Abstract
We designed and optimized tissue-responsive adhesive materials by matching material and tissue properties. A two-component material based on dextran aldehyde and dendrimer amine provides a cohesive gel through aldehyde–amine cross-linking and an adhesive interface created by a dextran aldehyde-selective reaction with tissue amines. By altering aldehyde–amine chemistry, we examined how variations in tissue surfaces (serosal amine density in the duodenum, jejunum, and ileum) affect interactions with adhesive materials of varied compositions (aldehyde content). Interestingly, the same adhesive formulation reacts differentially with the three regions of the small intestine as a result of variation in the tissue amine density along the intestinal tract, affecting the tissue–material interfacial morphology, adhesion strength, and adhesive mechanical properties. Whereas tissues provide chemical anchors for interaction with materials, we were able to tune the adhesion strength for each section of the small intestine tissue by altering the adhesive formulation using a two-component material with flexible variables aimed at controlling the aldehyde/amine ratio. This tissue-specific approach should be applied to the broad spectrum of biomaterials, taking into account specific microenvironmental conditions in material design.
INTRODUCTION
Property gradients at interfaces between tissues with different functionalities create more than physical barriers. Subtle chemical gradients enhance differential recognition and functionality even for adjacent tissues and can take on increased importance in the design of materials that interact chemically with tissue surfaces. Whereas materials and devices are designed to modulate specific functions for a given application and tissue bed, the definition of material–tissue interactions has rarely considered differences in target tissue.1 Tissues are composed of the same basic cells and components—this is part of physiologic conservation—yet subtle differences in the relative ratios and configuration of these materials allow for definitive discrimination between like and adjacent tissues. The main question that arises is, what is the role of the in vivo tissue microenvironment in determining material performance? Specifically, it is interesting to examine the effect of tissue type on material interaction. Nature employs gradients in tissue properties to impart a smooth transition between tissue regions and to facilitate differential tissue functions, chiefly by adjusting the composition and architecture.2,3 We examined whether differences in chemical composition between tissues would affect biomaterial function, specifically the adhesion capacity. Soft-tissue adhesive materials provide an ideal material class for the assessment of tissue–material interactions as a sealant adhesion, which can be rigorously quantified through a series of functional assays that supplement characterizations of tissue reactivity and material fate.4,5 A vast array of techniques in material design have been employed to regulate the adhesive binding potential to tissue surfaces, including material surface patterning6 and chemical modification.7,8 The next step in material design would be to consider natural differences that transpire between tissue surfaces, as relevant to a specific medical application, and only then to examine how material can be further tuned to control its performance, specifically adhesive interactions. We propose that natural variations in tissue surface chemistry affect the tissue–material interaction by way of adhesion and also affect the material properties, including cohesion and therefore the mechanical properties, morphology, and degradation kinetics. To begin to unravel the impact of natural variation between adjacent tissues, we have designed a new family of adhesive materials based on dendrimers and dextran and have defined their interactions within the continuous regions of the small intestine: the duodenum, jejunum, and ileum. We hypothesized that differences in organ function and structure play a key role in determining the tissue composition and herein the tissue amine content. It has been shown that the serosal duodenum and jejunum contain higher collagen contents whereas the ileum presents a higher elasticity indicative of a higher elastin content. These differences in the composition of the extracellular matrix will dictate the serosal amine density, requiring the development of a specific assay to determine tissue amines.9 Variance in the surface properties of small intestinal subjacent tissues in our model system enables us to study the extent to which natural gradients in tissue properties affect tissue–biomaterial interactions and to examine whether the same material performs distinctly when applied to different tissue surfaces within the same organ.
RESULTS
Biocompatibility and in Vivo Pathology
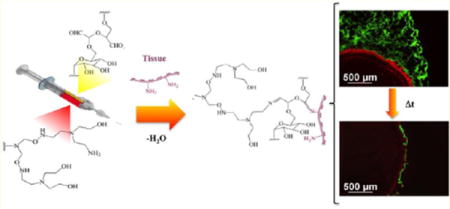
The dendrimer/dextran family of materials provides a model system for examining chemically directed adhesion. The synergy between dendrimer multifunctionality and size on the nanoscale (Figure 1a,b) enables the creation of smart materials with environmentally sensitive modalities.10 These materials support two competitive reactions: an external reaction between tissue amines and dextran aldehydes-promoting material–tissue adhesion (Figure 1c) and a second, internal reaction between dendrimer amines and dextran aldehydes that adds to gel cohesion and removes free aldehydes from potential tissue toxicity (Figure 1d). Multiarmed dendrimers with multiple amine groups per dendritic molecule should have a high capacity for absorbing these free aldehyde groups. When dendrimer generation and the oxidation level are optimized, the amine density determines if the material will gel, remain a liquid, or polymerize rapidly to form a plastic material. Whereas generation five dendrimer with 75% oxidation and 32 surface amine groups forms a hydrogel, generation three dendrimer with 32 surface amine molecules does not gel and remains as a liquid and generation five dendrimer with 128 amine groups polymerizes to form a white plastic that cannot retain water (Supporting Information Figure 1). Hence, we chose to work with generation five dendrimer that has a higher molecular weight than generation three and hence less steric hindrance for interaction with dextran, with only 25% of amines on the surface to support hydrogel formation.
Figure 1.
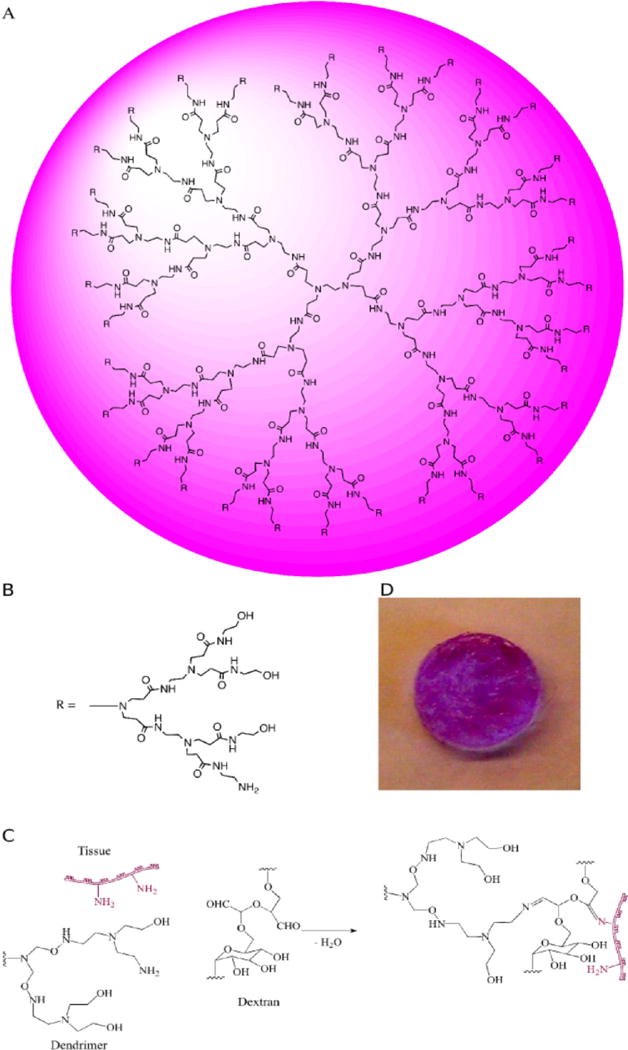
(A) Schematic of dendritic polyamidoamine dendrimer (PAMAM), presented as generation three with (B) two additional generations presented as the side R group having 75% oxidation—one amine group per three hydroxyls in each arm. (C) When applied to a tissue surface, the external reaction between dextran aldehyde and tissue amines imparts adhesive properties and an internal reaction between dextran aldehydes and (D) dendrimer amine imparts a cohesive gel.
A subset of materials that vary in dextran aldehyde solid content was chosen from a larger set of materials to examine the material biocompatibility in which excess aldehydes that remain free give rise to toxic cellular and tissue responses.11 Regardless of the dextran aldehyde solid content, cell survival was higher than 90% and supports the hypothesis that the multiple amine dendritic arms absorb excess aldehydes that might otherwise impart cytotoxicity (Figure 2). Following these experiments, materials were implanted in the dorsal subcutaneous space of a mouse that survived for 7 or 30 days (Figure 3a). Regardless of material formulation, the tissue response at day 7 was generally characterized by abundant dendrimer/dextran adhesive material in the subcutaneous region (hypodermis) surrounded by a mild to moderate zone of inflammation consisting primarily of histiocytic/macrophage infiltrates and interstitial edema with minimal scattered granulocytes (i.e., neutrophils and eosinophils) and early fibroplasia. Abundant dendrimer/dextran adhesive material was still present at day 30 but relative to that at day 7 appeared to be more vacuolated and associated with a relatively quiescent, decreased tissue response characterized by minimal cell infiltrates and the encapsulation by a thin band of fibrous connective tissue (specific regions delineated in Supporting Information Figure 2). Regardless of the time point or dose, there was no evidence of adverse pathology including hemorrhaging, necrosis, or aberrant neovascularization and no potentially adverse inflammation, supporting the potential use of such materials as adhesive materials. The inflammatory score (Figure 3b) did not exceed 2 (mild to moderate response) for any of the compositions or for the 7 or 30 day time points. Inflammatory cells such as histiocytes and neutrophils were present after 7 days as expected after any surgical manipulation, and their level diminished after 30 days, indicating a healing response.
Figure 2.
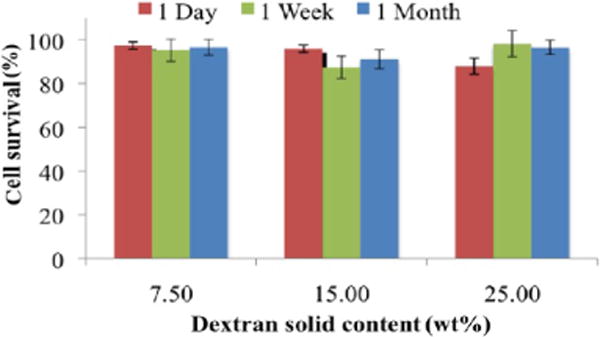
Material cytotoxicity examined in vitro using 3T3 fibroblasts. Cell survival in the presence of adhesive materials with increasing dextran aldehyde solid contents (7.5, 15, and 25 wt %) exceeds 90% regardless of the formulation or time elapsed (1, 7, or 30 days).
Figure 3.
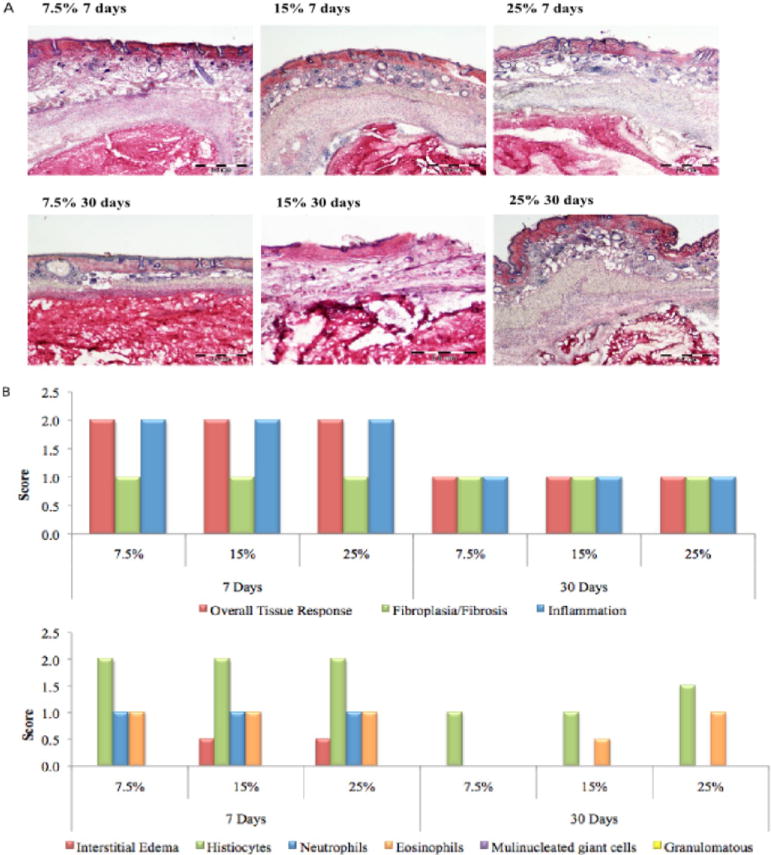
(A) In vivo response to adhesive materials with increasing (7.5, 15, and 25 wt %) showing minimal surrounding histiocytic/macrophage inflammation and minimal fibrous encapsulation, in agreement with the in vitro results. (B) On the basis of our scoring system, a mild response was evident at 7 days as expected after any tissue manipulation and implantation; however, this response became minimal after 30 days, regardless of the material formulation. Bar = 200 nm.
Material Tunability
We examined the extent to which we can control the material adhesive capacity to serosal rat small intestinal tissues by varying the material formulation. A difference of 17.5 wt % in dextran aldehyde solid content imparts a 115% change in the load required to disrupt the tissue–material bond and lead to failure. As expected, the lower the amount of aldehyde, the weaker the adhesive bond (Figure 4). Loading failure values for different material compositions were 0.2–0.4 N, which fall between the values reported for commercially available adhesive materials that are considered to be nonadhesive or extremely adherent, with loading failures of 0.15 N for fibrin glue and 0.6 N for cyanoacrylate under the same testing conditions.11 The aldehyde content is a key parameter determining not only the internal interaction with dendrimer amines but also the interaction with tissue amines. The interfacial regions between dendrimer/dextran and the excised rat jejunum were microscopically examined to add physical insight to adhesive interactions. Three distinct domains were evident with material adhesion, including the target tissue (T, red), bulk material (B, green), and an adhesive regime (I) interposed between the two (Figure 5a). The adhesive regime depicts the intermediate material structure resulting from the concurrent dextran aldehyde reactivity with dendrimer and tissue amines. The interfacial morphology of the adhesive regime varied with the material composition and reflected the strength of adhesion,4 appearing porous and discontinuous at low aldehyde content and more continuous at the higher contents. By quantifying the fluorescence intensity at the interface, we can evaluate the adhesive interactions with the tissue (Figure 5b). Indeed, there is a linear correlation (R = 0.98, Supporting Information Figure 3) between the maximum load as measured by the strength of materials in a mechanical tester (Figure 4) and the interfacial fluorescence signal (Figure 5b), supporting the notion that the interfacial morphology is a good indication of macroscopic failure. Similarly, the bulk of the material is more porous at the low aldehyde solid content (7.5 wt %) and gets denser as the content increases (up to 25 wt %), giving rise to a higher cross-linking density. This is supported by the smaller pore size and faster gelation as the dextran aldehyde solid content increases (Supporting Information Figure 4a,b).
Figure 4.
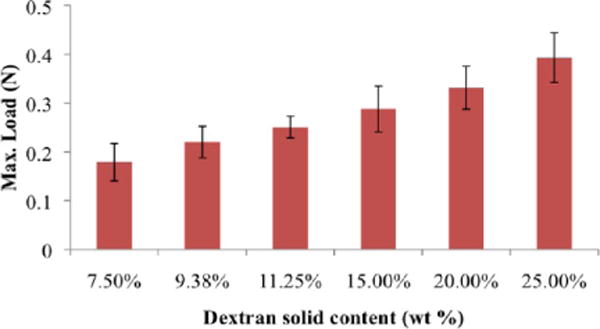
Material adhesion strength with respect to jejunal tissue can be mediated by formulation and increases with dextran aldehyde solid content. An increase of 17.5 wt % dextran aldehyde results in a 115% increase in adhesion strength. 7.5, 11.25 and 25 wt % loads are statistically significantly different, p < 0.5.
Figure 5.
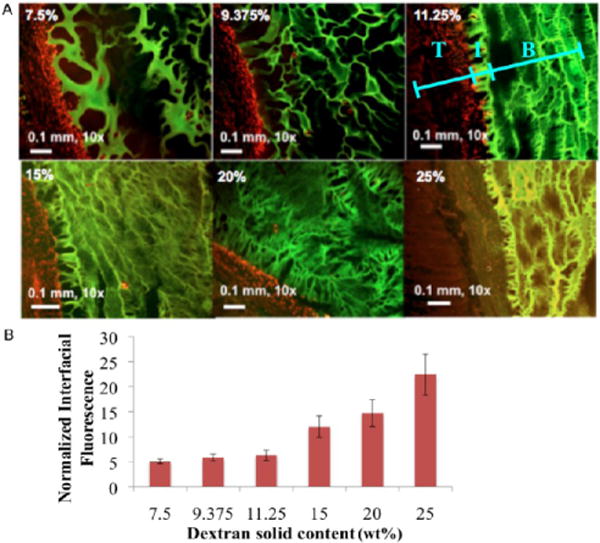
(A) Interaction between the adhesive material and tissue was examined by quantifying the fluorescence intensity at the interface between them (T = tissue, I = interface, and B = bulk material). Tissue was stained with propidium iodide (red) and material conjugated with fluorescein (green). When we increase the dextran aldehyde solid content, the interface becomes continuous and less porous, indicative of improved interaction with the tissues. (B) Interfacial fluorescence intensity increases with aldehyde solid content and has a linear correlation (R = 0.98) with the maximum load as measured by a mechanical tester (Figure 4). 7.5, 15, and 25 wt % interfacial fluorescence values are statistically significantly different, p < 0.5.
Tissue Microenvironment Alters Material Properties
We defined the interaction of dendrimer/dextran with the three regions of serosal rat small intestine—duodenum, jejunum, and ileum—using specific material formulations of D10-50-11.25 and G5-25-12.5. The nomenclature for this material reflects a composition that contains 10 kDa dextran with 50% oxidation and 11.25 wt % solid content mixed with generation five dendrimer with 75% oxidation (25% amine surface groups) and 12.5 wt % solid content. We hypothesized that a variation in tissue properties along the GI tract will support the diverse functionalities of these regions. Specifically, we were interested in examining whether there is a gradient in amine density along the gastrointestinal tract because these groups interact with aldehyde groups in our materials to form an adhesive bond. The surface amine chemistry of the three tissue types was quantified with fluorescent aldehyde-coated microspheres, providing a means of rapid evaluation of tissue–aldehyde reactivity (Figure 6a) and a mechanistic basis for the demonstrated variability in adhesive mechanics. We show that serosal ileum amines are at a much higher density than those of the duodenum and the jejunum (P < 0.05), indicating the adhesion capacity as corroborated by the quantification of interfacial fluorescence (Figure 6b) with a high correlation of R = 0.99 and by the tissue–material adhesion strength (Figure 6c) as measured by a mechanical tester (R = 0.96). These results demonstrate that the tissue amine density is a critical factor determining the tissue–material interaction and, more broadly, that one needs to consider that organ function and structure define the specific tissue microenvironment.
Figure 6.
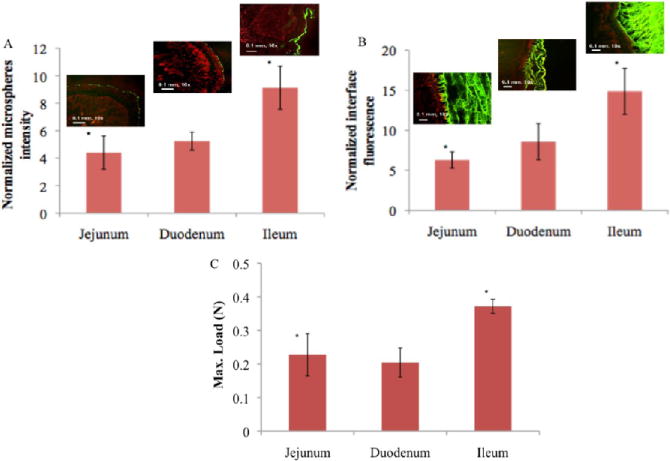
(A) The relative aldehyde reactivity of the jejunum and duodenum is statistically significantly different from that of the ileum, as assessed through the tissue-sample conjugation of fluorescent aldehyde-coated microspheres (p < 0.05 compared with the ileum). (B) The interface between dendrimer/dextran formulations (G5-25-12.5% and D10-50-11.25%, green) and the three distinct regions of the small intestine highlighted with propidium iodide (red) varies with the tissue type and is statistically significantly higher in the ileum (*p < 0.05 compared to that of the jejunum and duodenum). (C) The higher material reactivity with the ileum is further supported by the higher maximal load seen, as measured with a mechanical tester.
Whereas material formulation controls the adhesion and biocompatibility, it is interesting to study the effect of the tissue microenvironment and specifically the tissue amine density on adhesive material physicochemical properties, including the material morphology and degradation kinetics. The material residence time in vivo is important in determining the material capacity for providing support as a tissue heals and gains sufficient strength. We have applied a fast degrading formulation of adhesive material to tissue biopsies from the three regions of the small intestine to examine the effect of tissue type on the material degradation kinetics. Although the same material formulation was applied to the serosal side of the three intestinal regions, material degradation was slower when applied to the ileum compared to that on the jejunum and duodenum (Figure 7). Because the ileum provides more functional amines for the material, creating more points of interaction between them, the material stability is enhanced. Statistically significant differences are seen at early time points (1 and 2 h) whereas at late time points as the material loses much of its integrity the differences are less significant although on average they are still evident. This trend is supported by images of fluorescently labeled material and stained tissues after 0, 1, and 2 h (Supporting Information Figure 5). With time, less material is evident in the duodenum and jejunum and more material remains attached to the ileum. The dendrimer/dextran tunable family of materials shows great promise as surgical sealants with adequate adhesion, biocompatibility, and graded interaction with different tissues presenting a distinct density of surface chemical groups.
Figure 7.
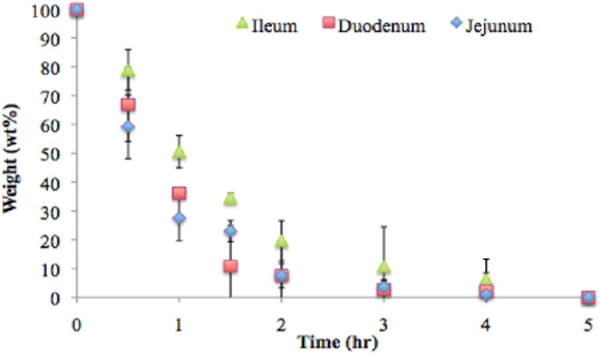
The tissue type affects the material residence time via the variation in the tissue–material interaction, as demonstrated by the weight loss of a quickly degrading material (D40-10-5% + G5-25-20%) when applied to the three regions of the small intestine.
Tissue-Specific Adhesive Formulations
Though the tissue type imposes constrains on the material, flexibility in material design enables control over material properties by providing a specific material formulation for each tissue. We show that changes in the material formulation can regulate the adhesion strength for each specific region of the rat intestine (Figure 8). By providing aldehydes for specific tissue amines, we modulated adhesion to an extent that depends on each tissue. Because the ileum has more amines, it is more responsive to changes in material formulation in the form of aldehydes. While increasing dextran aldehyde solid content from 11.25 to 15 wt % increased the maximal load in 15% for the three intestinal parts, further increase in dextran aldehyde to 25 wt % raised the load at failure to 55%. Providing more aldehydes similarly increases the load at failure for the three regions of the intestine. However, the higher number of tissue amines in the ileum absorb more aldehydes thus increasing the absolute value of the load to failure as the dextran-aldehyde content increases.
Figure 8.
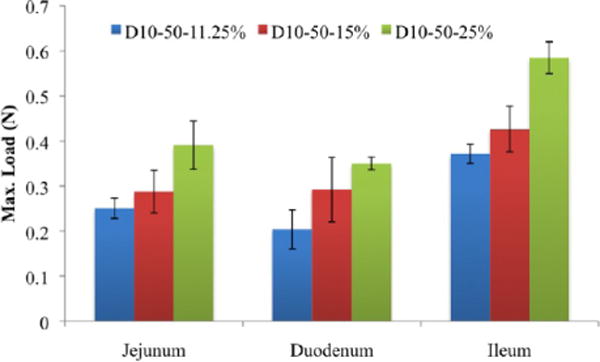
Tissues are responsive to changes in the dextran aldehyde solid content. By changing the material formulation, we can regulate the adhesion strength for each specific region of the intestine. For each composition, ileum load values are statistically significantly higher than those of the jejunum and duodenum, p < 0.5.
These results demonstrate that the specific tissue properties of the target organ, particularly its chemistry, must be considered when developing new materials, specifically adhesive materials, to facilitate the optimal tissue response and clinical outcome.
CONCLUSIONS
The optimization of tissue adhesive materials can minimize complications associated with internal leakage as seen, for example, in the gut, which is a frequent surgical complication that results in high morbidity and mortality. The variability in tissue structure and chemistry supports the diverse set of functions that tissues provide and should be seen as imparting differing microenvironmental conditions and reactivity to materials. Tissues and materials, specifically biodegradable materials, continuously influence each other. Although materials are subject to rigorous design criteria and thorough characterization, the properties of the target tissue, including tissue chemistry and morphology, must be considered when a material is to be used in a specific application and in a specific site. Herein, we utilized a model organ (the small intestine) and an adhesive (dendrimer/dextran) to demonstrate a wider concept: the formulation of biomaterials such as adhesive materials requires an understanding and tailoring based on the specific target tissue properties. The generalized use of an adhesive for a wide range of tissues will result in suboptimal clinical outcomes and in some cases device failure. Because material performance is a contextual state rather than just a constitutive property, it can be determined only within specific environments. Understanding biomaterials and tailoring them to elicit the desired in vivo effects is a crucial step in biomaterials research. A rational process in material design under controlled environments considering target tissue properties will allow the development of tailored biologically relevant materials that are site- and application-specific and therefore clinically superior.
EXPERIMENTAL DETAILS
Synthesis and Formation of Dendrimer/Dextran Hydrogels
Dendrimer/dextran networks were fabricated by dissolving generation five polyamidoamine (PAMAM) dendrimers with 25% amine surface groups in water (Dendritech Inc.) and mixing with a solution of oxidized dextran. Linear dextran (18.9 g, 10 kDa) is oxidized in water with sodium periodate (17.6 g) for 5 h to create dextran aldehyde (50% oxidation of glucose rings, 2 aldehyde groups per oxidized glucose ring). The reaction mixture is dialyzed (MEMBRA-CEL Dialysis Tubing, molecular weight cutoff of 3500 Da, Viskase Companies, Inc.). The two homogeneous polymer solutions were loaded into a dual-chamber syringe equipped with a 12-step mixing tip. Dendrimer/dextran network formation occurred within seconds to minutes, following the controlled mixing of dendrimer amine and dextran aldehyde via a Schiff base reaction between the constituent reactive groups (aldehydes and amines). The time required for gelation was recorded for each formulation.
Fluorescent Dendrimer Amine
To characterize the dendrimer/dextran morphology at the tissue–material interface as well as track gradual material release from the tissue surface, constituent dendrimer amine was labeled with fluorescein. Dendrimer amine (1 g) was dissolved in 50 mL of 0.2 M sodium carbonate buffer, followed by the addition of 0.020 g of 6-(fluorescein-5-carboxyamido) hexanoic acid (Invitrogen). The mixture was stirred at room temperature for 2 h, diluted in 100 mL of doubly distilled water, dialyzed, and lyophilized. Dendrimer amine solutions of 12.5 wt % solid content, 2% of which were fluorescently labeled, were then prepared and cross-linked with dextran aldehyde solutions in the established manner to yield fluorescent materials.
Adhesive Interface Morphology
To investigate the morphology of the interface between the adhesives and tissue surfaces, biopsied rat tissues were covered with 100 μL of fluorescently labeled dendrimer/dextran (fluorescein-conjugated dendrimer/dextran) that was allowed to cure for 4 min. Tissue samples were then snap frozen overnight, cryosectioned (16 μm sections), and stained with propidium iodide. The morphology of the tissue–material interface was quantified as the fluorescence intensity of fluorescein at the interface using image analysis (MetaMorph, Leica Microsystems). The pore size of the bulk adhesive material was also measured using MetaMorph.
Aldehyde Affinity of Soft-Tissue Surfaces
To determine the aldehyde affinity of various soft tissues, the conjugation of aldehyde-coated fluorescent microspheres (FluoSpheres aldehyde-sulfate micro-spheres, 0.02 μm, yellow-green fluorescence (505/515), 2% solids, aldehyde-coated f-MS (Molecular Probes)) to soft-tissue surfaces was quantified. Biopsies of rat duodenum, jejunum, and ileum were prepared with equal surface area (75 mm2) and submerged in 1 mL of 0.5% f-MS solutions for 20 min on a rocker at 37 °C. Tissue samples were thoroughly rinsed with 10 mL of PBS three times. Tissue specimens were then processed and analyzed to quantify the percent surface coverage by f-MS (Leica Microsystems, MetaMorph).
Adhesion Mechanics
The adhesion mechanics following dendrimer/dextran application to soft tissues was measured using an Instron mechanical tester. Adhesive test elements were created from a 100 μL application of dendrimer/dextran evenly distributed between two uniformly sized tissue biopsies (disks of 6 mm diameter, total test element thickness of 1 mm) of rat duodenum, jejunum, or ileum. Tissue surfaces were dry blotted with Kimwipes prior to material application. After applying dendrimer/dextran between tissue surfaces and allowing 5 min for material polymerization, adhesive test elements were displaced at a constant rate (0.05 mm s−1) and the load response was continuously recorded (200 measurements s−1). Recorded loads were normalized by the test element cross-sectional area and reported as an interfacial stress response to a change in thickness.
Material Retention
To track material loss following dendrimer/dextran adhesion to various soft tissues, 6-(fluorescein-5-carboxyamido) hexanoic acid fluorescently labeled materials were applied to small intestinal biopsies (75 mm2 sections). Identical volumes (50 μL) of dendrimer/dextran (G5-25-20% and D40-10-5 composition) were applied to tissue surfaces and allowed to polymerize for 5 min. Samples were then submerged in PBS at 37 °C, 50 μL aliquots were taken at 0, 0.5, 1, 1.5, 2, 3, and 4 h and diluted with 50 μL of PBS, and the material percentage weight loss was measured by fluorescence signal at 475/540 nm. In parallel, triplicates from each region of the small intestine were dry blotted at times of 0, 1, 2, 3, and 4 h, snap frozen in liquid nitrogen, and stored overnight at −80 °C. Then, tissue samples were cryosectioned (16 μm sections) and stained with propidium iodide. Tissue specimens were then processed and analyzed.
In Vitro Cytotoxicity
The in vitro cellular response to dendrimer/dextran materials was quantified via fluorometric colorimetric assays (Cytotox-ONE homogeneous membrane integrity assay, Promega). Cultures of rat 3T3 fibroblast cells were prepared in 24-well plates using standard techniques. At 70% culture confluence, adhesive degradation byproducts predegraded for 1, 7, or 30 days (dextran solid contents of 7.5, 15 and 25% with dendrimer 12.5%) of treatment were applied directly to the cell culture plate, allowing cell exposure to adhesive byproducts. Following material application, cultures were incubated for 24 h under standard conditions, followed by immediate analyses of the cellular response.
Subcutaneous Mouse Model
A subcutaneous implantation model of tissue response was used to evaluate the in vivo compatibility. A subcutaneous pocket was created in anesthetized SKH1 hairless mice, and a 100 μL preformed disk of dendrimer/dextran (G5-25-12.5 and D10-50-X, with X being 7.5, 15, and 25% solid contents) was introduced into the pocket. After 7 and 30 days, the mice were sacrificed and the skin and subcutaneous tissues were harvested. The samples were snap frozen in liquid nitrogen and stored at −80° until histological analysis. All experimental protocols were approved by the MIT Animal Care and Use Committee and were in compliance with NIH guidelines for animal use. Harvested tissue was cryosectioned to create 16-μm-thick sections. Hematoxylin and eosin staining was performed using standard methods. The fibrotic response was based on the morphology and was measured at multiple random locations in five images from tissue samples from each mouse. Histological slides were evaluated for their extent of inflammation using the following scores: 0, no observable change compared to the control; 1, a nearly imperceptible feature (minimal); 2, an easily identifiable or notable (mild/moderate) feature; 3, a prominent to overwhelming (marked/severe) feature.
Statistical Analyses
Data are presented as means ± standard deviations. To take multiple comparisons into account, all statistical comparisons were performed using one way ANOVA followed by the Tukey–Kramer test using InStat software (GraphPad, San Diego, CA, USA). A p value of <0.05 was considered to denote statistical significance.
Supplementary Material
Acknowledgments
N.A. and E.R.E. gratefully acknowledge support from the Deshpande Center at MIT. E.R.E. was supported by grants from the National Institutes of Health (RO1 GM 49039).
Footnotes
Supporting Information
Material physical properties and morphology change as a function of composition and target tissue site. This material is available free of charge via the Internet at http://pubs.acs.org.
The authors declare no competing financial interest.
References
- 1.Laschke MW, Vollmar B, Menger MD, Laschke MW, Vollmar B, Menger MD. The dorsal skinfold chamber: window into the dynamic interaction of biomaterials with their surrounding host tissue. Eur Cells Mater. 2011;22:147–167. doi: 10.22203/ecm.v022a12. [DOI] [PubMed] [Google Scholar]
- 2.Sun C, Waite JH, Sun C, Waite JH. Mapping chemical gradients within along a fibrous structural tissue mussel byssal threads. J Biol Chem. 2005;280:39332–39336. doi: 10.1074/jbc.M508674200. [DOI] [PubMed] [Google Scholar]
- 3.Messersmith PB, Messersmith PB. Materials science. Multitasking in tissues materials. Science. 2008;319:1767–1768. doi: 10.1126/science.1155122. [DOI] [PubMed] [Google Scholar]
- 4.Artzi N, Shazly T, Baker AB, Bon A, Edelman ER, Artzi N, Shazly T, Baker AB, Bon A, Edelman ER. Aldehyde-amine chemistry enables modulated biosealants with tissue-specific adhesion. Adv Mater. 2009;21:3399–3403. doi: 10.1002/adma.200900340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Artzi N, Oliva N, Puron C, Shitreet S, Artzi S, bon Ramos A, Groothuis A, Sahagian G, Edelman ER, Artzi N, Oliva N, Puron C, Shitreet S, Artzi S, bon Ramos A, Groothuis A, Sahagian G, Edelman ER. In vivo in vitro tracking of erosion in biodegradable materials using non-invasive fluorescence imaging. Nat Mater. 2011;10:704–709. doi: 10.1038/nmat3095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mahdavi A, Ferreira L, Sundback C, Nichol JW, Chan EP, Carter DJ, Bettinger CJ, Patanavanich S, Chignozha L, Ben-Joseph E, Galakatos A, Pryor H, Pomerantseva I, Masiakos PT, Faquin W, Zumbuehl A, Hong S, Borenstein J, Vacanti J, Langer R, Karp JM, Mahdavi A, Ferreira L, Sundback C, Nichol JW, Chan EP, Carter DJ, Bettinger CJ, Patanavanich S, Chignozha L, Ben-Joseph E, Galakatos A, Pryor H, Pomerantseva I, Masiakos PT, Faquin W, Zumbuehl A, Hong S, Borenstein J, Vacanti J, Langer R, Karp JM. A biodegradable biocompatible gecko-inspired tissue adhesive. Proc Natl Acad Sci USA. 2008;105:2307–2312. doi: 10.1073/pnas.0712117105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Stewart RJ, Ransom TC, Hlady V, Stewart RJ, Ransom TC, Hlady V. Natural underwater adhesives. J Polym Sci Part B: Polym Phys. 2011;49:757–771. doi: 10.1002/polb.22256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lee BP, Messersmith PB, Israelachvili JN, Waite JH, Lee BP, Messersmith PB, Israelachvili JN, Waite JH. Mussel-Inspired Adhesives Coatings. Annu Rev Mater Res. 2011;41:99–132. doi: 10.1146/annurev-matsci-062910-100429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Storkholm JH, Villadsen GE, Jensen SL, Gregersen H, Storkholm JH, Villadsen GE, Jensen SL, Gregersen H. Mechanical properties collagen content differ between isolated guinea pig duodenum jejunum and distal ileum. Dig Dis Sci. 1998;43:2034–2041. doi: 10.1023/a:1018855113849. [DOI] [PubMed] [Google Scholar]
- 10.Khandare J, Calderon M, Dagia NM, Haag R. Multifunctional dendritic polymers in nanomedicine: opportunities challenges. Chem Soc Rev. 2011;41:2824–2848. doi: 10.1039/c1cs15242d. [DOI] [PubMed] [Google Scholar]
- 11.Artzi N, Shazly T, Crespo C, Ramos AB, Chenault HK, Edelman ER, Artzi N, Shazly T, Crespo C, Ramos AB, Chenault HK, Edelman ER. Characterization of star adhesive sealants based on PEG/dextran hydrogels. Macromol Biosci. 2009;9:754–765. doi: 10.1002/mabi.200800355. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


