Abstract
Purpose
Some dry eye disease (DED) patients have sensitized responses to corneal stimulation, while others experience hypoalgesia. Many patients have normal tear production, suggesting that reduced tears are not always the cause of DED sensory dysfunction. In this study, we show that disruption of lacrimal innervation can produce hypoalgesia without changing basal tear production.
Methods
Injection of a saporin toxin conjugate into the extraorbital lacrimal gland of male Sprague-Dawley rats was used to disrupt cholinergic innervation to the gland. Tear production was assessed by phenol thread test. Corneal sensory responses to noxious stimuli were assessed using eye wipe behavior. Saporin DED animals were compared to animals treated with atropine to produce aqueous DED.
Results
Cholinergic innervation and acetylcholine content of the lacrimal gland were significantly reduced in saporin DED animals, yet basal tear production was normal. Saporin DED animals demonstrated normal eye wipe responses to corneal application of capsaicin, but showed hypoalgesia to corneal menthol. Corneal nerve fiber density was normal in saporin DED animals. Atropine-treated animals had reduced tear production but normal responses to ocular stimuli.
Conclusions
Because only menthol responses were impaired, cold-sensitive corneal afferents appear to be selectively altered in our saporin DED model. Hypoalgesia is not due to reduced tear production, since we did not observe hypoalgesia in an atropine DED model. Corneal fiber density is unaltered in saporin DED animals, suggesting that molecular mechanisms of nociceptive signaling may be impaired. The saporin DED model will be useful for exploring the mechanism underlying corneal hypoalgesia.
Keywords: corneal sensitivity, saporin toxin, cholinergic fibers, sensory responses, dry eye disease
Dry eye disease (DED) represents a group of disorders related to disruption of lacrimal function; a primary feature is an altered sensory perception of corneal stimuli. Patients with DED demonstrate either increased or decreased responses to noxious corneal stimulation and sometimes experience spontaneous pain, hyperalgesia, or allodynia.1–5 Changes in corneal sensory perception in DED have been postulated to be the result of sensitization of corneal sensory fibers due to an aqueous deficit at the ocular surface. Paradoxically, many DED patients do not have dry eyes or overt loss of lacrimal function. Numerous findings support the notion that basal tear production is not a good indicator of corneal sensory dysfunction.5,6 A recent study found that DED symptoms were significantly associated with nonocular pain and depression, but were not correlated with tear film measurements.7 In the present study we used two methods to disrupt the tear reflex circuit to determine the effect on sensory responses to noxious corneal stimulation.
Tear production, as well as pain, can be evoked by corneal stimulation. The reflex for tear production involves motor neurons within the superior salivatory nucleus (SSN),8 which send projections to parasympathetic cholinergic motor neurons in the pterygopalatine ganglion (PPG) that innervate the lacrimal gland and evoke tear production through stimulation of the acini within the gland (Fig. 1, dotted lines).9 In contrast, the reflex pathway involving the sensory perception of noxious corneal stimuli involves a pathway from the cornea to the trigeminal dorsal horn to neurons in the parabrachial nuclei10,11 and higher brain centers (Fig. 1, solid lines). The motor response to noxious stimulation of the cornea involves stereotypical eye wipe behaviors with the ipsilateral forelimb.12
Figure 1.
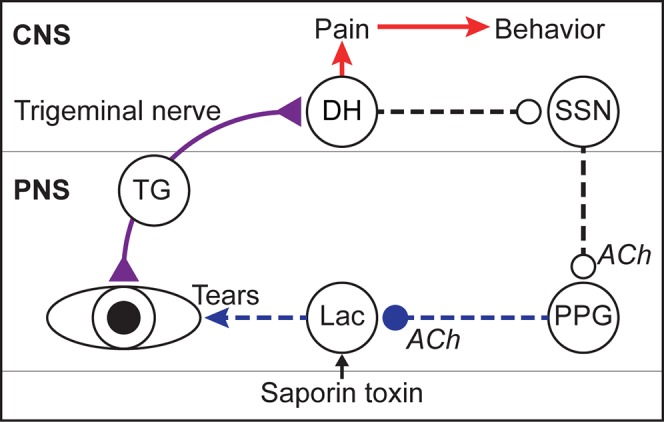
Schematic of sensory and reflex pathways regulating tear production and corneal sensation in the rat. Corneal sensation and tear film status are monitored by corneal afferents that innervate the corneal epithelium, have their cell bodies in the trigeminal ganglion (TG), and send projections via the trigeminal nerve (purple line) to the trigeminal dorsal horn (DH). Ascending pathways from the DH (red arrows) mediate pain and behavior in response to corneal stimulation. A separate reflex circuit from the DH (dotted lines) mediates tear production (Tears) via the parasympathetic reflex pathway through the superior salivatory nucleus (SSN) and pterygopalatine ganglion (PPG) to the lacrimal gland (Lac). In our novel DED rat model, a saporin toxin is microinjected into the extraorbital lacrimal gland (Lac) and ablates a portion of the innervating cholinergic nerve fibers (ACh) that originate in the PPG. ACh, acetylcholine; CNS, central nervous system; PNS, peripheral nervous system.
We will contrast two models, one that involves pharmacologic blockade of cholinergic receptors to reduce tear production, and a novel rat model of DED that we call saporin DED, which involves denervation of the extraorbital lacrimal gland using a saporin toxin conjugated to a monoclonal antibody that binds to the p75 neurotrophin receptor (p75NTR).13 The ribosome-inactivating saporin toxin is injected into the gland where it is taken up by nerves expressing p75NTR and transported to their cell bodies in the PPG, specifically ablating those nerves (Fig. 1).13 There are a variety of conditions in which lacrimal gland nerves are damaged by either an immune response or chemical exposure, and thus we felt that specifically damaging the nerves in the lacrimal gland might yield a scientifically interesting DED model by disrupting the lacrimal reflex pathway. To assess change in corneal nociception, we test responses to ocular stimuli that activate specific classes of nociceptors. Capsaicin activates sensory fibers that contain TRPV1, which is also responsive to noxious heat stimulation,14 and is present in corneal polymodal nociceptors.15 Menthol activates cold-sensitive fibers,16 which represent a smaller but distinct population of corneal nociceptors that have been implicated in dry eye mechanisms.17 Since responses to capsaicin and menthol are mediated by distinct molecular mechanisms, it is important to assess nociceptive responses to stimuli representing distinct modalities.
Methods
Experimental Animals
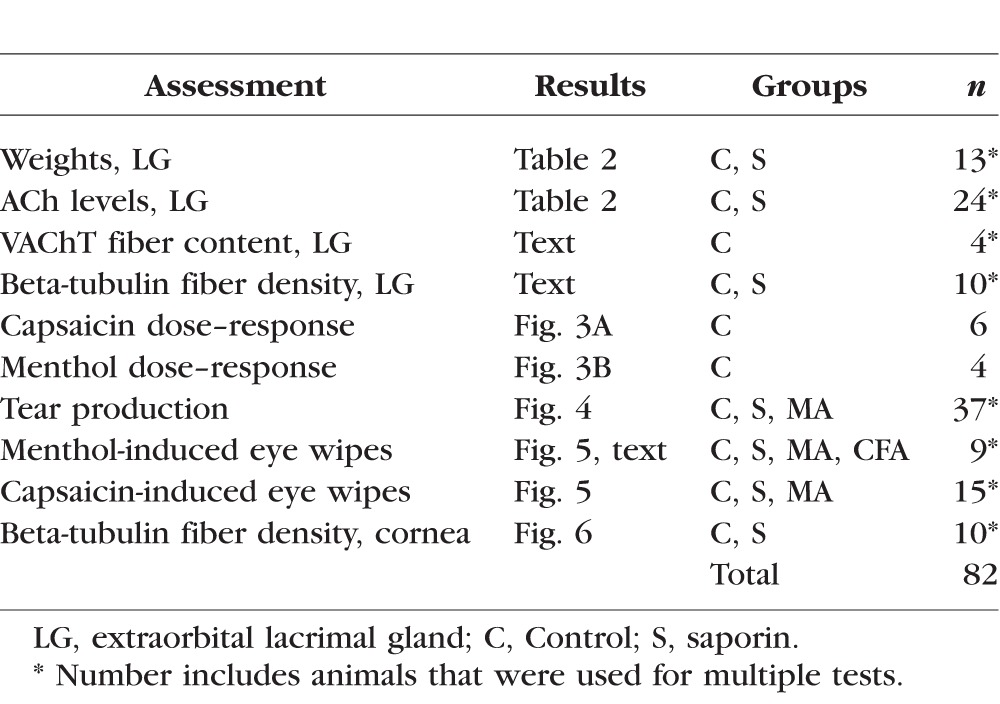
All protocols were approved by the Institutional Animal Care and Use Committee at Oregon Health & Science University, and all experiments adhered to the guidelines of the National Institutes of Health, the Committee for Research and Ethical Issues of the International Association for the Study of Pain (IASP), and the ARVO Statement for the Use of Animals in Ophthalmic and Vision Research for animal experimentation. Male Sprague-Dawley rats (250–450 g; Charles River Laboratories, Wilmington, MA, USA) were housed in pairs on a 12/12 light/dark cycle and were given access to food and water ad libitum. A total of 82 animals were used for all experiments (see Table 1); the specific number of animals used in each study is also indicated in the Results section. In some cases, the same animal could be used for measuring multiple endpoints, but other endpoints are mutually exclusive. For example, behavioral measures and histology of the gland can be performed in the same rat, but mass spectrometry and histology of the gland cannot be conducted in the same case and thus required separate groups.
Table 1.
Assessments, Experimental Groups, and Number of Animals Used for Each Component of This Study
Dry Eye Models
Methyl Atropine Model.
Rats were lightly restrained and given subcutaneous injections into the scapular scruff of 0.1% methyl atropine (1 mg/kg) twice daily (0900 and 1600 hours) for 2 days and once in the morning of the third day. Control animals were injected with equal volumes of saline using the same method on the same schedule.
Saporin Model.
Rats were deeply anesthetized with vaporized isoflurane in oxygen (5% for induction, 2%–3% maintenance), and the left extraorbital lacrimal gland was isolated. 192-IgG-saporin (5 μL, 0.5 μg/μL; Advanced Targeting Systems, San Diego, CA, USA) was microinjected into the gland through a glass pipette. Trypan blue was included in the solution to monitor the spread of the injectate, and cotton swabs were used to prevent spread of the saporin toxin to other tissues. The incision was closed with 3-0 monocryl suture (Ethicon, Cornelia, GA, USA) and covered with anesthetic ointment. The rats were returned to their home cage and monitored during recovery from anesthesia. Control rats were injected with vehicle (phosphate-buffered saline [PBS]) into the gland.
Inflammation Model.
To test the effects of inflammation of the lacrimal gland on ocular nociception, we injected 5 μL 1:1 Complete Freund's Adjuvant (CFA):saline solution into the left extraorbital gland (see above).
Phenol Thread Measurements
Tear production was measured using cotton threads embedded with phenol red (Zone-Quick; Oasis Medical, Glendora, CA, USA) that were placed in the lateral canthus of the anesthetized rat for 15 seconds. We used a within-animal analysis to compare phenol thread measurements at the endpoint of each study to baseline measurements. We also compared the endpoint measurements of the treatment groups to control animals.
Behavioral Measurement of Ocular Sensation
Awake rats were lightly restrained while 10 μL either capsaicin or menthol (Sigma-Aldrich Corp., St. Louis, MO, USA) was applied to the ocular surface. Ipsilateral eye wipes with the forelimb were counted for 3 minutes.12,18,19 Facial grooming behaviors or hind paw scratches were not included. Naïve animals were used to establish dose–response curves for both capsaicin and menthol. For our DED studies, we selected doses of capsaicin (0.01%) and menthol (20 mM) that produced intermediate levels of responses for each stimulus. Methyl atropine (MA) rats were assessed on the third day after the last injection of MA. Saporin rats were assessed 3 to 4 weeks after surgery. Control animals were tested in parallel on behavioral assays to identical stimuli.
Perfusion and Tissue Preparation for Microscopy
Rats were overdosed with pentobarbital sodium (150 mg/kg) and perfused transcardially as previously described11,20,21 through the ascending aorta with 10 mL heparinized saline (1000 units/mL) followed by 600 mL 4% paraformaldehyde (in 0.1 M phosphate buffer [PB], pH 7.4). Eyes were enucleated immediately after perfusion and placed in PB. Corneas were removed from each eye and placed in PB at 4°C until immunoprocessing (see below). Extraorbital lacrimal glands were removed after perfusion, placed in 4% paraformaldehyde in PB for 30 minutes at room temperature, and rinsed in PB. Glands were then placed into 30% sucrose in PB at 4°C until cryosectioning. Each lacrimal gland was frozen, sectioned (20 μm), mounted on slides, and stored at −20°C until immunocytochemistry was performed (see below).
Immunocytochemistry
All tissue was processed for immunocytochemistry as previously described.19 Slide-mounted lacrimal glands were incubated in a primary antibody cocktail of mouse anti-neuronal class III beta-tubulin (clone TUJ1, 1:500; Covance/BioLegend, Dedham, MA, USA), rabbit anti-dopamine beta-hydroxylase (DBH, 1:4000; Abcam, Cambridge, MA, USA), and guinea pig anti-vesicular acetylcholine transporter (VAChT, 1:100; EMD Millipore, Billerica, MA, USA) for two nights at 4°C. The specificity of the primary antibodies has been previously validated in our laboratory and others.22,23 Tissue sections were rinsed and incubated in a cocktail of fluorescent secondary antibodies (1:800; Alexa Fluor 488 donkey anti-rabbit, Alexa Fluor 647 donkey anti-guinea pig [Life Technologies, Grand Island, NY, USA]) and Cy3 donkey anti-mouse (Jackson ImmunoResearch Laboratories, Inc., West Grove, PA, USA) for 2 hours. Sections were air dried, coverslipped with Prolong Gold Antifade reagent (Life Technologies), and stored at −20°C. The specificity of the secondary antibodies has been confirmed by omitting the primary antibodies. Whole corneas were processed free-floating for beta-tubulin and clover-leafed onto slides and coverslipped as above.
Microscopy and Analysis
Extraorbital lacrimal gland sections were imaged on an Olympus BX51 microscope equipped with a DP71 camera (Olympus America, Center Valley, PA, USA). Immunocytochemistry was used to measure the innervation density of saporin-lesioned lacrimal glands. Beta-tubulin was used to assess overall nerve density, while VAChT and DBH were used to assess parasympathetic and sympathetic fibers, respectively. Low-magnification epifluorescent images were taken from three random regions of interest (ROIs) within each cryosection throughout each lacrimal gland. Regions centered over large empty ducts were avoided to reduce false negative results. Overall innervation of the lacrimal glands was analyzed in ImageJ (http://imagej.nih.gov/ij/; provided in the public domain by the National Institutes of Health, Bethesda, MD, USA) by taking each beta-tubulin image (nine images/animal) and converting it to a binary image. Each binary image was thresholded to include all labeled fibers and then analyzed for the percentage of pixels that were thresholded (area fraction). Area fractions were averaged for each animal to give one value per animal. To determine the extent of cholinergic and catecholaminergic innervation in the naïve extraorbital lacrimal gland, we used ImageJ to randomly trace 10 beta-tubulin–immunoreactive (-ir) fiber lengths per image (90 fibers/animal), saved the fiber lengths as ROIs, and then superimposed them onto the VAChT and DBH images. Each fiber length was then identified as containing VAChT or DBH or neither.
Corneal fibers detected with beta-tubulin were imaged on a Zeiss LSM 510 confocal microscope (Carl Zeiss MicroImaging, Thornwood, NY, USA). Z-stacks of 1 μm optical sections bounded by the vertical extent of beta-tubulin immunoreactivity through the epithelial and subbasal corneal layers were captured using the single-pass, multitracking format. Images were thresholded and converted to binary images as described above. A single area fraction was determined for each cornea.24
Acetylcholine Quantification by HPLC-MS
Fresh, whole extraorbital lacrimal glands were excised, weighed, and immediately transferred to prechilled tubes on dry ice and stored at −80°C until processed. Acetylcholine was measured by isotope dilution liquid chromatography tandem mass spectrometry (LC-MS/MS) using a Shimadzu Prominence UPLC with a Scherzo SS-C18 50 × 2-mm 3 μm column interfaced to a 4000 Q TRAP mass spectrometer (Applied Biosystems, Foster City, CA, USA) with an electrospray ionization source in positive mode. Multiple reaction monitoring was used monitoring the [M+H]+ transitions of m/z 146 → 87 and m/z 150 → 91 for acetylcholine and d4-acetylcholine internal standard, respectively. Standards containing 1 to 200 ng/mL acetylcholine in 0.1 M perchloric acid containing 10 ng/mL d4-acetylcholine were prepared for each sample set.
Statistical Analyses
Statistical analyses were performed using SigmaPlot 12.0 software (Systat Software, Inc., San Jose, CA, USA).
A one-way ANOVA with Holm-Sidak post hoc test was used to compare weights of left and right extraorbital lacrimal glands from saporin and control animals. The same test was used to compare acetylcholine (ACh) levels in saporin and control animals. This analysis allowed us to not only verify effectiveness of saporin lesions, but also determine if there were compensatory responses in the contralateral gland. An independent samples t-test was used to compare the mean area fractions of nerve fibers innervating the saporin-injected and naïve extraorbital lacrimal glands, as well as corneal fiber densities between saporin and control animals. This test was also used to compare the mean number of stimulus-evoked eye wipes of the saporin DED and MA DED models compared to controls. Paired t-tests were used for within-animal comparisons of phenol thread measurements taken prior to treatment (baseline) and at the endpoint of each DED model. We used a Kruskal-Wallis one-way ANOVA on ranks with Dunn's post hoc test to compare percent changes in phenol thread measurements among control, saporin, and MA DED rats. In all cases, a P value less than 0.05 was considered significant.
Results
Saporin Toxin-Induced Denervation Reduced Lacrimal Gland Weight, ACh Content, and Innervation
Injections of p75 conjugated saporin toxin into the extraorbital gland produced noticeable atrophy at 3 weeks; the ipsilateral glands of saporin DED animals weighed less than their contralateral gland or the glands from naïve animals (Table 2; one-way ANOVA, P = 0.004). Holm-Sidak post hoc analyses showed that the saporin-treated gland was significantly lighter than the contralateral gland in saporin-treated animals, as well as the glands in control animals. The contralateral saporin gland weight was not different than control gland weights. Acetylcholine levels in the extraorbital glands of saporin DED rats were compared to controls using a quantitative bioanalytical LC-MS/MS–based assay (Dennis Koop, Bioanalytical Shared Resources/Pharmacokinetics Core, Oregon Health & Science University). Acetylcholine was significantly reduced in the saporin DED animals compared to the contralateral gland in the same animal or on either side of the vehicle control animals (Table 2; one-way ANOVA, P < 0.001). Saporin-lesioned extraorbital glands had a 38% reduction in ACh content (Table 2).
Table 2.
Validation of Saporin Lesions of Cholinergic Fibers in Extraorbital Lacrimal Glands
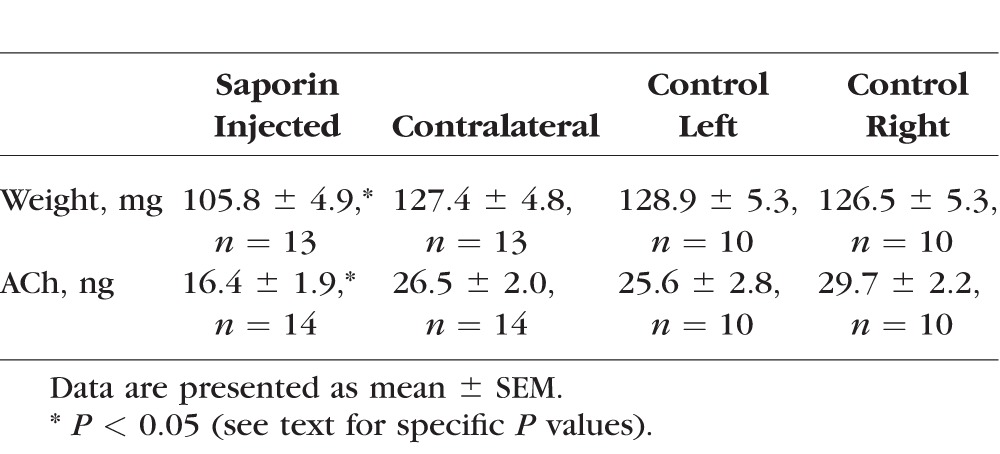
In a separate assay to verify denervation of the lacrimal gland, we first confirmed that 96% of beta-tubulin-ir fibers in the lacrimal glands of naïve animals contained VAChT immunoreactivity (Fig. 2, n = 4). While DBH innervation of the lacrimal gland was present, the density was nominal (<8% of beta-tubulin-ir fibers), so we could not accurately assess loss after saporin denervation. Thus, we used the beta-tubulin marker to quantify the degree of nerve loss in the glands of our saporin DED rats. The overall area fraction of nerve fibers in the extraorbital lacrimal gland was reduced by approximately 40% in saporin rats compared to naïve controls (saporin: 2.4 ± 0.3%, n = 6; naïve: 4.1 ± 0.3%, n = 4; t-test, P = 0.002). Together, these results indicate that glands were smaller, ACh content was reduced, and fiber density was reduced by saporin toxin injections into the lacrimal gland; and there was no compensatory response on the contralateral side.
Figure 2.
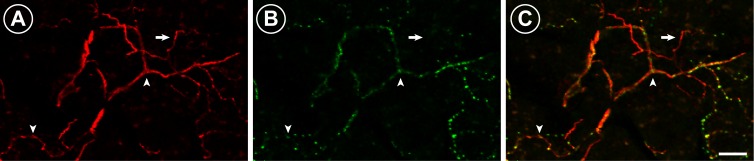
Representative micrographs of parasympathetic fibers innervating the extraorbital lacrimal gland in naïve animals. (A) Beta-tubulin-immunoreactive (-ir) fibers (red) run throughout the extraorbital lacrimal gland. (B) Vesicular acetylcholine transporter-immunoreactive fibers (green) are also present throughout the extraorbital lacrimal gland. (C) The vast majority of beta-tubulin-ir fibers also contain VAChT immunoreactivity (arrowheads), although there were a few examples of beta-tubulin-ir fibers that did not contain VAChT (arrow). Images presented are different channels from one 9 μm confocal projection. Scale bar: 20 μm.
Dose-Dependent Eye Wipe Behaviors Evoked by Corneal Stimulation With Capsaicin and Menthol
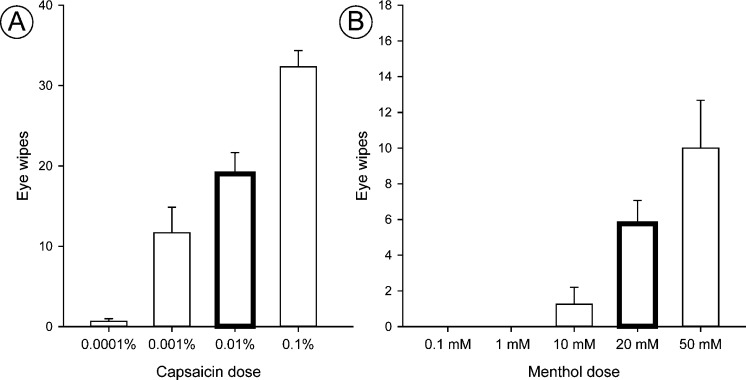
We tested two types of corneal stimuli that evoke nocifensive responses following ocular application: capsaicin and menthol. In naïve animals, we found that eye wipe responses increased dose-dependently in response to ocular application of either capsaicin (Fig. 3A) or menthol (Fig. 3B), supporting the use of this behavior as an indication of relative intensity of noxious stimuli.12 Stimulus-evoked eye blinks did not change dose-dependently with menthol (data not shown); therefore, only eye wipes were used in further studies as a nocifensive response in awake animals. For the remaining studies, we selected the 0.01% capsaicin and the 20 mM menthol doses (Fig. 3), which produced eye wipes at intermediate levels for each stimulus and would allow us to detect either increases or decreases in behavior for our DED rats.
Figure 3.
Ocular application of capsaicin and menthol dose-dependently increases eye wipes in naïve animals. Awake naïve animals received 10 μL capsaicin ([A] n = 6 total; 3/dose) or menthol ([B] n = 4 total; 4/dose) onto the ocular surface of one eye. Bars indicate mean ± SEM. Doses used for subsequent studies are bolded.
Tear Production Is Distinct Between Two DED Models
Methyl atropine produced a significant reduction in basal tear production as compared to pretreatment baselines (MA baseline: 11.0 ± 0.7 mm; MA endpoint: 3.6 ± 0.3 mm, n = 10; paired t-test, P = 0.000014). In contrast to MA, our saporin DED rats showed no change in their phenol thread measurements compared to their pretreatment baselines (saporin baseline: 14.0 ± 1.6 mm; saporin endpoint: 13.3 ± 1.6 mm, n = 8; paired t-test, P = 0.66). We found no change in phenol thread measurements in control animals that received either subcutaneous saline injections (saline baseline: 8.4 ± 0.9 mm; saline endpoint: 8.0 ± 1.0 mm, n = 9; paired t-test, P = 0.447) or PBS injections into their lacrimal gland (PBS baseline: 13.1 ± 1.8 mm; PBS endpoint: 13.1 ± 1.7 mm, n = 10; paired t-test, P = 1.0); thus control groups were pooled for further analysis (Fig. 4). The percent of baseline tear production was then calculated for each animal and averaged for each treatment group in order to compare between groups (Fig. 4). Using this analysis, tear production was significantly reduced in MA animals as compared to control animals, while tear production in saporin DED animals was not different from that in controls (Kruskal-Wallis one-way ANOVA with Dunn's post hoc analysis, P < 0.001) (Fig. 4).
Figure 4.
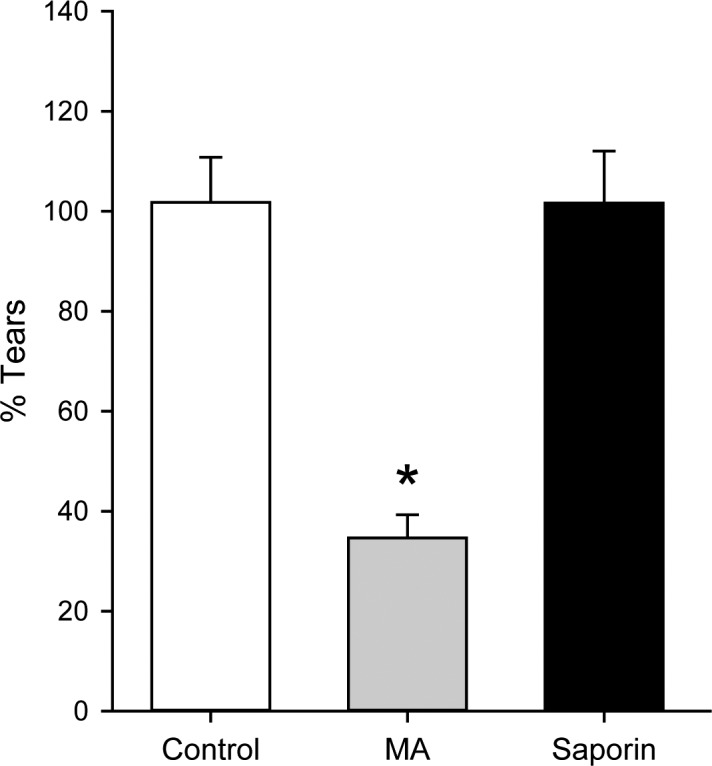
Dry eye disease models have distinct effects on tear production. Phenol thread measurements taken at the endpoint of each DED model are expressed as a percent of baseline measurements (% Tears). Systemic saline injections or PBS injections into the extraorbital lacrimal gland did not reduce tear volumes (groups pooled as Controls; white bar; n = 19). Tear production in methyl atropine (MA) animals (gray bar; n = 10) was significantly reduced. Saporin microinjections into the extraorbital lacrimal gland did not change tear volume as compared to that in control animals (black bar; n = 8). Bars indicate mean ± SEM. *P < 0.05.
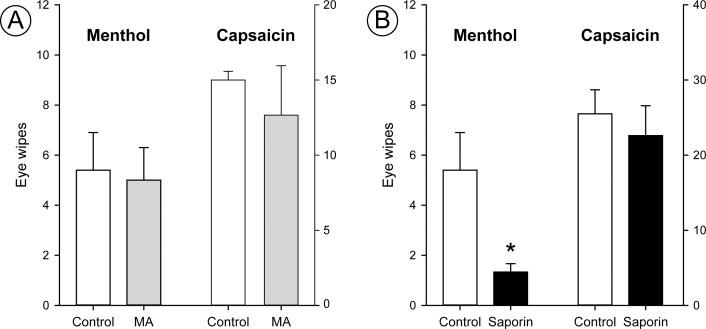
Saporin DED Rats Show Reduced Eye Wipes to Ocular Menthol, but Not Capsaicin
Methyl atropine produced no changes in the nocifensive eye wipe behavior (Fig. 5) despite producing significant deficits in tear production (Fig. 4). The number of eye wipes evoked by capsaicin or menthol were similar in MA animals compared to saline controls (Mann-Whitney rank sum test, P = 0.841). In contrast, saporin DED animals showed fewer eye wipes following ocular menthol (Fig. 5) compared to controls (t-test, P = 0.045). We did not see a significant effect of saporin treatment on eye wipe responses to ocular capsaicin compared to controls (Mann-Whitney rank sum test, P = 0.19). These results indicate that only menthol, not capsaicin, responses were altered in saporin DED animals.
Figure 5.
Saporin DED selectively reduces eye wipe responses to ocular menthol, while methyl atropine does not change nociceptive responses to menthol or capsaicin. Menthol eye wipes are shown on the scale on the left side of each graph; capsaicin data are scaled on the right side of each graph. (A) Methyl atropine (MA) animal (gray bars) eye wipe responses to menthol or capsaicin were similar to those in control animals (white bars) (n = 5 animals per treatment group). (B) Saporin animals (black bars) showed significantly fewer eye wipes to ocular menthol than control animals (white bars) (n = 3 saporin, 5 control animals). Saporin animals did not show any significant differences in eye wipe responses to capsaicin compared to control animals (n = 5 saporin, 4 control animals). The bar representing control animal eye wipes to menthol in both (A) and (B) is from the same set of animals and is repeated to facilitate direct comparisons of the groups. Bars indicate mean ± SEM. *P < 0.05.
Inflammation of the Lacrimal Gland Does Not Alter Eye Wipe Behavior
After lacrimal nerves are lesioned by the saporin toxin, the cellular debris within the gland will be removed by the immune system. We wanted to determine if a strong inflammatory response within the lacrimal gland could alter sensory responses in the cornea. We found that injections of CFA into the extraorbital lacrimal gland failed to alter the responses to corneal applications of menthol 3 weeks later (data not shown). Thus, a strong inflammation in the gland, which is likely to occur after our saporin lesions, is not sufficient to produce long-term hypoalgesia.
Corneal Fiber Density Is Not Changed in Saporin DED Rats
In order to determine if loss of corneal fibers could explain the reduced responses to menthol in our saporin DED animals, corneal fiber density was measured at the conclusion of the experiment (Fig. 6). There was no difference in the area fraction of corneal fiber density between control and saporin DED rats (t-test; P = 0.217).
Figure 6.
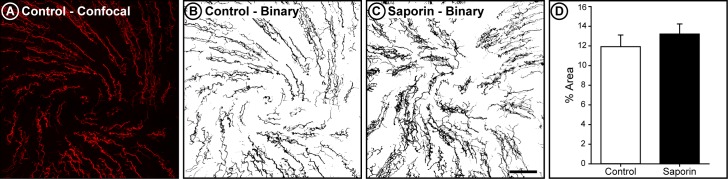
Corneal fiber density is not altered by lacrimal gland saporin toxin injections. (A) Confocal image of corneal fibers in a control animal. Image is a 27.54 μm–thick projection of the epithelial and subbasal corneal layers. (B) The binary representation of this image, used to calculate area fraction. (C) Example of a binary image of corneal fibers in a saporin DED rat. (D) Average area fraction of corneal fibers was similar in control (n = 5) and saporin DED (n = 5) animals. Bars indicate mean ± SEM.
Discussion
We have produced a novel model of DED that causes hypoalgesia to ocular stimulation without changing tear production. By partially lesioning the cholinergic innervation of the lacrimal gland, we have disrupted sensory responses to noxious ocular stimuli. The denervation is limited and does not alter the basal tear production, as assessed by the phenol thread test.25,26 Our findings suggest that the assessment of tear production is not always predictive of altered responses to noxious ocular stimulation. This is in agreement with clinical studies suggesting that DED symptoms do not consistently correlate with tear film measurements.7 Since altered sensory responses are often a complaint in DED patients and the potential source for both pain and injury, it is critical that we recognize that these endpoints (tear production and nocifensive behavioral responses) represent diverse efferent responses from a common afferent system (Fig. 1). Our findings suggest that we can dissociate the reflex pathways mediating basal tear production and those mediating nociceptive responses to ocular stimulation with our saporin DED model.
Targeting the Cholinergic Innervation in the Extraorbital Lacrimal Gland
Several animal models being used to simulate DED target the parasympathetic portion of the reflex pathway involved in tear production, including administration of cholinergic antagonists, such as scopolamine and atropine,26–28 and elimination of the preganglionic input onto the parasympathetic cholinergic neurons in the PPG.29 Another model involves the surgical excision of the lacrimal glands.30,31 Our novel saporin model allows us to selectively alter the cholinergic system13,32 within the extraorbital lacrimal gland rather than the whole body. This approach also leaves the gland in the body and causes a reduction but not complete loss of cholinergic innervation in the lacrimal gland.
We used three separate indices to confirm that the saporin toxin was denervating the cholinergic input to the injected extraorbital lacrimal gland, producing a partial lesion. The loss of cholinergic fibers from the extraorbital lacrimal gland impacts acinar cell function, since these fibers would normally release ACh that would bind to M3 muscarinic receptors localized on acinar cells in the lacrimal gland.33,34 These receptors are thought to be a target of autoantibodies produced in patients with Sjogren's syndrome.35 When stimulated, the acinar cells produce and secrete proteins and water to produce tear fluid that is then transported to the ocular surface. In the present studies we did not find any significant change in basal tear production following partial lesions of the lacrimal gland, showing that partial innervation is sufficient to maintain basal levels of tears. It is possible that tear volume might be reduced in response to a lacrimal challenge, but this would be confounded in most assessments that use corneal stimulation to evoke tearing. Our observation that behavioral responses to ocular stimulation with menthol are reduced suggests that evoked tearing to select ocular stimulation could also be reduced,36 but we would not be able to determine if this is due purely to damage to the efferent side of the pathway or due to the insensitivity of the corneal afferents to stimulation.
Behavioral Assessment of Corneal Sensation in DED
Several previous studies of DED models have demonstrated decreases in tear film measurements and increases in corneal surface deficits, but these studies did not evaluate changes in corneal sensory responses.26–29 Alterations in corneal sensory responses represent an important clinical consequence of DED that many animal DED models do not address.1–5 To this end, our study produced a unique DED model focused primarily on behavioral responses to noxious corneal stimulation.
We used eye wipe behavior to assess changes in ocular sensation in our saporin DED animals because previous studies have found that this particular behavioral response is evoked in rats by stimuli applied to trigeminal receptive fields that normally evoke a sensation of pain in humans.12,18,19,37 The behavioral response evoked by noxious stimuli is distinct from the behavioral response to stimuli that would evoke itch in humans.37,38 The notion that this behavior is nociceptive is also supported by recent findings that eye wipe behaviors are dose-dependently attenuated by morphine.12 Taken together, these studies confirm that eye wipe behavior is a sensitive indicator of ocular nociception.
Saporin DED Rats Are Hypoalgesic to Ocular Menthol, but Not Capsaicin
We found that saporin-induced denervation of the lacrimal gland attenuated eye wipe responses to corneal stimulation in a modality-specific manner; eye wipe responses to menthol were reduced while there was no change in responses to capsaicin. These results suggest that cold-sensitive nociceptors are most impacted on our saporin DED models, while polymodal nociceptors that would be responsive to capsaicin are not impaired.15 Polymodal nociceptors are the most prevalent type of nociceptors in the cornea, representing approximately 70% of afferents, and respond to both mechanical stimulation and high temperatures. Cold-sensitive fibers represent approximately 10% of corneal afferents but are thought to be responsive to evaporative stress.39 Our finding suggest that cold-sensitive fibers are most impacted in our saporin DED model, but it is difficult to make direct comparisons between the menthol and capsaicin stimuli because the mechanisms for transduction are so distinct, different populations of afferents are involved, and the stimuli are not psychophysically equivalent.
In many studies, changes in responses to noxious stimuli are seen across several modalities (heat, mechanical, cold), rather than in specific modalities as seen in the present study. However, modality-specific changes in response to cutaneous noxious stimuli have been reported clinically in extreme preterm infants who showed a decrease in thermal, but not mechanical, sensitivity.40 A recent model of neonatal inflammatory injury found an increase in sensitivity to mechanical stimulation but normal thermal sensitivity in adult rats that had been exposed to interplantar bee venom as neonates.41 Modality-specific changes have also been seen in vitro in trigeminal ganglia (TG) neurons cultured from animals that received an infraorbital nerve transection42; the TG neurons that had been transected were less responsive to cold and more responsive to heat stimuli. Therefore, the underlying mechanisms that lead to hypoalgesia for different nociceptive modalities may be distinct. In humans, contact lens wear has been shown to alter sensitivity to various classes of ocular stimuli.43
Mechanisms of Corneal Hypoalgesia Not Known
Our data are in contrast to recent studies that found increased, or hyperalgesic, responses to noxious corneal stimuli in a lacrimal gland excision model of DED. The glandectomy model involves the excision of one or more of the lacrimal glands, which can reduce tear production9,31,44–46 and has been reported to increase sensory responses to noxious corneal stimulation.30,31 In a recent study, Meng and colleagues30 found that excising both the infra- and extraorbital lacrimal glands increased corneal sensitivity to mechanical stimuli at certain time points and increased eye wipe time upon ocular application of hypertonic saline at 8 weeks post gland excision. In another study, excision of just the extraorbital lacrimal gland increased mustard oil-evoked eye blinks.31 It is likely that these various models induce distinct changes in corneal nociceptors or ascending pain pathways that are worthy of further exploration. In our current study, we found that MA caused substantial reduction in tear production, but no changes in nociceptive responses. The systemic atropine would block cholinergic receptors throughout the body and thus would impact not only the lacrimal gland, but also other ocular tissues with parasympathetic innervation. In spite of this systemic blockade of parasympathetic receptors, the nociceptive responses in our MA-treated animals were normal. It is possible that more sustained deficits in aqueous content of the tears are required to induce sensitization of corneal fibers, or the finding could indicate that molecular changes other than aqueous content are involved in inducing sensory changes in nociceptors. Animal models are excellent tools for the study of cellular and molecular mechanisms regulating corneal sensory systems, but there may be species-specific changes that must be validated and that may or may not generalize to humans.
The hypoalgesia seen in our saporin DED animals could be due to loss of corneal sensory fibers, which has been suggested in some clinical studies of DED but not by others.4,6,47 However, we found no changes in overall corneal nerve fiber density in our saporin DED animals. Thus, it is most likely that hypoalgesia stems from a change in the ability of these sensory fibers to encode certain stimuli or transduce signals to the nervous system. There may be alterations in channels and molecules within the sensory neurons that are being modified in our saporin model and mediating the menthol-specific hypoalgesia. One obvious candidate would be the transient receptor potential melastatin 8 (TRPM8) channel, which is the main transducer of cold and menthol stimuli.16,36,48 However, a recent study has suggested that high menthol doses, such as the one used in the current study, evoke nociceptive responses in a TRPM8-independent manner.49 It is possible that alterations in other TRP channels or other ion channels, such as potassium, sodium, and calcium channels, are changing the activation properties of menthol-sensitive corneal sensory neurons in our saporin DED animals.15,17,50,51 In order to elucidate the mechanisms that underlie the altered behavioral responses, future studies will explore peripheral and central sites of the corneal sensory pathway.
Conclusions
Denervation of the lacrimal gland reduced behavioral responses to the application of menthol to the corneal surface, without altering basal tear production or overall corneal nerve density. Our results indicate that our saporin DED model is able to dissociate the effects of DED on behavioral responses to nociceptive corneal stimulation from tear production. We contrast our saporin DED model with a commonly used rodent model, administration of the muscarinic receptor antagonist atropine, which caused an aqueous tear deficit but did not alter nociceptive responses to menthol or capsaicin. Perhaps the most exciting aspect of our model is that the saporin DED rats demonstrate corneal hypoalgesia, a symptom seen in many DED patients4,5 that has yet to be reported in other animal models.
Acknowledgments
The authors thank Dennis Koop and Jenny Luo of the Oregon Health & Science University Bioanalytical Shared Resources/Pharmacokinetics Core for their assistance with the acetylcholine assay.
Supported by National Institutes of Health Grants DE12640 (SAA, DMH, SMH) and P30 NS061800.
Disclosure: S.A. Aicher, None; S.M. Hermes, None; D.M. Hegarty, None
References
- 1. Rosenthal P,, Borsook D. The corneal pain system. Part I: the missing piece of the dry eye puzzle. Ocul Surf. 2012; 10: 2–14. [DOI] [PubMed] [Google Scholar]
- 2. Chao C,, Golebiowski B,, Stapleton F. The role of corneal innervation in LASIK-induced neuropathic dry eye. Ocul Surf. 2014; 12: 32–45. [DOI] [PubMed] [Google Scholar]
- 3. Nettune GR,, Pflugfelder SC. Post-LASIK tear dysfunction and dysesthesia. Ocul Surf. 2010; 8: 135–145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Labbe A,, Alalwani H,, Van Went C,, Brasnu E,, Georgescu D,, Baudouin C. The relationship between subbasal nerve morphology and corneal sensation in ocular surface disease. Invest Ophthalmol Vis Sci. 2012; 53: 4926–4931. [DOI] [PubMed] [Google Scholar]
- 5. Bourcier T,, Acosta MC,, Borderie V,, et al. Decreased corneal sensitivity in patients with dry eye. Invest Ophthalmol Vis Sci. 2005; 46: 2341–2345. [DOI] [PubMed] [Google Scholar]
- 6. Stapleton F,, Marfurt C,, Golebiowski B,, et al. The TFOS International Workshop on Contact Lens Discomfort: report of the subcommittee on neurobiology. Invest Ophthalmol Vis Sci. 2013; 54:TFOS71–TFOS97. [DOI] [PMC free article] [PubMed]
- 7. Galor A,, Felix ER,, Feuer W,, et al. Dry eye symptoms align more closely to non-ocular conditions than to tear film parameters. Br J Ophthalmol. 2015; 99: 1126–1129. [DOI] [PubMed] [Google Scholar]
- 8. Akerman S,, Goadsby PJ. A novel translational animal model of trigeminal autonomic cephalalgias. Headache. 2015; 55: 197–203. [DOI] [PubMed] [Google Scholar]
- 9. Meng ID,, Kurose M. The role of corneal afferent neurons in regulating tears under normal and dry eye conditions. Exp Eye Res. 2013; 117: 79–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Aicher SA,, Hegarty DM,, Hermes SM. Corneal pain activates a trigemino-parabrachial pathway in rats. Brain Res. 2014; 1550: 18–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Aicher SA,, Hermes SM,, Hegarty DM. Corneal afferents differentially target thalamic- and parabrachial-projecting neurons in spinal trigeminal nucleus caudalis. Neuroscience. 2013; 232: 182–193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Farazifard R,, Safarpour F,, Sheibani V,, Javan M. Eye-wiping test: a sensitive animal model for acute trigeminal pain studies. Brain Res Brain Res Protoc. 2005; 16: 44–49. [DOI] [PubMed] [Google Scholar]
- 13. Milner TA,, Hammel JR,, Ghorbani TT,, Wiley RG,, Pierce JP. Septal cholinergic deafferentation of the dentate gyrus results in a loss of a subset of neuropeptide Y somata and an increase in synaptic area on remaining neuropeptide Y dendrites. Brain Res. 1999; 831: 322–336. [DOI] [PubMed] [Google Scholar]
- 14. Caterina MJ,, Rosen TA,, Tominaga M,, Brake AJ,, Julius D. A capsaicin-receptor homologue with a high threshold for noxious heat. Nature. 1999; 398: 436–441. [DOI] [PubMed] [Google Scholar]
- 15. Belmonte C,, Acosta MC,, Merayo-Lloves J,, Gallar J. What causes eye pain? Curr Ophthalmol Rep. 2015; 3: 111–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. McKemy DD,, Neuhausser WM,, Julius D. Identification of a cold receptor reveals a general role for TRP channels in thermosensation. Nature. 2002; 416: 52–58. [DOI] [PubMed] [Google Scholar]
- 17. Belmonte C,, Brock JA,, Viana F. Converting cold into pain. Exp Brain Res. 2009; 196: 13–30. [DOI] [PubMed] [Google Scholar]
- 18. Price TJ,, Patwardhan A,, Akopian AN,, Hargreaves KM,, Flores CM. Modulation of trigeminal sensory neuron activity by the dual cannabinoid-vanilloid agonists anandamide N-arachidonoyl-dopamine and arachidonyl-2-chloroethylamide. Br J Pharmacol. 2004; 141: 1118–1130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Hegarty DM,, Hermes SM,, Largent-Milnes TM,, Aicher SA. Capsaicin-responsive corneal afferents do not contain TRPV1 at their central terminals in trigeminal nucleus caudalis in rats. J Chem Neuroanat. 2014; 61-62: 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Hermes SM,, Mitchell JL,, Aicher SA. Most neurons in the nucleus tractus solitarii do not send collateral projections to multiple autonomic targets in the rat brain. Exp Neurol. 2006; 198: 539–551. [DOI] [PubMed] [Google Scholar]
- 21. Hegarty DM,, Tonsfeldt K,, Hermes SM,, Helfand H,, Aicher SA. Differential localization of vesicular glutamate transporters and peptides in corneal afferents to trigeminal nucleus caudalis. J Comp Neurol. 2010; 518: 3557–3569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Suckow SK,, Deichsel EL,, Ingram SL,, Morgan MM,, Aicher SA. Columnar distribution of catecholaminergic neurons in the ventrolateral periaqueductal gray and their relationship to efferent pathways. Synapse. 2013; 67: 94–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Mousa SA,, Shaqura M,, Schaper J,, et al. Developmental expression of delta-opioid receptors during maturation of the parasympathetic, sympathetic, and sensory innervations of the neonatal heart: early targets for opioid regulation of autonomic control. J Comp Neurol. 2011; 519: 957–971. [DOI] [PubMed] [Google Scholar]
- 24. Dvorscak L,, Marfurt CF. Age-related changes in rat corneal epithelial nerve density. Invest Ophthalmol Vis Sci. 2008; 49: 910–916. [DOI] [PubMed] [Google Scholar]
- 25. Barabino S,, Dana MR. Animal models of dry eye: a critical assessment of opportunities and limitations. Invest Ophthalmol Vis Sci. 2004; 45: 1641–1646. [DOI] [PubMed] [Google Scholar]
- 26. Dursun D,, Wang M,, Monroy D,, et al. A mouse model of keratoconjunctivitis sicca. Invest Ophthalmol Vis Sci. 2002; 43: 632–638. [PubMed] [Google Scholar]
- 27. Viau S,, Maire MA,, Pasquis B,, et al. Time course of ocular surface and lacrimal gland changes in a new scopolamine-induced dry eye model. Graefes Arch Clin Exp Ophthalmol. 2008; 246: 857–867. [DOI] [PubMed] [Google Scholar]
- 28. Altinors DD,, Bozbeyoglu S,, Karabay G,, Akova YA. Evaluation of ocular surface changes in a rabbit dry eye model using a modified impression cytology technique. Curr Eye Res. 2007; 32: 301–307. [DOI] [PubMed] [Google Scholar]
- 29. Toshida H,, Nguyen DH,, Beuerman RW,, Murakami A. Evaluation of novel dry eye model: preganglionic parasympathetic denervation in rabbit. Invest Ophthalmol Vis Sci. 2007; 48: 4468–4475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Meng ID,, Barton ST,, Mecum NE,, Kurose M. Corneal sensitivity following lacrimal gland excision in the rat. Invest Ophthalmol Vis Sci. 2015; 56: 3347–3354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Katagiri A,, Thompson R,, Rahman M,, Okamoto K,, Bereiter DA. Evidence for TRPA1 involvement in central neural mechanisms in a rat model of dry eye. Neuroscience. 2015; 290: 204–213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Book AA Wiley RG, Schweitzer JB. 192 IgG-saporin: I. Specific lethality for cholinergic neurons in the basal forebrain of the rat. J Neuropathol Exp Neurol. 1994; 53: 95–102. [DOI] [PubMed] [Google Scholar]
- 33. Walcott B. The lacrimal gland and its veil of tears. News Physiol Sci. 1998; 13: 97–103. [DOI] [PubMed] [Google Scholar]
- 34. Dartt DA. Dysfunctional neural regulation of lacrimal gland secretion and its role in the pathogenesis of dry eye syndromes. Ocul Surf. 2004; 2: 76–91. [DOI] [PubMed] [Google Scholar]
- 35. Dartt DA. Neural regulation of lacrimal gland secretory processes: relevance in dry eye diseases. Prog Retin Eye Res. 2009; 28: 155–177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Parra A,, Madrid R,, Echevarria D,, et al. Ocular surface wetness is regulated by TRPM8-dependent cold thermoreceptors of the cornea. Nat Med. 2010; 16: 1396–1399. [DOI] [PubMed] [Google Scholar]
- 37. Klein A,, Carstens MI,, Carstens E. Facial injections of pruritogens or algogens elicit distinct behavior responses in rats and excite overlapping populations of primary sensory and trigeminal subnucleus caudalis neurons. J Neurophysiol. 2011; 106: 1078–1088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Shimada SG,, LaMotte RH. Behavioral differentiation between itch and pain in mouse. Pain. 2008; 139: 681–687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Belmonte C,, Gallar J. Cold thermoreceptors, unexpected players in tear production and ocular dryness sensations. Invest Ophthalmol Vis Sci. 2011; 52: 3888–3892. [DOI] [PubMed] [Google Scholar]
- 40. Walker SM,, Franck LS,, Fitzgerald M,, Myles J,, Stocks J,, Marlow N. Long-term impact of neonatal intensive care and surgery on somatosensory perception in children born extremely preterm. Pain. 2009; 141: 79–87. [DOI] [PubMed] [Google Scholar]
- 41. Li M,, Chen H,, Tang J,, Chen J. Neonatal bee venom exposure induces sensory modality-specific enhancement of nociceptive response in adult rats. Pain Med. 2014; 15: 986–997. [DOI] [PubMed] [Google Scholar]
- 42. Schmid D,, Messlinger K,, Belmonte C,, Fischer MJ. Altered thermal sensitivity in neurons injured by infraorbital nerve lesion. Neurosci Lett. 2011; 488: 168–172. [DOI] [PubMed] [Google Scholar]
- 43. Situ P,, Simpson TL,, Jones LW,, Fonn D. Effects of silicone hydrogel contact lens wear on ocular surface sensitivity to tactile, pneumatic mechanical, and chemical stimulation. Invest Ophthalmol Vis Sci. 2010; 51: 6111–6117. [DOI] [PubMed] [Google Scholar]
- 44. Fujihara T,, Murakami T,, Fujita H,, Nakamura M,, Nakata K. Improvement of corneal barrier function by the P2Y(2) agonist INS365 in a rat dry eye model. Invest Ophthalmol Vis Sci. 2001; 42: 96–100. [PubMed] [Google Scholar]
- 45. Kurose M,, Meng ID. Dry eye modifies the thermal and menthol responses in rat corneal primary afferent cool cells. J Neurophysiol. 2013; 110: 495–504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Nemet A,, Belkin M,, Rosner M. Transplantation of newborn lacrimal gland cells in a rat model of reduced tear secretion. Isr Med Assoc J. 2007; 9: 94–98. [PubMed] [Google Scholar]
- 47. Hosal BM,, Ornek N,, Zilelioglu G,, Elhan AH. Morphology of corneal nerves and corneal sensation in dry eye: a preliminary study. Eye (Lond). 2005; 19: 1276–1279. [DOI] [PubMed] [Google Scholar]
- 48. Peier AM,, Moqrich A,, Hergarden AC,, et al. A TRP channel that senses cold stimuli and menthol. Cell. 2002; 108: 705–715. [DOI] [PubMed] [Google Scholar]
- 49. Robbins A,, Kurose M,, Winterson BJ,, Meng ID. Menthol activation of corneal cool cells induces TRPM8-mediated lacrimation but not nociceptive responses in rodents. Invest Ophthalmol Vis Sci. 2012; 53: 7034–7042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Madrid R,, de la Pena E,, Donovan-Rodriguez T,, Belmonte C,, Viana F. Variable threshold of trigeminal cold-thermosensitive neurons is determined by a balance between TRPM8 and Kv1 potassium channels. J Neurosci. 2009; 29: 3120–3131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Viana F,, de la Pena E,, Belmonte C. Specificity of cold thermotransduction is determined by differential ionic channel expression. Nat Neurosci. 2002; 5: 254–260. [DOI] [PubMed] [Google Scholar]