Abstract
Rationale: Children’s Interstitial and Diffuse Lung Disease (chILD) is a heterogeneous group of disorders that is challenging to categorize. In previous study, a classification scheme was successfully applied to children 0 to 2 years of age who underwent lung biopsies for chILD. This classification scheme has not been evaluated in children 2 to 18 years of age.
Objectives: This multicenter interdisciplinary study sought to describe the spectrum of biopsy-proven chILD in North America and to apply a previously reported classification scheme in children 2 to 18 years of age. Mortality and risk factors for mortality were also assessed.
Methods: Patients 2 to 18 years of age who underwent lung biopsies for diffuse lung disease from 12 North American institutions were included. Demographic and clinical data were collected and described. The lung biopsies were reviewed by pediatric lung pathologists with expertise in diffuse lung disease and were classified by the chILD classification scheme. Logistic regression was used to determine risk factors for mortality.
Measurements and Main Results: A total of 191 cases were included in the final analysis. Number of biopsies varied by center (5–49 biopsies; mean, 15.8) and by age (2–18 yr; mean, 10.6 yr). The most common classification category in this cohort was Disorders of the Immunocompromised Host (40.8%), and the least common was Disorders of Infancy (4.7%). Immunocompromised patients suffered the highest mortality (52.8%). Additional associations with mortality included mechanical ventilation, worse clinical status at time of biopsy, tachypnea, hemoptysis, and crackles. Pulmonary hypertension was found to be a risk factor for mortality but only in the immunocompetent patients.
Conclusions: In patients 2 to 18 years of age who underwent lung biopsies for diffuse lung disease, there were far fewer diagnoses prevalent in infancy and more overlap with adult diagnoses. Immunocompromised patients with diffuse lung disease who underwent lung biopsies had less than 50% survival at time of last follow-up.
Keywords: rare pediatric lung disease, interstitial lung disease, pathology
The spectrum of interstitial lung disease (ILD) includes a large, heterogeneous group of mostly rare pulmonary disorders characterized by abnormalities of the distal lung units and disordered gas exchange (1, 2). Because many of the included disorders extend beyond or do not even involve the interstitium, ILD in children (chILD) is considered a syndrome of diffuse and interstitial lung disease (3). chILD is different from adult ILD in that idiopathic pulmonary fibrosis (IPF) with usual interstitial pneumonia (UIP) phenotype has not been convincingly reported in children and Disorders of Infancy, such as neuroendocrine cell hyperplasia in infancy (NEHI) and pulmonary interstitial glycogenosis, have not been reported in adults (4–6). In 2007, the Pathology Working Group representing the chILD Research Co-operative of North America applied a new classification scheme to the review of the lung biopsies of 187 infants younger than 2 years of age presenting with diffuse lung disease from 11 pediatric centers (7). Not surprisingly, disorders unique to or more prevalent in infancy accounted for more than half of the cases, and the classification scheme was successfully applied to this cohort.
The next phase has been to modify the classification to apply it to older children with chILD. The Pathology Working Group performed a systematic histologic review to determine the spectrum and distribution of chILD in children 2 to 18 years of age and to correlate the disorders found with clinical features and outcomes. Our hypothesis was that the spectrum and distribution of chILD in older children would be different from that found in children younger than 2 years age. Some of the results of this study have been published in the form of abstracts (8, 9).
Methods
Twelve participating children’s hospitals in North America (Appendix 1) provided pathologic material from all diagnostic lung biopsies performed in children, 2 to 18 years of age, with diffuse lung disease over a 4-year period (January 1, 2001 to December 31, 2004). The institutional review board of each participating institution approved this study.
Cases received in consultation, needle and transbronchial biopsies, lobectomies, segmental resections, and biopsies performed for focal pulmonary lesions were excluded. Cases were also excluded if they were missing relevant clinical data (i.e., if datasheets were missing or not filled out) or if slides were not available.
The lung biopsies were reviewed by the Pathology Working Group in a consensus conference format at two workshops. Pairs of pathologists with expertise in pediatric diffuse lung disease reviewed each case, reached consensus, and assigned a final pathologic diagnosis using a modification of the chILD Classification scheme developed for the previous infant study (see Table E1 in the online supplement) (7, 10, 11). In cases where there was more than one pathologic diagnosis, the dominant pattern was chosen. In one case, a patient was biopsied twice, and the first biopsy was chosen for review.
A standardized data collection form with background information on the clinical history, past medical history, and exam findings was completed for each case by chart review at each participating site. Information was reported as “yes,” “no,” or “unknown.” The clinicians reported ages as whole years or fractions of years. The clinician determined whether the patient, at the time of symptoms/illness onset, was clinically immunocompromised (ICM) or immunocompetent (ICT). Patients who were later determined to have a non-ICM diagnosis (groups I–III, V, and VI from Table E1) were reassigned to the ICM clinically, non-ICM–path diagnosis (Figure 1). Clinical status at the time of biopsy, patient symptoms (dyspnea, tachypnea, cough, exercise intolerance, and hemoptysis) and clinical findings (hypoxemia, retractions, crackles, wheezes, normal auscultation, and failure to thrive), and additional history (including pulmonary hypertension) were reported for each patient and gathered from each participating site. Attempts were made to fill in any missing data by a second chart review. The clinical data were reviewed and summarized by the Clinical Working Group of the chILD Research Cooperative and correlated with the pathologic diagnosis determined by the Pathology Working Group.
Figure 1.
Study cohort and clinical–pathologic classification in children 2 to 18 years of age with diffuse lung disease. ABCA = ATP-binding cassette, subfamily A, member 3; GVHD = graft versus host disease; ICM = immunocompromised; ICT = immunocompetent; ILD = interstitial lung disease; NEHI = Neuroendocrine Cell Hyperplasia of Infancy; PTLD = post-transplant lymphoproliferative disorder.
SAS version 9.3 (SAS Institute Inc., Cary, NC) statistical software package was used for all statistical analyses. To evaluate risk factors of mortality in this population, associations between each individual risk factor of interest and mortality were assessed using a single-predictor logistic regression analysis. The variables considered were ICM, sex, presence of pulmonary hypertension (PHTN), clinical status at time of biopsy, immunosuppressant therapy, steroid therapy, and clinical signs and symptoms (dyspnea, tachypnea, cough, exercise intolerance, hemoptysis, hypoxemia, retractions, crackles, wheezes, normal auscultation, and failure to thrive). To account for multiple comparisons, a false discovery rate adjustment was implemented. Fisher’s exact tests were used to evaluate whether mortality differed between the patients who were clinically ICM but found to have a pathologic diagnosis typical of ICT patients compared with those with consistent clinical and pathological diagnoses.
Results
Clinical Characteristics of Study Population
A total of 231 cases were submitted for review. Forty cases met exclusion criteria, leaving 191 cases that were used for the final analysis (Figure 1). The number of cases varied by center (n = 5–49; mean, 15.8) (Figure E1). Age at the time of biopsy ranged from 2 to 18 years (mean, 10.6 yr) (Figure E2). Almost twice as many biopsies were done in the 10- to 18-year group (n = 126) compared with the 2 to 9 year group (n = 65). Clinical features of the study population are shown in Table 1. Few patients in this cohort had perinatal respiratory symptoms: 11 required oxygen (out of 102 who reported), and nine were mechanically ventilated (out of 105 who reported data). Of those patients for whom treatment status was reported, almost half received systemic steroids and/or other immunomodulating therapy.
Table 1.
Clinical features of study population
| Characteristics | Number of patients (%) | Number Reported (maximum 191) |
|---|---|---|
| Male sex | 81 (43.8) | 185 |
| Intubation at birth | 9 (8.6) | 105 |
| Oxygen required at birth | 11 (10.8) | 102 |
| Immunocompromised | 90 (47.1) | 191 |
| Treatment at time of biopsy | ||
| Systemic steroids | 72 (46.8) | 154 |
| Immunomodulating therapy | 78 (46.4) | 168 |
The most common signs and symptoms at presentation included cough (63%), exercise intolerance (57%), dyspnea (54%), hypoxemia (52%), crackles (44%), and tachypnea (48%). Fewer patients presented with wheeze (15%), failure to thrive (23%), gastroesophageal reflux disease (23%), hemoptysis (8%), retractions (20%), and PHTN (18%). Five percent of patients presented with a normal exam (Figure E3).
In this study, 101 patients were ICT and 90 patients were ICM. Of the ICM patients, 78 had pathologic diagnoses that could be categorized within the Disorders of the Immunocompromised Host, whereas 12 had pathologic diagnoses that best fit in another category (Figure 1).
Distribution of Pathologic Diagnoses
In the current study, only 4.7% of patients were diagnosed with a Disorder of Infancy, the smallest of any of the diagnostic categories and earliest time of biopsy (Tables 2 and 3). Five patients had a growth abnormality, three had NEHI, and one had pathologic findings consistent with ATP-binding cassette, subfamily A, member 3 (ABCA3) genetic mutations.
Table 2.
chILD classification, comparing pathologic diagnoses for the groups 0–2* and 2–18 yr
| 0–2 yr* (5-yr period) |
2–18 yr (4-yr period) |
|||
|---|---|---|---|---|
| Cases (n) | % | Cases (n) | % | |
| Disorders of infancy | 102 | 54.5 | 9 | 4.7 |
| Developmental | 11 | 5.9 | 0 | 0 |
| Growth | 50 | 26.7 | 5 | 2.6 |
| PIG | 6 | 3.2 | 0 | 0 |
| NEHI | 17 | 9.1 | 3 | 1.6 |
| Surfactant | 18 | 9.6 | 1 | <1.0 |
| Primary lung disorders of the ICT host | 23 | 12.3 | 31 | 16.2 |
| Disorders related to systemic disease processes | 5 | 2.7 | 42 | 22.0 |
| Disorders of the ICM host | 28 | 15.0 | 78 | 40.8 |
| Vascular disorders masquerading as ILD | 9 | 4.8 | 14 | 7.3 |
| Unclassified | 20 | 10.7 | 17 | 8.9 |
| Total | 187 | 191 | ||
Definition of abbreviations: chILD = children’s interstitial and diffuse lung disease; ICM = immunocompromised; ICT = immunocompetent; ILD = interstitial lung disease; NEHI = Neuroendocrine Cell Hyperplasia of Infancy; PIG = pulmonary intersitital glycogenosis.
Data were reported by Deutsch and colleagues (7).
Table 3.
Age at biopsy, follow-up, and mortality data
| Categories | Age at Biopsy (yr) (range) | Median Follow-up (yr) (range) | Mortality† (%) |
|---|---|---|---|
| Disorders more common in infancy | 4.2 ± 2.9 (2–10)* | 3.0 (0.92–4.33) | 1/8 (12.5) |
| Primary lung disorders of the ICT host | 11.6 ± 4.5 (2.3–17.9) | 1.2 (0.07–4.0) | 2/28 (7.1) |
| Disorders related to systemic disease | 11.4 ± 3.8 (2.6–17) | 2.0 (0.08–6.0) | 8/40 (20.0) |
| Disorders of the ICM host | 10.6 ± 4.9 (2–17.8) | 1.0 (0.1–4.17) | 38/72 (52.8) |
| Vascular disorders masquerading as ILD | 9.6 ± 4.7 (2–16.8) | 1.5 (0.58–2.58) | 4/13 (30.8) |
| Unclassified | 11.0 ± 4.9 (2–17.8) | 1.0 (0.05–4.0) | 7/15 (46.7) |
Definition of abbreviations: ICM = immunocompromised; ICT = immunocompetent; ILD = interstitial lung disease.
Values are mean ± SD.
Number of patients who died/number reporting outcome.
Thirty-one patients (16.2%) had a primary lung disorder of the ICT host. The majority (52%) of these patients had an infectious or postinfectious etiology. The next most common diagnosis in this category was diseases related to environmental agents (13%), with one patient not further classified, two classified as hypersensitivity pneumonitis, and one classified as toxic inhalation. Less common diagnoses included aspiration (6%), eosinophilic pneumonia (10%), acute interstitial pneumonia/Hamman-Rich syndrome/idiopathic diffuse alveolar damage (10%), idiopathic pulmonary hemosiderosis (6%), and other (3%).
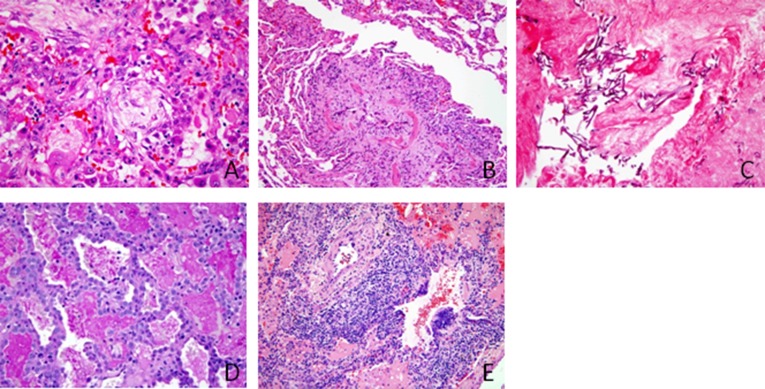
Disorders related to systemic disease was the second most common diagnostic group (n = 42 cases; 22%). Most of these patients had immune-mediated disorders (83%), including pulmonary vasculitis syndromes (n = 12) (Figure 2A), nonspecific interstitial pneumonia (n = 4) (Figure 2B), pulmonary hemorrhage (n = 3), autoimmune pulmonary alveolar proteinosis (n = 2), nonspecific pulmonary manifestations (n = 2), and other diagnoses (n = 12) (Figures 2C–2E). Three other patients had a nonimmune, systemic disease diagnosis, including a storage disorder (n = 1), sarcoidosis (n = 1), and “other” diagnosis (n = 5).
Figure 2.
Representative pathology findings in immunocompetent patients. (A) Pulmonary capillaritis demonstrating interstitial and alveolar neutrophils, admixed with acute hemorrhage and hemosiderin-laden macrophages, in a 4-year-old girl with anemia and hematuria (hematoxylin and eosin [H&E] stain; original magnification: ×50). (B) Nonspecific interstitial pneumonia pattern in a 10-year-old girl with systemic sclerosis (H&E stain; original magnification: ×50). (C) Pleural fibrosis and pleuritis with subpleural interstitial inflammation, fibrosis, and cholesterol clefts (endogenous lipoid pneumonia) in a patient with systemic sclerosis (Movat pentachrome stain; original magnification: ×50). (D) Mild interstitial fibrosis and severe pulmonary arterial disease in a 10-year-old girl with juvenile idiopathic arthritis (H&E; original magnification: ×10). (E) Severe pulmonary arteriopathy with medial hypertrophy and intimal proliferation in a patient with juvenile idiopathic arthritis (Movat pentachrome; original magnification: ×50).
Disorders of the ICM host was the most common diagnostic category in this study, accounting for 40.8% (n = 78) of the total cases. Opportunistic infections occurred in half the cases and included fungal (n = 12) or suspected (n = 13), Pneumocystis jiroveci (n = 4), viral (n = 4), and bacterial (n = 3) infections. Disorders related to treatment (including chemotherapy, radiation, and combined therapy) and drug hypersensitivity were found in 19% of the ICM patients. Disorders related to transplantation and rejection were found in 18% of the ICM patients, including two with rejection, three with graft-versus-host disease, and three with post-transplant lymphoproliferative disease. Diffuse alveolar damage of undetermined etiology and lymphoid infiltrates related to being ICM occurred in 10 and 12% of the ICM patients, respectively. Figure 3 demonstrates representative classic findings of these ICM patients, including viral-induced diffuse alveolar damage (Figure 3A), constrictive bronchiolitis (Figure 3B), fungal infection (Figure 3C), pulmonary alveolar proteinosis secondary to drug toxicity (Figure 3D), and lymphoproliferative disorder associated with Epstein-Barr virus (Figure 3E). Of the 12 ICM patients with pathologic diagnosis not directly related to immunocompromised status, one had a growth abnormality, three had pulmonary vascular disease, and eight had an unclassified diagnosis.
Figure 3.
Representative pathology findings in immunocompromised patients. (A) Diffuse alveolar damage and organizing pneumonia pattern secondary to respiratory syncytial virus infection in a 16-year-old girl with myelodysplastic syndrome and aplastic anemia, status after bone marrow transplant (hematoxylin and eosin [H&E] stain; original magnification: ×50). (B) Severe postinfectious constrictive bronchiolitis associated with organizing pneumonia in an 11-year-old boy on therapy for acute myeloid leukemia (H&E stain; original magnification: ×25). (C) Zygomycete infection with invasive fungal hyphae in a 14-year-old girl on therapy for Burkitt lymphoma (H&E stain; original magnification: ×50). (D) Acquired pulmonary alveolar proteinosis and reactive changes consistent with drug toxicity in a 4-year-old girl on therapy for acute myeloid leukemia (periodic acid Schiff stain; original magnification: ×50). (E) Epstein-Barr virus–associated lymphoproliferative disorder, polymorphous type, in a 10-year-old girl status after liver transplant (H&E stain; original magnification: ×25).
Vascular disorders masquerading as ILD were uncommon, accounting for only 7.3% of this cohort. Of these, congestive vasculopathy was found in eight patients, arterial hypertensive vasculopathy in one, lymphangiomatosis in two, pulmonary edema in two, and thromboembolic disease in one. Mortality in this group was moderately high (30.8%).
Seventeen biopsies (8.9%) were placed in the Unclassified category due to nondiagnostic biopsy (n = 13), end-stage disease (n = 2), inadequate tissue provided (n = 1), and insufficient information (n = 1).
Outcomes
Mortality data were available for over 92% (176 out of 191) of the patients, and mortality in each disease category is shown in Table 3. Patient follow-up data ranged from 0.05 to 6 years. Disorders of the ICM host had 52.8% mortality, followed by Unclassified diagnoses (46.7%) and vascular disorders masquerading as ILD (30.8%). Disorders of the normal host had the lowest mortality rate (7.1%). Additional outcome data were available for over 85% of patients, and an adapted disease severity score (12) was used to characterize the patients at time of last follow-up (Table 4). There were no differences in mortality for ICM patients with infection compared with those without (P = 0.9117).
Table 4.
Outcomes reported at last contact with patient
| Outcome | Number of Patients (% of total) | Number Reported (maximum 191) |
|---|---|---|
| Asymptomatic | 69 (40.8) | 169 |
| Symptomatic, normal room air saturation | 35 (21.2) | 165 |
| Symptomatic, abnormal saturation with sleep or exercise | 10 (6.1) | 163 |
| Symptomatic, abnormal saturation at rest | 1 (0.6) | 163 |
| Symptomatic, pulmonary hypertension | 6 (3.7) | 164 |
| Ventilator dependent | 1 (4.3) | 164 |
| Transplant | 3 (6.7) | 165 |
| Death | 60 (34.1) | 176 |
In the logistic regression models, three variables were found to be individually associated with mortality after false discovery rate adjustment for multiple comparisons: ICM host, mechanical ventilation, and immunosuppressant therapy (Table E2). PHTN was found not to have an association with mortality, which was contradictory to a similar study evaluating the association between PHTN and mortality within ICT patients. In our cohort, PHTN status was known for 141 patients (91 ICT patients and 50 ICM patients), and 26 patients were noted to have PHTN (24 ICT patients and two ICM patients). To evaluate whether PHTN was associated with mortality within the ICT patients, a further, exploratory analysis was conducted. PHTN was associated with mortality among the ICT patients (odds ratio, 3.808; 95% confidence interval, 1.287–11.264; P = 0.0157).
Twelve patients were clinically ICM but were found to have a pathologic diagnosis that is more typical of ICT patients (Figure 1). Of the 113 with ICT pathologic diagnoses, mortality data were available on 104 (92 of the clinically and pathologically ICT subjects and all 12 of the clinically ICM patients with ICT pathologic diagnoses). Of the patients with both ICT clinical status and pathologic diagnoses, 16.3% died, whereas 58.3% of the patients who were clinically ICM with ICT pathologic diagnoses died. There was a significant difference in mortality between these groups (P = 0.0030). Mortality data were available on 72 of the 101 patients clinically classified as ICM. Thirty-eight of the original ICM subjects (52.8%) died, and there was no significant difference in mortality between the two groups (P = 0.7648).
Discussion
This multicenter study represents the largest systematic analysis of the pathologic spectrum of chILD in children 2 to 18 years of age and the largest analysis of children of all ages with diffuse lung disease when combined with the previously reported study of infants younger than 2 years of age (378 total cases) (7). As in the infant study, the number of biopsies performed in older children varied greatly among the participating institutions (Figure E1). In contrast to the infant study in which more biopsies were done in the youngest infants, in this study more biopsies were performed in older children and adolescents in the 2 to 18 year study group (Figure E2).
The chILD Classification is a clinical–pathologic system designed to interpret lung pathology in a clinical context (7, 11). Because the distribution and spectrum of disease between the <2 and 2 to 18 groups were quite different, the original classification scheme used in the infant study required expansion to account for all the entities encountered in older children. Similarly, in a recent 11-year retrospective pathologic review of 211 chILD cases of all ages from the Royal Brompton Hospital, Rice and colleagues found that the original chILD classification provided a useful template for the diagnosis of children younger than 2 years, but expansion of the categories was necessary to make it applicable to older children (13). In contrast to the single-center Brompton study, which was strictly a pathologic review, our multicenter study included clinical and outcome information that was correlated with pathologic findings.
Patients included in our study had higher mortality than that of previously reported studies in which mortality ranged from 5.9 to 16.1% (12, 14–16). We propose two factors that explain these differences: (1) our study included only biopsied children and (2) our study included both ICM and ICT patients. In the 18-year retrospective review of 93 chILD cases from Vanderbilt Children’s Hospital, Soares and colleagues found that, with newer noninvasive techniques, almost one third could be diagnosed without lung biopsy (16). Compared with the Vanderbilt study, which included biopsied and nonbiopsied children of all ages, the mortality of biopsied children in our combined studies (<2 and 2–18) was twice as high (16.1 vs. 32.3%). We speculate that children with diffuse infiltrates requiring lung biopsy are likely to have greater disease severity than those not requiring biopsy. The inclusion of only biopsied children may have skewed the data to show a higher mortality compared with all children with diffuse lung disease. Previous studies that included only ICT patients had lower mortality (5.9–15.1%) (12, 14, 15, 17). Over 40% of our patients were classified as Disorders of the ICM Host, and these patients suffered greater than 50% mortality, compared with 16.3% mortality in ICT cases. Because Disorders of the ICM Host comprised the largest group with the highest mortality, a revision of the chILD Classification scheme to broadly separate ICM and ICT patients should be considered.
In this study, hypoxemia, tachypnea, and retractions occurred less commonly in children 2 to 18 years of age when compared with previously reported infants <2 years of age (7). This implies that older children may have more insidious symptoms and may present later in disease course than infants. Also, in contrast to the infant study where diffuse developmental and growth disorders, NEHI, pulmonary interstitial glycogenosis, and surfactant dysfunction disorders accounted for more than 50% of cases (7), only a few cases of growth abnormalities, NEHI, and surfactant disorders were found in older children, accounting for only 4% of cases.
The individual risk factors associated with mortality in this study included ICM host, mechanical ventilation, and immunosuppressant therapy. Although PHTN was not a risk factor for death when considering both ICM and ICT patients, it was associated with mortality in an exploratory subanalysis of ICT patients only. This observation is consistent with a previous report demonstrating that ICT children with ILD had a decreased probability of survival when they had PHTN (7, 12).
With the exception of a few cases found that could be classified in the Disorders of Infancy category, diagnoses found in this study overlap with those seen in adults. The one notable exception is that we had no cases of IPF with UIP phenotype. IPF with UIP phenotype is a lethal disease of unknown etiology in older adults characterized pathologically temporal heterogeneity and fibroblastic foci (18). Before the strict pathologic definition was established, there were many cases of IPF, UIP, and cryptogenic fibrosing alveolitis reported in the older pediatric literature (19–22). However, to our knowledge, there have only been three cases of UIP meeting pathologic criteria reported in children in the modern literature. Young and colleagues found UIP pattern in a child with ABCA3 mutations (23), and Campo and colleagues found UIP pattern in a kindred with ABCA3 mutations (24); however, this is not consistent with IPF with UIP phenotypes because of the known surfactant dysfunction mutation. In the Royal Brompton series, one case of UIP was found post mortem in an 18-year-old man before the availability of testing for surfactant mutations and with no clinical information (13). Similar to our study, no IPF/UIP cases were found in the Vanderbilt series (16). Therefore, IPF with UIP phenotype has not been convincingly demonstrated in a child.
This study suffers from its retrospective design. Only subjects who met all inclusion criteria were described within this study, and thus one should not use these findings to estimate incidence or prevalence of disorders within the more general population. Clinicians at each site determined patient characteristics such as PHTN and whether a patient was ICT or ICM rather than strict criteria. We were able to only estimate follow-up time using ages recorded by the clinician rather than using exact dates, and therefore it was not possible to perform survival analysis. We have described factors associated with mortality within this population and that are furthermore subject to the common retrospective study problem of missing information, and therefore we do not assert these findings should be used to estimate mortality risks in the general population. Multiple variables were “unknown.” We included only the predominant pathologic pattern to best classify the diagnoses, but, in doing so, we lost information regarding the minor pattern, which may have provided additional information.
In summary, in children 2 to 18 years of age, the pathologic spectrum of diffuse lung disease overlaps with that seen in adults with some exceptions but differs from that seen in infants. The original chILD Classification Scheme required expansion to account for the variations found in this age group. The most common indication for biopsy was diffuse infiltrates in an ICM host, and this group had the highest mortality. Because all similar studies have been retrospective in design, multicenter prospective studies are needed to address the natural history, approach to diagnosis (including diagnostic imaging and the need for lung biopsy), and potential treatments for these diverse disorders.
Appendix
Institutions contributing cases: Ann & Robert H Lurie Children’s Hospital of Chicago (Chicago, IL), Children’s Hospital Colorado (Aurora, CO), Children’s Hospital of Pittsburgh of UPMC (Pittsburgh, PA), Children’s of Alabama (Birmingham, AL), Cincinnati Children’s Medical Center (Cincinnati, OH), Hospital for Sick Children (Toronto, ON, Canada), Johns Hopkins Children’s Center (Baltimore, MD), Kosair Children’s Hospital (Louisville, KY), Nationwide Children’s Hospital (Columbus, OH), Seattle Children’s Hospital (Seattle, WA), St. Louis Children’s Hospital (St. Louis, MO), and Texas Children’s Hospital (Houston, TX). Members of the chILD Research Co-operative who participated in the current study:
Pathology Working Group
Claire Langston, Houston, TX, Chair
Eric Albright, Columbus, OH
Fred Askin, Baltimore, MD
Pauline Chou, Chicago, IL
Susan Coventry, Louisville, KY
Ernest Cutz, Toronto, ON, Canada
Gail Deutsch, Seattle, WA
Megan Dishop, Aurora, CO
Csaba Galambos, Aurora, CO
Kathleen Patterson, Seattle, WA
Judy Pugh, Birmingham, AL
Susan Wert, Cincinnati, OH
Frances White, St. Louis, MO
Imaging Working Group
Alan Brody, Cincinnati, OH, Chair
Eric Crotty, Cincinnati, OH
Eric Effmann, Seattle, WA
Paul Guillerman, Houston, TX
Fred Long, Columbus, OH
David Lynch, Denver, CO
Clinical Working Group
Robin Deterding, Aurora, CO, Chair
Robert Castile, Columbus, OH
Walter Castro, Houston, TX
Sharon Dell, Toronto, ON, Canada
Minh Doan, Houston, TX
Leland Fan, Aurora, CO
Danny Garcia, Houston, TX
Jim Hagood, San Diego, CA
Aaron Hamvas, St. Louis, MO
Gwen Kerby, Aurora, CO
Geoffrey Kurland, Pittsburgh, PA
Deborah Liptzin, Aurora, CO
George Mallory, Houston, TX
Susanna McColley, Chicago, IL
Ron Morton, Louisville, KY
Larry Nogee, Baltimore, MD
Adrienne Prestridge, Chicago, IL
Greg Redding, Seattle, WA
Kelly Smith, Houston, TX
Stuart Sweet, St. Louis, MO
Bruce Trapnell, Cincinnati, OH
Tim Vece, Houston, TX
Lisa Young, Nashville, TN
Footnotes
This work was supported by Rare Lung Diseases Consortium, National Institutes of Health grants U54 RR019498 (B.C.T.), U54 HL127672 (B.C.T.), and 5T32HL007670 (D.R.L.).
Author Contributions: L.L.F. designed the study, acquired data, interpreted data, wrote, revised, and approved the final manuscript. M.K.D., C.G., F.B.A., and F.V.W. designed the study, acquired data, participated in pathologic review, and approved the final manuscript. C.L. and B.C.T. designed the study and approved the final manuscript. D.R.L. and M.E.K. interpreted data and wrote, revised, and approved the final manuscript. G.H.D., L.R.Y., and R.R.D. designed the study, acquired data, and revised and approved the final manuscript. G.K., J.H., and S.D. designed the study, acquired data, and approved the final manuscript.
This article has an online supplement, which is accessible from this issue's table of contents at www.atsjournals.org
Originally Published in Press as DOI: 10.1513/AnnalsATS.201501-064OC on August 20, 2015
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Clement A, Nathan N, Epaud R, Fauroux B, Corvol H. Interstitial lung diseases in children. Orphanet J Rare Dis. 2010;5:22. doi: 10.1186/1750-1172-5-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Das S, Langston C, Fan LL. Interstitial lung disease in children. Curr Opin Pediatr. 2011;23:325–331. doi: 10.1097/MOP.0b013e3283464a37. [DOI] [PubMed] [Google Scholar]
- 3.Deterding R. Evaluating infants and children with interstitial lung disease. Semin Respir Crit Care Med. 2007;28:333–341. doi: 10.1055/s-2007-981654. [DOI] [PubMed] [Google Scholar]
- 4.Fan LL, Langston C. Pediatric interstitial lung disease: children are not small adults. Am J Respir Crit Care Med. 2002;165:1466–1467. doi: 10.1164/rccm.2204012. [DOI] [PubMed] [Google Scholar]
- 5.Deterding RR, Pye C, Fan LL, Langston C. Persistent tachypnea of infancy is associated with neuroendocrine cell hyperplasia. Pediatr Pulmonol. 2005;40:157–165. doi: 10.1002/ppul.20243. [DOI] [PubMed] [Google Scholar]
- 6.Canakis AM, Cutz E, Manson D, O’Brodovich H. Pulmonary interstitial glycogenosis: a new variant of neonatal interstitial lung disease. Am J Respir Crit Care Med. 2002;165:1557–1565. doi: 10.1164/rccm.2105139. [DOI] [PubMed] [Google Scholar]
- 7.Deutsch GH, Young LR, Deterding RR, Fan LL, Dell SD, Bean JA, Brody AS, Nogee LM, Trapnell BC, Langston C, et al. Pathology Cooperative Group; ChILD Research Co-operative. Diffuse lung disease in young children: application of a novel classification scheme. Am J Respir Crit Care Med. 2007;176:1120–1128. doi: 10.1164/rccm.200703-393OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Deterding RR, Young LR, Dishop MK, Fan LL, Dell S, Sweet SC, Hagood JS, Redding GJ, Castile R, Kurland G, et al. Diffuse lung disease in older children: report of the ChILD Network Review. San Francisco, CA: American Thoracic Society; 2007. [Google Scholar]
- 9.Galambos C, Dishop MK, White FV, Deterding RR, Young LR, Langston C, Group PC. Spectrum of lung disease in immunocompromised children (2–18 years): a multi-institutional of the Children’s Interstitial Lung Disease (ChILD) Research Cooperative. Mod Pathol. 2008;21:209. [Google Scholar]
- 10.Kurland G, Deterding RR, Hagood JS, Young LR, Brody AS, Castile RG, Dell S, Fan LL, Hamvas A, Hilman BC, et al. American Thoracic Society Committee on Childhood Interstitial Lung Disease (chILD) and the chILD Research Network. An official American Thoracic Society clinical practice guideline: classification, evaluation, and management of childhood interstitial lung disease in infancy. Am J Respir Crit Care Med. 2013;188:376–394. doi: 10.1164/rccm.201305-0923ST. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Langston C, Dishop MK. Diffuse lung disease in infancy: a proposed classification applied to 259 diagnostic biopsies. Pediatr Dev Pathol. 2009;12:421–437. doi: 10.2350/08-11-0559.1. [DOI] [PubMed] [Google Scholar]
- 12.Fan LL, Kozinetz CA. Factors influencing survival in children with chronic interstitial lung disease. Am J Respir Crit Care Med. 1997;156:939–942. doi: 10.1164/ajrccm.156.3.9703051. [DOI] [PubMed] [Google Scholar]
- 13.Rice A, Tran-Dang MA, Bush A, Nicholson AG. Diffuse lung disease in infancy and childhood: expanding the chILD classification. Histopathology. 2013;63:743–755. doi: 10.1111/his.12185. [DOI] [PubMed] [Google Scholar]
- 14.Clement A ERS Task Force. Task force on chronic interstitial lung disease in immunocompetent children. Eur Respir J. 2004;24:686–697. doi: 10.1183/09031936.04.00089803. [DOI] [PubMed] [Google Scholar]
- 15.Fan LL, Kozinetz CA, Deterding RR, Brugman SM. Evaluation of a diagnostic approach to pediatric interstitial lung disease. Pediatrics. 1998;101:82–85. doi: 10.1542/peds.101.1.82. [DOI] [PubMed] [Google Scholar]
- 16.Soares JJ, Deutsch GH, Moore PE, Fazili MF, Austin ED, Brown RF, Sokolow AG, Hilmes MA, Young LR. Childhood interstitial lung diseases: an 18-year retrospective analysis. Pediatrics. 2013;132:684–691. doi: 10.1542/peds.2013-1780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Griese M, Haug M, Brasch F, Freihorst A, Lohse P, von Kries R, Zimmermann T, Hartl D. Incidence and classification of pediatric diffuse parenchymal lung diseases in Germany. Orphanet J Rare Dis. 2009;4:26. doi: 10.1186/1750-1172-4-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Travis WD, Costabel U, Hansell DM, King TE, Jr, Lynch DA, Nicholson AG, Ryerson CJ, Ryu JH, Selman M, Wells AU, et al. ATS/ERS Committee on Idiopathic Interstitial Pneumonias. An official American Thoracic Society/European Respiratory Society statement: update of the international multidisciplinary classification of the idiopathic interstitial pneumonias. Am J Respir Crit Care Med. 2013;188:733–748. doi: 10.1164/rccm.201308-1483ST. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zapletal A, Houstĕk J, Samánek M, Copová M, Paul T. Lung function in children and adolescents with idiopathic interstitial pulmonary fibrosis. Pediatr Pulmonol. 1985;1:154–166. doi: 10.1002/ppul.1950010307. [DOI] [PubMed] [Google Scholar]
- 20.Hewitt CJ, Hull D, Keeling JW. Fibrosing alveolitis in infancy and childhood. Arch Dis Child. 1977;52:22–37. doi: 10.1136/adc.52.1.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chetty A, Bhuyan UN, Mitra DK, Roy S, Deorari A. Cryptogenic fibrosing alveolitis in children. Ann Allergy. 1987;58:336–340. [PubMed] [Google Scholar]
- 22.Steinkamp G, Müller KM, Schirg E, von der Hardt H. Fibrosing alveolitis in childhood: a long-term follow-up. Acta Paediatr Scand. 1990;79:823–831. doi: 10.1111/j.1651-2227.1990.tb11561.x. [DOI] [PubMed] [Google Scholar]
- 23.Young LR, Nogee LM, Barnett B, Panos RJ, Colby TV, Deutsch GH. Usual interstitial pneumonia in an adolescent with ABCA3 mutations. Chest. 2008;134:192–195. doi: 10.1378/chest.07-2652. [DOI] [PubMed] [Google Scholar]
- 24.Campo I, Zorzetto M, Mariani F, Kadija Z, Morbini P, Dore R, Kaltenborn E, Frixel S, Zarbock R, Liebisch G, et al. A large kindred of pulmonary fibrosis associated with a novel ABCA3 gene variant. Respir Res. 2014;15:43. doi: 10.1186/1465-9921-15-43. [DOI] [PMC free article] [PubMed] [Google Scholar]