Abstract
Objective
Although many perinatal factors have been linked to adverse neurodevelopmental outcomes in very premature infants, much of the variation in outcome remains unexplained. The impact on brain development of one potential factor, exposure to stressors in the Neonatal Intensive Care Unit, has not yet been studied in a systematic, prospective manner.
Methods
In this prospective cohort study of infants born at <30 weeks gestation, nurses were trained in recording procedures and cares. These recordings were used to derive Neonatal Infant Stressor Scale scores, which were employed to measure exposure to stressors. Magnetic resonance imaging (brain metrics, diffusion, and functional magnetic resonance imaging) and neurobehavioral examinations at term equivalent postmenstrual age were used to assess cerebral structure and function. Simple and partial correlations corrected for confounders including immaturity and severity of illness were used to explore these relationships.
Results
Exposure to stressors was highly variable, both between infants and throughout a single infant’s hospital course. Exposure to a greater number of stressors was associated with decreased frontal and parietal brain width, altered diffusion measures and functional connectivity in the temporal lobes, and abnormalities in motor behavior on neurobehavioral examination.
Interpretation
Exposure to stressors in the Neonatal Intensive Care Unit is associated with regional alterations in brain structure and function. Further research into interventions that may decrease or mitigate exposure to stressors in the Neonatal Intensive Care Unit is warranted.
Introduction
Survival rates for very preterm infants (born <30 weeks gestation) have improved dramatically in recent decades due to advances in perinatal and neonatal care. However, long-term neurodevelopmental outcomes remain a concern, with 5–10% of very preterm children having cerebral palsy and up to 40% displaying more mild motor deficits.1 The incidence of cognitive deficits is higher, with 30–60% of very preterm children experiencing cognitive impairments and social and emotional difficulties.2–4 Perinatal risk factors associated with adverse neurobehavioral outcomes include immaturity at birth, postnatal steroid use, prolonged ventilation, inotrope use, male gender, and cerebral injury such as white and grey matter abnormalities.4–8 However, these risk factors correlate only modestly with outcome implying that other neural mechanisms exist. Abnormalities in cerebral development, particularly in the frontal and temporal lobes, have been described in preterm infants9, 10 with few insights into the mechanisms that may be responsible.
The role of stress as a modifier of brain development in the preterm infant has been debated. Experimental animal data suggest that environmental factors in the Neonatal Intensive Care Unit (NICU), such as stress, may play a role.11 In addition, several interventional studies aimed at reducing stress in premature infants12, 13 have demonstrated improvements in short and long term outcomes. Although these studies suggest that exposure to stressors in the NICU may be harmful, there have been no studies examining the relationship of stressors in the NICU environment to brain injury, altered structural development, and functional outcome. This has been hampered by the lack of a systematic measure of stressful exposures in the NICU setting, as commonly used methods such as measurement of salivary cortisol have not been deemed as reliable indicators of stress in preterm infants. This limitation, however, was recently overcome.14 In this study we aimed to quantify stressful exposures among hospitalized preterm infants to determine their relationship to brain structure via magnetic resonance imaging (MRI) and functional outcome via neurobehavioral evaluations at term equivalent age.
Methods
An observational cohort study was conducted in preterm infants recruited within 24 hours of birth from November 2008 to December 2009. Exclusion criteria included infants >30 weeks gestation and infants who were moribund with severe sepsis or respiratory failure in the first 12 hours of life. The study was approved by the Human Studies Committee, and informed consent was obtained from parents prior to enrollment.
Stress was measured using the Neonatal Infant Stressor Scale (NISS).14 This scale consists of an easy-to-use single record sheet listing 36 procedures and interventions, ranging from diaper change to intubation, identified to contribute to infant stress. After procedures are documented over the course of days, weeks, or months, a cumulative stress score is determined. Nurses were trained in recording stressors as they occurred in a dedicated log, and documentation was cross-checked against the clinical record. Stressful exposures were measured from enrollment in the study until discharge from the NICU or term equivalent postmenstrual age (PMA). Clinical perinatal data from the medical records of mother and infant were also collected.
Magnetic Resonance Imaging (MRI)
MRI scans were performed at term equivalent age (36–44 weeks PMA) without sedation15 on a Siemens Magnetom Trio 3T scanner (Erlangen, Germany). Evaluation included an MP-RAGE T1-weighted sequence (TR/TE 1500/3 ms, voxel size 1 mm × 0.7 mm × 1 mm) and a turbo-spin echo (TSE) T2-weighted sequence (TR/TE 8600/160 ms, voxel size (1 mm)3, echo train length 17). A gradient echo, echo-planar-image (EPI) sequence sensitized to T2* blood oxygen dependent (BOLD) signal changes (TR/TE 2910/28 ms, voxel size (2.4 mm)3, flip angle 90°, FOV 160 mm, bandwidth 1662 Hz) was used to collect 200 frames over 10 minutes for functional connectivity MRI (fcMRI). Diffusion data were obtained using a spin-echo EPI sequence with 48 b values ranging from 0 to 1200 s/mm2 and spatial resolution (1.2 mm)3.
Brain injury was scored using 4 scales representing white matter, cortical grey matter, deep nuclear grey matter, and cerebellar injury, which were summed to produce a total MRI injury score.16 Eight measurements were made on tissue and fluid spaces including bifrontal, brain and bone biparietal, white matter, transverse cerebellar, and right and left lateral ventricular diameters and interhemispheric distance.17 Diffusion MRI analysis was performed using Analyze (Mayo Clinic, Rochester, MN) with regions of interest placed in the superior frontal, inferior frontal, and temporal lobes to generate apparent diffusion coefficient (ADC) and relative anisotropy (RA) values. fcMRI data were aligned, converted to atlas space, and spatially smoothed. Frames with significant motion were excluded. The BOLD time series for seed regions was then cross-correlated with all other voxels within the brain, generating correlation maps identifying regions with functional connection to the regions of interest.18 Ten infants with the higher and lowest NISS scores were chosen with a goal to match for gender and race and exclude infants with significant cerebral injury. Maps representing ten infants with lower NISS scores and ten infants with higher NISS scores were compared to a map representing ten healthy, term-born infants. Additionally, premature infants grouped by estimated gestational age at birth (> or <27 weeks) and length of ventilation (< 1 v > 10 days) were examined to explore their effect on the patterns of functional connectivity as to whether it resembled, and thus may explain, differences noted in relationship to stressful exposure. Though brain metrics, diffusion MRI, and functional connectivity analyses were performed on multiple regions of the brain, only those regions where differences were found to have a significant relationship to exposure to stressors are presented in the results.
Neurobehavioral Examination
Infants underwent neurobehavioral testing using the NICU Network Neurobehavioral Scale (NNNS)19 and Dubowitz exam at term equivalent PMA.20, 21 Examinations were completed by a single trained and certified examiner and were videotaped for reliability of scoring.
Statistical Analysis
The association of exposure to stressors (daily average cumulative NISS score for the first 14 and 28 days of life and from admission until term equivalent PMA or hospital discharge) with brain injury or abnormal brain development was evaluated using correlations (SPSS version 17, Chicago, IL). Simple bivariate correlations (r) were calculated, as were partial correlations (ρ) corrected for immaturity (estimated gestational age), initial severity of illness (CRIB score22), age at MRI scan, brain injury (total MRI injury score), and length of ventilation. Simple bivariate correlations were also used to assess the relationships between exposure to stress and continuous covariates (estimated gestational age, CRIB score, and length of ventilation) and binary logistic regression was used to assess the relationship between exposure to stressors and presence of cerebellar and intraventricular hemorrhage. For fcMRI analysis, correlation maps were compared qualitatively between groups, with central tendencies in strength of correlation between anatomic regions of interest and level of stress identified. Demographic characteristics of the three groups were explored using chi-squared (gender, race) and ANOVA (age at scan). Bonferroni adjusted thresholds were generated for multiple comparisons in relation to significant findings.
Role of Funding Sources
The sponsors had no role in study design, collection, analysis, or interpretation of the data, and writing or revisions of the manuscript.
Results
There were 65 eligible infants during the study period, with 55 recruited. Infants who withdrew from the study (n=3) or did not survive to term equivalent age (n=8) were not included in the analysis as no outcomes could be obtained. The characteristics of the 44 infants included in the study are listed in Table 1.
Table 1.
Characteristics of Cohort
| Demographic Characteristics | n=44 |
|---|---|
| Gestational Age at Birth (mean±SD) | 26.8±1.8 |
| Birth weight (g) (mean±SD) | 971±262 |
| Male, n (%) | 20 (44) |
| Singleton, n (%) | 26 (58) |
| Caucasian, African-American, Hispanic, Asian (n) | 20, 20, 2, 2 |
| IUGR*, n (%) | 4 (9) |
| CRIB score,22(mean±SD) | 3.2±3.2 |
| Antenatal Steroids, n (%) | 32 (71%) |
| Total Number of Days on Ventilator [median(range)] | 3 (0–92) |
| Oxygen therapy at 36 weeks, n (%) | 29 (66) |
| White matter injury (none, mild, moderate, severe) | 4, 24, 10, 4 |
| Intraventricular hemorrhage (none, I, II, III, IV) | 32, 6, 5, 0, 1 |
| Postnatal steroids, n (%) | 16 (36) |
| Dexamethasone only, hydrocortisone only, both | 2, 10, 4 |
| Inotropic support, n (%) | 13 (30) |
| Dopamine only; dopamine and dobutamine; dopamine and hydrocortisone; dopamine, dobutamine and hydrocortisone | 9, 2, 1, 1 |
| Confirmed sepsis | 6 (14) |
| Positive blood/CNS culture, necrotizing enterocolitis | 4, 2 |
| Patent ductus arteriosus, n (%) | 17 (39) |
| Treated with ibuprofen only, ibuprofen and surgical ligation | 13, 7 |
| Gestational Age at Term Scan (mean±SD) | 38.0±1.6 |
| Treated with laser surgery for retinopathy of prematurity, n (%) | 4 (91) |
Defined as >2SD below mean based on normative data from British 1990 growth reference for preterm data. (http://www.healthforallchildren.co.uk)
Exposure to Stressors
The average daily exposure to stressors was greatest in the first 14 days of life, with a similar pattern for the number of procedures (Table 2, Figure 1). The relationship of NISS scores to all MRI measures at term equivalent PMA is listed in Table 3. Infants with higher NISS scores over the total length of stay were more likely to be immature at birth (r=−.708, p<.001); have higher CRIB scores in the first 12 hours of life (r=.483, p<.001); receive prolonged ventilation during their neonatal stay (r=.801, p<.001). These relationships were consistent over all three time points. There was no relationship between weight gain (change in Z-score of weight from birth to term equivalent scan) and exposure to stressors at all three time points (Pearson’s correlation =−.086, −.191, and −.159 for 1st 14 days, 1st 28 days, and total stay respectively; p>.05 for all three time points). There were no other relationships with perinatal factors listed in Table 1.
Table 2.
Average daily neonatal Infant Stressor Scale (NISS) score and number of procedures in the first 14 and 28 days of life and from birth through term equivalent age.
| Average daily Neonatal Infant Stressor Scale scores | |
| First 14 days (mean±SD) | 106±13 |
| First 28 days (mean±SD) | 102±18 |
| Admission until term equivalent/discharge (mean±SD) | 80±12 |
| Range of daily Neonatal Infant Stress Scale scores | |
| First 14 days (min, max) | 85, 132 |
| First 28 days (min, max) | 71, 139 |
| Admission until term equivalent/discharge (min, max) | 61, 110 |
| Average daily number of procedures | |
| First 14 days (mean±SD) | 11±4 |
| First 28 days (mean±SD) | 10±5 |
| Admission until term equivalent/discharge (mean±SD) | 7±3 |
| Range of daily number of procedures | |
| First 14 days (min, max) | 4, 18 |
| First 28 days (min, max) | 3, 20 |
| Admission until term equivalent/discharge (min, max) | 3, 14 |
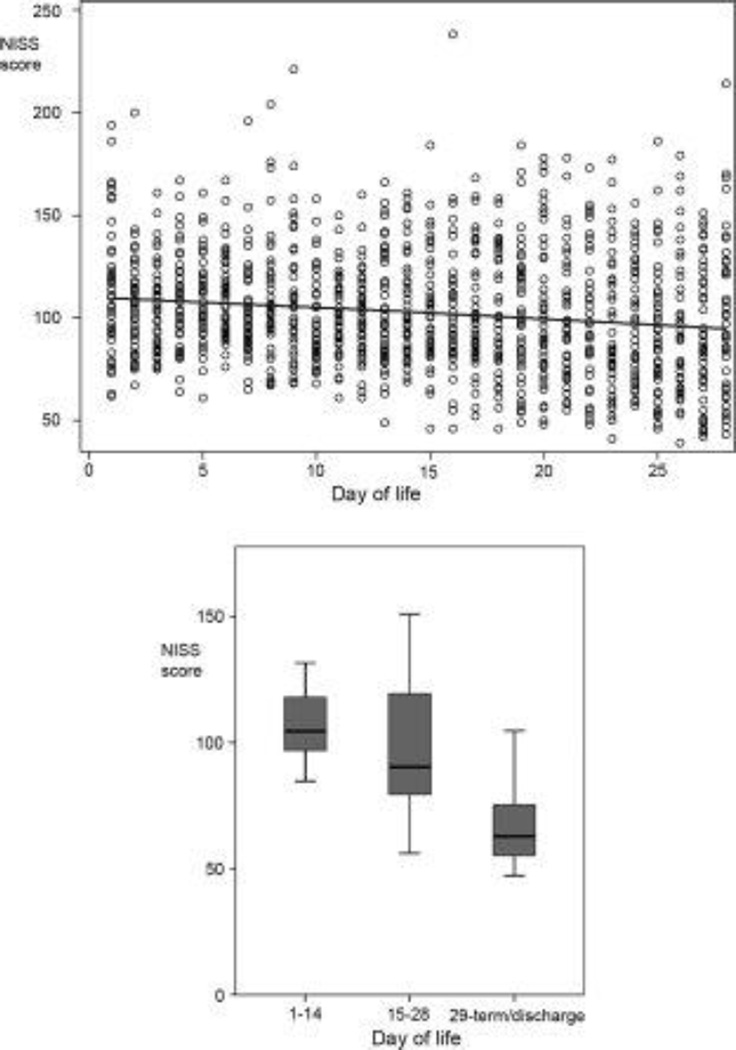
Figure 1.
Graphs showing average daily NISS score throughout admission to the NICU. The left pane depicts NISS score versus day of life for each infant for the first 28 days of life. Note that NISS score is higher and less variable between infants in the first days of life. The right pane depicts NISS score average daily NISS scores over the first and second 14 days of life and from 29 days. The black bar represents the median NISS score, the gray box the middle 50% of scores, and the whiskers the range of scores. Note that NISS score is greatest in the first 14 days and decreases thereafter.
Table 3.
The relationship of NISS scores from the total score in the first 14 and 28 days of life and average daily score from NICU stay in relation to brain metrics, diffusion measures and neurobehavior prior to and after adjustment for potential confounders.
| 14 day | 14 day* | 14 day† | 28 day | 28 day* | 28 day† | Total | Total* | Total† | |
|---|---|---|---|---|---|---|---|---|---|
| Brain Metrics (n=42) | |||||||||
| BFD | −.423 .005 |
−.254 .123 |
−.252 .133 |
−.567 <.001 |
−.364 .025 |
−.367 .035 |
−.533 <.001 |
−.308 .060 |
−.347 .035 |
| BPD | −.338 .029 |
−.261 .114 |
−.273 .101 |
−.482 .001 |
−.454 .004 |
−.486 .002 |
−.385 .012 |
−.257 .119 |
−.351 .033 |
| BBPD | −.447 .003 |
−.371 .022 |
−.356 .030 |
−.615 <.001 |
−.596 <.001 |
−.585 <.001 |
−.535 <.001 |
−.418 .009 |
−.413 .011 |
| WM | −.368 .016 |
−.386 .017 |
−.398 .015 |
−.272 .081 |
−.355 .029 |
−.378 .021 |
−.150 .344 |
−.124 .458 |
−.175 .300 |
| TCD | −.245 .118 |
−.062 .713 |
.001 .997 |
−.452 .003 |
−.255 .122 |
−.173 .306 |
−.454 .003 |
−.263 .110 |
−.061 .722 |
| Diffusion Measures (n=26) | |||||||||
| Right Temporal ADC | .378 .057 |
.404 .056 |
.391 .072 |
.207 .311 |
.208 .341 |
.191 .395 |
.128 .533 |
.093 .672 |
.020 .931 |
| Left Temporal ADC | .366 .066 |
.354 .097 |
.330 .133 |
.249 .220 |
.292 .176 |
.264 .235 |
.253 .213 |
.323 .132 |
.190 .397 |
| Right Temporal RA | −.157 .445 |
−.083 .708 |
−.054 .811 |
−.352 .078 |
−.307 .155 |
−.286 .197 |
−.439 .025 |
−.417 .048 |
−.362 .097 |
| Left Temporal RA | −.320 .112 |
−.298 .167 |
−.279 .208 |
−.323 .108 |
−.186 .396 |
−.164 .466 |
−.303 .133 |
−.143 .514 |
−.057 .802 |
| Dubowitz (n=40) | |||||||||
| Total | −.307 .054 |
−.290 .086 |
−.269 .119 |
−.182 .261 |
−.042 .809 |
−.002 .990 |
−.138 .397 |
−.030 .862 |
.156 .372 |
| Reflexes | −.283 .077 |
−.416 .012 |
−.403 .016 |
−.077 .635 |
−.167 .331 |
−.140 .424 |
−.023 .886 |
−.058 .738 |
−.019 .915 |
| Abnormal Patterns | −.422 .007 |
−.358 .032 |
−.337 .047 |
−.265 .098 |
−.050 .773 |
−.003 .987 |
−.221 .170 |
−.002 .990 |
.127 .468 |
| NNNS (n=43) | |||||||||
| Nonoptimal Reflexes | .265 .086 |
.347 .030 |
.343 .035 |
.135 .389 |
.216 .187 |
.208 .209 |
.073 .641 |
.087 .597 |
.042 .800 |
Corrected for EGA, CRIB score, and total MRI Injury score (+age at scan for brain metrics)
BFD-bifrontal diameter; BPD-brain to brain biparietal diameter; BBPD-bone to bone biparietal diameter; WM-white matter diameter; TCD-transverse cerebellar diameter; ADC-apparent diffusion coefficient; RA-relative anisotropy
Brain Injury
There was no relationship between white and gray matter injury scores and stressful exposures. There was a positive correlation between cerebellar hemorrhage and stressful exposures only over total length of stay (OR 1.155 [1.055–1.265] p=.002), which persisted when corrected for immaturity. There was also a relationship between total brain injury score and stressful exposures (28 days: r=0.362, p=0.02; total average to discharge/term: r=0.35, p=0.023), which persisted after adjustment for immaturity (28 days: r=0.391, p=0.01; total average to discharge/term: r=0.351, p=0.02). There was no relationship of intraventricular hemorrhage to stressful exposures (p>0.05 for all time points).
Brain Metrics
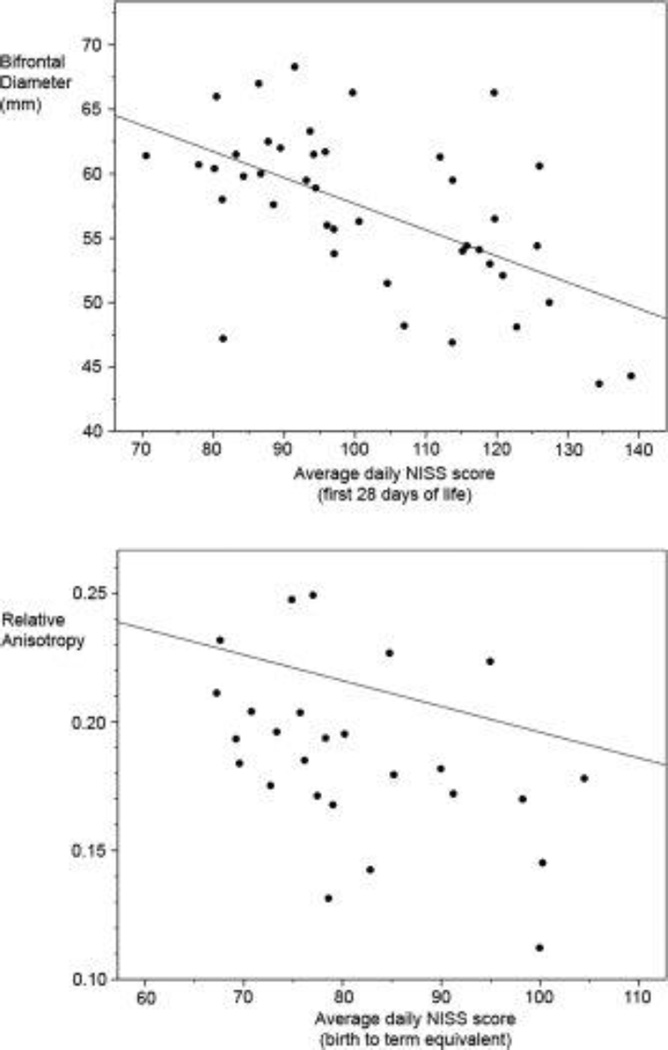
There was no relationship of ventricular diameter to any stress measures. In contrast, bifrontal and biparietal (including brain and bone measures) and white matter diameters were reduced with increasing stressful exposures (Fig 1). Correction for PMA at scan was included in all analyses. Correction for immaturity at birth, early severity of illness [CRIB score], MRI injury score, and length of ventilation diminished the relationship of stressful exposures in the first 14 days to brain metrics, though correlations of exposures in the first 28 days persisted. Transcerebellar diameter was associated with increased stressful exposure only when uncorrected for immaturity and severity of illness. Using a Bonferroni correction, our adjusted threshold for significance is 0.01.
Diffusion Tensor Imaging (DTI)
Two infants were not scanned and 16 scans were not of sufficient quality for DTI analysis. Superior and inferior frontal lobe DTI measures [ADC and RA] were not correlated with stressful exposures at 14 and 28 days, although the trend was towards increasing ADC and decreasing RA with increasing exposure to stressors. The right temporal lobe demonstrated decreased anisotropy with increased exposure to stressors in the NICU (r=−0.439, p=0.025), which persisted when corrected for immaturity at birth, early severity of illness [CRIB score], and MRI injury score. There was also a trend towards decreased anisotropy in the left temporal lobe and increased ADC in both temporal lobes associated with increasing exposure to stressors (table 3).
Functional connectivity MRI
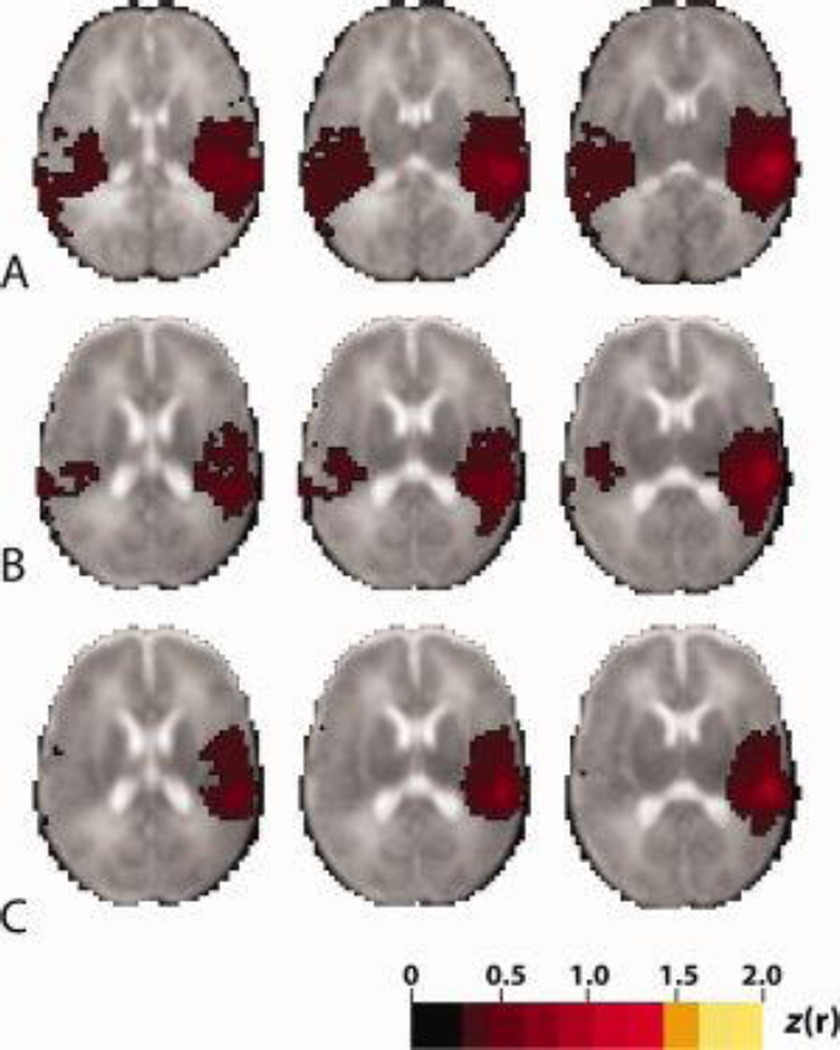
Discrepancies in fcMRI measures were noted between the designated groups, with the most prominent differences in neural networks identified using a seed in the right temporal lobe (Fig 3), where interhemispheric correlations, resembling that in term control infants, were identified in the temporal lobes of the low stress group but not in the high stress group. No differences in functional networks were noted in relationship to immaturity (EGA [< > 27 weeks] or prolonged ventilation [< 1 v > 10 days]), with particular note of a lack of any difference in the pattern of temporal lobe networks due to these factors. Thus, immaturity or prolonged ventilation did not appear to account for this regional disturbance in temporal lobe networks
Figure 3.
Mean functional connectivity correlation maps generated using the right temporal lobe seed in (A) term control infants (n=10) (B) low stress infants (n=10), (C) high stress infants (n=10). Illustrated quantity is Fisher z-transformed correlation coefficient (threshold value = 0.3). Note the average correlation map in the infants with high stress exposures does not demonstrate the interhemispheric correlation between the temporal lobes identified in low stress and term born control infants. The right side of image corresponds to the right side of brain. Age at scan, race, and gender were not significantly different between groups.
Neurobehavior
The relationship of NISS measures to neurobehavioral outcomes at term equivalent are listed in Table 3. Abnormal movement pattern (r=−0.422, p=0.04) and reflex (−0.283, p=0.08) subscales of the Dubowitz trended or were lower among infants with higher stress exposure in the first 14 days, which persisted when corrected for immaturity at birth, early severity of illness [CRIB score], MRI injury score, and length of ventilation (r=−0.403, p=0.02 and r=−0.337, p=0.05 respectively). A similar trend of increased non-optimal reflexes on the NNNS (r=0.265, p=0.09) was observed in infants with more stressful exposures, which also persisted after correction (r=0.343, p=0.04). There was no clear relationship between 28 day and total stressful exposures with neurobehavioral measures at term.
Discussion
Our study demonstrated that preterm infants in the NICU are exposed to many potential stressors, and that the amount of exposure varies between infants and over the course of a single infant’s admission to the NICU. Increased exposure to stressors in the NICU was associated with decreased brain size in the frontal and parietal regions and altered brain microstructure and functional connectivity within the temporal lobes. Alterations in neurobehavior at term equivalent were also associated with increased early exposure to stress.
Brain metrics, including bifrontal and biparietal diameters, have been shown to be highly correlated with brain tissue volume, in particular total brain and cortical gray matter volumes.17 Thus, the decreased bifrontal and biparietal diameters documented in our study in association with higher exposure to stressors represent reduced brain size. Although preterm infants in the NICU tend to be dolichocephalic, it has been shown that metrics such as bifrontal and biparietal diameter are correlated with head circumference such that a decrease in diameter represents a decrease in total brain size that is not compensated for by an increase in fronto-occipital diameter.17 Although long-term consequences of these reductions in brain size are unknown, data suggest that brain volumes at term equivalent PMA are predictors of neurodevelopmental outcomes later in childhood.23
We found a trend towards increased ADC and decreased RA with increasing exposure to stressors, representing altered cerebral white matter microstructure. Though this is the first study to examine the relationship between exposure to stressors and alterations in brain development, there have been several interventional studies aimed at decreasing stress in the NICU by delivery of developmentally appropriate care that have demonstrated positive effects on developmental and clinical outcomes.24–26 One study examined the influence of stress-reduction interventions on DTI measures and found that infants with the intervention had improved RA values in the frontal white matter suggesting more mature fiber tract development.24 Consistent with those findings, our data showed a trend towards decreased RA and increased ADC in the frontal lobes associated with greater stress exposure, but more significant differences were seen in the temporal lobes, in particular the right temporal lobe. Hemispheric asymmetries in the temporal lobe in preterm infants have previously been described, which is consistent with a differential developmental vulnerability.23
Alterations in functional connectivity related to exposure to stressors were also seen. Recent studies have shown differences in functional connectivity between term and preterm infants at term equivalent age, with preterm infants demonstrating decreased correlation, decreased long range connectivity, and lack of emergence of the default mode network compared to term infants.18 We found a specific association of high stress exposure with altered interhemispheric connections between the temporal lobes. Thus, neural network development in the temporal lobes in preterm infants exposed to more stressors appeared to be less mature and more poorly developed than in infants exposed to fewer stressors. Differences between the two groups cannot be explained by immaturity or length of ventilation, as infants grouped by these factors did not demonstrate these discrepancies in functional connections.
The alterations in the temporal lobes associated with high stress exposure detected in this study have not been observed previously. Chronic stress has been found to selectively reduce hippocampal volume in rats27, and studies on post-traumatic stress disorder in humans support this finding.28 Although this study did not specifically examine the effects of exposure to stressors on the hippocampus, it is possible that some of the changes seen in temporal lobe microstructure and connectivity may also be reflected in altered hippocampal size and structure.
Examining brain metrics, diffusion MRI, and functional connectivity MRI together allows for a more complete picture of altered brain development in response to stress exposure. Our findings that high stress exposure is associated with differences in the brain on both an anatomic and functional level indicate that the differences associated with stress are not only structural but also alter the function of the brain and thus potentially affect neurodevelopmental outcomes.
Differences in neurobehavioral function in relation to stress exposure were also observed. In particular, altered reflex development and abnormal patterns of movement were found in infants exposed to greater stress in the first 14 days of life. Our findings are consistent with a previous study that detected improved neurobehavioral performance among infants receiving developmental care and stress reduction in the NICU. This study also described persistent benefits at 9 months24 and 8 years.26 Although the mechanisms for these associations are unknown, it is possible that infants exposed to more stressors in the NICU are unable to develop mature patterns of movement and instead express abnormal and more immature patterns in response to their environment. Neonatal reflexes are very important as they form the foundation for later movement acquisition, and abnormal reflex development in the neonatal period can impede development and signal an abnormal developmental trajectory. Further investigations into whether infants with significant stress exposure have higher rates of long term neurodevelopmental sequelae are warranted.
A potential limitation implicit within this study is our attribution of the changes in brain size, diffusion measures, functional connectivity, and behavior to stressful exposures rather than simply severity of illness. We corrected for the effects of clinical condition at birth, immaturity, underlying brain injury at term equivalent PMA, and length of ventilation. Even after correcting for these covariates, there are other clinical factors related both to amount of stressful exposures and brain development. Although correction for these factors is necessary and important for teasing out the independent influence of stress, it also eliminates a significant portion of stressful exposures – particularly those related to respiratory illness and ventilation such changing tape on endotracheal tubes, frequent suctioning, and blood draws. We believe that stress, rather than simply being a marker of severe illness, is in the pathway from severe illness to altered brain development. As a result, relationships between stressful exposures and brain structure persisted despite correction for these measures of severe illness. Despite this, we cannot completely untangle the effect of severe illness from the effects of exposure to stressors in the NICU and cannot exclude the possibility that some of the associations found are related to severity of illness rather than or in addition to exposure to stressors in the NICU. In addition, multiple comparisons are made with adjusted thresholds for significance generated using Bonferroni methods, but reassuringly findings by differing methods reveal similar patterns in region and nature.
A further limitation of this study is the consistency and completeness of data collection. Over 200 NICU nurses completed stress records, which were cross-checked daily with the clinical and nursing charts for consistency. Due to the number of nurses and the long duration of NICU admissions, stress was not recorded during every shift for every subject, and thus completeness of the data was variable. Clinical and nursing charts were used to supplement data provided by nurses on stress records. A sensitivity analysis showed similar results when subjects with less data filled out using stress records were excluded. A third limitation is the small number of subjects available for analysis. Despite enrolling 55 subjects in the study, only 44 survived to term equivalent PMA and only 26 had MRI scans of high enough quality for diffusion analysis. Finally, the outcome data for these infants is not yet known. This cohort will be followed through childhood to determine the effects of stressful exposures in the NICU on subsequent neurocognitive, behavioral, and motor functioning.
This study demonstrates for the first time the systematic quantification and investigation of the effects of stress in the NICU environment on brain development in preterm infants. We found the NISS a useful tool in quantifying stressful exposures that could be beneficial in future studies. Our data suggest an important vulnerability of the preterm brain to stressful exposures, independent of measures of severity of illness that is highly worthy of research focus.
Figure 2.
Graphs showing relationships between average daily NISS score and (left pane) bifrontal diameter and (right pane) right temporal lobe relative anisotropy.
Acknowledgements
This study was supported by the National Institute of Child Health and Development (NICHD HD057512), the National Center for Research Resources (NCRR UL1 RR024992 and TL1 RR024995) of the National Institutes of Health (NIH) and the Doris Duke Distinguished Clinical Scientist Award (Doris Duke Charitable Foundation). The authors also wish to acknowledge the assistance of Drs. Amit Mathur and Joshua Shimony and research staff Karen Lukas, Jennifer Walker, Jayne Sicard-Su, Anthony Barton, Joseph Ackerman, and Terri Devault. Finally, we wish to thank the families and infants whose generosity in their participation in this research allows these scientific insights.
Abbreviations
- NICU
Neonatal Intensive Care Unit
- PMA
Post menstrual age
- NISS
Neonatal Infant Stressor Scale
- MRI
Magnetic Resonance Imaging
- ADC
Apparent Diffusion Coefficient
- RA
Relative Anisotropy
- BFD
Bifrontal Diameter
- BPD
Biparietal Diameter
- BBPD
Bone Biparietal Diameter
- NNNS
NICU Network Neurobehavioral Scale
- fcMRI
Functional Connectivity Magnetic Resonance Imaging
- DTI
Diffusion Tensor Imaging
REFERENCES
- 1.Holsti L, Grunau RV, Whitfield MF. Developmental coordination disorder in extremely low birth weight children at nine years. J Dev Behav Pediatr. 2002;23:9–15. doi: 10.1097/00004703-200202000-00002. [DOI] [PubMed] [Google Scholar]
- 2.Taylor HG, Minich NM, Klein N, Hack M. Longitudinal outcomes of very low birth weight: neuropsychological findings. J Int Neuropsychol Soc. 2004;10:149–163. doi: 10.1017/S1355617704102038. [DOI] [PubMed] [Google Scholar]
- 3.Anderson PJ, Doyle LW. Executive functioning in school-aged children who were born very preterm or with extremely low birth weight in the 1990s. Pediatrics. 2004;114:50–57. doi: 10.1542/peds.114.1.50. [DOI] [PubMed] [Google Scholar]
- 4.Laptook AR, O'Shea TM, Shankaran S, Bhaskar B. Adverse neurodevelopmental outcomes among extremely low birth weight infants with a normal head ultrasound: prevalence and antecedents. Pediatrics. 2005;115:673–680. doi: 10.1542/peds.2004-0667. [DOI] [PubMed] [Google Scholar]
- 5.Ambalavanan N, Baibergenova A, Carlo WA, et al. Early prediction of poor outcome in extremely low birth weight infants by classification tree analysis. J Pediatr. 2006;148:438–444. doi: 10.1016/j.jpeds.2005.11.042. [DOI] [PubMed] [Google Scholar]
- 6.Fily A, Pierrat V, Delporte V, et al. Factors associated with neurodevelopmental outcome at 2 years after very preterm birth: the population-based Nord-Pas-de-Calais EPIPAGE cohort. Pediatrics. 2006;117:357–366. doi: 10.1542/peds.2005-0236. [DOI] [PubMed] [Google Scholar]
- 7.Vollmer B, Roth S, Riley K, et al. Neurodevelopmental outcome of preterm infants with ventricular dilatation with and without associated haemorrhage. Dev Med Child Neurol. 2006;48:348–352. doi: 10.1017/S0012162206000764. [DOI] [PubMed] [Google Scholar]
- 8.Boardman JP, Craven C, Valappil S, et al. A common neonatal image phenotype predicts adverse neurodevelopmental outcome in children born preterm. Neuroimage. 2010;52:409–414. doi: 10.1016/j.neuroimage.2010.04.261. [DOI] [PubMed] [Google Scholar]
- 9.Thompson DK, Warfield SK, Carlin JB, et al. Perinatal risk factors altering regional brain structure in the preterm infant. Brain. 2007;130:667–677. doi: 10.1093/brain/awl277. [DOI] [PubMed] [Google Scholar]
- 10.Peterson BS, Vohr B, Staib LH, et al. Regional brain volume abnormalities and long-term cognitive outcome in preterm infants. JAMA. 2000;284:1939–1947. doi: 10.1001/jama.284.15.1939. [DOI] [PubMed] [Google Scholar]
- 11.Rangon CM, Fortes S, Lelievre V, et al. Chronic mild stress during gestation worsens neonatal brain lesions in mice. J Neurosci. 2007;27:7532–7540. doi: 10.1523/JNEUROSCI.5330-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Als H. Newborn Individualized Developmental Care and Assessment Program (NIDCAP): An Education and Training Program for Health Care Professionals. Boston: NIDCAP Federation International; 1986. [Google Scholar]
- 13.Goldson E, editor. Reading the Premature Infant. New York: Oxford University Press; 1999. [Google Scholar]
- 14.Newnham CA, Inder TE, Milgrom J. Measuring preterm cumulative stressors within the NICU: the Neonatal Infant Stressor Scale. Early Hum Dev. 2009;85:549–555. doi: 10.1016/j.earlhumdev.2009.05.002. [DOI] [PubMed] [Google Scholar]
- 15.Mathur AM, Neil JJ, McKinstry RC, Inder TE. Transport, monitoring, and successful brain MR imaging in unsedated neonates. Pediatr Radiol. 2008;38:260–264. doi: 10.1007/s00247-007-0705-9. [DOI] [PubMed] [Google Scholar]
- 16.Woodward LJ, Anderson PJ, Austin NC, et al. Neonatal MRI to predict neurodevelopmental outcomes in preterm infants. N Engl J Med. 2006;355:685–694. doi: 10.1056/NEJMoa053792. [DOI] [PubMed] [Google Scholar]
- 17.Nguyen The Tich S, Anderson PJ, Shimony JS, et al. A novel quantitative simple brain metric using MR imaging for preterm infants. AJNR Am J Neuroradiol. 2009;30:125–131. doi: 10.3174/ajnr.A1309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Smyser CD, Inder TE, Shimony JS, et al. Longitudinal Analysis of Neural Network Development in Preterm Infants. Cereb Cortex. 2010 doi: 10.1093/cercor/bhq035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Salisbury AL, Fallone MD, Lester B. Neurobehavioral assessment from fetus to infant: the NICU Network Neurobehavioral Scale and the Fetal Neurobehavior Coding Scale. Ment Retard Dev Disabil Res Rev. 2005;11:14–20. doi: 10.1002/mrdd.20058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dubowitz L, Ricciw D, Mercuri E. The Dubowitz neurological examination of the full-term newborn. Me. nt Retard Dev Disabil Res Rev. 2005;11:52–60. doi: 10.1002/mrdd.20048. [DOI] [PubMed] [Google Scholar]
- 21.Lester BM, Tronick EZ, Brazelton TB. The Neonatal Intensive Care Unit Network Neurobehavioral Scale procedures. Pediatrics. 2004;113:641–667. [PubMed] [Google Scholar]
- 22.The CRIB (clinical risk index for babies) score: a tool for assessing initial neonatal risk and comparing performance of neonatal intensive care units. The International Neonatal Network. Lancet. 1993;342:193–198. [PubMed] [Google Scholar]
- 23.Peterson BS, Anderson AW, Ehrenkranz R, et al. Regional brain volumes and their later neurodevelopmental correlates in term and preterm infants. Pediatrics. 2003;111:939–948. doi: 10.1542/peds.111.5.939. [DOI] [PubMed] [Google Scholar]
- 24.Als H, Duffy FH, McAnulty GB, et al. Early experience alters brain function and structure. Pediatrics. 2004;113:846–857. doi: 10.1542/peds.113.4.846. [DOI] [PubMed] [Google Scholar]
- 25.Newnham CA, Milgrom J, Skouteris H. Effectiveness of a modified Mother-Infant Transaction Program on outcomes for preterm infants from 3 to 24 months of age. Infant Behav Dev. 2009;32:17–26. doi: 10.1016/j.infbeh.2008.09.004. [DOI] [PubMed] [Google Scholar]
- 26.McAnulty GB, Butler SC, Bernstein JH, et al. Effects of the Newborn Individualized Developmental Care and Assessment Program (NIDCAP) at age 8 years: preliminary data. Clin Pediatr (Phila) 2010;49:258–270. doi: 10.1177/0009922809335668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lee T, Jarome T, Li SJ, et al. Chronic stress selectively reduces hippocampal volume in rats: a longitudinal magnetic resonance imaging study. Neuroreport. 2009;20:1554–1558. doi: 10.1097/WNR.0b013e328332bb09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gurvits TV, Shenton ME, Hokama H, et al. Magnetic resonance imaging study of hippocampal volume in chronic, combat-related posttraumatic stress disorder. Biol Psychiatry. 1996;40:1091–1099. doi: 10.1016/S0006-3223(96)00229-6. [DOI] [PMC free article] [PubMed] [Google Scholar]