Abstract
Background
To identify patient and procedural characteristics associated with postoperative respiratory depression or sedation that required naloxone intervention.
Methods
We identified patients who received naloxone to reverse opioid-induced respiratory depression or sedation within 48 hours after discharge from anesthetic care (transfer from the post anesthesia care unit, or transfer from the operating room to postoperative areas) between July 1, 2008 and June 30, 2010. Patients were matched to two controls based on age, sex, and exact type of procedure performed during the same year. A chart review was performed to identify patient, anesthetic and surgical factors that may be associated with risk for intervention requiring naloxone. In addition, we identified all patients who developed adverse respiratory events [hypoventilation, apnea, oxyhemoglobin desaturation, pain/sedation mismatch] during Phase I anesthesia recovery. We performed conditional logistic regression taking into account the 1:2 matched set case-control study design to assess patient and procedural characteristics associated with naloxone use.
Results
We identified 134 naloxone administrations, 58% within 12 hours of discharge from anesthesia care, with incidence of 1.6 per 1,000 (95% CI 1.3 – 1.9) anesthetics. Presence of obstructive sleep apnea (odds ratio = 2.45, 95%CI 1.27-4.66, P = 0.008), and diagnosis of adverse respiratory event in postanesthesia recovery room (odds ratio = 5.11, 95%CI 2.32-11.27, P < 0.001) were associated with increased risk for requiring naloxone to treat respiratory depression or sedation following discharge from anesthesia care. Following discharge from anesthesia care, patients administered naloxone used a greater median dose of opioids (10 [interquartile range 0, 47.1] vs. 5 [0, 24.8] intravenous morphine equivalents, P = 0.020) and more medications with sedating side effects (N = 41 [31%] vs. 24 [9%], P<0.001).
Conclusion
Obstructive sleep apnea and adverse respiratory events in recovery room are harbingers of increased risk for respiratory depression or sedation requiring naloxone after discharge from anesthesia care. Also, patients administered naloxone received more opioids and other sedating medications after discharge from anesthetic care. Our findings suggest that these patients may benefit from more careful monitoring after being discharged from anesthesia care.
Keywords: Postoperative complications: respiratory depression, respiratory specific events, obstructive sleep apnea, naloxone, opioids
Introduction
Etiologies for postoperative respiratory failure can be broadly categorized into either hypoventilatory or hypoxic respiratory failure. In the general surgical adult patient, hypoventilatory respiratory failure is typically thought to be secondary to oversedation from anesthetic and analgesic medications, preexisting sleep disordered breathing conditions (e.g., obstructive sleep apnea), or a combination as patients with OSA are more sensitive to respiratory depression from these medications. OSA is a common sleep breathing disorder, with an estimated prevalence of 17% and 9% among middle-aged men and women, respectively,1 with approximately 90% of cases undiagnosed.2 Mirroring the obesity epidemic, there has been a substantial increase in the prevalence of OSA in the general population.1
Pain management standards set by the Joint Commission on Accreditation of Healthcare Organizations3 have emphasized the importance of adequate pain control in hospitalized patients. These standards have resulted in substantial increase in perioperative opioid administration to control postoperative pain,4 as well as an increase in the incidence of opioid-induced oversedation.5 The Institute for Safe Medication Practices has raised concerns that this practice shift has resulted in increased number of cases of oversedation and fatal respiratory depression events.6 Thus clinicians are faced with the need to balance the competing priorities of delivering adequate postoperative pain control with avoidance of opioid induced side effects7 especially in patients with a high prevalence of OSA.
Though there are numerous methods for assessing opioid induced respiratory depression,8 naloxone administration has been proposed as a surrogate marker for an episode of over-narcotization.9 Several studies have examined preoperative risk factors and the administration of naloxone in the postoperative period since the publication of Joint Commission standards.9-11 These have found that older age, presence of comorbidities such as obstructive sleep apnea (OSA), and use of medications such as benzodiazepines were all associated with increased risk for postoperative naloxone administration.
As an added layer of safety for patients recovering from anesthesia during Phase I recovery, our institution has adopted a formal screening process for 4 types of adverse respiratory events: hypoventilation, apnea, oxyhemoglobin desaturation, and pain/sedation mismatch and termed them respiratory specific events.12-13 In our institution, patients who experience these events were found to have more postoperative respiratory complications and more episodes of oxyhemoglobin desaturations.12-13 However, whether or not these events are associated with increased rate of respiratory depression or excessive sedation has not been studied. Our primary aim was to assess whether preclinical characteristics and various perioperative variables, including respiratory specific events during Phase I recovery, were associated with naloxone administration following discharge from anesthesia care (within in 48 hours of dismissal from post anesthesia care unit or transfer from the operating room to postoperative area).
Methods
This study was approved by the Mayo Clinic, Rochester MN, Institutional Review Board. Consistent with Minnesota Statute 144.295, we included only patients who have provided authorization for research use of their medical records (historically >95% of Mayo Clinic patients).14
Study design
This study employed a retrospective case control design that assessed patient and procedural characteristics associated with the requirement of postoperative naloxone administration.
Study setting
This study was set at a large academic tertiary care facility.
Population studied
An electronic search was performed of the institutional medical records from July 1, 2008 to June 30, 2010 to identify adult patients who: 1) underwent general anesthesia whose tracheas were extubated either in the operating room or in post anesthesia recovery unit, and 2) received naloxone within 48 hours of dismissal from anesthetic care (dismissal from post anesthesia care unit or transfer from the operating room to a postoperative area [i.e., postsurgical ward, intensive care unit, progressive care unit]).15-16 Patients administered naloxone in the operating room, procedural room or postanesthesia care unit were excluded. Patients who remained intubated following dismissal from anesthetic care and transferred to an intensive care unit were also excluded. Patients were also excluded if naloxone was administered to treat opioid-induced pruritus. The 48-hour time window was selected to allow identification of risk factors directly related to the perioperative course. For each patient who was administered naloxone, we used our medical record database to identify two control patients who underwent general anesthesia in the same year, but who did not receive naloxone in the first 48 hours following dismissal from anesthesia care. The cases and controls were matched on age, sex and exact type of procedure (based on ICD-9 procedure code).
Data abstraction
Electronic medical records were abstracted for demographics, comorbid conditions, preoperative, intraoperative and postoperative variables, postoperative course and complications. Overall physical status was assessed from the American Society of Anesthesiologists Physical Status Score (ASA-PS). Comorbid conditions were defined as cardiovascular disease: coronary artery disease (myocardial infarction, coronary stent placement or cardiac bypass surgery), congestive heart failure (or EF <40%), moderate to severe valvular disease, or peripheral vascular disease; OSA; pulmonary disease: chronic obstructive pulmonary disease or asthma; use of home oxygen; or other severe pulmonary disease (i.e., malignancy, restrictive pulmonary disease, or pulmonary hypertension); neurologic disease: cerebrovascular disease (i.e., stroke, previous carotid surgery), dementia (i.e., Alzheimer's disease), movement disorders (i.e., Parkinson's disease), or malignancy; diabetes mellitus (on oral agents or insulin); and preoperative use of benzodiazepines or opioids. Preoperative use of benzodiazepines and opioids were defined as regular (chronic) use of these medications on the medical record, and does not include limited use of these medications for immediate preoperative anxiolysis or analgesia.
The anesthetic and surgical records were reviewed for surgical procedure; anesthetic technique (general anesthesia with or without neuraxial analgesia) and duration of anesthesia; benzodiazepine, ketamine use and systemic opioid analgesics were recorded (during procedure and phase I recovery). The doses of systemic opioid analgesics were converted to intravenous morphine equivalents (ME) using published guidelines.17-18 If used, the dose of remifentanil was not considered for calculating intravenous morphine equivalents because of its ultrashort duration. Because of different pharmacokinetic profiles between the lipophilic opioid fentanyl and other commonly used opioids, a separate variable was created to indicate those patients who received any “long-acting” opioids (i.e., hydromorphone, morphine, or oxymorphone); however in these patients fentanyl was also taken into account for ME calculations. For those patients who were admitted to the postanesthesia care unit (PACU) for phase I recovery, we reviewed the duration of PACU stay for the occurrence of respiratory specific events. During Phase I recovery, registered nurses continuously monitored patients for 4 types of respiratory specific events: hypoventilation (3 episodes of < 8 respirations/minute); apnea (episode of apnea ≥ 10 seconds); hypoxemia (3 episodes of oxyhemoglobin desaturations as measured by pulse oximetry [<90% with or without nasal cannula]); and “pain/sedation mismatch” (defined as Richmond Agitation Sedation Score19 = -3 – -5 and a numeric pain score > 5 [from a scale 0 to 10 {worst pain imaginable}]).12-13 Any patient who has a respiratory specific event must have a subsequent sixty minute period free of further events in order to be transferred to a non-monitored ward. Patients who had repeated events were discharged to an advanced monitored care setting or were continuously monitored for oxyhemoglobin desaturation via a pulse oximeter monitored by telemetry. Otherwise discharge criteria for Phase I recovery were based on standard discharge criteria.20
For patients who received naloxone, postoperative opioids were recorded from the time of discharge from anesthetic control to the time of naloxone administration. For control patients, the perioperative opioid administration from the time of anesthesia discharge to the time naloxone administration for the matched case was recorded. Postoperative opioids were then converted to intravenous ME and adjusted for time by dividing by the number of hours between anesthesia discharge to naloxone administration (or corresponding time for control patients) (IV ME mg/hr). In addition to postoperative opioids, the postoperative administration of other medications with sedating properties (benzodiazepines, gabapentinoids [gabapentin, pregabalin], sleep aides [zolpidem, tricyclic or sedating antidepressants], spasmolytics [cyclobenzaprine], and antihistamines) was recorded. If naloxone administration was recorded, primary indication for administration was recorded and categorized as treatment for isolated respiratory depression, over sedation, or cardiovascular collapse. Other interventions in addition to administration of naloxone were also recorded.
Outcomes
Postoperative complications that occurred within the first 30 postoperative days of the surgery were reported. Information was obtained from medical records from index hospitalization, re-hospitalization, or outpatient visits. Postoperative complications included: myocardial infarction, cerebrovascular event, sepsis or multiorgan failure, or death. Hospital length of stay and 30-day mortality was recorded.
Statistical Analyses
Data are summarized using mean ± SD or median (25th, 75th percentiles) for continuous variables and frequency percentages for nominal variables. Of patients who received naloxone, characteristics were compared between full unit (0.4 mg) and titrated doses (<0.4 mg) using Fisher's exact test or Chi Square test. Analyses to assess characteristics potentially associated with naloxone use were performed using conditional logistic regression taking into account the 1:2 matched set case-control study design. Preoperative characteristics listed in Table 1 with evidence of an association in the univariate analysis (P ≤ 0.05) and all perioperative characteristics listed in Table 2 were assessed with the exception of age, sex and type of procedure since these were matching variables in the study design. Body mass index, anesthesia duration, and opioid dose (intraoperative and recovery room) were modeled as continuous variables and all other characteristics were modeled as nominal variables. The variable indicating intravenous opioid dose represents the ME in mg administered intraoperatively and during Phase I recovery and includes both fentanyl and long-acting opioids. To account for differences in the pharmacokinetics of fentanyl and long-acting opioids, an additional variable was introduced indicating whether patients received any long-acting opioid. For the continuous variables, the assumption of linearity in the logit was assessed using the Box-Tidwell test, and no evidence of non-linearity was detected. In addition to assessing each characteristic individually (univariate analyses), two multivariable analyses were performed. For the first multivariable analysis, all 134 matched sets were included and all preoperative characteristics that were found to be statistically significant on univariate analysis were included as explanatory variables in the model along with anesthetic technique and duration, any use of benzodiazepines, any use of ketamine, opioid dose, and whether any long-acting opioid was used. The 2nd multivariable analysis was restricted to 122 matched sets where the case and at least 1 matched control went to the PACU and included an additional binary explanatory variable indicating whether or not the patient experienced respiratory events in the PACU. Two-tailed P-values are reported and are not adjusted for multiple comparisons. Analyses were performed using SAS statistical software (Version 9.2, SAS Institute, Inc., Cary, NC).
Table 1.
Preoperative characteristics of patients who received naloxone within 48 hours of discharge from anesthetic care and matched controls.
| Naloxone N = 134 | No Naloxone N = 268 | P‡ | |
|---|---|---|---|
| Age (years) | 65.4 ± 14.7 | 64.6 ± 14.1 | ---- |
| Male sex | 56 (41.8) | 112 (41.8) | ---- |
| ASA physical status | 3 [2, 3] | 2 [2, 3] | 0.066 |
| Body mass index (kg/m2) | 28.9 ± 6.8 | 27.6 ± 5.9 | 0.040 |
| Cardiovascular disease | 46 (34.3) | 64 (23.9) | 0.013 |
| Coronary artery disease | 33 | 45 | |
| Congestive heart failure | 7 | 11 | |
| Valvular disease | 4 | 5 | |
| Peripheral vascular disease | 13 | 20 | |
| Obstructive sleep apnea | 35 (26.1) | 32 (11.9) | <0.001 |
| Pulmonary disease | 33 (24.6) | 37 (13.8) | 0.005 |
| Chronic obstructive pulmonary disease or asthma | 24 | 22 | |
| Use of home oxygen | 6 | 5 | |
| Other pulmonary disease* | 10 | 18 | |
| Neurologic disease | 22 (16.4) | 25 (9.3) | 0.034 |
| Stroke | 7 | 14 | |
| Dementia | 8 | 2 | |
| Movement disorders | 6 | 7 | |
| Malignancy | 40 (29.9) | 92 (34.3) | 0.203 |
| Diabetes mellitus | 32 (23.9) | 48 (17.9) | 0.146 |
| Preoperative chronic use of benzodiazepines | 20 (14.9) | 20 (7.5) | 0.023 |
| Preoperative chronic use of opioids | 32 (23.9) | 51 (19.0) | 0.229 |
Data presented as mean ± standard deviation, median [interquartile range], or number (percentage) as appropriate.
P-value from conditional logistic regression taking into account the 1:2 matched set study design. P-values are not provided for age and sex since these were matching variables.
Pulmonary malignancy, severe restrictive lung disease, pulmonary hypertension.
Abbreviation: ASA = American Society of Anesthesiologists
Table 2.
Characteristics of patients who received naloxone within 48 hours of discharge from anesthetic care and matched controls.
| Naloxone N = 134 | No Naloxone N = 268 | P* | |
|---|---|---|---|
|
| |||
| Surgical specialty† | ---- | ||
|
| |||
| General | 42 (31.3) | 84 (31.3) | |
|
| |||
| Urological/gynecological | 18 (13.4) | 36 (13.4) | |
|
| |||
| Neurosurgical | 5 (3.7) | 10 (3.7) | |
|
| |||
| Orthopedic | 43 (32.1) | 86 (32.1) | |
|
| |||
| Thoracic | 20 (14.9) | 40 (14.9) | |
|
| |||
| Vascular | 6 (4.5) | 12 (4.5) | |
|
| |||
| Anesthetic technique | 0.065 | ||
|
| |||
| General | 116 (86.6) | 244 (91.0) | |
|
| |||
| General with neuraxial analgesia | 18 (13.4) | 24 (9.0) | |
|
| |||
| Anesthetic duration (minutes) | 233 [170,328] | 209 [159,282] | 0.012 |
|
| |||
| Medication administration‡ | |||
|
| |||
| Midazolam | 84 (62.7) | 185 (69.0) | 0.150 |
| Midazolam, mg | 2 [2, 2] | 2 [2, 2] | 0.613 |
|
| |||
| Ketamine | 17 (12.7) | 23 (8.6) | 0.179 |
| Ketamine, mg | 10 [10, 25] | 10 [10, 20] | 0.721 |
|
| |||
| Long-acting opioids§ | 108 (80.6) | 194 (72.4) | 0.040 |
| Intraoperative and Phase I recovery opioid ME, mg‖ | 40.3 [28.0, 51.8] | 38 [25.0, 53.0] | 0.346 |
| Intraoperative opioid ME, mg | 32.75 [20.8, 40.3] | 31.0 [20.5, 44.1] | 0.807 |
| Phase I recovery opioid ME, mg | 7.5 [0, 15] | 5 [0, 12.5] | 0.022 |
|
| |||
| Transferred to PACU** | 123 (91.8) | 256 (95.5) | 0.138 |
|
| |||
| Respiratory specific eventsin PACUopioids†† | 31 (25.2) | 26 (10.2) | <0.001 |
|
| |||
| Hypoventilation | 18 | 15 | |
|
| |||
| Apnea | 16 | 10 | |
|
| |||
| Oxyhemoglobin desaturation | 13 | 9 | |
|
| |||
| Pain/sedation mismatch | 10 | 10 | |
Data presented as median [interquartile range], or number (percentage).
P-value from conditional logistic regression taking into account the 1:2 matched set study design. A P-value is not provided for the type of surgical procedure since this was a matching variable.
Within each specialty, cases and controls were matched on exact procedure.
Includes all medications administered during surgery and Phase I recovery.
Indicates patients who received opioids other than fentanyl such as hydromorphone, morphine or oxymorphone.
ME equivalents were calculated from all opioids administered (no patients received alfentanil or sufentanil) except remifentanil.
All patients bypassing the PACU were direct transfers from the operating room to the ICU except 2 patients in the naloxone group and 4 patients in the control group who were discharged to the standard postsurgical ward.
Percentages of respiratory specific event frequencies were calculated using number of patients transferred to the PACU as the denominator. Sum of individual respiratory events exceeds the number of patients because patients may have experienced more than a single event.
Abbreviations: ME = morphine equivalents; PACU = post anesthesia care unit.
Results
During the study period 84,533 patients underwent general anesthesia. Of those, 135 patients received naloxone for respiratory depression following general anesthesia and after tracheal extubation, and dismissal from anesthetic care. One patient did not provide authorization for study inclusion in retrospective studies and was excluded from analysis. The estimated rate of naloxone administration was 1.6 per 1,000 (95% CI 1.3 – 1.9) anesthetics.
Tables 1 and 2 summarize the baseline clinical presentation and the perioperative course, respectively, of cases and controls. Results from multivariable models assessing clinical and procedural characteristics potentially associated with naloxone administration are presented in Table 3. From the multivariable analysis that includes all cases and controls the presence of OSA (OR= 2.45, 95%CI 1.27-4.66, P = 0.008) was associated with an increased risk for receiving naloxone. When restricted to patients who received Phase I recovery in PACU those experiencing respiratory specific events had higher risk for subsequently receiving naloxone (OR 5.11, 95%CI 2.32-11.27, P < 0.001). Following discharge, patients who received naloxone were administered greater doses of opioids and were more frequently administered other sedating medications (Table 4). Patients who were regularly using opioid medications preoperatively had increased use of postoperative opioids.
Table 3. Multivariable conditional logistic regression.
| Characteristics | Model 1 | Model 2 | ||||
|---|---|---|---|---|---|---|
| OR | 95% CI | P | OR | 95% CI | P | |
| Preoperative | ||||||
| Body mass index (per kg/m2) | 1.01 | (0.97, 1.05) | 0.509 | 1.03 | (0.98, 1.07) | 0.254 |
| Cardiovascular disease | 1.95 | (1.08, 3.53) | 0.027 | 2.56 | (1.28, 5.11) | 0.008 |
| Obstructive sleep apnea | 2.43 | (1.27, 4.66) | 0.008 | 2.44 | (1.15, 5.19) | 0.021 |
| Pulmonary disease | 1.60 | (0.83, 3.11) | 0.164 | 2.02 | (0.92, 4.46) | 0.081 |
| Neurologic disease | 2.42 | (1.15, 5.07) | 0.020 | 4.05 | (1.61, 10.17) | 0.003 |
| Preoperative (chronic) use of benzodiazepines | 1.64 | (0.77, 3.49) | 0.196 | 2.28 | (0.98, 5.33) | 0.056 |
| Intraoperative | ||||||
| Anesthetic technique | 0.185 | 0.472 | ||||
| General | 1.00 | Reference | 1.00 | Reference | ||
| General with neuraxial analgesia | 1.98 | (0.72, 5.43) | 1.48 | (0.51, 4.35) | ||
| Anesthetic duration (per 30 minutes) | 1.09 | (1.00, 1.19) | 0.051 | 1.07 | (1.00, 1.22) | 0.043 |
| Use of midazolam‡ | 0.61 | (0.34, 1.07) | 0.082 | 0.59 | (0.30, 1.13) | 0.111 |
| Use of ketamine‡ | 1.16 | (0.51, 2.63) | 0.720 | 1.09 | (0.45, 2.66) | 0.852 |
| Opioid dose (per 10 mg IV ME) | 1.02 | (0.91, 1.14) | 0.740 | 1.01 | (0.89, 1.15) | 0.848 |
| Use of long-acting opioids | 1.97 | (0.96, 4.03) | 0.063 | 2.48 | (1.05,5.88) | 0.039 |
| PACU | ||||||
| Respiratory Event | 5.11 | (2.32,11.27) | <0.001 | |||
Body mass index, anesthesia duration, and opioid dose were modeled as continuous variables with odds ratios presented for increases of 1 kg/m2, 30 minutes and 10 mg IV ME respectively.
Model 1 includes all patients, including those who did not go to the PACU. Model 2 is restricted to 122 matched sets where the case and at least 1 matched control went to the PACU and includes an additional binary explanatory variable indicating whether or not the patient experienced respiratory events in the PACU. A series of exploratory analyses were performed to assess for potential two-way interaction between the preoperative comorbidities and PACU respiratory events (e.g. OSA-by-respiratory events) and no significant two-way interactions were detected.
Abbreviations: IV ME = intravenous morphine equivalents; PACU = postanesthesia care unit.
Table 4.
Medication administration following discharge from anesthesia care and outcomes between patients who received naloxone within 48 hours of discharge from anesthetic care and matched controls.
| Naloxone N = 134 | No Naloxone N = 268 | P value | |
|---|---|---|---|
| Medication administration following discharge from anesthesia care | |||
| Opioids administered | 89 (66.4) | 162 (60.4) | 0.275 |
| Postdischarge opioid ME, mg* | 10 [0, 47.1] | 5 [0, 24.8] | 0.020 |
| Time adjusted postdischarge opioid ME, mg/hr | 1.5 [0, 3.8] | 0.7 [0.0, 2.8] | 0.050 |
| Administration of other sedating medications† | 41 (30.6) | 24 (9.0) | < 0.001 |
| Outcomes | |||
| Hospital length of stay, days | 5 [3, 8] | 4 [2, 7] | < 0.001 |
| Event within 30 days of surgery | |||
| Stroke | 0 (0) | 1 (0.4) | 1.00 |
| Myocardial infarction | 1 (0.7) | 2 (0.7) | 1.00 |
| Sepsis | 2 (1.5) | 4 (1.5) | 1.00 |
| Death | 6 (4.5) | 2 (0.7) | 0.078 |
Data presented as median [interquartile range] or number (percentage).
Patients who regularly used opioids preoperatively had greater postoperative opioid consumption (20 [1.3, 68.7] vs. 5 [0, 24.0] ME mg, P < 0.0001) while those regularly using benzodiazepines did not (7.4 [0, 35.8] vs. 5 [0, 28.1], P = 0.249).
The most frequently administered postoperative sedating medications were benzodiazepines (18 cases and 8 controls) followed by gabapentinoids (15 and 5), sleep aides (9 and 7), antihistamines (5 and 2), and spasmolytics (4 and 1).
The majority (N = 78, 58%) of naloxone administrations occurred during the first 12 hours following surgery, and 110 (82%) occurred within the first 24 hours (Figure 1). The mean dose of naloxone was 0.32 ± 0.23 mg, with 64 (48%) receiving 0.4 mg of naloxone. Naloxone was administered on a standard postoperative ward to 111 (83%) of cases and in an advance monitored setting (i.e., intensive care unit) 23 (17%) of cases. The indications for its administration included treatment of respiratory depression (N= 70, 52%) or excessive sedation (N = 58, 43%), as part of cardiopulmonary resuscitation (N = 2, 2%) or for unclear indication (N = 4, 3%). There was no correlation between the dose of opioid administered during surgery and postanesthesia recovery and dose of administered naloxone (Figure 2). Median length of stay was longer in the naloxone group, but the rate of serious morbidity and mortality were similar between cases and controls (Table 4).
Figure 1.
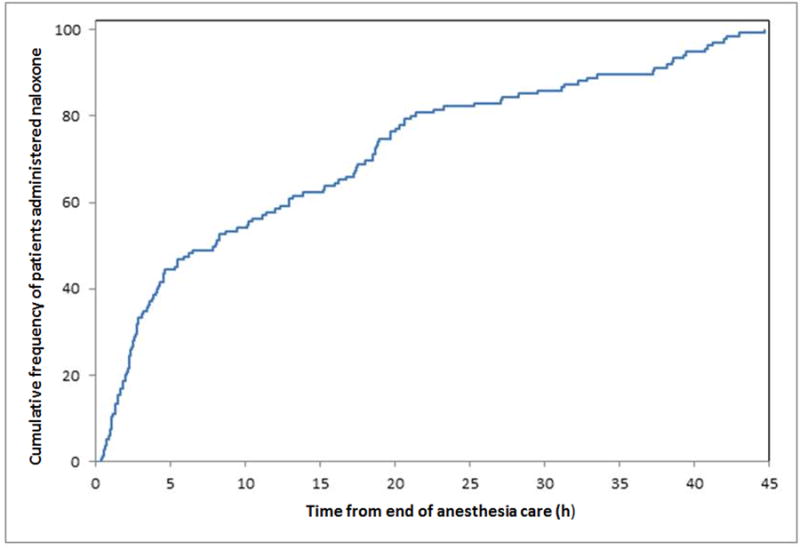
Cumulative frequency of time following discharge from anesthesia care to naloxone administration.
Figure 2.
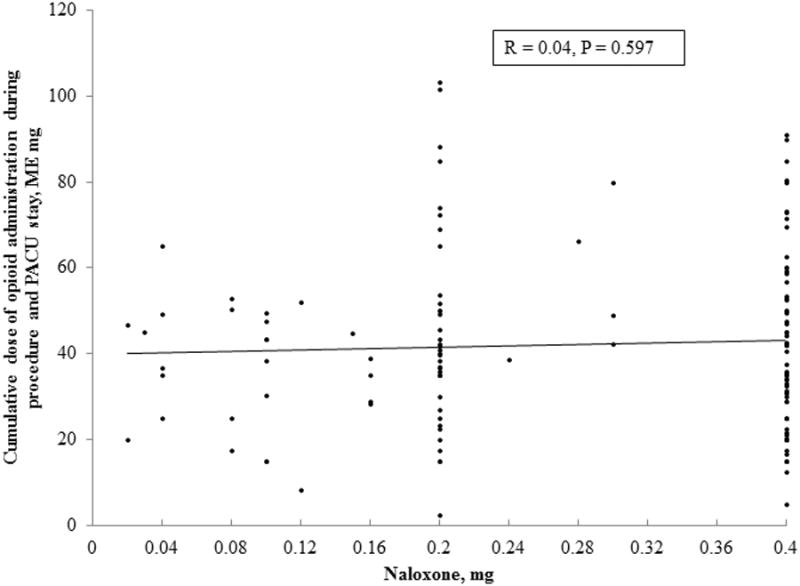
The relationship between the cumulative dose of opioids administered intraoperatively and during postanesthesia recovery and naloxone dose.
Abbreviation: PACU = postanesthesia care unit; ME = intravenous morphine equivalents.
Discussion
Our most important finding was that the patients who developed specific respiratory events in the recovery room had approximately a five-fold increased likelihood for receiving naloxone after discharge from anesthesia care. Another important finding is that preoperative diagnosis of OSA was also associated with respiratory depression or sedation that required naloxone administration. Our findings suggest that these patients may benefit from more careful monitoring after being discharged from anesthesia care.
The rate of naloxone administration in our study was similar to the lower estimate of postoperative naloxone administration reported in a meta-analysis that included 10 studies and 55,404 patients (0.3%, 95%CI 0.1 to 1.3%).8 However, these studies were published prior to 2000,8 thus predated Joint Commission pain standards, which changed the way postoperative pain is managed.4 Also that meta-analysis was heavily weighted by patients who received epidural analgesia (91.4%) compared to our study where less than 15% of our cases and controls received a form of neuraxial analgesia. A more recent study from the current era of Joint Commission pain standards reported 4.3 per 1,000 anesthetics (95%CI 3.0 to 5.4) received naloxone within 2 days following discharge from anesthetic care (the rate of epidural use in that cohort was 19.7%).9 Though speculative, one reason that our naloxone administration was lower may be because patients in our practice who experience respiratory specific events during Phase I recovery must have an additional 60 minute evaluation and must be free of further events prior to discharge to a standard postoperative ward.12-13 This practice could be an additional layer of safety. Nonetheless, it is important to note that the majority of naloxone administrations occurred within 12 hours of discharge from anesthetic care, and those who experienced respiratory events in the PACU were at increased risk for requiring naloxone. This suggests that our added layer of safety in PACU is certainly not foolproof in preventing subsequent severe respiratory depression after discharge from anesthesia care. Others have also found that naloxone is typically administered in the earlier postoperative period.9-11
It is not surprising that our patients with preoperative diagnosis of OSA had higher risk for developing postoperative respiratory depression requiring naloxone. Similarly, patients who had respiratory specific events in recovery room were also at increased risk. The rate of postoperative opioid administration among patients in the naloxone group was greater than in case controls, which was observed before by Gordon et al.9 The rate of administration of other medications with sedating side effects were also greater in the naloxone group. Though this study is not designed to examine potential associations with these medications and naloxone administration, it does raise concerns for a potential association between perioperative polypharmacy and postoperative respiratory depression and oversedation. It is notable that patients who regularly used preoperative opioids had greater postoperative opioid use; however, this patient subset was not found to be at increased risk for naloxone administration.
Patients who were administered naloxone in this study had longer hospital stays than controls. Others have observed that patients who experience opioid-induced adverse events have longer hospital length of stay and greater hospitalization costs.21 However, in this study we cannot determine if the longer hospitalization was a direct result of the opioid-induced respiratory depression or other patient or surgical factors.
A substantial proportion of patients in this cohort received a 0.4 mg of naloxone, which is the unit dose of naloxone stored in standard emergency drug kits (i.e., code carts). In this retrospective study it is difficult to ascertain all the specific circumstances under which naloxone was administered and how providers decided to administer a specific dose. However, the high percentage of patients receiving the full 0.4 mg naloxone dose may suggest unfamiliarity that this drug should ideally be titrated to effect. A 0.4 mg dose of naloxone given as a bolus is sufficient to produce an abstinence syndrome leaving patients in severe pain that may induce sympathetic surge which can be associated with pulmonary edema, asystole, and seizures.22-25 In order to avoid these complications gradual titration of naloxone by 0.04 mg increments to reverse opioid induced respiratory depression without untoward effects on analgesia has been recommended.26
Limitations
This study has all the inherent limitations of a retrospective research design. Importantly, we relied on the administration of naloxone as a surrogate marker for opioid-induced respiratory depression or severe sedation. Undoubtedly, less severe cases may have been treated with more conservative methods such as withholding opioids until the patient became more alert or alternatively by using noninvasive respiratory ventilation devices. Therefore our study does not report the rate at which postoperative respiratory depression occurs in our surgical population. It is plausible that our practice of prolonging the duration of Phase I recovery for patients experiencing respiratory specific events could have resulted in a relatively low incidence of postoperative naloxone administrations. The pharmacokinetic profiles of various opioids used in the perioperative period differ substantially and adds uncertainty to calculating morphine equivalents. To address this shortcoming, we introduced a dichotomous variable to indicate whether a patient received a long-acting opioid (i.e., hydromorphone, oxymorphone, morphine) during the perioperative period, and included this in the multivariable analysis. This study is not powered to evaluate if patients requiring naloxone have increased major postoperative morbidity or mortality, and therefore the lack of association in this study should not be used as evidence that a possible association exists. Another limitation of this study is lack of detailed records to determine the chronicity of outpatient opioid and benzodiazepine use. The assumption was made that the use of these medications were regular if they were documented in the patient's medical record and were not recently prescribed for an acute condition (i.e., recent traumatic injury now undergoing surgical repair).
In conclusion, OSA and respiratory specific events during Phase I recovery are associated with an increased likelihood of naloxone administration for opioid induced respiratory depression. Detecting respiratory specific events in the recovery room may be a method for identifying patients who may require an increased level of post-discharge monitoring. The potential benefits of this preemptive approach will need to be assessed in future studies.
Acknowledgments
Funding: From the Department of Anesthesiology, College of Medicine, Mayo Clinic, Rochester, MN, 55905. Research reported in this publication was also supported by the National Institute on Aging of the National Institutes of Health under Award Number R01AG034676. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
IRB: This study was approved by the Institutional Review Board (IRB) of Mayo Clinic, Rochester, MN, contact: 507-266-4000 (Pamela Kay Jones)
Contribution: Toby N. Weingarten, This author helped design the study, conduct the study, collect data, review the analysis reported in this manuscript, and prepare the manuscript. Vitaly Herasevich, This author helped design the study, conduct the study, collect data, review the analysis reported in this manuscript, and prepare the manuscript. Maria C. McGlinch, This author helped collect data and prepare the manuscript. Nicole C. Beatty, This author helped collect data and prepare the manuscript. Erin D. Christensen, This author helped collect data and prepare the manuscript. Susan K. Hannifan, This author helped collect data and prepare the manuscript. Amy E. Koenig, This author helped collect data and prepare the manuscript. Justin Klanke, This author helped collect data and prepare the manuscript. Xun Zhu, This author helped collect data and prepare the manuscript. Bhargavi Gali, This author helped review the analysis reported in this manuscript and prepare the manuscript. Darrell R. Schroeder, This author helped design the study, conduct the study, review the analysis reported in this manuscript, and prepare the manuscript. Juraj Sprung, This author helped design the study, review the analysis reported in this manuscript, and prepare the manuscript.
Attestation: Toby Weingarten approved the final manuscript. Toby Weingarten attests to the integrity of the original data and the analysis reported in this manuscript. Toby Weingarten is the archival author. Vitaly Herasevich approved the final manuscript. Vitaly Herasevich attests to the integrity of the original data and the analysis reported in this manuscript. Maria McGlinch approved the final manuscript. Nicole Beatty approved the final manuscript. Erin Christensen approved the final manuscript. Susan Hannifan approved the final manuscript. Amy Koenig approved the final manuscript. Justin Klanke approved the final manuscript. Xun Zhu approved the final manuscript. Bhargavi Gali approved the final manuscript. Darrell Schroeder approved the final manuscript. Juraj Sprung approved the final manuscript. Juraj Sprung attests to the integrity of the original data and the analysis reported in this manuscript.
Conflicts of Interest: None
Contributor Information
Toby N. Weingarten, Department of Anesthesiology, Mayo Clinic, Rochester, MN/USA.
Vitaly Herasevich, Email: Herasevich.Vitaly@mayo.edu, Department of Anesthesiology, Mayo Clinic, Rochester, MN/USA.
Maria C. McGlinch, Email: mcmcglinch@gmail.com, Department of Anesthesiology, Mayo Clinic, Rochester, MN/USA.
Nicole C. Beatty, Email: ncwbeatty@gmail.com, Department of Anesthesiology, Mayo Clinic, Rochester, MN/USA.
Erin D. Christensen, Email: christenson.erin@mayo.edu, Nursing Thoracic/Vascular ICU and Mayo School of Health Sciences, Mayo Clinic, Rochester, MN/USA.
Susan K. Hannifan, Email: hannifan.susan@mayo.edu, Mayo School of Health Sciences, Mayo Clinic, Rochester, MN/USA.
Amy E. Koenig, Email: koenig.amy@mayo.edu, Nursing Pediatric ICU and Mayo School of Health Sciences, Mayo Clinic, Rochester, MN/USA.
Justin Klanke, Email: klankej@yahoo.com, Department of Anesthesiology, Mayo Clinic, Rochester, MN/USA.
Xun Zhu, Email: zhu.xun@mayo.edu, Department of Anesthesiology, Mayo Clinic, Rochester, MN/USA.
Bhargavi Gali, Email: gali.bhargavi@mayo.edu, Departments of Anesthesiology, Mayo Clinic, Rochester, MN/USA.
Darrell R. Schroeder, Email: schroedd@mayo.edu, Biomedical Statistics and Informatics, Mayo Clinic, Rochester, MN/USA.
Juraj Sprung, Email: sprung.juraj@mayo.edu, Department of Anesthesiology, Mayo Clinic, Rochester, MN/USA.
References
- 1.Peppard PE, Young T, Barnet JH, Palta M, Hagen EW, Hla KM. Increased prevalence of sleep-disordered breathing in adults. Am J Epidemiol. 2013;177:1006–14. doi: 10.1093/aje/kws342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Young T, Evans L, Finn L, Palta M. Estimation of the clinically diagnosed proportion of sleep apnea syndrome in middle-aged men and women. Sleep. 1997;20:705–6. doi: 10.1093/sleep/20.9.705. [DOI] [PubMed] [Google Scholar]
- 3.Phillips DM. JCAHO pain management standards are unveiled. Joint Commission on Accreditation of Healthcare Organizations. JAMA. 2000;284:428–9. doi: 10.1001/jama.284.4.423b. [DOI] [PubMed] [Google Scholar]
- 4.Frasco PE, Sprung J, Trentman TL. The impact of the joint commission for accreditation of healthcare organizations pain initiative on perioperative opiate consumption and recovery room length of stay. Anesth Analg. 2005;100:162–8. doi: 10.1213/01.ANE.0000139354.26208.1C. [DOI] [PubMed] [Google Scholar]
- 5.Vila H, Jr, Smith RA, Augustyniak MJ, Nagi PA, Soto RG, Ross TW, Cantor AB, Strickland JM, Miguel RV. The efficacy and safety of pain management before and after implementation of hospital-wide pain management standards: is patient safety compromised by treatment based solely on numerical pain ratings? Anesth Analg. 2005;101:474–80. doi: 10.1213/01.ANE.0000155970.45321.A8. [DOI] [PubMed] [Google Scholar]
- 6.Medication Safety Alert Pain scales don't weigh every risk. Huntington Valley, PA: Institute of Safe Medications Practices; 2002. [Google Scholar]
- 7.White PF, Kehlet H. Improving pain management: are we jumping from the frying pan into the fire? Anesth Analg. 2007;105:10–2. doi: 10.1213/01.ane.0000268392.05157.a8. [DOI] [PubMed] [Google Scholar]
- 8.Cashman JN, Dolin SJ. Respiratory and haemodynamic effects of acute postoperative pain management: evidence from published data. Br J Anaesth. 2004;93:212–23. doi: 10.1093/bja/aeh180. [DOI] [PubMed] [Google Scholar]
- 9.Gordon DB, Pellino TA. Incidence and characteristics of naloxone use in postoperative pain management: a critical examination of naloxone use as a potential quality measure. Pain Manag Nurs. 2005;6:30–6. doi: 10.1016/j.pmn.2004.12.003. [DOI] [PubMed] [Google Scholar]
- 10.Ramachandran SK, Haider N, Saran KA, Mathis M, Kim J, Morris M, O'Reilly M. Life-threatening critical respiratory events: a retrospective study of postoperative patients found unresponsive during analgesic therapy. J Clin Anesth. 2011;23:207–13. doi: 10.1016/j.jclinane.2010.09.003. [DOI] [PubMed] [Google Scholar]
- 11.Taylor S, Kirton OC, Staff I, Kozol RA. Postoperative day one: a high risk period for respiratory events. Am J Surg. 2005;190:752–6. doi: 10.1016/j.amjsurg.2005.07.015. [DOI] [PubMed] [Google Scholar]
- 12.Gali B, Whalen FX, Jr, Gay PC, Olson EJ, Schroeder DR, Plevak DJ, Morgenthaler TI. Management plan to reduce risks in perioperative care of patients with presumed obstructive sleep apnea syndrome. J Clin Sleep Med. 2007;3:582–8. [PMC free article] [PubMed] [Google Scholar]
- 13.Gali B, Whalen FX, Schroeder DR, Gay PC, Plevak DJ. Identification of patients at risk for postoperative respiratory complications using a preoperative obstructive sleep apnea screening tool and postanesthesia care assessment. Anesthesiology. 2009;110:869–77. doi: 10.1097/ALN.0b013e31819b5d70. [DOI] [PubMed] [Google Scholar]
- 14.Jacobsen SJ, Xia Z, Campion ME, Darby CH, Plevak MF, Seltman KD, Melton LJ., 3rd Potential effect of authorization bias on medical record research. Mayo Clin Proc. 1999;74:330–8. doi: 10.4065/74.4.330. [DOI] [PubMed] [Google Scholar]
- 15.Herasevich V, Kor DJ, Li M, Pickering BW. ICU data mart: a non-iT approach. A team of clinicians, researchers and informatics personnel at the Mayo Clinic have taken a homegrown approach to building an ICU data mart. Healthc Inform. 2011;28:42, 44–5. [PubMed] [Google Scholar]
- 16.Herasevich V, Pickering BW, Dong Y, Peters SG, Gajic O. Informatics infrastructure for syndrome surveillance, decision support, reporting, and modeling of critical illness. Mayo Clin Proc. 2010;85:247–54. doi: 10.4065/mcp.2009.0479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.AfHCPaRPN, editor. Management of Cancer Pain. Clinical Practice Guideline Number 9. U.S. Dept. of Health and Human Services; 1994. 94-0592. [Google Scholar]
- 18.Principles of Analgesic Use in the Treatment of Acute Pain and Cancer Pain. Skokie, Illinois: American Pain Society; 1999. [Google Scholar]
- 19.Sessler CN, Gosnell MS, Grap MJ, Brophy GM, O'Neal PV, Keane KA, Tesoro EP, Elswick RK. The Richmond Agitation-Sedation Scale: validity and reliability in adult intensive care unit patients. Am J Respir Crit Care Med. 2002;166:1338–44. doi: 10.1164/rccm.2107138. [DOI] [PubMed] [Google Scholar]
- 20.Aldrete JA, Kroulik D. A postanesthetic recovery score. Anesth Analg. 1970;49:924–34. [PubMed] [Google Scholar]
- 21.Oderda GM, Said Q, Evans RS, Stoddard GJ, Lloyd J, Jackson K, Rublee D, Samore MH. Opioid-related adverse drug events in surgical hospitalizations: impact on costs and length of stay. Ann Pharmacother. 2007;41:400–6. doi: 10.1345/aph.1H386. [DOI] [PubMed] [Google Scholar]
- 22.Osterwalder JJ. Naloxone--for intoxications with intravenous heroin and heroin mixtures--harmless or hazardous? A prospective clinical study. J Toxicol Clin Toxicol. 1996;34:409–16. doi: 10.3109/15563659609013811. [DOI] [PubMed] [Google Scholar]
- 23.Horng HC, Ho MT, Huang CH, Yeh CC, Cherng CH. Negative pressure pulmonary edema following naloxone administration in a patient with fentanyl-induced respiratory depression. Acta Anaesthesiol Taiwan. 2010;48:155–7. doi: 10.1016/S1875-4597(10)60050-1. [DOI] [PubMed] [Google Scholar]
- 24.Neal JM, Owens BD. Hazards of antagonizing narcotic sedation with naloxone. Ann Emerg Med. 1993;22:145–6. doi: 10.1016/s0196-0644(05)80283-5. [DOI] [PubMed] [Google Scholar]
- 25.Brimacombe J, Archdeacon J, Newell S, Martin J. Two cases of naloxone-induced pulmonary oedema--the possible use of phentolamine in management. Anaesth Intensive Care. 1991;19:578–80. doi: 10.1177/0310057X9101900418. [DOI] [PubMed] [Google Scholar]
- 26.Boyer EW. Management of opioid analgesic overdose. N Engl J Med. 2012;367:146–55. doi: 10.1056/NEJMra1202561. [DOI] [PMC free article] [PubMed] [Google Scholar]


