 | 0 | 0 | 0 | 0 | 0 | 0 |
aData are tabulated over time to the last specimen collection that any subject had measurable drug content at the assay's LOQ. Specimens thereafter were uniformly negative.
Figure 2.
Mean concentrations of THC in oral fluid and blood specimens for nonsmokers and smokers following Session 2.
Whole blood analyses of smoker specimens
Whole blood specimens were collected from smokers over 8 h following each session before discharge. Data from the analyses of whole blood specimens by LC–MS-MS for THC, 11-OH-THC and THCCOOH are given in Table IV. Baseline specimens were generally positive for THC, 11-OH-THC and THCCOOH. The mean (range) concentrations of THC, 11-OH-THC and THCCOOH prior to smoking were—Session 1: THC, 1.6 (0.8–2.1) ng/mL, 11-OH-THC, 0.8 (0–1.2) ng/mL, THCCOOH, 37.2 (6.1–66.0) ng/mL; Session 2: THC, 4.1 (1.1–16.0) ng/mL, 11-OH-THC, 2.2 (0–9.2) ng/mL, THCCOOH, 78.9 (9.2–232.0) ng/mL and Session 3: THC, 5.0 (1.9–14.0) ng/mL, 11-OH-THC, 2.5 (1.3–8.1) ng/mL, THCCOOH, 89.8 (25.0–252.0) ng/mL.
Table IV.
LC–MS-MS Analyses of Smokers' Whole Blood Specimens Before and After Smoking Cannabis
| Time (h) | THC (ng/mL) | 11-OH-THC (ng/mL) | THCCOOH (ng/mL) | THC (ng/mL) | 11-OH-THC (ng/mL) | THCCOOH (ng/mL) | THC (ng/mL) | 11-OH-THC (ng/mL) | THCCOOH (ng/mL) |
|---|---|---|---|---|---|---|---|---|---|
| Session 1 | |||||||||
| Subject | S3 | S5 | S9 | ||||||
| −1 | 1.8 | 1.2 | 46 | 2.1 | 0.7 | 66 | 1.6 | 0.8 | 26 |
| 0.25 | 12 | 5.7 | 65 | 36 | 7.5 | 115 | 15 | 4.1 | 56 |
| 0.5 | 9.7 | 5.5 | 65 | 26 | 5.9 | 135 | 11 | 3.4 | 55 |
| 1 | 7.0 | 4.2 | 57 | 19 | 4.8 | 113 | 10 | 2.8 | 54 |
| 1.5 | 3.7 | 3.0 | 55 | 17 | 4.2 | 104 | 8.7 | 2.7 | 48 |
| 2 | 3.5 | 3.0 | 50 | 13 | 3.8 | 104 | 7.4 | 2.3 | 46 |
| 3 | 2.7 | 2.2 | 46 | 6.0 | 2.1 | 78 | 4.1 | 1.7 | 46 |
| 4 | 2.5 | 1.8 | 42 | 3.9 | 1.4 | 87 | 3.7 | 1.6 | 38 |
| 6 | 2.1 | 1.3 | 34 | 3.4 | 1.2 | 76 | 2.8 | 1.4 | 32 |
| 8 | 1.6 | 1.4 | 33 | 3.2 | 0.9 | 79 | 2.4 | 1.0 | 27 |
| Subject | S17 | S18 | S20 | ||||||
| −1 | 1.6 | 1.0 | 58 | 1.7 | 1.0 | 21 | 0.8 | 0 | 6.1 |
| 0.25 | 22 | 3.4 | 69 | 14 | 2.2 | 32 | 14 | 1.3 | 25 |
| 0.5 | 19 | 3.3 | 85 | 9.6 | 1.9 | 32 | 8.7 | 1.1 | 20 |
| 1 | MS | MS | MS | MS | MS | MS | 7.4 | 1.0 | 19 |
| 1.5 | 9.6 | 2.3 | 66 | 9.0 | 2.0 | 35 | 6.0 | 1.0 | 19 |
| 2 | 6.2 | 2.0 | 65 | 5.3 | 1.6 | 27 | 4.3 | 0.8 | 15 |
| 3 | 3.8 | 1.6 | 57 | 2.9 | 1.3 | 22 | 1.6 | 0.6 | 8.6 |
| 4 | 2.8 | 1.1 | 43 | 2.9 | 1.3 | 23 | 1.4 | 0.6 | 7.9 |
| 6 | 2.8 | 1.3 | 47 | 3.1 | 1.3 | 22 | 1.1 | 0.6 | 5.8 |
| 8 | 1.7 | 1.0 | 41 | 3.1 | 1.1 | 19 | 1.1 | 0.6 | 8.1 |
| Session 2 | |||||||||
| Subject | S1 | S5 | S9 | ||||||
| −1 | 16 | 9.2 | 232 | 3.0 | 1.0 | 99 | 2.2 | 1.5 | 38 |
| 0.25 | 48 | 17 | 245 | 20 | 4.7 | 136 | 19 | 5.2 | 57 |
| 0.5 | 36 | 14 | 237 | 13 | 3.7 | 129 | 13 | 4.3 | 57 |
| 1 | 27 | 13 | 221 | 9.3 | 2.6 | 109 | 10 | 3.7 | 52 |
| 1.5 | 24 | 11 | 208 | 7.9 | 2.5 | 94 | 3.6 | 1.9 | 44 |
| 2 | 19 | 10 | 216 | 6.8 | 2.0 | 103 | 3.4 | 1.7 | 40 |
| 3 | 12 | 8.2 | 243 | 4.5 | 1.4 | 92 | 3.2 | 1.8 | 48 |
| 4 | 11 | 7.6 | 226 | 3.5 | 1.2 | 94 | 2.5 | 1.5 | 42 |
| 6 | 9.8 | 5.8 | 239 | 2.9 | 1.0 | 85 | 2.1 | 1.3 | 38 |
| 8 | 9.6 | 6.0 | 235 | 3.0 | 0.9 | 83 | 2.3 | 1.3 | 36 |
| Subject | S17 | S18 | S20 | ||||||
| −1 | 1.1 | 0.7 | 61 | 1.1 | 0.6 | 34 | 1.1 | 0 | 9.2 |
| 0.25 | 17 | 4.2 | 63 | 7.8 | 2.1 | 44 | 11 | 2.3 | 26 |
| 0.5 | 9.4 | 3.1 | 55 | 6.2 | 2.0 | 44 | 8.0 | 1.7 | 25 |
| 1 | 6.1 | 2.3 | 52 | 4.0 | 1.5 | 42 | 4.3 | 1.1 | 21 |
| 1.5 | 5.8 | 2.3 | 50 | 2.2 | 1.0 | 25 | 3.5 | 1.0 | 18 |
| 2 | 2.9 | 1.3 | 26 | 2.6 | 1.0 | 31 | 2.7 | 0.8 | 15 |
| 3 | 2.1 | 1.0 | 38 | 1.6 | 0.7 | 27 | 1.6 | 0.6 | 14 |
| 4 | 2.3 | 1.1 | 44 | 1.0 | 0.6 | 25 | 1.7 | 0.6 | 11 |
| 6 | 1.8 | 0.8 | 33 | 2.2 | 0.8 | 37 | 1.3 | 0 | 11 |
| 8 | 2.0 | 0.9 | 42 | 1.3 | 0.5 | 22 | 1.4 | 0 | 11 |
| Session 3 | |||||||||
| Subject | S1 | S5 | S9 | ||||||
| −1 | 14 | 8.1 | 252 | 4.4 | 1.3 | 99 | 2.0 | 1.6 | 31 |
| 0.25 | 37 | 11 | 270 | 26 | 6.1 | 146 | 19 | 5.7 | 52 |
| 0.5 | 27 | 11 | 276 | 11 | 3.6 | 109 | 13 | 4.3 | 48 |
| 1 | 16 | 9.4 | 233 | 8.7 | 2.8 | 102 | 9.3 | 3.4 | 44 |
| 1.5 | 15 | 9.4 | 250 | 11 | 3.2 | 118 | 8.9 | 3.2 | 41 |
| 2 | 12 | 7.8 | 245 | 9.2 | 2.6 | 107 | 6.0 | 2.3 | 34 |
| 3 | 10 | 8.2 | 268 | 6.5 | 2.1 | 96 | 3.9 | 1.9 | 33 |
| 4 | 10 | 7.2 | 229 | 5.4 | 1.6 | 90 | 3.2 | 1.6 | 31 |
| 6 | 11 | 8.1 | 272 | 3.5 | 1.0 | 76 | 2.3 | 1.2 | 24 |
| 8 | 11 | 6.2 | 243 | 3.8 | 1.0 | 86 | 2.6 | 1.1 | 25 |
| Subject | S10 | S17 | S18 | ||||||
| −1 | 1.9 | 1.5 | 25 | 2.7 | 1.3 | 56 | 5.0 | 1.4 | 76 |
| 0.25 | 9.4 | 3.1 | 35 | 19 | 3.4 | 73 | 15 | 2.3 | 82 |
| 0.5 | 6.4 | 3.5 | 36 | 16 | 3.3 | 78 | 12 | 2.2 | 83 |
| 1 | 4.8 | 3.9 | 27 | 10 | 2.8 | 76 | 9.1 | 1.9 | 74 |
| 1.5 | 3.8 | 1.3 | 27 | 9.5 | 2.5 | 75 | 8.3 | 1.7 | 69 |
| 2 | 3.0 | 1.3 | 26 | 6.3 | 1.8 | 53 | 6.3 | 1.4 | 57 |
| 3 | 2.5 | 1.2 | 23 | 4.5 | 1.5 | 45 | 4.1 | 1.2 | 60 |
| 4 | 2.1 | 1.2 | 24 | 3.8 | 1.3 | 42 | 5.1 | 1.3 | 56 |
| 6 | 1.5 | 1.0 | 21 | 3.6 | 1.1 | 40 | 3.9 | 1.0 | 59 |
| 8 | 1.8 | 1.0 | 22 | 3.0 | 1.0 | 36 | 3.6 | 0.9 | 56 |
MS, missing specimen.
Following smoking, maximum THC, 11-OH-THC and THCCOOH concentrations (Cmax) by LC–MS-MS in blood generally occurred in the first collected specimen (Tmax = 0.25 h). Mean (range) Cmax concentrations of THC, 11-OH-THC and THCCOOH were—Session 1: THC, 18.8 (12.0–36.0) ng/mL, 11-OH-THC, 4.0 (1.3–7.5) ng/mL, THCCOOH, 66.8 (25.0–135.0) ng/mL; Session 2: THC, 20.5 (7.8–48.0) ng/mL, 11-OH-THC, 5.9 (2.1–17.0) ng/mL, THCOOH, 95.2 (26.0–245.0) ng/mL and Session 3: THC, 20.9 (9.4–37.0) ng/mL, 11-OH-THC, 5.4 (2.3–11.0) ng/mL, THCCOOH, 111.8 (36.0–276.0) ng/mL.
Correlation of cannabinoid analytes between oral fluid and whole blood
Correlations of paired individual nonsmoker subjects' THC concentrations in oral fluid and blood were not performed because of the small number of specimens per participant that contained measurable quantities. However, a composite correlation of all nonsmoker participants (n = 44) individually paired specimens for THC in oral fluid versus THC in blood yielded a Pearson's product–moment correlation coefficient (r) equal to 0.385 (d.f. = 42); significance of the correlation (nondirectional) was P < 0.01. A composite correlation of all smoker participants (n = 175) individually paired specimens for THC in oral fluid versus THC in blood yielded a Pearson's product–moment correlation coefficient (r) equal to 0.340 (d.f. = 173); significance of the correlation (nondirectional) was P < 0.001. A composite correlation of all smoker participants (n = 176) for THCCOOH in oral fluid versus THCCOOH in blood yielded a Pearson's product–moment correlation coefficient (r) equal to 0.503 (d.f. = 174); significance of the correlation (nondirectional) was P < 0.001.
Individual correlations of the same analyte (THC or THCCOOH) between oral fluid and blood for the six smokers individually paired specimens in each session are listed in Table V along with d.f. and significance (P-values). THC concentrations in oral fluid generally were significantly correlated with those in blood with the exceptions of S18 (Session 1) and S5 (Session 2). In contrast, individual smoker correlations of THCCOOH in oral fluid to THCCOOH in blood generally were not significant.
Table V.
Pearson's Product–Moment Correlations Between Matrices of THC and THCOOH in Nominally Paired Smokers' Oral Fluid and Blood Specimens
| Session 1 |
Session 2 |
Session 3 |
|||
|---|---|---|---|---|---|
| THC in oral fluid versus THC in blood | |||||
| Subject | r (d.f.), P | Subject | r (d.f.), P | Subject | r (d.f.), P |
| S3 | 0.968 (8), 0.001 | S1 | 0.881 (8), 0.001 | S1 | 0.847 (8), 0.01 |
| S5 | 0.968 (8), 0.001 | S5 | 0.563 (8), ns | S5 | 0.705 (8), 0.05 |
| S9 | 0.772 (8), 0.01 | S9 | 0.852 (8), 0.01 | S9 | 0.893 (8), 0.001 |
| S17 | 0.951 (7), 0.001 | S17 | 0.913 (8), 0.001 | S17 | 0.720 (8), 0.05 |
| S18 | 0.514 (7), ns | S18 | 0.866 (8), 0.01 | S18 | 0.898 (8), 0.001 |
| S20 | 0.899 (8), 0.001 | S20 | 0.968 (8), 0.001 | S10 | 0.925 (8), 0.001 |
| Mean, r | 0.845 | Mean, r | 0.841 | Mean, r | 0.831 |
| Range, r | 0.514–0.968 | Range, r | 0.563–0.968 | Range, r | 0.705–0.925 |
| THCCOOH in oral fluid versus THCCOOH in blood | |||||
| Subject | r (d.f.), P | Subject | r (d.f.), P | Subject | r (d.f.), P |
| S3 | 0.056 (7), ns | S1 | 0.401 (8), ns | S1 | 0.491 (8), ns |
| S5 | 0.478 (8), ns | S5 | 0.139 (8), ns | S5 | 0.250 (8), ns |
| S9 | 0.518 (8), ns | S9 | 0.666 (8), 0.05 | S9 | 0.465 (8), ns |
| S17 | 0.026 (8), ns | S17 | 0.535 (8), ns | S17 | 0.120 (8), ns |
| S18 | 0.316 (8), ns | S18 | 0.236 (8), ns | S18 | 0.439 (8), ns |
| S20 | 0.371 (8), ns | S20 | 0.414 (8), ns | S10 | 0.363 (8), ns |
| Mean, r | 0.294 | Mean, r | 0.398 | Mean, r | 0.355 |
| Range, r | 0.026–0.518 | Range, r | 0.139–0.666 | Range, r | 0.120–0.491 |
r, Pearson's correlation coefficient; d.f., degrees of freedom; P, level of significance; ns, not significant at P < 0.05.
Correlation between cannabinoid analytes in the same matrix
Individual correlations between analyte (THC and THCCOOH) within the same matrix (oral fluid or blood) for the six smokers in each session are listed in Table VI along with d.f. and significance (P-values). Correlations between THC concentration and THCCOOH in oral fluid generally were variable with approximately one-half of the subjects being significantly correlated. In contrast, individual correlations of THC in blood to THCCOOH in blood generally were highly significant with the exception of S1 (Sessions 2 and 3).
Table VI.
Pearson's Product–Moment Correlations Within Matrix of THC and THCOOH in Nominally Paired Smokers' Oral Fluid and Blood Specimens
| Session 1 |
Session 2 |
Session 3 |
|||
|---|---|---|---|---|---|
| THC in oral fluid versus THCCOOH in oral fluid | |||||
| Subject | r (d.f.), P | Subject | r (d.f.), P | Subject | r (d.f.), P |
| S3 | 0.386 (8), ns | S1 | 0.934 (9), 0.001 | S1 | 0.914 (9), 0.001 |
| S5 | 0.640 (9), 0.05 | S5 | 0.619 (9), 0.05 | S5 | 0.097 (9), ns |
| S9 | 0.778 (9), 0.01 | S9 | 0.937 (9), 0.001 | S9 | 0.821 (9), 0.010 |
| S17 | 0.062 (9), ns | S17 | 0.763 (9), 0.01 | S17 | 0.375 (9), ns |
| S18 | 0.709 (6), 0.05 | S18 | 0.325 (8), ns | S18 | 0.787 (9), 0.01 |
| S20 | 0.490 (9), ns | S20 | 0.436 (9), ns | S10 | 0.400 (9), ns |
| Mean, r | 0.511 | Mean, r | 0.669 | Mean, r | 0.566 |
| Range, r | 0.062–0.778 | Range, r | 0.325–0.937 | Range, r | 0.097–0.914 |
| THC in blood versus THCCOOH in blood | |||||
| Subject | r (d.f.), P | Subject | r (d.f.), P | Subject | r (d.f.), P |
| S3 | 0.877 (8), 0.001 | S1 | 0.124 (8), ns | S1 | 0.488 (8), ns |
| S5 | 0.873 (8), 0.001 | S5 | 0.930 (8), 0.001 | S5 | 0.947 (8), 0.001 |
| S9 | 0.906 (8), 0.001 | S9 | 0.895 (8), 0.001 | S9 | 0.945 (8), 0.001 |
| S17 | 0.806 (7), 0.01 | S17 | 0.597 (8), ns | S17 | 0.822 (8), 0.01 |
| S18 | 0.879 (7), 0.01 | S18 | 0.817 (8), 0.01 | S18 | 0.822 (8), 0.01 |
| S20 | 0.961 (8), 0.001 | S20 | 0.934 (8), 0.001 | S10 | 0.921 (8), 0.001 |
| Mean, r | 0.884 | Mean, r | 0.716 | Mean, r | 0.824 |
| Range, r | 0.806–0.961 | Range, r | 0.124–0.934 | Range, r | 0.488–0.947 |
r, Pearson's correlation coefficient; d.f., degrees of freedom; P, level of significance; ns, not significant at P < 0.05.
Self-reported drug effects
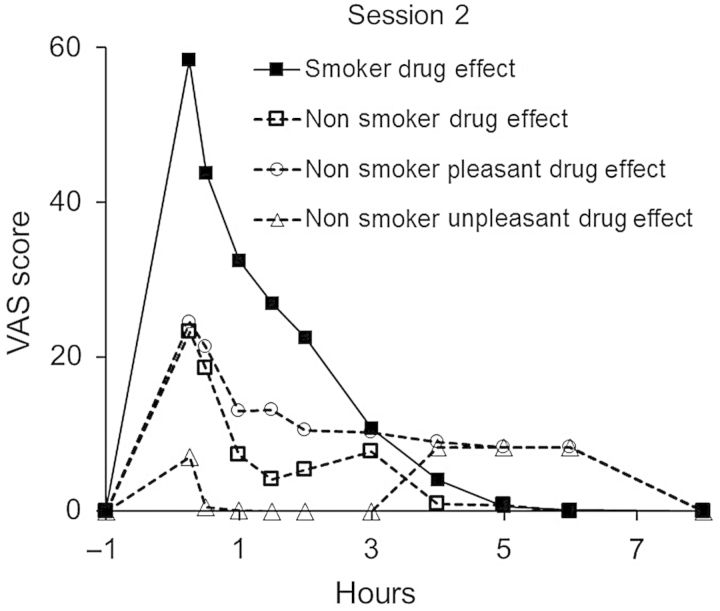
Nonsmokers reported zero or low responses (relative to smokers) in Sessions 1 and 3 on VAS ratings for ‘drug effect’, ‘pleasant drug effect’ and ‘unpleasant drug effect’. Responses by nonsmokers in Session 2 were substantially higher for ‘drug effect’ and ‘pleasant drug effect’ and slightly elevated for ‘unpleasant drug effect’. Mean responses on these measures for Session 2 are shown in Figure 3. Smoker responses on ‘drug effect’ in Session 2 are included in Figure 3 for comparison.
Figure 3.
Mean self-reported ratings of drug effects by nonsmokers in Session 2 (n = 6). The mean drug effect response of smokers (n = 6) is included for comparison.
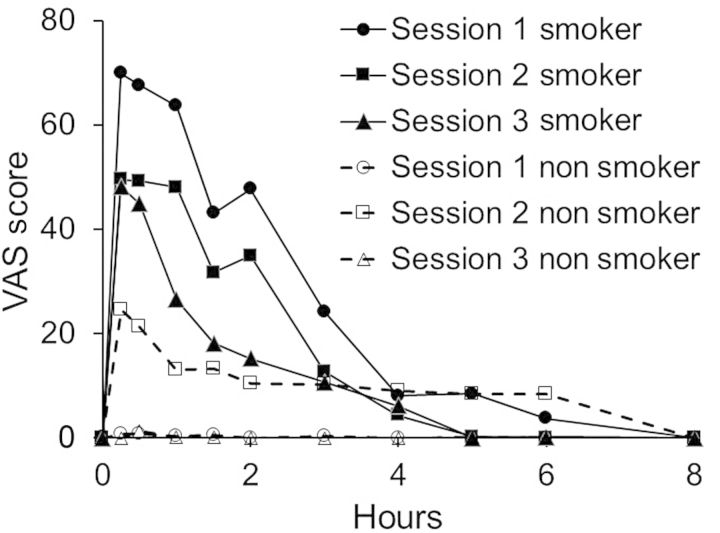
An important finding in this study was the subjective reports of ‘pleasant drug effect’ by nonsmokers in Session 2, which suggested that they not only experienced a drug effect from passive exposure, but also experienced a ‘pleasant’ effect. Of the six nonsmokers, four of six reported a mean (range) increase of 37.5 (27–54) on the 100-point scale (S8, S23, S37 and S38), whereas S40 and S41 reported maximum scores of <10. The mean peak ratings by nonsmokers and smokers of pleasant drug effect occurred at the first VAS rating (∼15 min) post-exposure and declined to baseline over the next 1–4 h. The mean ratings for pleasant drug effects by nonsmokers and smokers across the three sessions are illustrated in Figure 4.
Figure 4.
Mean self-reported ratings of ‘pleasant drug effect’ by nonsmokers and smokers (n = 6).
Estimation of THC ‘body-load’ (relative dose) for nonsmokers compared with smokers
The amount of THC inhaled by nonsmokers (relative to smokers), estimated based on comparisons of mean AUC0−t measures (n = 6) of THC in oral fluid and blood, is summarized in Table VII alongside VAS ratings of pleasant drug effect. The relative amounts of THC in oral fluid and blood, respectively, for nonsmokers compared with smokers were—(%nonsmokers/smokers): Session 1, 4.7%, 2.4%; Session 2, 11.2%, 5.9% and Session 3, 1.4%, 0.3%. The amounts of total THCCOOH excreted in urine (relative to 0.1974 mg) were—(%nonsmokers/smokers): Session 1, 3.1%; Session 2, 17.9% and Session 3, 3.8%. The VAS measures of ‘pleasant drug effect’ for nonsmokers (with an n = 5 for Session 2, S37 was identified as an outlier and removed) compared with smokers (n = 6) were—(%nonsmokers/smokers): Session 1, 0.8%; Session 2, 18.5% and Session 3, 0.8%. Without removal of the outlier subject for Session 2, the VAS response for ‘good drug effect’ relative to smokers was 65.0%.
Table VII.
Estimation of the Relative Dose of THC Inhaled by Nonsmokers Compared with Smokers
| Session 1 |
Session 2 |
Session 3 |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Nonsmoker |
Smoker |
Nonsmoker |
Smoker |
Nonsmoker |
Smoker |
||||||
| Oral fluid | |||||||||||
| Subject | AUC0−ta | Subject | AUC0−t | Subject | AUC0−t | Subject | AUC0−t | Subject | AUC0−t | Subject | AUC0−t |
| S7 | 29.7 | S3 | 1919.2 | S8 | 25.2 | S1 | 318.2 | S25 | 0.4 | S1 | 142.2 |
| S11 | 6.0 | S5 | 424.9 | S23 | 50.3 | S5 | 120.8 | S26 | 13.1 | S5 | 272.2 |
| S13 | 47.1 | S9 | 444.9 | S37 | 30.3 | S9 | 554.5 | S27 | 2.9 | S9 | 1000.2 |
| S14 | 39.4 | S17 | 203.0 | S38 | 118.0 | S17 | 597.9 | S28 | 0.5 | S17 | 1097.8 |
| S15 | 28.4 | S18 | 66.5 | S40 | 10.6 | S18 | 215.0 | S29 | 1.1 | S18 | 422.9 |
| S16 | 11.0 | S20 | 390.9 | S41 | 6.3 | S20 | 345.2 | S36 | 26.7 | S10 | 187.9 |
| Mean | 26.9 | Mean | 574.9 | Mean | 40.1 | Mean | 358.6 | Mean | 7.5 | Mean | 520.5 |
| %NS/S | 4.7 | %NS/S | 11.2 | %NS/S | 1.4 | ||||||
| Blood | |||||||||||
| Subject | AUC0−t | Subject | AUC0−t | Subject | AUC0−t | Subject | AUC0−t | Subject | AUC0−t | Subject | AUC0−t |
| S7 | 1.2 | S3 | 24.4 | S8 | 1.7 | S1 | 101.4 | S25 | 0.0 | S1 | 83.1 |
| S11 | 1.2 | S5 | 71.9 | S23 | 4.3 | S5 | 44.1 | S26 | 0.2 | S5 | 54.4 |
| S13 | 1.4 | S9 | 43.1 | S37 | 1.0 | S9 | 34.1 | S27 | 0.2 | S9 | 41.6 |
| S14 | 0.8 | S17 | 39.0 | S38 | 2.6 | S17 | 29.6 | S28 | 0.2 | S17 | 48.0 |
| S15 | 0.2 | S18 | 36.6 | S40 | 3.7 | S18 | 19.2 | S29 | 0.0 | S18 | 45.3 |
| S16 | 1.0 | S20 | 23.7 | S41 | 1.3 | S20 | 21.1 | S36 | 0.2 | S10 | 22.8 |
| Mean | 0.9 | Mean | 39.8 | Mean | 2.4 | Mean | 41.6 | Mean | 0.1 | Mean | 49.2 |
| %NS/S | 2.4 | %NS/S | 5.8 | %NS/S | 0.3 | ||||||
| Urine, %THCCOOH excreted | |||||||||||
| Subject | THCCOOH (mg) | %NS/Sb | Subject | THCCOOH (mg) | %NS/S | Subject | THCCOOH (mg) | %NS/S | |||
| S7 | 0.0074 | 3.7 | S8 | 0.0159 | 8.1 | S25 | 0.0040 | 2.1 | |||
| S11 | 0.0042 | 2.1 | S23 | 0.0625 | 31.6 | S26 | 0.0120 | 6.1 | |||
| S13 | 0.0132 | 6.7 | S37 | 0.0362 | 18.3 | S27 | 0.0070 | 3.5 | |||
| S14 | 0.0051 | 2.6 | S38 | 0.0353 | 17.9 | S28 | 0.0072 | 3.7 | |||
| S15 | 0.0019 | 1.0 | S40 | 0.0083 | 4.2 | S29 | 0.0019 | 1.0 | |||
| S16 | 0.0052 | 2.6 | S41 | 0.0543 | 27.5 | S36 | 0.0128 | 6.5 | |||
| Mean | 0.0062 | 3.1 | Mean | 0.0354 | 17.9 | Mean | 0.0075 | 3.8 | |||
| VAS rating of ‘Pleasant Drug Effect’ | |||||||||||
| Subject | AUC0−t | Subject | AUC0−t | Subject | AUC0−t | Subject | AUC0−t | Subject | AUC0−t | Subject | AUC0−t |
| S7 | 0.0 | S3 | 113.9 | S8 | 23.0 | S1 | 32.5 | S25 | 0.0 | S1 | 37.5 |
| S11 | 0.0 | S5 | 147.5 | S23 | 11.6 | S5 | 167.3 | S26 | 0.5 | S5 | 67.8 |
| S13 | 7.6 | S9 | 77.4 | S37 | 345.1 | S9 | 10.5 | S27 | 0.0 | S9 | 11.5 |
| S14 | 0.0 | S17 | 453.6 | S38 | 70.8 | S17 | 208.6 | S28 | 0.0 | S17 | 207.1 |
| S15 | 0.0 | S18 | 51.5 | S40 | 1.5 | S18 | 100.0 | S29 | 0.0 | S18 | 144.4 |
| S16 | 0.0 | S20 | 154.9 | S41 | 0.5 | S20 | 176.8 | S36 | 3.5 | S10 | 28.6 |
| Mean | 1.3 | 166.5 | Mean | 75.4 | 115.9 | Mean | 0.7 | 82.8 | |||
| %NS/S | 0.8 | %NS/S | 65.0 | %NS/S | 0.8 | ||||||
| Mean (n = 5) | 21.5 | ||||||||||
| % NS/S | 18.5 | ||||||||||
%NS/S, relative percentage of nonsmoker to smoker response.
aAUC calculations of THC concentrations in oral fluid and blood were calculated by the linear trapezoidal rule. Individual AUC0−t calculations were made from time 0 to the time of the last detectable concentration (nonsmokers) or to the last collected specimen (8 h for smokers).
bThe cumulative total amount of THCCOOH excreted by each nonsmoker was compared with 0.1974 mg of THCCOOH; the amount of THCCOOH reported to be excreted in urine over a 7-day period by smokers who smoked a single 3.55% THC cigarette (24).
Discussion
This study was designed to simulate extreme secondhand, cannabis smoke exposure that could be experienced by drug-free individuals. The secondhand smoke was created in close proximity to nonsmokers by six experienced cannabis smokers. In Sessions 1 and 2, cannabis of moderate (5.3% THC) and high (11.3%) potencies were consumed by smoking participants under ad libitum conditions that might occur naturalistically. No ventilation was employed in these 1-h exposure sessions (Sessions 1 and 2) and there was visible, heavy build-up of smoke inside the room. Subjects who did not wear goggles (supplied by the investigators) reported eye and mucous membrane irritation. Session 3 was a repeated exposure session, similar to Session 2, but with ventilation conditions simulating home air-conditioning. There was notably less smoke build-up in Session 3 and less eye irritation was noted than in prior sessions.
Analyses of oral fluid and whole blood specimens from nonsmokers by LC–MS-MS provided important evidence regarding the degree of exposure that occurred from secondhand smoke under the different session conditions. THC was detected in individual nonsmoker specimens and attained or exceeded an administratively determined confirmatory cutoff concentration of 2 ng/mL for up to 12 h following exposure. However, IA for THC by ELISA revealed that only one oral fluid specimen (S23, Session 2) tested positive through 3 h after exposure; thereafter, all specimens tested uniformly negative. Additionally, it should be noted that most nonsmokers tested positive for <3 h. The cutoff concentrations employed for the IA (4 ng/mL) and confirmatory tests (2 ng/mL THC) were those proposed in the 2004 Mandatory Guidelines (15). Importantly, none of the nonsmokers' oral fluid specimens had measurable concentrations of THCCOOH. Hence, in cases where there was extreme secondhand smoke exposure, it appears that testing for THCCOOH might serve to distinguish actual drug use from secondhand exposure. It should be noted that conditions produced in the current study were designed to represent extreme acute exposure and could not be considered to be “unknowing” in nature.
Analyses of oral fluid specimens following nonsmokers' exposure to extreme secondhand smoke indicated that inhalation of environmental cannabis smoke led to the presence of THC in oral fluid. This study does not reveal whether the basis for that presence involved topical mucosal deposition (with possible transmucosal absorption) or was solely via absorption from the respiratory tract into the bloodstream. Analyses of whole blood from the nonsmokers confirmed that THC was rapidly absorbed into blood. It seems likely that inhalation of THC-laden smoke deep into the respiratory tract would account for most of the THC measured in blood. As indicated in Figure 2, there was obvious rapid uptake of THC into blood for both nonsmokers and smokers with both groups of subjects displaying a similar time course of THC appearance in the assays.
Understanding the relationship of THC in oral fluid with THC in blood remains somewhat uncertain, as is the origin of low concentrations of THCCOOH and conjugated-THCCOOH in oral fluid from smokers' specimens (25). Early studies by Perez-Reyes (3) with radio-labeled drug appeared to have established that diffusion of THC from blood into oral fluid does not occur appreciably, if at all, and that the origin of THC in oral fluid was from direct deposition of THC-laden smoke particles in the oral cavity. However, in the present study, there was clear evidence of THC deposition in nonsmokers from environmental cannabis smoke generated by smokers in the immediate vicinity. Once THC was deposited in the oral cavity via smoking or inhalation of cannabis smoke, the question remains of the dispositional outcome of mouth-deposited THC. The data from this study suggest that deposited THC is eventually absorbed into blood, possibly by the sublingual and/or transmucosal route. Earlier reports of sublingual administration have indicated that THC is effectively absorbed by this route (26–28). For example, sublingual administration of a crushed Namisol® tablet (oral formulation containing 5 mg of THC) to 13 subjects resulted in a maximum plasma concentration (Cmax) of 2.3 ng/mL of THC with a peak time of 74.5 min (Tmax) (29).
Another potential route of absorption for orally deposited THC would be by swallowing followed by absorption from the gastrointestinal tract. However, the rate of absorption of orally consumed cannabis is slow and its bioavailability is quite low (estimated to be ∼6%) (30); consequently, the oral route was not likely to be a significant contributing factor in this study to THC blood concentrations of nonsmokers.
The presence of low concentrations of THCCOOH and conjugated-THCCOOH in oral fluid cannot be accounted for by cannabis smoke deposition as studies have not detected these analytes in cannabis smoke (19, 31). In contrast to the prior study by Perez-Reyes (3), we believe it is likely that these metabolites in oral fluid arise from diffusion from blood into oral fluid. A second possible explanation would be metabolism of THC to THCCOOH by mucosal membrane enzymes. Further research is needed for a clearer understanding of the origin and source of THCCOOH and conjugated-THCCOOH in oral fluid.
Assessment of the relationship of drug and metabolite in oral fluid with that in blood could offer clues to these complex dispositional questions related to the disposition of orally deposited THC. In the current study, nominally paired oral fluid and blood specimens allowed an examination of the relationship by correlational analyses of drug concentrations in these different matrices. Correlations of specimens for all nonsmoker paired specimens and all smoker paired specimens were significant for THC in oral fluid compared with THC in blood. However, it should be noted that these data were highly variable and would not allow accurate predictions of concentrations between these matrices.
More noteworthy, correlations of THC in oral fluid to blood for individual smokers were generally significant in the three smoking sessions. These correlations included baseline concentrations and for 8 h following cessation of smoking. As illustrated in Figure 2, THC concentrations in smokers' oral fluid were elevated immediately following smoking and then followed a parallel time course to blood over the 8-h collection period. These data suggest that THC deposited in the oral cavity of smokers undergoes an initial rapid clearance (possibly swallowing of excess THC) followed by a sustained mucosal depot that contributed to blood concentrations via transmucosal absorption. For nonsmokers, THC from environmentally inhaled cannabis smoke followed a similar pattern of rapid clearance followed by dropping to undetectable concentrations in a few hours.
Interestingly, correlations of THCCOOH concentrations in oral fluid with THCCOOH in blood were generally not significant for smokers. This would be surprising if one assumed that THCCOOH in oral fluid originated primarily by diffusion from blood.
Examination of the relationship between THC and THCCOOH in smokers' oral fluid also indicated only weak associations, but there were strong associations between these two analytes in blood. If THCCOOH is present in oral fluid primarily from transmucosal metabolism of THC, a stronger association might be expected. However, a limitation of this study was that the blood was not hydrolyzed prior to analysis. Consequently, the data for THCCOOH in blood are for the free unconjugated form of THCCOOH; conjugated-THCCOOH in blood was not measured. In contrast, oral fluid concentrations of THCCOOH represent ‘total’ concentrations. With hydrolysis of the blood specimens, it is possible that concentrations of total THCCOOH in blood would have shown a stronger relationship with total oral fluid concentrations of THCCOOH.
Regardless of the exact mechanism of THC and THCCOOH disposition in blood that occurred for nonsmokers in this study, it is important to note that significant pharmacological effects were noted in these participants. In an earlier report of this study, Herrmann et al. (21) showed that significant subjective effects occurred for nonsmokers after exposure, particularly in the most extreme exposure session (Session 2; high-potency cannabis, no ventilation). Nonsmoker participants reported significantly higher ratings of ‘drug effect’ and ‘pleasant drug effect’ in Session 2 (compared with baseline during the hour post-exposure). The magnitude of peak effects for nonsmoker ratings on the drug effect and pleasant drug effect in Session 2 was ∼40% of smoker ratings.
Further estimates were made in this study of the amounts (dose) of THC (oral fluid and blood) and THCCOOH (urine) in nonsmoker specimens relative to smokers. The traditional approach to estimation of absolute bioavailability (amount of delivered dose) is by comparison of AUC measures of a test dose administered by a specified route compared with a standard dose delivered by an intravenous injection. Determination of relative bioavailability is performed in a similar manner but by comparing a test dose with a standard dose administered by the same or similar route. Drug concentrations in blood are traditionally used for determining bioavailability, but urine concentrations and pharmacological measures may also be used.
Calculations of AUC measures over time for THC in oral fluid and blood and self-reported pleasant drug effects for nonsmokers and smokers provided relative estimates of the ‘dose’ of THC experienced by nonsmokers. Total THCCOOH excreted in urine by nonsmokers was compared with the amount of THCCOOH (0.1974 mg) reported to be excreted in urine over a 7-day period by smokers who smoked a single 3.55% THC cigarette (24). These biological and pharmacological ‘dose’ estimates provided consistent indications that the dose delivered to nonsmokers (relative to smokers) was generally low (<5%) in Session 1 (5.3% THC, no ventilation) and Session 3 (11.3% THC, ventilation), but was substantially higher (6–18%) in Session 2 (11.3% THC, no ventilation) across all measures. These findings indicated that extreme passive cannabis exposure delivered a sufficient dose of THC to a nonsmoker that mimicked, albeit to a lesser extent, active cannabis smoking. Extreme passive exposure resulted in positive oral fluid tests, urinary excretion of THCCOOH, blood concentrations of THC up to 5 ng/mL and significant subjective effects of intoxication. During informal conversations, several participants indicated that they had experienced similar conditions at some point in the past, suggesting that this type of extreme secondhand smoke exposure may occur in social situations. Consequently, individuals who place themselves in such exposure conditions may test positive for cannabis use and exhibit pharmacological effects similar to effects produced from smoked cannabis. The extent of exposure and number of resulting positive tests were shown to be influenced by various factors, key among them being room ventilation, cannabis potency and amount of cannabis consumed by smokers. In the unventilated study conditions, the smoke was clearly visible and irritated the eyes of anyone not using the provided goggles. Thus, it seems likely that exposure under less extreme conditions, such as casual encounters with cannabis smoke and in situations in which an individual was not aware of smoke exposure, would be very unlikely to result in positive tests and behavioral changes.
Study limitations
The results from this study are limited by the standardized experimental conditions employed. Only two variations in potencies of smoked cannabis (5.3 and 11.3% THC) and two variations in room exposure conditions (no ventilation and ventilation) were evaluated. Many variations in exposure conditions (e.g., cannabis potency, length of exposure, number of smokers, chronic low dose exposure and room size) are possible. Extrapolation of these study results to other situations and conditions of exposure should be undertaken with sufficient caution. It is also difficult to judge the relative effect of potency because less cannabis was consumed in Session 1 (moderate potency) compared with Session 2 (high potency). An additional study with fixed cannabis consumption by smokers or use of a smoking machine would better isolate the effect of cannabis potency. Finally, there was no placebo cannabis condition. Thus, we cannot rule out the effect of expectancy on the subjective drug effect ratings provided by smokers and nonsmokers alike.
Conclusions
This study demonstrated that extreme exposure to environmental cannabis smoke by nonsmokers situated in close proximity to smokers led to deposition of THC in oral fluid. Oral fluid specimens for nonsmokers in the two sessions conducted without ventilation tested positive by IA (4 ng/mL cutoff concentrations) and were confirmed for THC by LC–MS-MS (2 ng/mL) for up to 3 h following cessation of exposure. In the third session of this study, conducted with ventilation that simulated air-conditioning, some initial positive tests occurred, but the number was substantially diminished. Importantly, no THCCOOH was detectable in oral fluid by LC–MS-MS at the LOQ (0.02 ng/mL) in any exposure session for nonsmokers. Concurrent concentrations of THC and THCCOOH appeared in blood of nonsmokers over a similar time course as oral fluid, indicating that rapid absorption and metabolism of THC had occurred as a result of exposure. Similar tests of oral fluid and blood specimens from the participating smokers revealed significant correlations of THC in oral fluid with THC in concurrent blood specimens, whereas THCCOOH in oral fluid was not generally correlated with THCCOOH in blood. There was significant correlation of THC in oral fluid with THCCOOH in both oral fluid and blood. Although the absorptive mechanisms involved in the disposition and subsequent metabolism of orally deposited THC remain unclear, it is plausible that respiratory absorption and transmucosal absorption played an important role in linking THC concentration in oral fluid to blood as demonstrated in both nonsmokers and smokers. The rapid absorption of THC into blood led to functional pharmacologic effects in nonsmokers as a result of environmental exposure. The amount of THC delivered to nonsmokers compared with cannabis smokers in these exposure conditions was low (<5%) for Sessions 1 and 3, but was substantially higher in Session 2 (6–18% of smokers' doses). Thus, in the most extreme exposure condition, the effects of passive exposure mimicked, to a lesser extent, active smoking effects. This combined body of data suggest that environmental exposure to cannabis smoke should be avoided by nonsmokers and potentially has implications for those who undergo drug testing and those engaged in safety-sensitive activities (e.g., driving). Extreme exposure of nonsmokers could lead to positive drug tests and drug-induced behavioral changes not unlike those produced by active cannabis smoking.
Funding
E.J.C. is a consultant to the Division of Workplace Programs, Substance Abuse and Mental Health Services Administration (SAMHSA) and has an Adjunct Professor appointment with Johns Hopkins University School of Medicine, Baltimore, MD, USA. R.V., G.E.B. and E.S.H. are with Johns Hopkins School of Medicine, Baltimore, MD, USA. J.M.M. is an employee of RTI International and C.L. and R.F. are employees of SAMHSA. SAMHSA provided financial support for the study. NIDA provided material support for the study (cannabis) and financial support for the participation of E.S.H. (T32-DA07209). Support for the Johns Hopkins Bayview residential research unit provided from grant UL1-RR025005 from the National Center for Research Resources, a component of the National Institutes of Health and NIH Roadmap for Medical Research.
References
- 1.Substance Abuse and Mental Health Administration and Substance. Results from the 2013 National Survey on Drug Use and Health: Summary of National Findings. NSDUH Series H-48, HHS Publication No. (SMA) 14-4863. Substance Abuse and Mental Health Administration and Substance: Rockville, MD, 2014. [Google Scholar]
- 2.Davis K.H., McDaniel I.A., Cadwell L.W., Moody P.L. (1984) Some smoking characteristics of marijuana cigarettes. In: Agurell S., Dewey W.L., Willette R.E. (eds). The Cannabinoids: Chemical, Pharmacologic, and Therapeutic Aspects. Academic Press: Orlando, FL, pp. 97–109. [Google Scholar]
- 3.Perez-Reyes M. (1990) Marijuana smoking: factors that influence the bioavailability of tetrahydrocannabinol. In: Chiang C.N., Hawks R.L. (eds). Research Findings on Smoking of Abused Substances, NIDA Research Monograph #99. U.S. Government Printing Office: Rockville, MD, pp. 42–62. [PubMed] [Google Scholar]
- 4.Sparacino C.M., Hyldburg P.A., Hughes T.J. (1990) Chemical and biological analysis of marijuana smoke condensate. NIDA Research Monograph, 99, 121–140. [PubMed] [Google Scholar]
- 5.Moir D., Rickert W.S., Levasseur G., Larose Y., Maertens R., White P., et al. (2008) A comparison of mainstream and sidestream marijuana and tobacco cigarette smoke produced under two machine smoking conditions. Chemical Research in Toxicology, 21, 494–502. [DOI] [PubMed] [Google Scholar]
- 6.Cone E.J., Bigelow G.E., Herrmann E.S., Mitchell J.M., LoDico C., Flegel R., et al. (2015) Non-smoker exposure to secondhand cannabis smoke. I. Urine screening and confirmation results. Journal of Analytical Toxicology, 39, 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Anizan S., Milman G., Desrosiers N., Barnes A.J., Gorelick D.A., Huestis M.A. (2013) Oral fluid cannabinoid concentrations following controlled smoked cannabis in chronic frequent and occasional smokers. Analytical and Bioanalytical Chemistry, 405, 8451–8461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Concheiro M., Lee D., Lendoiro E., Huestis M.A. (2013) Simultaneous quantification of Delta(9)-tetrahydrocannabinol, 11-nor-9-carboxy-tetrahydrocannabinol, cannabidiol and cannabinol in oral fluid by microflow-liquid chromatography-high resolution mass spectrometry. Journal of Chromatography A, 1297, 123–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kauert G.F., Ramaekers J.G., Schneider E., Moeller M.R., Toennes S.W. (2007) Pharmacokinetic properties of delta9-tetrahydrocannabinol in serum and oral fluid. Journal of Analytical Toxicology, 31, 288–293. [DOI] [PubMed] [Google Scholar]
- 10.Lee D., Schwope D.M., Milman G., Barnes A.J., Gorelick D.A., Huestis M.A. (2012) Cannabinoid disposition in oral fluid after controlled smoked cannabis. Clinical Chemistry, 58, 748–756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lee D., Milman G., Barnes A.J., Goodwin R.S., Hirvonen J., Huestis M.A. (2011) Oral fluid cannabinoids in chronic, daily cannabis smokers during sustained, monitored abstinence. Clinical Chemistry, 57, 1127–1136. [DOI] [PubMed] [Google Scholar]
- 12.Milman G., Schwope D.M., Gorelick D.A., Huestis M.A. (2012) Cannabinoids and metabolites in expectorated oral fluid following controlled smoked cannabis. Clinica Chimica Acta, 413, 765–770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Scheidweiler K.B., Himes S.K., Chen X., Liu H.F., Huestis M.A. (2013) 11-Nor-9-carboxy-9-tetrahydrocannabinol quantification in human oral fluid by liquid chromatography-tandem mass spectrometry. Analytical and Bioanalytical Chemistry, 405, 6019–6027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Moore C., Ross W., Coulter C., Adams L., Rana S., Vincent M., et al. (2006) Detection of the marijuana metabolite 11-nor-Delta9-tetrahydrocannabinol-9-carboxylic acid in oral fluid specimens and its contribution to positive results in screening assays. Journal of Analytical Toxicology, 30, 413–418. [DOI] [PubMed] [Google Scholar]
- 15.DHHS. (2004) Proposed revisions to mandatory guidelines for federal workplace drug testing programs. Federal Register, 69, 19673–19732. [Google Scholar]
- 16.Niedbala R.S., Kardos K.W., Fritch D.F., Kardos S., Fries T., Waga J., et al. (2001) Detection of marijuana use by oral fluid and urine analysis following single-dose administration of smoked and oral marijuana. Journal of Analytical Toxicology, 25, 289–303. [DOI] [PubMed] [Google Scholar]
- 17.Niedbala S., Kardos K., Salamone S., Fritch D., Bronsgeest M., Cone E.J. (2004) Passive cannabis smoke exposure and oral fluid testing. Journal of Analytical Toxicology, 28, 546–552. [DOI] [PubMed] [Google Scholar]
- 18.Niedbala R.S., Kardos K.W., Fritch E.F., Kunsman K.P., Blum K.A., Newland G.A., et al. (2005) Passive cannabis smoke exposure and oral fluid testing. II. Two studies of extreme cannabis smoke exposure in a motor vehicle. Journal of Analytical Toxicology, 29, 607–615. [DOI] [PubMed] [Google Scholar]
- 19.Moore C., Coulter C., Uges D., Tuyay J., van der Linde S., van Leeuwen A., et al. (2011) Cannabinoids in oral fluid following passive exposure to marijuana smoke. Forensic Science International, 212, 227–230. [DOI] [PubMed] [Google Scholar]
- 20.Office of National Drug Control Policy. National Drug Control Strategy; Data Supplement 2013; Office of National Drug Control Policy: Washington, DC, 2013. [Google Scholar]
- 21.Herrmann E.S., Cone E.J., Mitchell J.M., Bigelow G.E., LoDico C., Flegel R., et al. (2015) Non-smoker exposure to secondhand cannabis smoke II: effect of room ventilation on the physiological, subjective, and behavioral/cognitive effects. Drug and Alcohol Dependence, 151, 194–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Coulter C., Garnier M., Moore C. (2012) Analysis of tetrahydrocannabinol and its metabolite, 11-nor-Delta(9)-tetrahydrocannabinol-9-carboxylic acid, in oral fluid using liquid chromatography with tandem mass spectrometry. Journal of Analytical Toxicology, 36, 413–417. [DOI] [PubMed] [Google Scholar]
- 23.Coulter C., Miller E., Crompton K., Moore C. (2008) Tetrahydrocannabinol and two of its metabolites in whole blood using liquid chromatography-tandem mass spectrometry. Journal of Analytical Toxicology, 32, 653–658. [DOI] [PubMed] [Google Scholar]
- 24.Huestis M.A., Mitchell J.M., Cone E.J. (1996) Urinary excretion profiles of 11-nor-9-carboxy-Delta9-tetrahydrocannabinol in humans after single smoked doses of marijuana. Journal of Analytical Toxicology, 20, 441–452. [DOI] [PubMed] [Google Scholar]
- 25.Moore C., Rana S., Coulter C., Day D., Vincent M., Soares J. (2007) Detection of conjugated 11-nor-Delta9-tetrahydrocannabinol-9-carboxylic acid in oral fluid. Journal of Analytical Toxicology, 31, 187–194. [DOI] [PubMed] [Google Scholar]
- 26.Grotenhermen F. (2003) Pharmacokinetics and pharmacodynamics of cannabinoids. Clinical Pharmacokinetics, 42, 327–360. [DOI] [PubMed] [Google Scholar]
- 27.Anonymous. (2003) Cannabis-based medicines—GW pharmaceuticals: high CBD, high THC, medicinal cannabis—GW pharmaceuticals, THC:CBD. Drugs in R&D, 4, 306–309. [DOI] [PubMed] [Google Scholar]
- 28.Mattes R.D., Engelman K., Shaw L.M., ElSohly M.A. (1994) Cannabinoids and appetite stimulation. Pharmacology, Biochemistry, and Behavior, 49, 187–195. [DOI] [PubMed] [Google Scholar]
- 29.Klumpers L.E., Beumer T.L., van Hasselt J.G., Lipplaa A., Karger L.B., Kleinloog H.D., et al. (2012) Novel Delta(9)-tetrahydrocannabinol formulation Namisol(R) has beneficial pharmacokinetics and promising pharmacodynamic effects. British Journal of Clinical Pharmacology, 74, 42–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ohlsson A., Lindgren J.E., Wahlen A., Agurell S., Hollister L.E., Gillespie H.K. (1980) Plasma delta-9-tetrahydrocannabinol concentrations and clinical effects after oral and intravenous administration and smoking. Clinical Pharmacology and Therapeutics, 28, 409–416. [DOI] [PubMed] [Google Scholar]
- 31.Day D., Kuntz D.J., Feldman M., Presley L. (2006) Detection of THCA in oral fluid by GC-MS-MS. Journal of Analytical Toxicology, 30, 645–650. [DOI] [PubMed] [Google Scholar]