Abstract
Purpose
We have shown previously that normal observers detect dark targets faster and more accurately than light targets, when presented in noisy backgrounds. We investigated how these differences in detection time and accuracy are affected by age and ganglion cell pathology associated with glaucoma.
Methods
We asked 21 glaucoma patients, 21 age-similar controls, and 5 young control observers to report as fast as possible the number of 1 to 3 light or dark targets. The targets were positioned at random in a binary noise background, within the central 30° of the visual field.
Results
We replicate previous findings that darks are detected faster and more accurately than lights. We extend these findings by demonstrating that differences in detection of darks and lights are found reliably across different ages and in observers with glaucoma. We show that differences in detection time increase at a rate of approximately 55 msec/dB at early stages of glaucoma and then remain constant at later stages at approximately 800 msec. In normal subjects, differences in detection time increase with age at a rate of approximately 8 msec/y. We also demonstrate that the accuracy to detect lights and darks is significantly correlated with the severity of glaucoma and that the mean detection time is significantly longer for subjects with glaucoma than age-similar controls.
Conclusions
We conclude that differences in detection of darks and lights can be demonstrated over a wide range of ages, and asymmetries in dark/light detection increase with age and early stages of glaucoma.
Keywords: retina, thalamo-cortical, light–dark, perimetry, psychophysics
Visual information travels from the eye to the rest of the brain through two major pathways that signal light increments (ON) and decrements (OFF) in local regions of visual space. In mammals, ON and OFF channels remain segregated in the thalamus and combine for the first time in visual cortex. However, ON-OFF cortical mixing is incomplete and unbalanced. Although single cortical neurons receive input from both channels, ON and OFF thalamic afferents segregate in different cortical domains1–4 and cortical current sinks generated by OFF thalamic afferents are stronger and occupy larger territory than those generated by ON afferents.4 Moreover, cortical responses to dark stimuli are stronger, faster, more linearly related to luminance contrast, and have better spatial and temporal resolution than responses to light stimuli.5–16 Consistent with these physiological differences, dark targets are detected faster and more accurately than light targets on noisy backgrounds,12,17 and dark pixels have a more important role in judgments of texture variance than light pixels.18,19
Although dark/light asymmetries are most pronounced in visual cortex,4,6–8,10–13 they also are significant in the retina9,13,20–22 and possibly originate in photoreceptor outputs.13 Therefore, diseases that disrupt retinal function, such as glaucoma, could affect the dark/light asymmetries in visual perception. Glaucoma is a progressive disease that affects retinal ganglion cells and often results in loss of sensitivity in the visual field, especially within the central 30° of fixation.23,24 Glaucoma also has been shown to affect temporal processing25,26 and can have a profound effect on quality of life.27–30 To investigate if dark/light asymmetries are affected by glaucoma within the central 30° of fixation, we asked human observers to report the number of dark or light targets presented in binary noise on a monitor screen. Our results demonstrated that darks are detected more accurately and faster than lights in control observers and observers with glaucoma. Moreover, we showed that these dark/light asymmetries increase with age and in the early stages of glaucoma.
Methods
We recruited 21 patients with open angle glaucoma (48–83 years old; mean, 64.7 ± 7.5 years old), 21 control observers with a similar age range (49–74 years old; mean, 62.2 ± 7.3 years old), and 5 young control observers (21–25 years old). The study was performed following the principles outlined in the Declaration of Helsinki. The inclusion criteria for all groups were: best corrected visual acuity of at least 0.2 logMAR units (approximately 20/30), spherical equivalent refractive error within −6 to +2 diopters (D), cylinder correction within 3 D, clear ocular media, and absence of known eye disease following a comprehensive eye examination (except for glaucoma in the patient group). The exclusion criteria for all groups were: ocular or systemic disease known to affect the visual field, such as diabetic retinopathy (except glaucoma in the patient group), history of intraocular surgery (except uncomplicated cataract surgery more than 1 year before enrollment or glaucoma surgery in the patient group), and use of medications known to affect vision. Additional exclusion criteria for control observers were a self-reported, first-degree relative with glaucoma and intraocular pressure > 21 mm Hg for two or more clinic visits. No exclusions were based on sex or race. The degree of glaucoma in each patient was based upon the results of static automated perimetry testing performed with the Humphrey Visual Field Analyzer II (Carl Zeiss Meditec, Inc., Dublin, CA, USA), using 24-2 SITA Standard algorithm. It was quantified as the total mean deviation of visual sensitivity in decibels and varied from normal to severe (1.24 to −22.21 dB; mean, −4.76 ± 5.8 dB). We did not measure sensitivity at the fovea and our most central stimulus was 3° away from fixation. In an initial recruitment of 11 glaucoma subjects, we selected a wide range of visual field defects spanning from normal to severe, and found significant differences in accuracy and reaction time between glaucoma subjects and age-similar controls. Based on this sample and power analysis (“sampsizepwr,” MATLAB; MathWorks, Natick, MA, USA), we estimated that we would need a sample of 32 subjects (16 control and 16 glaucoma) to reveal significant differences between glaucoma and age-similar controls (e.g., effect size for reduction in dark/light accuracy, 7.1 ± 5.96/10.69 ± 11.54%; power, 0.9; alpha, 0.05; sample size required, 31). To fulfill the requirements of the power analysis, we selected a sample of 42 subjects (21 age-similar controls and 21 glaucomatous patients).
Observers were asked to report as fast as possible the number of square targets embedded in a background of binary white noise consisting of equal numbers of dark and light elements. The number of targets could be one, two, or three, and were either all dark or all light. Stimuli were presented on a monitor screen and observers had to press a key to indicate the number of targets that they saw (Fig. 1). Each time a key press was registered, an auditory tone signaled the progression onto the next trial. Therefore, the duration of each screen was determined by the observer's reaction time. Stimuli were presented using MATLAB and Psych-toolbox31 on a gamma calibrated monitor (Mitsubishi DP2070SB or Display ++ LCD). The monitor covered 23.0° × 30.3° of visual angle at a distance of 1 m and each stimulus target was 1.0° × 1.0° in size. The mean luminance of the monitor was kept constant at 50 candelas per square meter. Each observer was tested monocularly after being properly refracted. Experiments were conducted in a dark room. Before the testing started, observers were visually adapted to a gray screen for 15 seconds.
Figure 1.

Stimulus. Observers were asked to report as fast as possible the number of dark (top row) and light (bottom row) targets embedded in a white noise background.
A total of 600 to 800 reaction times was collected for each observer in an hour-long session. Observers were given a series of 100 trials at a time followed by a short break. Reaction time histograms (bin size 0.2 s) were averaged and fitted using an exponential-Gaussian function.32 This function assumes that reaction times result from taking the sum of independent Gaussian and exponential random variables, implying that the probability distribution characterizing reaction times is the convolution of an exponential (exp) and a Gaussian (ϕ) function given by the following equation:
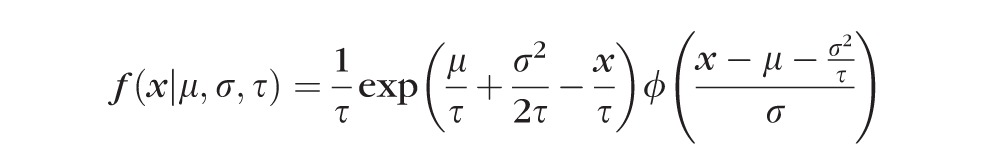 |
The parameters of the equation are the mean (μ) and standard deviation (σ) of the Gaussian function and the mean of the exponential function (τ).
Results
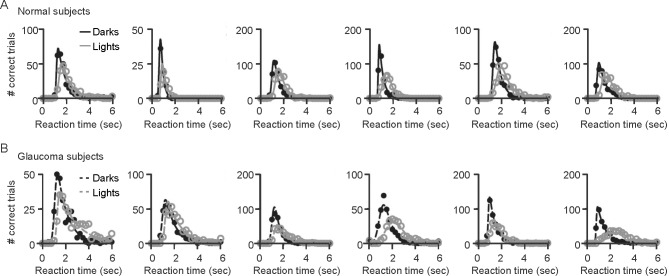
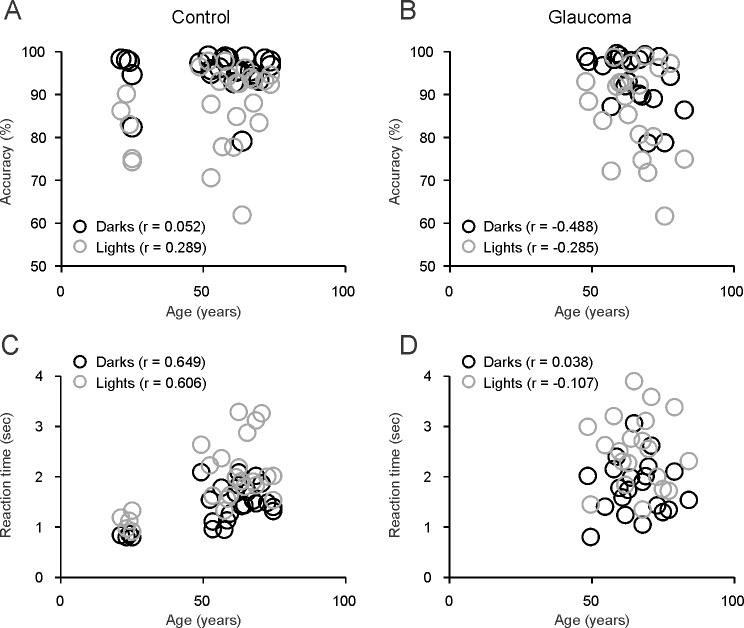
Previous studies17 demonstrated that observers are faster and more accurate at counting dark than light targets embedded in binary noise (Fig. 1). We used the same method to investigate the extent in which this dark/light asymmetry is affected by age and glaucoma. To measure observer performance, we plotted the number of correct trials as a function of the reaction time and fitted the distributions with a Gaussian-Exponential function (see Methods). Control observers (Fig. 2A) and observers with glaucoma (Fig. 2B) were faster and more accurate at counting dark than light targets. In control observers, accuracy was not correlated with age (Fig. 3A; accuracy versus age for darks, r = 0.052, P = 0.799; lights, r = 0.289, P = 0.270; darks-lights, r = −0.359, P = 0.072). In glaucomatous observers, we found a weak correlation between accuracy and age but only for dark targets (Fig. 3B; accuracy versus age for darks, r = −0.488, P = 0.025; lights, r = −0.285, P = 0.210; darks-lights, r = −0.033, P = 0.888).
Figure 2.
Observer performance. Observer's performances were evaluated by plotting the number of correct trials as a function of reaction time, when the targets to be detected were dark (dark circles and lines) or light (gray circles and lines). (A, B) In both groups of observers, those with normal vision (A) and those with glaucoma (B), the responses were faster and the number of correct trials higher for dark than light targets.
Figure 3.
Correlations between age and task performance. Age was weakly correlated with accuracy and reaction time. (A) The correlations between age and accuracy were not significant in control subjects. (B) In glaucomatous subjects, the correlations were only significant for dark targets (r = −0.488, P = 0.025). (C, D) The correlations between age and reaction time were significant for lights (r = 0.649, P < 0.001) and darks (r = 0.606, P = 0.001) in control observers (C) but not in glaucomatous observers (D) or in control observers that were >49 years old (C).
Reaction time was correlated with age in control observers (Fig. 3C; darks, r = 0.649, P = 0.0003; lights, r = 0.606, P = 0.001) but not in observers >49 years old (Fig. 3C; darks, r = 0.120, P = 0.603; lights, r = 0.136, P = 0.556) or in glaucoma observers (Fig. 3D; darks, r = 0.038, P = 0.869; lights, r = −0.107, P = 0.645). Differences in reaction time between lights and darks also were correlated significantly with age in control observers (lights-darks, r = 0.422, P = 0.032) but not in observers older than 49 years (lights-darks, r = 0.117, P = 0.613) or glaucomatous observers (r = −0.248, P = 0.279).
On average, observers were more accurate at detecting darks than lights. The difference in accuracy between darks and lights was 8.08% in control observers (Fig. 4A; darks, 95.59% ± 4.69%; lights, 87.51% ± 9.4%, P = 0.0002, Wilcoxon test), 7.01% in age-similar controls (darks, 95.85% ± 4.23% versus lights, 88.84% ± 0.57%, P = 0.0003, Wilcoxon test) and 7.05% in glaucoma observers (darks, 93.06% ± 6.55%; lights, 86.55% ± 10.6%, P = 0.015, Wilcoxon test). The accuracy was only 2.2% better in age-similar controls than glaucomatous observers (Fig. 4A; darks, 95.85% ± 4.23% vs. 93.06% ± 6.55%, P = 0.579; lights, 88.84% ± 0.57% vs. 86.55% ± 10.6%, P = 0.443, Wilcoxon tests), a finding that is not surprising given that most of the glaucoma subjects were at early stages of the disease. If we selected glaucoma subjects with the greatest visual field loss (mean deviation < −6), their accuracy was 6.6% lower than the age-similar controls for dark targets (95.85% ± 4.23% vs. 95.59% ± 4.69%, P = 0.02, Wilcoxon test) and 15.75% lower for light targets (87.51^ ± 9.4% vs. 73.09% ± 26.85%, P = 0.03, Wilcoxon test).
Figure 4.
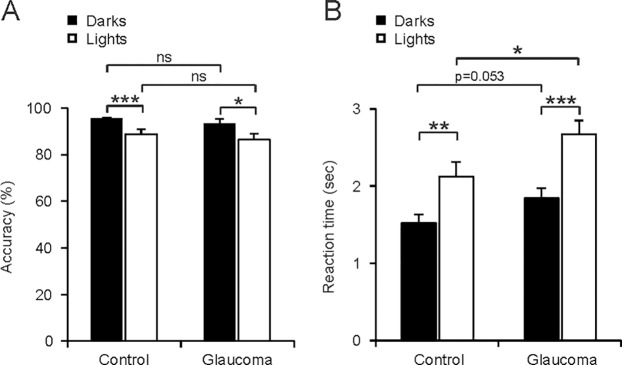
Darks are perceived more accurately and faster than lights in observers with normal vision and observers with glaucoma. (A) Accuracy (percent of correct responses) was higher for darks (dark bars) than lights (light bars). (B) Reaction time was faster for darks than lights. The difference in reaction time between observers with normal vision and glaucoma reached significance only for light targets. The histograms illustrate means and standard errors of the mean. ***P < 0.001, **P < 0.01, *P < 0.05, not significant (ns) P > 0.05. Wilcoxon tests.
Differences in detecting darks and lights also could be demonstrated in measurements of reaction times (Fig. 4B). The difference in reaction time between darks and lights was 0.53 seconds in control observers (darks, 1.39 ± 0.41 seconds; lights, 1.92 ± 0.66 seconds; P = 0.002, Wilcoxon test), 0.6 seconds in age-similar controls (darks, 1.52 ± 0.34 seconds; lights, 2.12 ± 0.58 seconds; P = 0.011, Wilcoxon test), and 0.82 seconds in glaucomatous observers (darks, 1.84 ± 0.54 seconds; lights, 2.66 ± 0.84 seconds; P = 0.0009, Wilcoxon test). The differences between control and glaucomatous observers were significant for light targets and approached significance for dark targets (Fig. 4B; darks, 1.52 ± 0.34 vs. 1.84 ± 0.54 seconds; P = 0.053; lights, 2.12 ± 0.58 vs. 2.66 ± 0.84 seconds; P = 0.036, Wilcoxon tests). Moreover, if we selected glaucomatous subjects with the greatest visual field loss (mean deviation < −6), the differences in reaction time became more pronounced and were significant for light and dark targets (darks, 1.52 ± 0.34 vs. 2.07 ± 0.43 seconds; P = 0.019; lights, 2.12 ± 0.58 vs. 3.09 ± 0.38 seconds; P = 0.009, Wilcoxon tests). The severity of the disease was significantly correlated with detection accuracy (Fig. 5A; darks, r = 0.531, P = 0.013; lights, 0.491, P = 0.024) but not with reaction time (Fig. 5B; darks, r = −0.379, P = 0.089; lights, r = −0.348, P = 0.122). However, the correlations with reaction time reached significance if we selected the glaucomatous subjects with the most limited visual field loss (mean deviation > −3; darks, r = −0.57, P = 0.014; lights, r = −0.529, P = 0.023).
Figure 5.
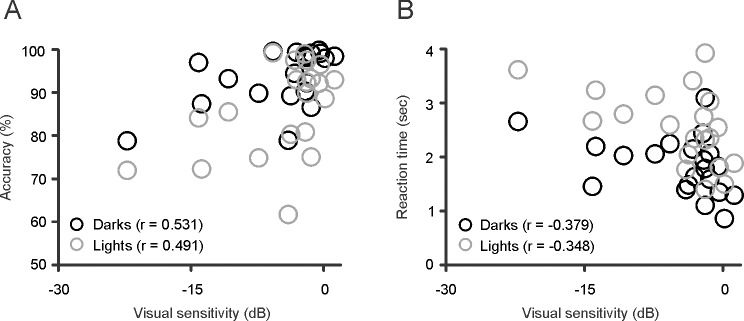
Correlations between visual sensitivity and task performance in observers with glaucoma. (A) Visual sensitivity was correlated with accuracy for dark (r = 0.531, P = 0.013) and light (r = 0.491, P = 0.024) targets. (B) Visual sensitivity also was weakly correlated with reaction time but the correlations did not reach significance.
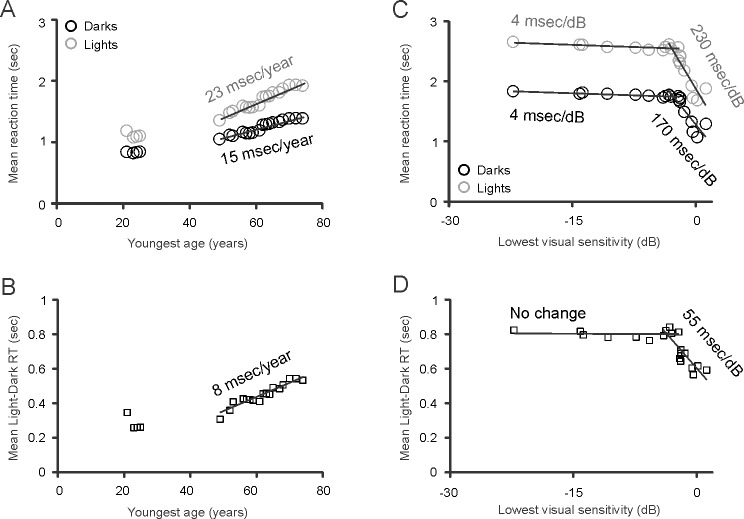
The analyses described above revealed pronounced differences in accuracy and detection time between dark and light targets in all subject groups, significant differences between glaucoma subjects and age-similar controls and a correlation between the severity of glaucoma and visual performance, for accuracy and reaction time. To further investigate changes in the dark/light asymmetry with age and glaucoma, we used a method of moving average. To study the effect of age, we used an average sliding window with a fixed border at the oldest age and another border that moved with each increase in age within our sample. To study the effect of glaucoma progression, we placed the fixed border at the highest value of visual sensitivity and the other border moved with each reduction in sensitivity within our sample. These analyses measured how the average reaction time changed as we narrowed the range of ages (or visual sensitivities) from a full range to a range without one of the values, two of the values, and so forth. By fitting the average values obtained with these analyses to linear functions, we found that the reaction time increased faster with age for light than dark targets (Fig. 6A; lights, 23 msec/y, r2 = 0.9495; darks, 15 msec/y, r2 = 0.9344) with an average difference of 8 msec/y (Fig. 6B, r2 = 0.9065). Notably, reaction times also increased faster for light than dark targets with glaucoma progression (Fig. 6C; lights, 230 msec/dB, r2 = 0.8127; darks, 170 msec/y, r2 = 0.765) with an average difference of 55 msec/dB (r2 = 0.7448), but only at early stages of glaucoma (>−4 dB of mean deviation in visual sensitivity). At later stages, the change in reaction time slowed down by more than one order of magnitude for lights and darks (lights, 4 msec/dB, r2 = 0.5269; darks, 4 msec/dB, r2 = 0.8376).
Figure 6.
Prediction of change in reaction time (RT) as a function of age and glaucoma. All figures show mean reaction times (A, C) and light-dark differences in reaction time (B, D) calculated with a sliding edge that has a fixed boundary at the youngest age (A, B) or lowest visual sensitivity (C, D) and another sliding boundary at the value of the x-axis. This analysis indicate that the reaction time increases with age 8 msec/y faster for light than dark targets (A, B). Interestingly, in glaucomatous subjects, reaction time also increases 55 msec/dB faster for light than dark targets (C, D). However, this difference disappears when the mean deviation of visual sensitivity becomes more negative than −3 dB.
In summary, our results indicated that the difference in reaction time between lights and darks increases at a rate of approximately 55 msec/dB at early stages of glaucoma (Fig. 6D) and then remains constant at later stages at approximately 800 msec difference. It should be noted that a similar analysis revealed a negligible reduction in accuracy with age in age-similar controls (lights, 0.1%/y, r2 = 0.6731; darks, 0.04%/y, r2 = 0.0428) and a similar reduction in accuracy for darks and lights in subjects at early stages of glaucoma (darks, 0.7%/dB, r2 = 0.7898, lights, 0.5%/dB, r2 = 0.3869; mean deviation of visual sensitivity > −4 dB). At later stages of glaucoma, the reduction in accuracy was still limited but seemed more pronounced for light than dark targets (darks, 0.08%/dB, r2 = 0.9679; lights, 0.2%/dB, r2 = 0.9148).
Discussion
We demonstrated that dark targets are perceived faster and more accurately than light targets in subjects spanning a wide range of ages (21–83 years old) that had normal vision or glaucoma. We also demonstrated that glaucoma alters this dark/light asymmetry by affecting the time and accuracy of detection. The reduction in detection accuracy was found mostly in subjects with advanced glaucoma and could be accounted for, in part, by the presence of visual field scotomas. Since the locations of our stimuli were selected randomly, targets that fell by chance within a scotoma had less of a chance of being perceived than targets falling in unaffected regions. Our findings support the notion that darks are processed faster,11,12 have access to more neuronal resources than light targets,4,6–10,12,13,20,21 and that dark/light asymmetries in sensory processing are present across different ages and in retinal disease. It also should be noted that, although response asymmetries in visual search have been studied extensively33–37 and are known to be influenced by the background,38,39 the use of a random-noise background (with only target and distractor colors)17,40 is important because it rules out asymmetries due to experimental design.
Glaucoma is a disease that causes degeneration of retinal ganglion cells frequently in association with increased intraocular pressure.23,41–44 The finding that dark/light asymmetries become more pronounced in glaucoma indicates that the disease may affect differently the ON and OFF pathways. There is strong evidence indicating that the detection of dark targets is mediated mostly by the OFF pathway and the detection of light targets mostly by the ON pathway.12 Visual responses are stronger, faster, and more temporally precise in OFF- than ON-center neurons if the targets are dark and vice versa if the targets are light. Also, ON and OFF pathways are known to remain segregated in visual cortex2–4,10 and, without the ON pathway, monkeys and humans fail to detect light targets but not dark targets.45,46
Early studies claimed that large ganglion cells were more affected in glaucoma.42 However, other studies found evidence for cell shrinkage in multiple cell types rather than selective cell-type loss.47–49 Because ON retinal ganglion cells have larger dendritic fields than OFF retinal ganglion cells,22,50–53 the ON pathway would be expected to be more affected if the largest cells were more vulnerable in glaucoma. While the OFF pathway responds faster than the ON pathway in central retina,11,12,54 previous studies indicate that ON cells may be faster than OFF cells in peripheral retina.55 Therefore, if glaucoma caused selective degeneration of the larger ON cells in peripheral retina, it would be expected to make the reaction time increase more for light than dark targets. Consistent with this hypothesis, visual evoked potentials are more affected in glaucoma subjects when measured with positive than negative luminance contrast56,57 and our results also revealed the strongest increase in reaction time when using light targets. On the other hand, because cortical responses to dark stimuli are stronger and have better spatial resolution than cortical responses to light stimuli,5–16 dark stimuli may be more appropriate to map the borders between glaucomatous and normal regions in the visual field.58–60
It is important to note that measurements of reaction time and accuracy are closely related. Our results suggested that, as ganglion cell pathology progresses within an early stage of the disease, the reaction times increase very rapidly (170–230 msec/dB), keeping the accuracy loss restricted to less than 1%/dB. However, as the disease progresses even further, the reaction times stop increasing and the accuracy loss becomes more noticeable (15.75% for light targets). It also is important to emphasize that our measurements were obtained in observers >48 years old that had lived with glaucoma over several years. A recent study has demonstrated that, a few days after inducing elevated intraocular pressure in mice, the most affected retinal ganglion cells have dendrites in the OFF sublamina of the inner plexiform layer.49 In the future, it will be interesting to investigate the differences in the detection of darks and lights near the onset of the disease. However, this study will require a large sample of subjects. To meet this challenge, we have developed a mobile app, “eye speed,” that will allow any clinician across the world to measure dark/light asymmetries and monitor disease progression using our visual test.61 The mobile app is freely available and takes advantage of the fact that our stimuli are binary (black or white) and do not require screen calibration. That is, the mid-gray background adaptation used in the experiments reported here is equivalent to the background of the mobile app, which is made of an equal number of black and white squares. The response time does vary across mobile devices, but differences between darks and lights can be reproduced with android and iOS versions of the app. Therefore, while more work is still needed, we hope that measurements with mobile devices will facilitate the study of dark/light asymmetries in the future and help to investigate the functional roles of ON and OFF pathways in visual disease.
Acknowledgments
Supported by Grants T35EY020481, EY005253, EY007556, EY0133312, and the SUNY Brain Summer Scholars Program.
Disclosure: L. Zhao, None; C. Sendek, None; V. Davoodnia, None; R. Lashgari, None; M.W. Dul, None; Q. Zaidi, None; J.-M. Alonso, None
References
- 1. Zahs KR,, Stryker MP. Segregation of ON and OFF afferents to ferret visual cortex. J Neurophysiol. 1988; 59: 1410–1429. [DOI] [PubMed] [Google Scholar]
- 2. Norton TT,, Rager G,, Kretz R. ON and OFF regions in layer IV of striate cortex. Brain Res. 1985; 327: 319–323. [DOI] [PubMed] [Google Scholar]
- 3. McConnell SK,, LeVay S. Segregation of on- and off-center afferents in mink visual cortex. Proc Natl Acad Sci U S A. 1984; 81: 1590–1593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Jin JZ,, Weng C,, Yeh CI,, et al. On and off domains of geniculate afferents in cat primary visual cortex. Nature Neurosci. 2008; 11: 88–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Zemon V,, Gordon J,, Welch J. Asymmetries in ON and OFF visual pathways of humans revealed using contrast-evoked cortical potentials. Vis Neurosci. 1988; 1: 145–150. [DOI] [PubMed] [Google Scholar]
- 6. Yeh CI,, Xing D,, Shapley RM. “Black” responses dominate macaque primary visual cortex v1. J Neurosci. 2009; 29: 11753–11760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Xing D,, Yeh CI,, Shapley RM. Generation of black-dominant responses in V1 cortex. J Neurosci. 2010; 30: 13504–13512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Xing D,, Yeh CI,, Gordon J,, Shapley RM. Cortical brightness adaptation when darkness and brightness produce different dynamical states in the visual cortex. Proc Natl Acad Sci U S A. 2014; 111: 1210–1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Pandarinath C,, Victor JD,, Nirenberg S. Symmetry breakdown in the ON and OFF pathways of the retina at night: functional implications. J Neurosci. 2010; 30: 10006–10014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Jin J,, Wang Y,, Swadlow HA,, Alonso JM. Population receptive fields of ON and OFF thalamic inputs to an orientation column in visual cortex. Nature Neurosci. 2011; 14: 232–238. [DOI] [PubMed] [Google Scholar]
- 11. Jin J,, Wang Y,, Lashgari R,, Swadlow HA,, Alonso JM. Faster thalamocortical processing for dark than light visual targets. J Neurosci. 2011; 31: 17471–17479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Komban SJ,, Kremkow J,, Jin J,, et al. Neuronal and perceptual differences in the temporal processing of darks and lights. Neuron. 2014. ; 82: 224–234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Kremkow J,, Jin J,, Komban SJ,, et al. Neuronal nonlinearity explains greater visual spatial resolution for darks than lights. Proc Natl Acad Sci U S A. 2014; 111: 3170–3175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Samonds JM,, Potetz BR,, Lee TS. Relative luminance and binocular disparity preferences are correlated in macaque primary visual cortex matching natural scene statistics. Proc Natl Acad Sci U S A. 2012; 109: 6313–6318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Veit J,, Bhattacharyya A,, Kretz R,, Rainer G. On the relation between receptive field structure and stimulus selectivity in the tree shrew primary visual cortex. Cereb Cortex. 2014; 24: 2761–2771. [DOI] [PubMed] [Google Scholar]
- 16. Liu K,, Yao H. Contrast-dependent OFF-dominance in cat primary visual cortex facilitates discrimination of stimuli with natural contrast statistics. Eur J Neurosci. 2014; 39: 2060–2070. [DOI] [PubMed] [Google Scholar]
- 17. Komban SJ,, Alonso JM,, Zaidi Q. Darks are processed faster than lights. J Neurosci. 2011; 31: 8654–8658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Chubb C,, Nam JH. Variance of high contrast textures is sensed using negative half-wave rectification. Vision Res. 2000; 40: 1677–1694. [DOI] [PubMed] [Google Scholar]
- 19. Lu ZL,, Sperling G. Black-white asymmetry in visual perception. J Vis. 2012; 12: 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Ratliff CP,, Borghuis BG,, Kao YH,, Sterling P,, Balasubramanian V. Retina is structured to process an excess of darkness in natural scenes. Proc Natl Acad Sci U S A. 2010; 107: 17368–17373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Balasubramanian V,, Sterling P. Receptive fields and functional architecture in the retina. J Physiol. 2009; 587: 2753–2767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Ahmad KM,, Klug K,, Herr S,, Sterling P,, Schein S. Cell density ratios in a foveal patch in macaque retina. Vis Neurosci. 2003; 20: 189–209. [DOI] [PubMed] [Google Scholar]
- 23. Harwerth RS,, Quigley HA. Visual field defects and retinal ganglion cell losses in patients with glaucoma. Arch Ophthalmol. 2006; 124: 853–859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Harwerth RS,, Carter-Dawson L,, Shen F,, Smith EL,, III,, Crawford ML. Ganglion cell losses underlying visual field defects from experimental glaucoma. Invest Ophthalmol Vis Sci. 1999; 40: 2242–2250. [PubMed] [Google Scholar]
- 25. O'Hare F,, Rance G,, Crowston JG,, McKendrick AM. Auditory and visual temporal processing disruption in open angle glaucoma. Invest Ophthalmol Vis Sci. 2012; 53: 6512–6518. [DOI] [PubMed] [Google Scholar]
- 26. McKendrick AM,, Denniss J,, Turpin A. Response times across the visual field: empirical observations and application to threshold determination. Vision Res. 2014; 101: 1–10. [DOI] [PubMed] [Google Scholar]
- 27. Burton R,, Crabb DP,, Smith ND,, Glen FC,, Garway-Heath DF. Glaucoma and reading: exploring the effects of contrast lowering of text. Optom Vis Sci. 2012; 89: 1282–1287. [DOI] [PubMed] [Google Scholar]
- 28. Crabb DP,, Smith ND,, Glen FC,, Burton R,, Garway-Heath DF. How does glaucoma look?: patient perception of visual field loss. Ophthalmology. 2013; 120: 1120–1126. [DOI] [PubMed] [Google Scholar]
- 29. Glen FC,, Crabb DP,, Smith ND,, Burton R,, Garway-Heath DF. Do patients with glaucoma have difficulty recognizing faces? Invest Ophthalmol Vis Sci. 2012; 53: 3629–3637. [DOI] [PubMed] [Google Scholar]
- 30. Owen VM,, Crabb DP,, White ET,, Viswanathan AC,, Garway-Heath DF,, Hitchings RA. Glaucoma and fitness to drive: using binocular visual fields to predict a milestone to blindness. Invest Ophthalmol Vis Sci. 2008; 49: 2449–2455. [DOI] [PubMed] [Google Scholar]
- 31. Brainard DH. The psychophysics toolbox. Spatial Vis. 1997; 10: 433–436. [PubMed] [Google Scholar]
- 32. Cousineau D,, Lacouture Y. How to use MATLAB to fit the ex-Gaussian and other probability functions to a distribution of response times. Tutorials Quant Meth Psych. 2008; 4: 35–45. [Google Scholar]
- 33. Wolfe JM. Asymmetries in visual search: an introduction. Percept Psychophys. 2001; 63: 381–389. [DOI] [PubMed] [Google Scholar]
- 34. Treisman A,, Gormican S. Feature analysis in early vision: evidence from search asymmetries. Psychol Rev. 1988; 95: 15–48. [DOI] [PubMed] [Google Scholar]
- 35. D'Zmura M. Color in visual search. Vision Res. 1991; 31: 951–966. [DOI] [PubMed] [Google Scholar]
- 36. Nagy A,, Cone SM. Asymmetries in simple feature searches for color. Vision Res. 1996; 36: 2837–2847. [DOI] [PubMed] [Google Scholar]
- 37. Nagy AL,, Sanchez RR. Critical color differences determined with a visual search task. J Opt Soc Am A. 1990; 7: 1209–1217. [DOI] [PubMed] [Google Scholar]
- 38. Rosenholtz R,, Nagy AL,, Bell NR. The effect of background color on asymmetries in color search. J Vis. 2004; 4: 224–240. [DOI] [PubMed] [Google Scholar]
- 39. Rosenholtz R. Search asymmetries? What search asymmetries? Percept Psychophys. 2001; 63: 476–489. [DOI] [PubMed] [Google Scholar]
- 40. Wool LE,, Komban SJ,, Kremkow J,, et al. Salience of unique hues and implications for color theory. J Vis. 2015; 15: 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Quigley HA,, Sanchez RM,, Dunkelberger GR,, L'Hernault NL,, Baginski TA. Chronic glaucoma selectively damages large optic nerve fibers. Invest Ophthalmol Vis Sci. 1987; 28: 913–920. [PubMed] [Google Scholar]
- 42. Quigley HA. Neuronal death in glaucoma. Prog Retin Eye Res. 1999; 18: 39–57. [DOI] [PubMed] [Google Scholar]
- 43. Libby RT,, Gould DB,, Anderson MG,, John SW. Complex genetics of glaucoma susceptibility. Ann Rev Genom Hum Genet. 2005; 6: 15–44. [DOI] [PubMed] [Google Scholar]
- 44. Whitmore AV,, Libby RT,, John SW. Glaucoma: thinking in new ways-a role for autonomous axonal self-destruction and other compartmentalised processes? Prog Retin Eye Res. 2005; 24: 639–662. [DOI] [PubMed] [Google Scholar]
- 45. Dolan RP,, Schiller PH. Effects of ON channel blockade with 2-amino-4-phosphonobutyrate (APB) on brightness and contrast perception in monkeys. Vis Neurosci. 1994; 11: 23–32. [DOI] [PubMed] [Google Scholar]
- 46. Dryja TP,, McGee TL,, Berson EL,, et al. Night blindness and abnormal cone electroretinogram ON responses in patients with mutations in the GRM6 gene encoding mGluR6. Proc Natl Acad Sci U S A. 2005; 102: 4884–4889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Morgan JE. Optic nerve head structure in glaucoma: astrocytes as mediators of axonal damage. Eye. 2000; 14: 437–444. [DOI] [PubMed] [Google Scholar]
- 48. Jakobs TC,, Libby RT,, Ben Y,, John SW,, Masland RH. Retinal ganglion cell degeneration is topological but not cell type specific in DBA/2J mice. J Cell Biol. 2005; 171: 313–325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. El-Danaf RN,, Huberman AD. Characteristic patterns of dendritic remodeling in early-stage glaucoma: evidence from genetically identified retinal ganglion cell types. J Neurosci. 2015; 35: 2329–2343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Peichl L. Alpha and delta ganglion cells in the rat retina. J Comp Neurol. 1989; 286: 120–139. [DOI] [PubMed] [Google Scholar]
- 51. Peichl L,, Ott H,, Boycott BB. Alpha ganglion cells in mammalian retinae. Procj Roy Soc Lond B. 1987; 231: 169–197. [DOI] [PubMed] [Google Scholar]
- 52. Dacey DM,, Petersen MR. Dendritic field size and morphology of midget and parasol ganglion cells of the human retina. Proc Natl Acad Sci U S A. 1992; 89: 9666–9670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Tauchi M,, Morigiwa K,, Fukuda Y. Morphological comparisons between outer and inner ramifying alpha cells of the albino rat retina. Exp Brain Res. 1992; 88: 67–77. [DOI] [PubMed] [Google Scholar]
- 54. Nichols Z,, Nirenberg S,, Victor J. Interacting linear and nonlinear characteristics produce population coding asymmetries between ON and OFF cells in the retina. J Neurosci. 2013; 33: 14958–14973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Chichilnisky EJ,, Kalmar RS. Functional asymmetries in ON and OFF ganglion cells of primate retina. J Neurosci. 2002; 22: 2737–2747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Greenstein VC,, Seliger S,, Zemon V,, Ritch R. Visual evoked potential assessment of the effects of glaucoma on visual subsystems. Vision Res. 1998; 38: 1901–1911. [DOI] [PubMed] [Google Scholar]
- 57. Zemon V,, Tsai JC,, Forbes M,, et al. Novel electrophysiological instrument for rapid and objective assessment of magnocellular deficits associated with glaucoma. Doc Ophthalmol. 2008; 117: 233–243. [DOI] [PubMed] [Google Scholar]
- 58. Mutlukan E. Computerised campimetry with static dark-on-bright stimuli. Doc Ophthalmol. 1993; 84: 335–350. [DOI] [PubMed] [Google Scholar]
- 59. Mutlukan E. A comparison of automated static dark stimuli with the Humphrey STATPAC program in glaucomatous visual field loss. Br J Ophthalmol. 1994; 78: 175–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Sampson GP,, Badcock DR,, Walland MJ,, McKendrick AM. Foveal contrast processing of increment and decrement targets is equivalently reduced in glaucoma. Br J Ophthalmol. 2008; 92: 1287–1292. [DOI] [PubMed] [Google Scholar]
- 61. Davoodnia V,, Lashgari R,, Alonso JM. Eye speed. Google Play (first version available); App Store (first version available). 2015. Available at: https://play.google.com/store/apps/details?id=vandad.testtrial&hl=en.