Abstract
Background
Cervical cancer is the 2nd most frequent and top killer cancer among women in Ethiopia. Prevalence and factors associated with visual inspection with acetic acid (VIA) positive result is not studied yet at the study area.
Methods
A cross-sectional study was conducted at Jimma model clinic of Family Guidance Association of Ethiopia, from September 11, 2013 to October 11, 2013. Pertinent data of 334 screened clients were transferred to Epidata version3.1 using checklist, double data entry verification done and exported to SPSS version16.0. After cleaning the data, descriptive analysis was done and logistic regression model employed to identify predictors of VIA positive result. Statistical significance was declared at P < 0.05.
Results
Out of 334 screened clients, 43 (12.9 %) had VIA positive result. Initiation of sexual intercourse earlier than 16 years was found to be an independent predictor increasing the risk of VIA positive by 2.2 times as compared to clients who started at the age of 16 or more years (AOR [95 % CI] = 2.2 [1.1, 4.3]).
Conclusions
Early initiation of sexual intercourse was an independent predictor of VIA positive result in this study. Thus, any cervical cancer prevention and control effort at the study area should address the problem of early initiation of sexual intercourse.
Electronic supplementary material
The online version of this article (doi:10.1186/s13104-015-1594-x) contains supplementary material, which is available to authorized users.
Keywords: Cervical cancer, Visual inspection with acetic acid, VIA positive result, Cryotherapy
Background
Cervical cancer is a disease in which the cells of the cervix become abnormal and start to grow uncontrollably, forming tumors [1]. It is caused by the sexually transmitted human papilloma virus (HPV) infection which has been detected in up to 99 % of women with squamous cervical carcinoma [2]. Young age at first intercourse (<16 years), multiple sexual partners, cigarette smoking and high parity are risk factors for HPV acquisition and cervical cancer.
Cancer of the cervix is the second most common cancer among women worldwide, with about 530,000 new patients diagnosed and over 270,000 deaths every year. It is a major cause of morbidity and mortality among women in low and middle income countries (LMICs) where more than 85 % of the global burden and deaths occur because of poor access to screening and treatment services [3, 4].
In Africa, according to most recent estimates, 80,400 women are diagnosed with cervical cancer every year, the second most frequent cancer. 50,300 die from the disease every year, the leading cause of cancer death. Rates vary substantially across regions, with the incidence and death rates in East Africa, the region Ethiopia belongs to, and West Africa five times as high as the rates in North Africa [5].
In Ethiopia, the annual number of new cervical cancer cases was 4648 and 3235 (69.6 %) die from the disease making it the 2nd most frequent and top killer cancer among women according to an estimate by International agency for research on cancer (IARC) [4]. However, the figures most likely under-represent actual number of cases and deaths, given the low level of awareness, cost, limited access to screening and treatment services and lack of a national cancer registry [6]. In order to address the problem, visual inspection with acetic acid (VIA) and cryotherapy for cervical cancer prevention among people living with HIV/AIDS (PLWHA) had been started in Ethiopia on September 2010 with collaborative effort of Pathfinder International, Federal Ministry of Health (FMOH) of Ethiopia and the Stanford University Program for International Reproductive Education and Services (SPIRES). However, studies on prevalence and factors associated with VIA positive were limited at the study area and this research was primarily designed to address that.
Methods
Family Guidance Association of Ethiopia (FGAE) is one of the leading non-governmental providers of sexual and reproductive health (SRH) care in Ethiopia. FGAE has 20 medium SRH clinics and 27 youth centers across Ethiopia. This study was conducted at Jimma model clinic (JMC), one of the 20 medium SRH clinics of FGAE, Jimma town, 350 km southwest of Addis Ababa, Ethiopia’s capital. The catchment area of the clinic is Jimma town and surrounding districts. The clinic started opportunistic screening of females aged 25–45 years on September 2012 as per cervical cancer prevention guideline for low-resource settings [7]. Thus, after proper counseling of clients aged 25–45 years who came for medical or reproductive health services, those with free will were screened with 5 % acetic acid and test positive cryotherapy eligible clients were treated with cryotherapy while cryotherapy ineligible clients and those with lesions suspicious for cancer were referred to Jimma University specialized hospital (JUSH). Diagnostic criteria were as per cervical cancer prevention guideline for low-resource settings and screening results were defined as:
VIA positive: presence of raised and thickened white plaques or acetowhite epithelium, usually near the Squamo-columnar junction (SCJ).
VIA negative: presence of smooth, pink, uniform and featureless cervix; cervical ectropion; polyp; cervicitis; inflammation; and/or nabothian cyst after applying a dilute solution of acetic acid.
Eligible for cryotherapy: acetowhite lesion <75 % of cervix; lesion does not extend onto the vaginal wall; and lesion extends <2 mm beyond the diameter of the cryoprobe.
Ineligible for cryotherapy: acetowhite lesion >75 % of cervix; lesion extends into the vaginal wall; lesion extends >2 mm beyond the diameter of the cryotip and lesion suspicious for cancer.
Suspicious for cancer: presence of cauliflower-like growth or ulcer; fungating and bleeding mass.
Primary data was registered on standard client evaluation form for cervical cancer prevention service by trained general practioner and nurse. Ethical approval was obtained from ethical review board of Jimma University. A letter of support was obtained from JMC. Client records were treated confidentially and name of clients was not included in the data collection. After checking for integrity and plausibility, data was collected from standard client evaluation form for cervical cancer prevention service on checklist for retrieving data from September 11, 2013 to October 11, 2013 and transferred to Epidata. Double entry verification was also made and the entered data was exported to SPSS version 16.0 for analysis. Descriptive analysis of variables involved was done and Logistic regression was employed for identifying predictors of VIA positive result.
Results
Baseline characteristics of screened clients
A total of 334 clients aged 25–45 years were screened by (VIA) from September 2012 to October 11, 2013 at the study clinic. More than half (51.5 %) of them were in the age range 25–30 years with mean age of 32.4 (SD5.4) years. Most of them were married (73.7 %), multiparous (69.2 %), having only one sexual partner (73.1 %) and had no history of STI (84.7 %). More than half of them were HIV negative (51.2 %) and initiated sexual intercourse at 16 years or older (52.4 %) with mean age at initiation 16.7 (SD3) years. None of the screened clients had ever smoked, used steroid chronically or had abnormal Pap smear previously. Squamo-columnar junction (SCJ) was visible in all the screened clients (Table 1).
Table 1.
Baseline characteristics of clients screened using VIA at Family Guidance Association of Ethiopia, south west area office, Jimma model clinic, Jimma, 2013
| Characteristics | Number (%) |
|---|---|
| Age in years | |
| 25–30 | 172 (51.5) |
| 31–35 | 79 (23.7) |
| 36–40 | 61 (18.3) |
| 41–45 | 22 (6.6) |
| Educational status | |
| Illiterate | 73 (21.9) |
| Primary | 105 (31.4) |
| Secondary | 105 (31.4) |
| Tertiary | 51 (15.3) |
| Marital status | |
| Single | 11 (21.9) |
| Married | 246 (73.7) |
| Divorced | 38 (11.4) |
| Widowed | 23 (6.9) |
| Separated | 16 (4.8) |
| Parity | |
| Nulliparous | 31 (9.3) |
| Primiparous | 72 (21.6) |
| Multiparous | 231 (69.2) |
| Age at first intercourse | |
| <16 | 148 (44.3) |
| 16 | 175 (52.4) |
| Unknown | 11 (3.3) |
| Current contraceptive | |
| OCP | 20 (6) |
| DEPO | 57 (17.1) |
| Implanon | 9 (2.7) |
| Jadelle | 6 (1.8) |
| IUCD | 6 (1.8) |
| Condom | 7 (2.1) |
| BTL | 2 (0.6) |
| Dual contraception | 8 (1.5) |
| None | 222 (66.5) |
| STI history | |
| Yes | 51 (15.3) |
| No | 283 (84.7) |
| HIV sero-status | |
| Unknown | 28 (8.4) |
| Negative | 171 (51.2) |
| Positive | 135 (40.4) |
| HIV positives | |
| On HAART | 110 (81.5) |
| Not on HAART | 25 (18.5) |
| No of sexual partners | |
| One | 244 (73.1) |
| Multiple | 90 (26.9) |
| Yes | 334 (100) |
| SCJ seen | |
| No | 0 |
Prevalence of VIA positive result
Of 334 screened clients, 43 (12.9 %) were found to have VIA positive result while 287 (85.9 %) had negative test result. The remaining four (1.2 %) were found to have lesions suspicious for cancer. Fourty-two of the 43 (97.7 %) clients with positive VIA test result were eligible for cryotherapy while one of the clients with positive VIA test result had lesion larger than cryoprobe by greater than 2 mm and thus not eligible. Thus, 42 clients with positive VIA test result who were eligible for cryotherapy were treated at the clinic while one of the clients who is ineligible for cryotherapy is referred to JUSH along with the four patients who had lesions suspicious for cancer (Table 2).
Table 2.
VIA test result, cryotherapy eligibility and reasons for referral among clients screened at Family Guidance Association of Ethiopia, south west area office, Jimma model clinic, Jimma, 2013
| Characteristics | Number (%) |
|---|---|
| VIA test result | |
| Positive | 43 (12.9) |
| Negative | 287 (85.9) |
| Suspicious for cancer | 4 (1.2) |
| Cryotherapy eligibility | |
| Eligible | 42 (97.7) |
| Ineligible | 1 (2.3) |
| Reasons for referral | |
| Suspicious for cancer | 4 (80) |
| Lesion larger than cryoprobe >2 mm | 1 (20) |
Factors associated with VIA positive result
Age at onset of intercourse and HIV-status were unknown in 11 and 28 screened clients respectively while both age at onset of intercourse and HIV-status was unknown in 3 resulting in exclusion of 36 screened clients from logistic regression analysis. Thus, of the 334 clients screened at the clinic, only 298 were eligible for logistic regression analysis (Fig. 1). Significant association was observed on bivariate logistic regression between VIA positive and age of clients and age at first intercourse. On multivariable logistic regression, clients who started intercourse at less than 16 years were 2.2 times (AOR [95 % CI] 2.2 [1.1, 4.3]) more likely to have VIA positive as compared to those who started intercourse at the age of 16 or more years (Table 3). However, history of STI, number of sexual partners, HIV-status and HAART-status were not found to be predictive of VIA positive in this study.
Fig. 1.
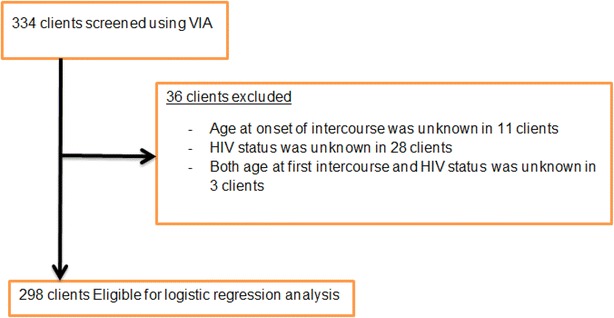
Profile of clients screened using VIA at Family Guidance Association of Ethiopia, south west area office, Jimma model clinic, Jimma, 2013. Three hundred and thirty-four clients were screened using VIA at Jimma model clinic. Age at onset of intercourse and HIV-status were unknown in 11 and 28 screened clients respectively while both age at onset of intercourse and HIV-status were unknown in 3 resulting in exclusion of a total of 36 screened clients from logistic regression analysis. Thus, only 298 were eligible for logistic regression analysis
Table 3.
Logistic regression analysis of factors associated with VIA positive at Family Guidance Association of Ethiopia, south west area office, Jimma model clinic, Jimma, 2013
| Covariates | VIA result | |||
|---|---|---|---|---|
| Positive, no. (%) | Negative, no. (%) | COR [95 % CI] | AOR [95 % CI] | |
| Age | ||||
| 25–30 | 30 (17.8) | 139 (82.2) | 1 | 1 |
| 31–35 | 8 (10.1) | 71 (89.9) | 0.5 [0.2, 1.1] | 0.5 [0.2, 1.2] |
| 36–40 | 3 (5) | 57 (95) | 0.3 [0.1, 0.9]* | 0.4 [0.1, 1.1] |
| 41–45 | 2 (9.1) | 20 (90.9) | 0.4 [0.1, 1.9] | 0.5 [0.1, 2.3] |
| Age at first intercourse | ||||
| 16 years | 17 (9.7) | 158 (90.3) | 1 | 1 |
| <16 years | 22 (15.3) | 122 (84.7) | 2 [1.1, 4.0]* | 2.2 [1.1, 4.3]* |
| Current use contraceptive | ||||
| No | 25 (11.4) | 194 (88.6) | 1 | 1 |
| Yes | 18 (16.2) | 93 (83.8) | 1.4 [0.8, 2.7] | 1.7 [0.9, 3.3] |
| History of STI | ||||
| No | 35 (12.5) | 245 (87.5) | 1 | 1 |
| Yes | 8 (16) | 42 (84) | 1.3 [0.6, 3.1] | 1.6 [0.7, 3.6] |
| Number of sexual partners | ||||
| One | 32 (13.3) | 209 (86.7) | 1 | 1 |
| Multiple | 11 (12.4) | 78 (87.6) | 0.9 [0.5, 2.9] | 0.7 [0.3, 1.6] |
| HIV sero-status | ||||
| Negative | 20 (11.9) | 148 (88.1) | 1 | 1 |
| Positive | 21 (15.7) | 113 (84.3) | 0.8 [0.4, 1.5] | 1.2 [0.6, 2.6] |
| HIV positives | ||||
| Not on HAART | 2 (8.3) | 22 (91.7) | 1 | 1 |
| On HAART | 19 (17.3) | 91 (82.7) | 0.7 [0.2, 2.4] | 0.7 [0.2, 2.6] |
Discussion
Of 334 screened clients, 12.9 % had VIA positive and those who started sexual intercourse earlier than 16 years were 2.2 times at higher risk as compared to those who started sex at the age of 16 or more. The mean age at initiation of intercourse was 16.7 (SD = 3) years and this is comparable with median age at first intercourse for women aged 25–49 years both in Oromia (17 years) and Ethiopia (16.6 years) [8].
The prevalence of VIA positive at the study clinic (12.9 %) was similar to study finding from central Ethiopia among PLWHA’s (11 %) [9] but lower than study finding from southern Ethiopia among PLWHA’s (22.1 %) [10]. It was also similar to study finding from Madagascar (11.3 %) [11], Malawi (12.4 %) [11], Latin America (12 %) [12] and Thailand (13.3 %) [13] though it is lower than study findings from Nigeria (16 %) [14], Sudan (16 %) [15] and Bangladesh (18 %) [16]. However, it is higher than study findings from Uganda (7.8 %) [11] and Tanzania (9.7 %) [11]. The difference in prevalence could be due to the differences in the age of study populations [10], as evidenced by the two Ethiopian studies among PLWHA in which one used the age group 30–45 years [9] while the other used 18 years and older [10]. It may also be due to differences in test providers skills [17, 18] and underlying prevalence of other sexually transmitted infections [18]. The higher prevalence in Sudan [15], Nigeria [14] and Bangladesh [16] is most probably due to a lower sample size in the study, 100, 125 and 44 respectively, although provision of the service by laywomen, poor test providers skills, has also contributed to the finding of Bangladesh study. Further, VIA also inherently suffers from the same challenges as other visual interpretation methods including colposcopy and cytology as evidenced by Indian study where VIA positivity rate varied from 4 % to 31 % among the six gynecologists who performed the test [17].
Early initiation of intercourse increased the risk of VIA positive by 2.2 times which is similar to study from Brazil (OR [95 % CI] 1.97 [1.18–3.3]) [19], Nigeria (OR [95 % CI] 3.7 [1.07–12.8]) [14] and India (OR [95 % CI] 3.5 [1.1–10.9]) [20]. Early onset of sexual activity is thought to be associated with high risk because, during puberty, cervical tissue undergoes physiologic changes, transformation zone on the ectocervix is enlarged, and exposure to HPV at such times may facilitate infection which may make this area more vulnerable to development of dysplasia, a cervical squamous precancer [21]. Kenyan study has also showed higher risk of VIA positive among HIV patients (AOR [95 % CI] 4.8 [1.8–12.4]), those having multiple sexual partners (AOR [95 % CI] 3.8 [1.1–13.5]) [22] and HIV patients not on antiretroviral therapy (HAART) (AOR [95 % CI] 2.21 [1.28–3.83]) [23]. Tanzanian study showed higher risk of VIA positive among widowed/separated (OR [95 % CI] 1.41 [1.17–1.66]) and grand multipara women (OR [95 % CI] 3.19 [1.84–5.48]) [24]. South Ethiopian study reported higher risk among those with history of sexually transmitted disease (AOR [95 % CI] 2.30 [1.23, 4.29]) [10]. However, age of the client, history of STI, number of sexual partners, HIV-status and HAART-status were not found to be predictive of VIA positive in this study.
The role of HIV in precancerous cervical lesion is thought to be mediated through immune suppression [1]. Thus, prompt initiation of HAART through an early enrollment into care has an impact on reducing the prevalence and progression of cervical precancerous lesions [23]. Women who are separated or widowed may have higher number of lifetime sexual partners in comparison with married women and as number of lifetime sexual partners increases, the risk of HPV infection increases and thus they are more susceptible for developing precancerous lesions [25]. High parity increases the risk of precancerous cervical lesions most likely due to repeated cervical trauma during consecutive births and hormonal adjustment during and after pregnancies which may create an entry point for the HPV virus [26]. History of sexually transmitted disease increases the risk of precancerous cervical lesions due to the sexually transmitted nature of HPV infection [27].
Conclusions
In this study, 12.9 % of screened clients had VIA positive and early initiation of intercourse was found to be an independent predictor increasing the risk by 2.2 times. Thus, there is a need to introduce HPV vaccination for girls aged 9–13 years, advocate for the norm of virginity till marriage, avoid early age at marriage, promote delaying of age at initiation of sexual intercourse, give sexuality education tailored to age and culture and promote and provide condom for those engaged in sexual activity in addressing early initiation of sexual intercourse (Additional files 1, 2).
Authors’ contributions
ZM conceived and designed the study, collected data, did statistical analysis and wrote the manuscript. FA and HA designed the study and reviewed the manuscript. All authors read and approved the final manuscript.
Acknowledgements
Our heartfelt thanks goes to Mr. Ahmed Kedir, Jimma model clinic manager, for giving a letter of support to conduct the research and facilitating the data collection from standard client evaluation form for cervical cancer prevention service.
Competing interests
The authors declare that they have no competing interests.
Abbreviations
- AIDS
acquired immuno-deficiency syndrome
- AOR
adjusted odds ratio
- CI
confidence interval
- COR
crude odds ratio
- FGAE
Family Guidance Association of Ethiopia
- FMOH
Federal Ministry of Health
- HAART
highly active antiretroviral therapy
- HIV
human immunodeficiency virus
- HPV
human papilloma virus
- JMC
Jimma model clinic
- JUSH
Jimma University specialized hospital
- LMICs
low and middle income countries
- OR
odds ratio
- PLWHA
people living with HIV/AIDS
- SCJ
squamo-columnar junction
- SD
standard deviation
- SPIRES
Stanford University Program for International Reproductive Education and Services
- STI
sexually transmitted infections
- VIA
visual inspection with acetic acid
Additional files
10.1186/s13104-015-1594-x Standard Client Evaluation form for Cervical Cancer Prevention service.
10.1186/s13104-015-1594-x Family Guidance Association of Ethiopia (FGAE), Jimma model clinic, checklist for retrieving data from standard Client Evaluation form for Cervical Cancer Prevention service: September 2013.
Contributor Information
Zewdie Mulissa Deksissa, Email: zmulissa@yahoo.com.
Fessahaye Alemseged Tesfamichael, Email: fessahayeat@yahoo.com.
Henok Assefa Ferede, Email: menothen@gmail.com.
References
- 1.Berek JS, editor. Berek and Novak’s gynecology. 14th ed. Stanford: Lippincott Williams & Wilkins; 2007.
- 2.Walboomers JMJ, Manos MM. Human papillomavirus is a necessary cause of invasive cervical cancer worldwide. J Pathol. 1999;189:12–19. doi: 10.1002/(SICI)1096-9896(199909)189:1<12::AID-PATH431>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- 3.WHO. Comprehensive cervical cancer prevention and control: a healthier future for girls and women. Geneva, Switzerland; 2013.
- 4.Ferlay JS, Bray F, Forman D, Mathers C. GLOBOCAN 2008: cancer Incidence and Mortality Worldwide in 2008. Lyon, France: International Agency for Research on Cancer. 2010; 2013. Available from: http://globocan.iarc.f.
- 5.Castellsagué XS, Aguado T, Louie KS, Bruni L, Muñoz J, et al. HPV and Cervical Cancer in the World: WHO/ICO Information Centre on HPV and Cervical Cancer (HPV Information Centre); 2007.
- 6.Addis Tesfa: May 2011 Project Brief. Pathfinder International; 2011.
- 7.Jhpiego. Cervical cancer prevention guidelines for low-resource settings. Baltimore, Maryland; 2005.
- 8.Ethiopia demographic and health survey 2011. Addis Ababa, Ethiopia: central statistical agency, Ethiopia, ICF international calverton, Maryland; 2012.
- 9.Adnew SS-D, Blumenthal PD, Shiferaw N, Ansel J, Sisay G. Introducing cervical cancer prevention using visual inspection with acetic acid (VIA) and cryotherapy among HIV-positive women: new hope for Ethiopian women. International AIDS conference Washington DC; 2012.
- 10.Gedefaw A, Astatkie A, Tesema GA. The prevalence of precancerous cervical cancer lesion among HIV-infected women in Southern Ethiopia: a cross-sectional study. PLoS One. 2013;8(12):e84519. doi: 10.1371/journal.pone.0084519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.WHO. Prevention of cervical cancer through screening using visual inspection with acetic acid (VIA) and treatment with cryotherapy. A demonstration project in six African countries: (Malawi, Madagascar, Nigeria, Uganda, the United Republic of Tanzania, and Zambia). Geneva; 2012.
- 12.Sarian LOD, Naud P, Roteli-Martins C, Longatto-Filho A, Tatti S, et al. Evaluation of visual inspection with acetic acid (VIA), Lugol’s iodine (VILI), cervical cytology and HPV testing as cervical screening tools in latin America. J Med Screen. 2005;12(3):142–149. doi: 10.1258/0969141054855328. [DOI] [PubMed] [Google Scholar]
- 13.RTCOG/JHPIEGO Safety, acceptability, and feasibility of a single-visit approach to cervical-cancer prevention in rural Thailand: a demonstration project. Lancet. 2003;61:814–820. doi: 10.1016/s0140-6736(03)12707-9. [DOI] [PubMed] [Google Scholar]
- 14.Ogunbowale TL. Cervical cancer risk factors and predictors of cervical dysplasia among women in south-west Nigeria. Austral J Rural Health. 2008;16(6):338–342. doi: 10.1111/j.1440-1584.2008.01013.x. [DOI] [PubMed] [Google Scholar]
- 15.Ibrahim AR, Pukkala E, Aro AR. Cervical cancer risk factors and feasibility of visual inspection with acetic acid screening in Sudan. Int J Womens Health. 2011;3:117–122. doi: 10.2147/IJWH.S14189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bradford LSD, Hussain SMA, Begum SR, Hussain F, Hoque S, et al. Development of a cervical cancer screening program in a slum setting using visual inspection with acetic acid: analysis of feasibility and cost. Open J Obstet Gynecol. 2012;2:140–146. doi: 10.4236/ojog.2012.22027. [DOI] [Google Scholar]
- 17.Vedantham HS, Kalpana B, Rekha C, Karuna BP, Vidyadhari K, et al. Determinants of VIA (Visual inspection of the cervix after acetic acid application) positivity in cervical cancer screening of women in a peri-urban area in Andhra Pradesh, India. Cancer Epidemiol Biomark Prev. 2010;19(5):1373–1380. doi: 10.1158/1055-9965.EPI-09-1282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mahé CG. Screening test accuracy studies: how valid are our conclusions? Application to visual inspection methods for cervical screening. Cancer Causes Control. 2005;16(6):657–666. doi: 10.1007/s10552-005-0296-4. [DOI] [PubMed] [Google Scholar]
- 19.Ferreirada SIK, Quinto SSC, Ferreirade ANO, Koifman S. TP53 genetic polymorphisms and environmental risk factors associated with cervical carcinogenesis in a cohort of Brazilian women with cervical lesions. Toxicol Environtal Health. 2010;73(13–14):888–900. doi: 10.1080/15287391003744823. [DOI] [PubMed] [Google Scholar]
- 20.Biswas LNM, Maiti PK, engupta S. Sexual risk factors for cervical cancer among rural Indian women: a case–control study. Int J Epidemiol. 1997;26(3):491–495. doi: 10.1093/ije/26.3.491. [DOI] [PubMed] [Google Scholar]
- 21.WHO. Comprehensive cervical cancer control: a guide to essential practice. Geneva; 2006.
- 22.Temmerman MT, Kidula N, Claeys P, Muchiri L, Quint W. Risk factors for human papilloma virus and cervical precancerous lesions, and the role of concurrent HIV-1 infection. Int J Gynaecol Obstet. 1999;65(2):171–181. doi: 10.1016/S0020-7292(99)00043-0. [DOI] [PubMed] [Google Scholar]
- 23.Memiah PM, Kiiru G, Agbor S, Odhiambo F, Ojoo S et al. Prevalence and risk factors associated with precancerous cervical cancer lesions among HIV-infected women in resource-limited settings. AIDS Res Treat. 2012. [DOI] [PMC free article] [PubMed]
- 24.Kahesa CK, Ngoma T, Mwaisejage J, Dartell M, Iftner T, et al. Risk factors for VIA positivity and determinants of screening attendances in Dar es Salaam, Tanzania. BMC Public Health. 2012;12:1055. doi: 10.1186/1471-2458-12-1055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ho, G YFS, Bierman R, Burk RD. Natural history of human papillomavirus type 16 virus-like particle antibodies in young women. Cancer Epidemiol Biomark Prev. 2004;13(1):110–116. doi: 10.1158/1055-9965.EPI-03-0191. [DOI] [PubMed] [Google Scholar]
- 26.Maribel AC, Miguel G, Manuel DJ, Hilary BC, Silvana L, et al. Risk factors for high-risk human papillomavirus infection and cofactors for high-grade cervical disease in Peru. Int J Gynecol Cancer. 2011;21(9):1654–1663. doi: 10.1097/IGC.0b013e3182288104. [DOI] [PubMed] [Google Scholar]
- 27.Mbulaiteye SMB, Adebamowo C, Sasco AJ. HIV and cancer in Africa: mutual collaboration between HIV and cancer programs may provide timely research and public health data. Infect Agent Cancer. 2011;6(1):16–18. doi: 10.1186/1750-9378-6-16. [DOI] [PMC free article] [PubMed] [Google Scholar]


