Abstract
Smad proteins convey canonical intracellular signals for activated receptors in the TGFβ superfamily, but the activity of Smads and their impact on target genes is further regulated by a wide variety of cofactors and partner proteins. We have identified a new Smad1 partner, a GTPase named Gtpbp2 that is a distant relative of the translation factor eEf1a. Gtpbp2 affects canonical signaling in the BMP branch of the TGFβ superfamily, as morpholino knockdown of Gtpbp2 decreases, and overexpression of Gtpbp2 enhances, animal cap responses to BMP4. During Xenopus development, gtpbp2 transcripts are maternally expressed and localized to the egg animal pole, and partitioned into the nascent ectodermal and mesodermal cells during cleavage and early gastrulation stages. Subsequently, gtpbp2 is expressed in the neural folds, and in early tadpoles undergoing organogenesis gtpbp2 is expressed prominently in the brain, eyes, somites, ventral blood island and branchial arches. Consistent with its expression, morpholino knockdown of Gtpbp2 causes defects in ventral-posterior germ layer patterning, gastrulation and tadpole morphology. Overexpressed Gtpbp2 can induce ventral-posterior marker genes and localize to cell nuclei in Xenopus animal caps, highlighting its role in regulating BMP signaling in the early embryo. Here, we introduce this large GTPase as a novel factor in BMP signaling and ventral-posterior patterning.
Keywords: BMP signaling, GTPase, Gtpbp2, Smad1, ventral patterning, Xenopus embryo
Introduction
The Transforming Growth Factor β (TGFβ) superfamily regulates a diverse set of biological processes, such as cell proliferation, adhesion, migration, apoptosis, differentiation embryonic pattern formation and organogenesis (ten Dijke et al., 2002; Shi and Massague, 2003; Feng and Derynck, 2005; Schier and Talbot, 2005). Ligands in the TGFβ superfamily bind to particular combinations of serine/threonine kinase receptors that signal through Smad and non-Smad-dependent pathways (Moustakas and Heldin, 2005, 2012). In canonical mode, ligand-bound receptors activate R-Smads to signal downstream to target genes. R-Smad signaling is grouped into two distinct branches, with Smads1/5/8 conveying BMP/GDF signals and Smads2/3 operating under Activin/Nodal/ TGFβ.
Genes encoding TGFβ signaling components, and many of their functions, are well conserved throughout the metazoa, regulating embryonic development in animals as diverse as ancient diploblast lineages (cnidaria and ctenophora) through complex triploblasts (chordata). In vertebrate embryos in particular, Nodal/Vg1 and BMP-related pathways provide essential signals that induce and pattern the primary germ layers, regulate tissue morphogenesis and left-right asymmetry, and affect cellular pluripotency, differentiation, growth and death. TGFβ signals often act in concert with FGF and Wnt signaling in these developmental processes. In Xenopus embryos in particular, mesoderm and endoderm are induced by Nodals, Vg1 and Derriere ligands, acting together with FGFs, and early tissue patterning is achieved by BMPs alongside Wnt and FGF signals (Heasman, 2006; Kimelman, 2006; Itasaki and Hoppler, 2010). In the ectoderm, different levels of BMP signaling triggers differentiation of the epidermis, neural crest, sensory placodes and neural tissues (DeRobertis and Kuroda, 2004; Vonica and Brivanlou, 2006; Rogers et al., 2009).
Because of their importance in embryonic development and tissue homeostasis, a variety of mechanisms have evolved to regulate the activity of TGFβ pathways at all levels, from ligand production and extracellular regulation, through receptors, signal transducers and transcriptional cofactors (Itoh, and Dijke 2007). To explore regulation at the level of signal transduction, we sought to identify new partners of BMP/Smad signaling by performing yeast two-hybrid screens with Smad1 (Zhu et. al. 1999), which retrieved several TGFβ signaling regulators, including Smurf1 (Zhu et al. 1999, Thomsen 2013), Eps15r (Callery et al., 2013), and XMan (Osada et al., 2003; our unpublished results). Another factor we retrieved is Gtpbp2, a large GTPase distantly related to the translational regulators eEf1a1, Gspt1 (eRF3) and Hbs1-like (Kudo et al., 2000). No function has been ascribed to Gtpbp2, although it has shown to be expressed in developing mouse embryos (Watanabe et al., 2000; Kudo et al., 2000). Gtpbp2 has a conserved yet distinct paralog, Gtpbp1, that regulates mRNA 3’ end processing, but Gtpbp2 appears to lack that function (Woo et al., 2011). Here we show that Gtpbp2 interacts directly with Smad1, can potentiate BMP signaling and activate BMP target genes, is required for embryonic responses to BMP signaling, and is essential for normal ventral-posterior mesodermal patterning.
Materials and Methods
cDNA isolation and constructs
A partial clone corresponding to the C terminus of Gtpbp2 was retrieved from a yeast two-hybrid screen done with a Xenopus oocyte cDNA library (Clontech) using Smad1 as bait (Zhu et al., 1999). Full length EST for gtpbp2 (DT061674) was obtained from Resgen Inc. For mRNA synthesis and expression in cultured cells, Gtpbp2 isoforms including one with mismatches at morpholino binding sites were amplified by PCR and subcloned into pCS2-HA or pCS2. The HindIII-XbaI fragment of pCS2-HA for each construct was then subcloned into pCDNA3.1NotI. Final constructs were linearized with NotI, and mRNAs were made using mMESSAGE mMACHINE T7 Kit (Ambion). Deletion constructs for Gtpbp2ΔN and Gtpbp2ΔC were made by PCR from parent vector; 3xHA-Gtpbp2-pcDNA3.1NotI. N’ Cherry tagged versions of Gtpbp2 isoforms were made by cloning Cherry sequence into XhoI sites of 3xHA-Gtpbp2 parent vectors thereby replacing the 3xHA tag. C’-flag-Xenopus smad1 was cloned by PCR addition of a C terminal flag tag into pCS2. Flag-MH1 and Flag-MH2 were derived from this parental construct by PCR deletion of excluded sequences. All PCRs were performed using Platinum Pfx polymerase (Invitrogen) with low cycle number (< 18 cycles). Flag-xsmad1, and flag-xsmad4 were previously described. (Thomsen et. al, 1996; Zhu et al., 1999).
Morpholino design and injection
Xenopus embryos were collected and microinjected as described previously (Alexandrova and Thomsen, 2006). Morpholino Oligonucleotides (MOs) were supplied by GeneTools Inc., as follows, M2: TCCCCCTGACTGGCACGGAATGCCC, M1: CGCGGCTCCATCCCACCGGCCCTG, 5mis-to-M1: CcCGGgTCCATgCCACCcGCCgTG. Xenopus is an allotetroploid organism in which most of the genes, if not all, are coded from duplicated copies (Uno et al, 2013). Morpholinos were designed to target both copies of Gtpbp2.
Immunoprecipitation and western blots
Full-length and deletion constructs of HA tagged Gtpbp2 isoform b were co-transfected with full length Flag-tagged Smad1, or deletion constructs of Flag-Smad1, to Hek293T cells using transfection reagent Fugene6 (Roche). Cells were lysed 24 hours after transfection with PBS containing 1% Triton X-100, 2mM EDTA, 1mM Na3VO4 and complete protease inhibitors (Roche). Immunocomplexes were precipitated and washed according to the Flag-M2 Beads protocol (Sigma). Anti-HA-HRP (Roche) (1:500) and anti-Flag M2 (Sigma) (1:2000) followed by anti-mouse-HRP antibody (Sigma) (1:5000) were used to detect HA-Gtpbp2 forms and Flag-Smad1 constructs, respectively. A Flag-GFP construct was used a balancer in DNA transfections into cultured cells.
In Situ Hybridization and developmental RT-PCR
A fragment corresponding to positions 609 to 1510 of Xenopus gtpbp2 reference sequence NM_001099909 was PCR amplified, and cloned into pGEMT-Easy (Promega). The gtpbp2 RNA in situ probe was made from the pGEMT-Easy construct linearized with ApaI, transcribed with SP6 RNA polymerase, and labeled with digoxygenin-UTP (Roche). Whole mount in situ RNA hybridization was performed as described (Harland, 1991). In situ hybridization on sections was performed as described (Ciau-Uitz et al., 2000). Real-time quantitative PCR was performed with a LightCycler 480 System (Roche) to determine the developmental expression of gtpbp2, using primer pair: GTACGCTCTGGAGCCTGATG and TGTCTGCACCGACCTTCTCT. Digoxygenin (dig) labeled in situ probes for analysis of morphant embryos were made as described previously (Alexandrova and Thomsen, 2006).
Xenopus animal cap assays and quantitative RT-PCR
Synthetic mRNAs or MOs were injected into the animal pole of 2-cell stage embryos at doses indicated in the Figures and text. The total amount of synthetic mRNA injected was held constant by balancing with GFP mRNA. Animal caps were excised at blastula stage 8, cultured in 0.5x MMR, and harvested at Nieuwkoop and Faber stage 11 (mid-gastrula) or 18 (late neurula). Ten animal caps per each treatment were pooled and total RNA was extracted as described (Alexandrova and Thomsen, 2006), followed by cDNA synthesis with Superscript II Reverse Transcriptase (Invitrogen) using oligo-d(T)16–20 primers (Invitrogen). Real-time quantitative PCR (qPCR) was performed with the LightCycler 480 (Roche), using primer sequences and conditions as described (Xantos et al., 2002; http://www.hhmi.ucla.edu/derobertis/). Marker gene expression levels in cultured animal cap explants were normalized to an endogenous control gene (ornithine decarboxylase, ODC), and then plotted as a percentage of the level of endogenous gene expression in one embryo (set as 100%).
Luciferase reporter gene assay
Reporter assays were done by injecting 100ng Vent2-Luc reporter (Hata et al., 2000) together with 50ng TK-RL reporter as interrnal control to normalize the reporter activity, and 20–40ng of M1 or 40ng 5-base mismatched (5mis), morpholinos designed against Gtpbp2. Animal caps were explanted at stage 8 and harvested at stage 11. Ten animal caps or embryos per each treatment were pooled and extracts were prepared and analyzed using Dual-Glo Luciferase Assay System (Promega).
Immunoflourescence and imaging
mCherry-tagged gtpbp2a (2 ng) and membrane localized GFP (5 pg) synthetic mRNA were co-injected into the animal pole of 2 cell embryos, and then gently fixed at gastrula stage 11 in 1% paraformaldehyde in PBS for 15 minutes, washed 3x in PBS, and then costained with 2 mg/ml 4,6-diamino-2-phenylindole (DAPI). Embryos were then imaged on using a 10x objective on a Zeiss fluorescence microscope (Motorized Axio Imager Z1) with ApoTome attachment.
Results
Identification of Xenopus Gtpbp2
We sought to identify new regulators of Smad signaling by performing a yeast two-hybrid screen using Xenopus Smad1 as bait to probe an oocyte cDNA library. One of the cDNAs we retrieved encodes a predicted C-terminal, 144 amino acid fragment with significant homology to human and mouse Gtpbp2 (Fig. 1A), a large GTPase that, together with a homolog named Gtpbp1, define a small yet distinct family of GTPases with closest homology to translation elongation factor eEf1a (eEF1a). Gtpbp1/2 orthologs are conserved throughout the metazoa (Kudo et al., 2000; Watanabe et al., 2000) and feature three conserved domains: an N-terminal portion harboring a GTP-binding/GTPase domain with sequence conservation that places these GTPases (and eEf1a) within the greater ras superfamily. This domain binds Guanine nucleotides, and in eEf1a and Gtpbp1 it has been shown to possess GTPase activity (Riis et al., 1990; Woo et al., 2011). The C-terminal halves of Gtpbp1 and 2 feature two conserved sequence blocks, referred to as EF-Tu (or Gtpbp) domains II and III (Fig. 1A), and while the function of those domains in Gtpbp1/2 are not known, in eEf1a and EF-Tu those regions bind to GTP/GDP and aminoacyl-tRNAs and facilitate delivery of charged tRNAs to actively translating ribosomes (Sasikumar et al., 2012).
Figure 1. Gtpbp2 interacts with Smad proteins.

A. Schematic representation of Gtpbp2 and its conserved domains. Partial-length proteins isolated by yeast two-hybrid (Y2H) or constructed for interaction assays (ΔC and ΔN) are delineated by horizontal lines. Symbols A and B indicate the position of ATG start codons in the corresponding two isoforms of human Gtpbp2 protein. B. Flag-Smad1 construct was co-expressed with HA-Gtpbp2B in Hek293T cells, lysed, immunoprecipitated (IP) with Flag-agarose beads, and analyzed by western blot (WB) using α-HA-HRP and α-flag M2 antibodies. Lower panel shows Gtpbp2B levels in total cell lysates (TCL); top two panels show co-immunoprecipitated proteins. C. Xenopus GTPBP1, GTPBP2 and Smurf1, or GFP were individually translated in vitro in rabbit reticulocyte lysates, and labeled with 35S-Methionine. GST and GST-xMad1 (Smad1) proteins were expressed in and purified from E. coli. Purified GST tagged proteins were incubated with each in vitro translated protein and pulled down by GST beads. Gtpbp2 interacts GST-Smad1 in vitro whereas GTPBP1 does not. Smurf1 was used as a positive control and GFP as a negative control. D. Flag-Smad1, Flag-MH1 domain, and Flag-MH2 domain constructs (all Xenopus) were co-expressed with HA-Gtpbp2b and analyzed as in panel B. E. HA-tagged Gtpbp2b (full-length), ΔN-Gtpbp2b and ΔC-Gtpbp2b were co-expressed with Flag-tagged Xenopus Smad1 in Hek293T cells and analysed by IP as in panel B.
The Human gtpbp2 locus codes for two isoforms of Gtpbp2 protein (UniProtKB Q9BX10.1): a 602 amino acid (aa) “long form” Gtpbp2a (NP_061969.3), and a shorter 514 aa variant, Gtpbp2b (NP_001273145.1). These human isoforms share a common open reading frame (ORF), but isoform b transcripts are initiated within the ORF of isoform a, resulting in use of an alternative start codon in isoform b and a lack of the first 88 amino acids found in isoform a (Fig. 1A, and S. Fig. 1). This is consistent with original cloning reports indicating different protein sizes for human and mouse Gtpbp2 (Watanabe et al., 2000; Kudo et al., 2000). A search for corresponding transcripts in Xenopus laevis and Xenopus tropicalis EST databases yielded multiple cDNAs that encode the Gtpbp2a isoform, but just one X. laevis cDNA (genbank ID BI449029) that would correspond to the mammalian gtpbp2b transcript and protein. While we cannot rule out the possibility that this Xenopus transcript is a partial cDNA, we used it in functional tests (below) since it encodes the equivalent of the mammalian Gtpbp2b isoform and was the first cDNA we isolated for expression analysis and functional testing. This clone also showed more robust protein expression than the full-length gtpbp2a when the mRNAs were injected into embryos (below and Fig. 6). The Xenopus laevis long form is 81% identical to human Gtpbp2a, and since the mRNA encoding this protein appears to be the predominant form expressed in early Xenopus laevis embryos, we refer to this as Gtpbp2 henceforth, unless stated otherwise.
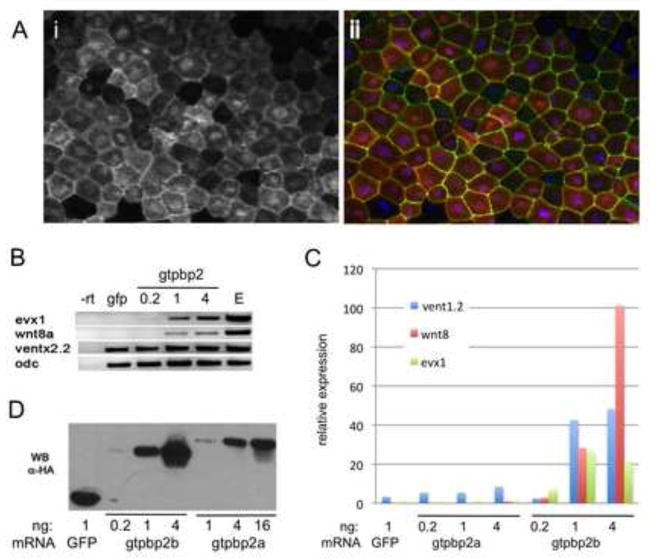
Figure 6. Gtpbp2 subcellular localization and induction of BMP target genes.
A. mCherry-tagged Xenopus gtpbp2a mRNA (2ng) co-injected with a membrane-localized GFP (mem-GFP) mRNA (10 pg), and costained with DAPI, shows nuclear and cytoplasmic localization in gastrula stage 11 animal cap cells; (i) mCherry-Gtpbp2 signal only, (ii) merged signals for mCherry-Gtpbp2b, mem-GFP and DAPI. B. Xenopus laevis Gtpbp2b can induce BMP marker genes evx1, wnt8a, vent2.2 in animal caps. gtpbp2b mRNA was injected at increasing doses (0.2ng, 1ng, and 4ng) into the animal pole of two cell stage embryos, animal caps were excised at stage 8, and cultured to stage 11 before gel-based RT-PCR with primers to indicated genes (otx as positive control for RT and loading). C. Q-PCR analysis of the bmp targets vent1.2, wnt8, and evx1 in animal caps injected at the 2 cell stage with increasing doses of gtpbp2a or gtpbp2b mRNA, excised at the 8 cell stage, and cultured to stage 11. D. Comparison of accumulation of HA-tagged Xenopus laevis Gtpbp2a versus Gtpbp2b isoforms in embryos injected with increasing doses of mRNA at the 2 cell stage and lysed at stage 11 for western blotting using an anti-HA antibody.
Gtpbp2 interacts with Smad1
We isolated Gtpbp2 as an interactor with Smad1 in a yeast 2-hybrid screen, so to validate this interaction, we performed co-immunoprecipitation (co-IP) assays in mammalian cells by cotransfecting HA-tagged Gtpbp2 together with Flag-tagged Smad1 into human Hek293T cells. Fig. 1B shows that HA-Gtpbp2 co-immunoprecipitates with the flag-tagged Smad1. To confirm whether Gtpbp2 interacts directly with Smad1, we incubated a purified GST-Smad1 fusion protein together with in vitro synthesized and 35S-labelled proteins for Gtpbp2, Gtpbp1, GFP or Smurf1 (as a positive control) and found that both Smurf1 and Gtpbp2 bind to Smad1, while Gtpbp1 did not (Fig. 1C). These results show that the interaction between Smad1 and Gtpbp2 is direct, and also indicate that Smad1 interacts selectively with Gtpbp2 but not its closest homolog Gtpbp1 (also verified in a separate study of Gtpbp1; D. Ki and W. Gillis unpublished observations).
To further characterize how Gtpbp2 and Smad proteins interact, we defined the interacting domains of Gtpbp2 and Smad1 by co-immunoprecipitation (co-IP) from COS cells. The predicted functional regions of Gtpbp2 include the GTP-binding/GTPase domain encompassing most of the N-terminal half of the protein, and the EF-Tu_II (GTPBP_II) and EF-Tu_III (GTPBP_III) domains in the C-terminal half (Fig. 1A). We generated expression constructs for N- and C-terminal domains of Gtpbp2 as well as MH1 and MH2 domains of Smad1. Results in Fig. 1D demonstrate that the Smad1 MH1 domain interacts with Gtpbp2 much more robustly than the MH2 domain. In the converse direction, full-length Smad1 was able to form a complex with Gtpbp2 lacking the GTPase domain (ΔNGtpbp2), but not Gtpbp2 lacking domains II and III (ΔC Gtpbp2; Fig. 1E). These results demonstrate that the putative effector domain of Gtpbp2 interacts with the MH1 domain of Smad1. These findings provide a foundation for future detailed structure-function and mechanistic studies of Gtpbp2-Smad interactions and support a role for Gtpbp2 in BMP signaling, investigated below.
Expression profile of Gtpbp2 in Xenopus embryos
To assess the roles of Gtpbp2 in developmental processes, we first determined the temporal and spatial expression of gtpbp2 mRNA in developing Xenopus embryos, by in situ hybridization on whole and histologically-sectioned embryos, as well as by real-time quantitative RT-PCR (qPCR; Fig. 2 and S. Fig. 2). We found gtpbp2 expressed maternally (egg, cleaving blastula) at levels that were nearly the maximum observed among all stages measured (Fig. 2A, S. Fig. 2). The high level of maternal transcripts persisted into early gastrulation (stage 10) but rapidly decreased by mid-late gastrulation, stages 11–12. Gtpbp2 expression was lowest in early to mid-neurulae, but transcript levels gradually increased toward the end of neurulation and in early tadpoles undergoing organogenesis (Fig. 2D, and S. Fig. 2). The maternal transcripts were found localized to the animal pole of egg and cleavage stage blastula embryos (Fig. 2A, S. Fig. 2), and at the onset of gastrulation gtpbp2 transcripts were detected primarily in the mesoderm and inner cell layer of the ectoderm (Fig. 2B). Although transcript levels were at their lowest in neurula stages, they were slightly enriched in the neural folds (S. Fig. 2). At tailbud and swimming tadpole stages, gtpbp2 transcripts were expressed prominently in the ventral blood island, somites, brain and eye (Fig. 2C, S. Fig. 2). These tadpole expression patterns are congruent with the expression of adult mouse gtpbp2 transcripts, which were found in the brain, skeletal muscle and blood (Kudo et al., 2000). In conclusion, Xenopus gtpbp2 is expressed as a maternal mRNA in the egg, is zygotically expressed early during gastrulation in the ectoderm and mesoderm, and later becomes expressed in specific tissues during organogenesis in the early tadpole.
Fig. 2. Temporal and tissue specific expression of Gtpbp2 during Xenopus development.
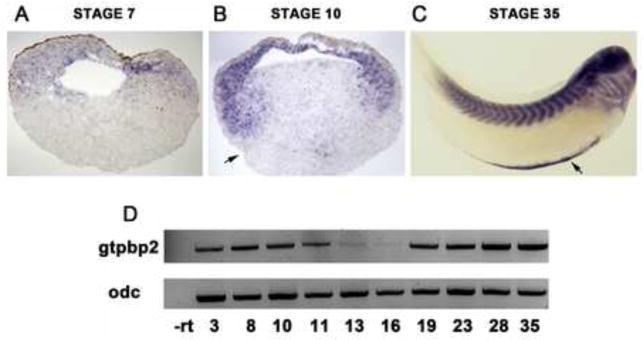
A. Maternal gtpbp2 transcripts were detected primarily in the animal pole at blastula stages (in sectioned stage 7 embryos). B. At the onset of gastrulation (stage 10) gtpbp2 is expressed in the inner layer of ectoderm and mesoderm. Arrow shows dorsal blastopore lip. C. At swimming tadpole stage (stage 35) gtpbp2 is strongly expressed in the somites, as well as in the brain, branchial arches, and ventral blood islands (VBI). Arrow shows VBI-specific expression of gtpbp2. D. Quantitative RT-PCR showing gtpbp2 levels across stages. The gtpbp2 signal significantly diminishes as gastrulation progresses, but is re-expressed at tadpole stages.
Gtpbp2 is required for responses to BMP signaling
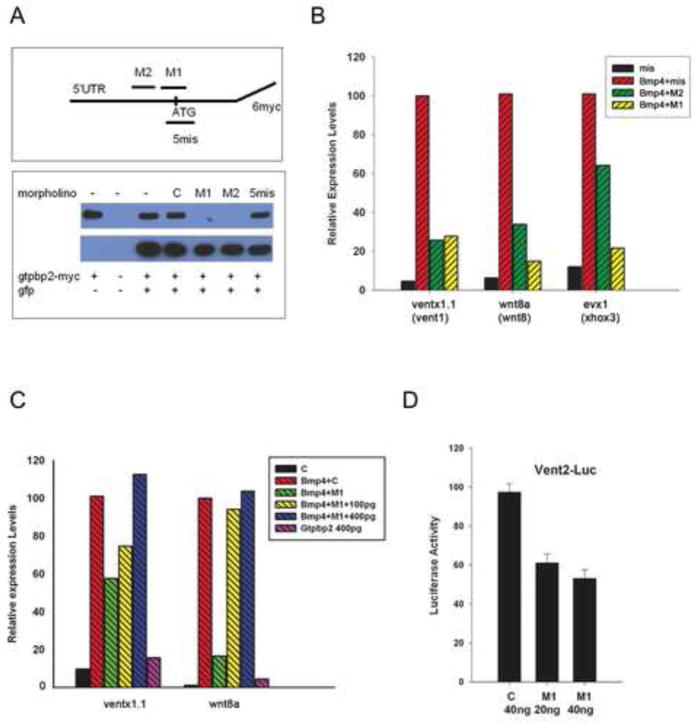
To determine whether Gtpbp2 provides any essential functions in BMP/Smad signaling and in Xenopus development, we designed translation-blocking antisense morpholino oligonucleotides (MOs) and implemented them in gene knockdown experiments. Two translation blocking morpholinos targeted the predicted initiation codon of Xenopus gtpbp2, corresponding to the AUG of the human gtpbp2a isoform (S. Fig. 1B). Gtpbp2 morpholino M1 overlaps this AUG, and a non-overlapping morpholino M2 is positioned just upstream of M1 (Fig. 3A). We verified that these morpholinos inhibit translation from a reporter mRNA construct consisting of the gtpbp2a ORF fused to a C-terminal myc tag. When mRNA and MO were coinjected into Xenopus embryos, the gtpbp2 M1 and M2 morpholinos each blocked expression of this reporter construct, while a control morpholino (5mis), which has 5 mismatches to M1 morpholino binding site, did not (Fig. 3A).
Figure 3. Gtpbp2 is required for BMP signaling in Xenopus animal cap tissue culture.
A. Two non-overlapping morpholinos are designed against the start site of gtpbp2 mRNA (M1 and M2), along with a control morpholino which has 5 mismatches to M1 binding site. Western blot shows Gtpbp2 morpholinos, M1 and M2, block translation of a C-terminally myc-tagged partial Gtpbp2 reporter construct injected into Xenopus embryos whereas a 5-mismatch to M1 control morpholino does not interfere with the expression of this reporter construct. A gtpbp2-myc mRNA (1 ng) harboring the 5’UTR and first few amino acid preceding the conserved domains of Gtpbp2 was coinjected with gfp mRNA (1 ng) and the morpholinos. Gtpbp2-myc protein levels were assayed by western blotting using anti-myc and anti-GFP antibodies, in embryo lysates harvested at mid-gastrulation (stage 11). B. Loss of Gtpbp2 inhibited BMP signaling. Embryos were injected at two-cell stage with 500 pg bmp4 mRNA, and 20 ng M1, or 40 ng M2, or 40 ng 5 mismatched-to-M1 morpholino (mis). Animal cap tissue was dissected at Stage 8, and cultured to Stage 11. BMP responsive genes ventx1.1 (vent1), wnt8a (wnt8), evx1 (xhox3) were assayed by quantitative RT-PCR (qPCR). C. Co-injection of 100 – 400 ng of a Gtpbp2 mRNA without morpholino target sites along with M1 morpholino restores BMP signaling in cultured animal cap explants. BMP signaling was assayed by the response of BMP target genes. D. BMP signaling was assayed with a BMP/Smad1-responsive vent2:luciferase reporter in animal caps. This reporter is significantly expressed when injected into animal caps with control morpholino, but including 20 or 40 ng of Gtpbp2 morpholino M1 reduces Vent2-Luciferase reporter activity to about half that of the control level (standard error indicated). Endogenous BMP response genes: evx (Xhox3), ventx1.1 (vent1) and wnt8a (wnt8). Assays in B and C performed on two or more biological replicates.
We isolated Gtpbp2 as a Smad1 interacting protein, and transcripts of both gtpbp2 (Fig. 1) and smad1 (Thomsen, 1996) are expressed in the ectodermal germ layer, or animal cap region, of blastula and gastrula stage embryos. Animal cap tissue is pluripotent and can be induced to form different lineages, depending on the dose and type of growth factor applied (Green et al., 1992; Asashima et al, 2009), so animal cap explant culture has long been used as a simple and effective system to probe activities of natural and candidate induction signals and downstream pathway components. Thus, the animal cap is an appropriate, natural tissue in which to ask whether Gtpbp2 is required for endogenous or ectopic BMP signaling in a reductive manner similar to mammalian cultured cell lines.
To determine whether Gtpbp2 is required for response to ectopic BMP signaling, two cell stage embryos were injected into the animal pole with 500 pg of bmp4 mRNA, together with either control or Gtpbp2 morpholinos (M1 or M2) and animal caps were explanted at midblastula stage 7–8, cultured and harvested when control embryos reached stage 11. Coinjection of control morpholino and bmp4 mRNA into caps induced several ventral mesodermal genes (ventx1.1, wnt8a, and evx1) that are known targets of BMP signaling. However, caps coinjected with bmp4 and Gtpbp2 M1 or M2 morpholinos showed a severely weakened induction of these BMP4 targets (Fig. 3B,C). These effects of Gtpbp2 knockdown in animal caps are specific, because reduced response to Bmp4, caused by mopholino M1, can be reversed by injection of a gtpbp2 mRNA harboring a mismatched morpholino binding target site (Fig. 3C).
Animal cap tissue has a low level of endogenous BMP signaling (Wilson et al, 1997; Kuroda et al., 2005) which allowed us to address whether Gtpbp2 is required for endogenous BMP signaling in animal cap explants using an alternative, luciferase reporter assay employing a vent2 promoter, a direct target of BMP signaling (Henningfeld et al., 2000). Indeed, Gtpbp2 morpholino co-injected into caps along with the reporter plasmid reduced activation of the vent2-Luc reporter by about 50% (Fig. 3D). When cultured to neurula stages animal caps differentiate into epidermis, an outcome that can be altered to cement gland and neural fates upon inhibition of BMP signaling, whereby intermediate levels of BMP signaling will lead to induction of cement gland genes, and in the absence of BMP signaling the cap tissue differentiates to neural progenitors (Wilson et al., 1997). Gtpbp2 morphant animal caps expressed decreased levels of the epidermal marker, cytokeratin (xk81a1) when cultured to early neurula (stage 15), consistent with a decrease in BMP signaling. However, Gtpbp2 morphant caps showed no induction of cement gland (ag1), or neural markers (sox2, and ncam1) (S. Fig.1C). Thus, although Gtpbp2 is required for optimal endogenous, as well as ectopic/elevated, BMP signaling in the Xenopus ectoderm, it does not appear to be required for BMP signaling that represses neural fate.
Gtpbp2 is required for ventral mesoderm patterning
Next, we proceeded with morpholino knockdown experiments in Xenopus embryos, to evaluate phenotypes and assess the potential contribution of putative Gtpbp2 isoforms to early development. Following bilateral injection of MOs at the two-cell stage, abnormalities in Gtpbp2 morphants were first observable in the late gastrula as a delay in blastopore closure (not shown). This suggested that early germ layer specification, patterning or morphogenetic processes, particularly those affecting the mesoderm, might be disturbed in the morphants. Since Gtpbp2 is required for BMP signaling, we expected that interfering with Gtpbp2 protein expression with morpholinos might cause body patterning defects. We observed that injection of M1 into the ventral-posterior marginal zone (VMZ) of 4-cell blastulae inhibited formation of the tail, posterior trunk and somites (Fig. 4A), which confirms a requirement for Gtpbp2 in the formation of ventral-posterior tissues. The loss of posterior structures in Gtpbp2 morphants could be partially rescued by co-injection of a gtpbp2a rescue mRNA (with 5 synonymous mismatches to the morpholino sequence; S. Fig. 3).
Figure 4. Gtpbp2 is required for embryonic ventrolateral mesoderm patterning.
A. Gtpbp2 is required for posterior tissue development. Diagram depicts the way 4-cell embryos were injected. Colored figure is shown to illustrate how dorsal (D) and ventrally-fated blastomeres (V) are identified and injected. Dorsal is the lighter pigmented half. Grayscale figure is the lateral view of the embryo. Embryos were injected with 30ng Gtpbp2 morpholino into the two ventral blastomeres at the 4-cell stage, placed in the ventral marginal zone (VMZ) and cultured to stage 35. Loss of Gtpbp2 leads to the complete loss of tail and posterior tissues. B. Morpholino knockdown of Gtpbp2 results in a severe reduction of post (xpo), wnt8, and myod1 (myf3) expression. Vent1 expression domain is more restricted to ventral tissues compare to controls. Embryos were injected with 30ng Gtpbp2 morpholino at 4 cell stage targeting the VMZ; the future posterior ventral tissues. C. Gtpbp2 is not required for mesendodermal induction. Sox17a, mixer, and mix1 expression are not affected. Embryos were injected bilaterally with 40ng Gtpbp2 morpholino at the 2-cell stage. Three independent sets of at least 20 embryos were analyzed in B and C, resulting in similar effects on marker gene expression.
To further investigate the ventral-posterior defects in Gtpbp2 morphants, we scored expression of region-specific patterning markers by whole-mount in situ hybridization (WISH) and found abnormal expression of several key genes that influence mesoderm development in the gastrula. VMZ targeting of the Gtpbp2 morpholino, as above (Fig. 4A), resulted in complete loss of the ventral-posterior marker genes post (xpo), wnt8a, and myod1 (myf3), and also inhibited lateral expression of ventx1.1, restricting it to the ventral-most region of the embryo (Fig. 4B). In contrast, the expression patterns of the mesendodermal marker genes, mix.2 and mixer, and the endoderm-specific marker gene sox17a, were not appreciably affected by Gtpbp2 knockdown in embryos bilaterally injected at the 2-cell stage (Fig. 4C).
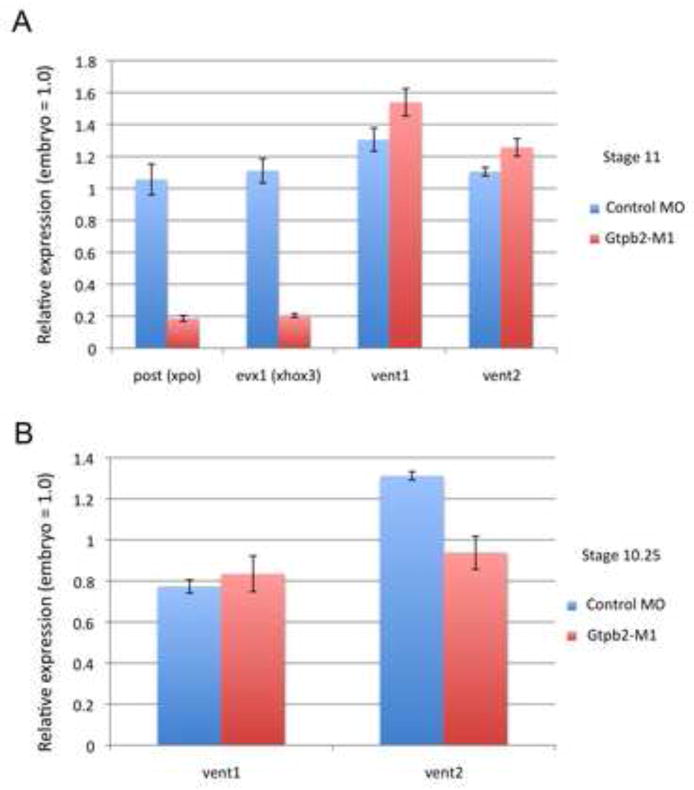
To substantiate the effects we observed on molecular markers by the WISH analysis, above (Fig. 4), and to more accurately determine the relative expression levels of patterning genes in Gtpbp2 morphants, we performed qPCR on embryos injected bilaterally at the 2-cell stage with 40 ng of control or Gtpbp2 MO1 (Fig. 5). In agreement with the WISH results above, we detected significantly reduced expression of ventral posterior markers, post (xpo), and evx1 (xhox3) in Gtpbp2 MO injected embryos at mid gastrulation, stage 11 (Fig. 5A). Curiously, while vent1 expression appears restricted to the ventral most regions in the embryos in Gtpbp2 MO injected embryos (Fig. 4B), and is reduced in animal cap assays, we did not observe a consistent effect on expression levels of vent2 or vent1 by qPCR, either at early (stage 10) or mid gastrula (stage 11) (Fig. 5A,B). Consistent with WISH results, expression of pan-endodermal marker sox17a remained unchanged in early to mid-gastrula embryos, stages 10 and 11 (data not shown). These results demonstrate that Gtpbp2 is required for normal mesodermal ventral-posterior patterning. Moreover, the decreased expression of BMP-controlled, ventral-posterior genes wnt8a, post, and evx1, supports our results with animal cap assays that Gtpbp2 is required for aspects of BMP signaling in the Xenopus embryo.
Figure 5. Gtpbp2 is required for embryonic ventrolateral mesoderm patterning gene expression.
Quantitative RT-PCR analysis of gene expression in control and Gtpbp2 morphant embryos. A. early gastrulation (stage 10.25) and B. mid gastrulation (stage 11), embryos have severely decreased expression levels of ventral lateral genes post (xpo) and evx1 (xhox3), but mild or no change in vent1 or vent2 expression. Ventral genes; ventx1.1 (vent1), wnt8a (wnt8), evx1 (xhox3) were assayed by quantitative RT-PCR (qPCR). Relative expression is normalized to control morpholino-injected embryos. Black bars indicate standard error from triplicate biological replicates.
Gtpbp2 overexpression induces mesodermal markers in animal cap explants
Our loss of function experiments support the hypothesis that Gtpbp2 is required for BMP signaling, but to gain more insight into the activities of Gtpbp2, we analyzed the subcellular localization and effects on gene expression of overexpressed Xenopus equivalents of the human Gtpbp2a (long) and Gtpbp2b (short) variant proteins in Xenopus animal caps (Figure 6). These variants are generated by alternative transcript initiation and first exon splicing (S. Fig. 1), but it is unclear whether the Xenopus genome encodes such short and long Gtpbp2 isoforms. However, as noted above, we obtained one Xenopus cDNA that fortuitously encodes the equivalent of Gtpbp2b, as well as a cDNA encoding Xenopus Gtpbp2a, which corresponds to all other Xenopus gtpbp2 transcripts annotated in the genbank database. We constructed HA epitope and mCherry tagged forms of these Xenopus gtpbp2 variants, and analyzed where the proteins are located when expressed in Xenopus animal cap ectodermal cells and human cultured (COS) cells. We found that the HA- or mCherry-tagged Gtpbp2a/b isoforms can exhibit both nuclear and cytoplasmic localization, with higher expression levels driving nuclear localization (Fig. 6A, S. Fig. 5D, S. Fig. 6).
With animal cap assays, we scored marker gene induction by the Gtpbp2a/b isoforms, using conventional gel-based RT-PCR and qPCR, and we found that Gtpbp2b readily induced the ventro-posterior mesodermal genes vent1.2, evx1, and wnt8a (Fig. 6C,D), while Gtpbp2a induced weak to modest expression of these genes. Levels of gene activation by the Gtpbp2 variants were proportional to mRNA dose, and on an mRNA basis, Gtpbp2a consistently appeared to be less potent than Gtpbp2b. To investigate the basis of this difference, we examined the relative expression levels of the two proteins by western blot, and found that Gtbpb2b accumulated to much higher steady state levels than Gtpbp2a, when the same amount of mRNA was injected into embryos (Fig. 6D). HA-Gtpbp2b protein levels always exceeded those of HA-Gtpbp2a, even when four times as much HA-gtpbp2a mRNA was injected into sibling batches of embryos. The reason for these differences in protein levels (e.g. translation efficiency, protein stability) is unknown. However, when the protein levels are normalized, Gtpbp2a still appears to be less active than Gtpbp2b in animal cap gene activation assays. For example, injecting either 1.0 or 4.0 ng of HA-gtpbp2a mRNA produced about the same amount of protein as 0.2 or 1.0 ng of HA-gtpbp2b, respectively, yet gene induction by Gtpbp2b was much greater than Gtpbp2a at either protein level. The molecular basis for these activity differences is unknown, but it may reflect differences between the N-termini of the a and b isoforms.
Discussion
In this study, we have performed some of the first functional analyses of a rather enigmatic large GTPase, Gtpbp2. We have identified Gtpbp2 as a novel binding partner to Smad1, and have shown that the Smad1 MH1 domain interacts with the Gtpbp2 C-terminal effector region. Both loss and gain of function analyses show that GTPBP2 is required for complete induction of BMP gene targets in ligand-injected animal caps. Furthermore, we found that depletion of Gtpbp2 protein in ventral tissues resulted in both a reduction in BMP signaling targets and defects in ventral/posterior structures, consistent with a reduction in Smad1 activity, and consistent with expression of the gtpbp2 gene in the nascent ectoderm and mesoderm. Overexpression of Gtpbp2 can also induce endogenous BMP response genes and other mesodermal marker genes in Xenopus animal cap explants. Our findings point to a role for Gtpbp2 in BMP signaling, although the exact mechanism is not known.
BMP signaling is required for patterning of the ventral-posterior mesendoderm, acting together with, but upstream of Wnt signals (Kimelman, 2006; Wills et al., 2008; Itasaki and Hoppler, 2010). BMP/Smad1 signaling also counteracts an intrinsic neural differentiation program in the nascent ectoderm (Wilson et al., 1997). In Xenopus, as in other vertebrates and bilaterians in general, BMP signaling provides information to pattern the early embryo along the dorsal-ventral body axis, and concomitantly specify posterior mesodermal fates (Muñoz-Sanjuán and Brivanlou 2001; Lane et al., 2004; Szeto and Kimelman, 2004; Zakin et al. 2005). In our present study, we found that morpholino knockdown of Gtpbp2 truncates posterior embryonic structures in the Xenopus embryo, and severely reduces the expression of ventral-posterior mesodermal marker genes, specifically post, wnt8a, myod1, and evx1 (Figures 4 and 5), consistent with a role in ventral-posterior patterning through BMP/Smad1 signaling.
Our initial results showing that Gtpbp2 morphant embryos have significantly decreased BMP target gene expression and posterior morphological defects, was confirmed in tests on animal caps, in which we observed that knockdown of Gtpbp2 blocked responses to endogenous BMP ligands or exogenous (overexpressed) BMP4. Thus, Gtpbp2 is required for response to BMP ligand. However, Gtpbp2 morphant caps do not express neural markers that would be expected from a complete inhibition of BMP signaling. Additionally, while Gtpbp2 appears to be required for BMP related induction of vent1 in animal caps, vent1 or vent2 showed little or no decrease in whole embryos injected with Gtpbp2 morpholinos. These findings lead us to suggest that Gtpbp2 is a potentiating, but not obligate, factor for BMP signaling responses, although it is quite conceivable (even likely) that small levels of Gtpbp2 protein remain after morpholino knockdown to perform any essential roles. Assessments in a true loss-of-function animal or cell line will help addresss such issues and answer other mechanistic questions. Nevertheless, we conclude Gtpbp2 is required for posterior mesodermal patterning of the Xenopus embryo through its role in BMP signaling.
Expression of the gtpbp2 gene suggests it has an early, general role in mesodermal and ectodermal patterning, followed by tissue specific roles in later development during organogenesis. There is a significant amount of maternal gtpbp2 mRNA localized to the blastula animal hemisphere, which appear to be augmented by zygotic gtpbp2 expression in the early mesoderm (Figure 2). Based on our functional tests, these maternal and early zygotic phases of Gtpbp2 expression appear to potentiate BMP signals, yet we find no consistent effect of Gtpbp2 knockdown on animal cap responses to Nodal (supplied as Xnr2, nodal2; results not shown). Potential roles for Gtpbp2 in other pathways cannot be ruled out, however, particularly since orthologs exist in non-metazoans that lack some or all BMP and TGFβ signaling pathways (e.g fungi, S. Fig. 4).
As development proceeds past blastula stages, Gtpbp2 expression falls precipitously at the onset gastrulation, then slowly rises during neurulation in a pattern that is slightly enriched in neural folds (S. Fig. 2). In tailbud through hatching tadpole development, gtpbp2 transcripts are predominantly expressed in the brain, somites, and ventral blood island, all of which are regulated to some degree by BMP signals. The expression in the blood island is particularly noteworthy since the VBI is the site of primitive hematopoiesis under the direction of BMPs (Schmerer and Evans, 2003; Myers and Krieg, 2013). Gtpbp2 was originally isolated as an interferon inducible gene in monocytes, but its early, essential role in mesodermal germ layer formation has prevented us from analyzing its potential role in blood development using morpholino technology.
The precise molecular mechanism by which Gtpbp2 regulates BMP signaling, or activate BMP response genes when overexpressed, is currently unclear, however Gtpbp2 appears to function downstream of both Smad1 protein stabilization processes and Smad1 activation via phosphorylation by Bmp Type I receptors. For instance, we found that embryos overexpressing tagged Gtpbp2, or injected with Gtpbp2 morpholino, showed no apparent effects on endogenous levels of total Smad1 protein, or activated phospho-Smad1 (S. Fig. 5A). Knockdown of Gtpbp2 also does not appear to affect the formation of Smad1-Smad4 complexes, as Gtpbp2 morphants and controls show no difference in the levels of tagged-Smad1 that co-immunoprecipitates with myc-Smad4 (S. Fig. 5B). In animal cap assays, Gtpbp2a appears to be less potent than Gtpbp2b at inducing BMP target genes, when overexpressed. While the molecular basis of this difference remains to be determined, this observation could have functional implications in systems where gtpbp2 transcripts may be differentially spliced to produce one or the other isoform (particularly in human). However, in Xenopus embryos gtpbp2 transcript profiles indicate that Gtpbp2a is the predominantly expressed form, and this likelihood is supported by our findings that targeting the ATG start codon of potential gtpbp2b transcripts with a morpholino causes no significant embryonic phenotype (results not shown).
Subcellular localization experiments indicate Gtpbp2 has the potential for cytoplasmic and nuclear functions. We found overexpressed, tagged Gtpbp2a protein localizing to the nucleus of either intact or disaggregated Xenopus ectodermal cells at early gastrulation (S. Fig. 5C, S. Fig. 6). Consistent with this data, we found overexpressed Gtpbp2b Smad1 proteins colocalizing to nuclear foci in COS cells (S. Fig. 5E), which may reflect a role in the formation of transcriptional complexes. The localization of Smads to subnuclear foci in similar speckled patterns has been observed previously (Janknecht et al., 1998; Yoshida et al., 2000), including activated Smad1/5 and a transcriptional partner, Runx (Zaidi et al., 2002). Hence, it is possible that Gtpbp2-Smad1 complexes are engaging in transcriptional activities in these nuclear foci. However, since we observe that nuclear localization of GFP-Smad1 is unaffected in Gtpbp2 morphant cells (S. Fig. 5C), Gtpbp2 does not appear to be required to redirect nuclear localization of Smad1. Furthermore, the subcellular location of endogenous Gtpbp2 protein described in the Human Protein Atlas appears cytoplasmic, but not nuclear, in the tissues examined (Uhlen et al., 2010). We also find that increasing the level of Xenopus Gtpbp2a in mammalian cells can shift its localization from cytoplasm to nucleus, but that the Xenopus version of human Gtpbp2b readily localizes to the nucleus (S. Fig. 5D). The subcellular localization of Gtpbp2 therefore may depend on dose, isoform type (a versus b) or interacting partner. These differences in localization might affect differences in the ability to induce BMP target genes.
Gtpbp2 is part of a superfamily of large GTPases that include eEf1a (elongation factor 1 alpha). The closest homolog of Gtpbp2, Gtpbp1, has been shown to target mRNAs and enhance their turnover through its interaction with 3’ RNA end-processing exosome complex (Woo et al., 2011). Not only are Gtpbp2 and Gtpbp1 distantly related to translation elongation factors, they have homology with other proteins that are involved in mRNA turnover, such as Hbs1 (Tsuboi et al., 2012). Thus, it is conceivable that Gtpbp2 might function in mRNA turnover as well. However, unlike Gtpbp1, Gtpbp2 does not bind to exosome components, nor does it promote similar decay of mRNA targets (Woo et al, 2011). Additionally, the finding that Gtpbp2 knockdown has an effect on Vent2 reporter but not endogenous vent1 or vent2 mRNA steady state levels in caps, strongly suggests that Gtpbp2 promotes Smad1 activity, rather than stability of Smad1-induced transcripts. Conversely, purified Gtpbp2, but not Gtpbp1, interacts with Smad1 in an in vitro binding assay, and Gtpbp1 does not show similar activity in promoting BMP signaling in animal caps (data not shown), providing further evidence that Gtpbp1 and Gtpbp2 do not overlap in function. Indeed, the Gtpbp1 and Gtpbp2 paralogs appear to be present in all animals, and even in the mold Neurospora crassa, suggesting these genes arose from an ancient duplication predating the emergence of animals, as well as the TGFβ signaling system, and therefore it is not at all surprising that these would have non-redundant functions, or that Gtpbp2 might have additional cellular functions besides BMP signaling. Finally, embryo-wide or dorsal targeted knockdown of Gtpbp2 results in a complex phenotype not explained by effects on BMP or Nodal, indicating that Gtpbp2 likely functions in other signaling pathways . These phenotypes and associated analyses will be presented in a separate study (in preparation).
Conclusions
In summary, we have discovered that Gtpbp2, a large GTPase of unknown function, is a novel binding partner of Smad1 and is required for BMP signaling in the Xenopus embryo. Gtpbp2 is expressed in the nascent ectoderm and mesoderm, and in major sites of organogenesis, during Xenopus development, and gain and loss of function analyses show that Gtpbp2 is required for BMP target gene induction and development of BMP-dependent ventral-posterior body structures. Gtpbp2 appears to operate in BMP signaling downstream of Smad1 activation, and has the potential to act in nuclear or cytoplasmic compartments.
Supplementary Material
Highlights.
A little understood Gtpase, named Gtpbp2, interacts with Smad1 and is nuclear
Gtpbp2 is required for, and can enhance, BMP signaling in Xenopus embryos
Knockdown of Gtpbp2 impairs embryonic ventro-posterior patterning
Blastula-gastrula Gtpbp2 transcripts are localized to the animal pole and mesoderm
Larval Gtpbp2 is expressed in somites, brain, branchial arches and blood
Acknowledgments
We thank the Thomsen lab and local colleagues for comments on the manuscript, and S. Sokol for reporter plasmids. We dedicate this work to the memory of John Miller, who participated in early efforts on the project. This work was supported by NIH grant 5R01GM080462 to GHT. Support for WQG was from NIDDK 2T32DK007521 and NIGMS 1K12GM102778.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Agius E, Oelgeschlager M, Wessely O, Kemp C, De Robertis EM. Endodermal Nodal-related signals and mesoderm induction in Xenopus. Development. 2000;127:1173–1183. doi: 10.1242/dev.127.6.1173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexandrova EM, Thomsen GH. Smurf1 regulates neural patterning and folding in Xenopus embryos by antagonizing the BMP/Smad1 pathway. Dev Biol. 2006;299:398–410. doi: 10.1016/j.ydbio.2006.08.009. http://dx.doi.org/10.1016/j.ydbio.2006.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asashima M, Ito Y, Chan T, Michiue T, Nakanishi M, Suzuki K, Hitachi K, Okabayashi K, Kondow A, Ariizumi T. In vitro organogenesis from undifferentiated cells in Xenopus. Dev Dyn. 2009;238:1309–1320. doi: 10.1002/dvdy.21979. http://dx.doi.org/10.1002/dvdy.21979. [DOI] [PubMed] [Google Scholar]
- Bates TJ, Vonica A, Heasman J, Brivanlou AH, Bell E. Coco regulates dorsoventral specification of germ layers via inhibition of TGFbeta signaling. Development. 2013;140:4177–4181. doi: 10.1242/dev.095521. http://dx.doi.org/10.1242/dev.095521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brannon M, Kimelman D. Activation of Siamois by the Wnt pathway. Dev Biol. 1996;180:344–347. doi: 10.1006/dbio.1996.0306. http://dx.doi.org/10.1006/dbio.1996.0306. [DOI] [PubMed] [Google Scholar]
- Bregman A, Avraham-Kelbert M, Barkai O, Duek L, Guterman A, Choder M. Promoter elements regulate cytoplasmic mRNA decay. Cell. 2011;147:1473–1483. doi: 10.1016/j.cell.2011.12.005. http://dx.doi.org/10.1016/j.cell.2011.12.005. [DOI] [PubMed] [Google Scholar]
- Callery EM, Park CY, Xu X, Zhu H, Smith JC, Thomsen GH. Eps15R is required for bone morphogenetic protein signaling and differentially compartmentalizes with Smad proteins. Open Biol. 2012;4:120060. doi: 10.1098/rsob.120060. http://dx.doi.org/10.1098/rsob.120060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carnac G, Kodjabachian L, Gurdon JB, Lemaire P. The homeobox gene Siamois is a target of the Wnt dorsalisation pathway and triggers organiser activity in the absence of mesoderm. Development. 1996;122:3055–3065. doi: 10.1242/dev.122.10.3055. [DOI] [PubMed] [Google Scholar]
- Ciau-Uitz A, Walmsley M, Patient R. Distinct origins of adult and embryonic blood in Xenopus. Cell. 2000;102:787–796. doi: 10.1016/s0092-8674(00)00067-2. http://dx.doi.org/10.1016/S0092-8674(00)00067-2. [DOI] [PubMed] [Google Scholar]
- Constance Lane M, Davidson L, Sheets MD. BMP antagonism by Spemann’s organizer regulates rostral-caudal fate of mesoderm. Dev Biol. 2004;275:356–74. doi: 10.1016/j.ydbio.2004.08.012. http://dx.doi.org/10.1016/j.ydbio.2004.08.012. [DOI] [PubMed] [Google Scholar]
- Davis BN, Hilyard AC, Lagna G, Hata A. SMAD proteins control DROSHA-mediated microRNA maturation. Nature. 2008;454:56–61. doi: 10.1038/nature07086. http://dx.doi.org/10.1038/nature07086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis BN, Hilyard AC, Nguyen PH, Lagna G, Hata A. Smad proteins bind a conserved RNA sequence to promote microRNA maturation by Drosha. Mol Cell. 2010;39:373–384. doi: 10.1016/j.molcel.2010.07.011. http://dx.doi.org/10.1016/j.molcel.2010.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dennler S, Itoh S, Vivien D, ten Dijke P, Huet S, Gauthier JM. Direct binding of Smad3 and Smad4 to critical TGF beta-inducible elements in the promoter of human plasminogen activator inhibitor-type 1 gene. EMBO J. 1998;17:3091–3100. doi: 10.1093/emboj/17.11.3091. http://dx.doi.org/10.1093/emboj/17.11.3091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeRobertis EM, Kuroda H. Dorsal-ventral patterning and neural induction in Xenopus embryos. Annu Rev Cell Dev Biol. 2004;20:285–308. doi: 10.1146/annurev.cellbio.20.011403.154124. http://dx.doi.org/10.1146/annurev.cellbio.20.011403.154124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorey K, Hill CS. A novel Cripto-related protein reveals an essential role for EGF-CFCs in Nodal signalling in Xenopus embryos. Dev Biol. 2006;292:303–316. doi: 10.1016/j.ydbio.2006.01.006. http://dx.doi.org/10.1016/j.ydbio.2006.01.006. [DOI] [PubMed] [Google Scholar]
- Dunn NR, Vincent SD, Oxburgh L, Robertson EJ, Bikoff EK. Combinatorial activities of Smad2 and Smad3 regulate mesoderm formation and patterning in the mouse embryo. Development. 2004;131:1717–1728. doi: 10.1242/dev.01072. http://dx.doi.org/10.1242/dev.01072. [DOI] [PubMed] [Google Scholar]
- Enssle J, Kugler W, Hentze MW, Kulozik AE. Determination of mRNA fate by different RNA polymerase II promoters. Proc Natl Acad Sci. 1993;90:10091–10095. doi: 10.1073/pnas.90.21.10091. http://dx.doi.org/10.1073/pnas.90.21.10091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng XH, Derynck R. Specificity and versatility in tgf-beta signaling through Smads. Annu Rev Cell Dev Biol. 2005;21:659–693. doi: 10.1146/annurev.cellbio.21.022404.142018. http://dx.doi.org/10.1146/annurev.cellbio.21.022404.142018. [DOI] [PubMed] [Google Scholar]
- Frontelo P, Leader JE, Yoo N, Potocki AC, Crawford M, Kulik M, Lechleider RJ. Suv39h histone methyltransferases interact with Smads and cooperate in BMP-induced repression. Oncogene. 2004;23:5242–5251. doi: 10.1038/sj.onc.1207660. http://dx.doi.org/10.1038/sj.onc.1207660. [DOI] [PubMed] [Google Scholar]
- Furtado MB, Solloway MJ, Jones VJ, Costa MW, Biben C, Wolstein O, Preis JI, Sparrow DB, Saga Y, Dunwoodie SL, Robertson EJ, Tam PP, Harvey RP. BMP/SMAD1 signaling sets a threshold for the left/right pathway in lateral plate mesoderm and limits availability of SMAD4. Genes Dev. 2008;22:3037–3049. doi: 10.1101/gad.1682108. http://dx.doi.org/10.1101/gad.1682108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green JB, New HV, Smith JC. Responses of embryonic Xenopus cells to activin and FGF are separated by multiple dose thresholds and correspond to distinct axes of the mesoderm. Cell. 1992;71:731–739. doi: 10.1016/0092-8674(92)90550-v. http://dx.doi.org/10.1016/0092-8674(92)90550-V. [DOI] [PubMed] [Google Scholar]
- Gronroos E, Kingston IJ, Ramachandran A, Randall RA, Vizan P, Hill CS. Transforming growth factor beta inhibits bone morphogenetic protein-induced transcription through novel phosphorylated Smad1/5-Smad3 complexes. Mol Cell Biol. 2012;32:2904–2916. doi: 10.1128/MCB.00231-12. http://dx.doi.org/10.1128/MCB.00231-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haimovich G, Medina DA, Causse SZ, Garber M, Millan-Zambrano G, Barkai O, Chavez S, Perez-Ortin JE, Darzacq X, Choder M. Gene expression is circular: factors for mRNA degradation also foster mRNA synthesis. Cell. 2013;153:1000–1011. doi: 10.1016/j.cell.2013.05.012. http://dx.doi.org/10.1016/j.cell.2013.05.012. [DOI] [PubMed] [Google Scholar]
- Harland R, Gerhart J. Formation and function of Spemann’s organizer. Annu Rev Cell Dev Biol. 1997;13:611–667. doi: 10.1146/annurev.cellbio.13.1.611. http://dx.doi.org/10.1146/annurev.cellbio.13.1.611. [DOI] [PubMed] [Google Scholar]
- Harland RM. In situ hybridization: an improved whole-mount method for Xenopus embryos. Methods Cell Biol. 1991;36:685–695. doi: 10.1016/s0091-679x(08)60307-6. http://dx.doi.org/10.1016/S0091-679X(08)60307-6. [DOI] [PubMed] [Google Scholar]
- Harvey SA, Tumpel S, Dubrulle J, Schier AF, Smith JC. no tail integrates two modes of mesoderm induction. Development. 2010;137:1127–1135. doi: 10.1242/dev.046318. http://dx.doi.org/10.1242/dev.046318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hata A, Lagna G, Massague J, Hemmati-Brivanlou A. Smad6 inhibits BMP/Smad1 signaling by specifically competing with the Smad4 tumor suppressor. Genes Dev. 1998;12:186–197. doi: 10.1101/gad.12.2.186. http://dx.doi.org/10.1101/gad.12.2.186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heasman J. Patterning the early Xenopus embryo. Development. 2006;133:1205–1217. doi: 10.1242/dev.02304. http://dx.doi.org/10.1242/dev.02304. [DOI] [PubMed] [Google Scholar]
- Henningfeld KA, Rastegar S, Adler G, Knochel W. Smad1 and Smad4 are components of the bone morphogenetic protein-4 (BMP-4)-induced transcription complex of the Xvent-2B promoter. J Biol Chem. 2000;275:21827–21835. doi: 10.1074/jbc.M000978200. http://dx.doi.org/10.1074/jbc.M000978200. [DOI] [PubMed] [Google Scholar]
- Howell M, Mohun TJ, Hill CS. Xenopus Smad3 is specifically expressed in the chordoneural hinge, notochord and in the endocardium of the developing heart. Mech Dev. 2001;104:147–150. doi: 10.1016/s0925-4773(01)00365-3. http://dx.doi.org/10.1016/S0925-4773(01)00365-3. [DOI] [PubMed] [Google Scholar]
- Itasaki N, Hoppler S. Crosstalk between Wnt and bone morphogenic protein signaling: a turbulent relationship. Dev Dyn. 2010;239:16–33. doi: 10.1002/dvdy.22009. http://dx.doi.org/10.1002/dvdy.22009. [DOI] [PubMed] [Google Scholar]
- Itoh S, ten Dijke P. Negative regulation of TGF-beta receptor/Smad signal transduction. Curr Opin Cell Biol. 2007;19:176–184. doi: 10.1016/j.ceb.2007.02.015. http://dx.doi.org/10.1016/j.ceb.2007.02.015. [DOI] [PubMed] [Google Scholar]
- Janknecht R, Wells NJ, Hunter T. TGF-beta-stimulated cooperation of smad proteins with the coactivators CBP/p300. Genes Dev. 1998;12:2114–2119. doi: 10.1101/gad.12.14.2114. http://dx.doi.org/10.1101/gad.12.14.2114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimelman D. Mesoderm induction: from caps to chips. Nat Rev Genet. 2006;7:360–372. doi: 10.1038/nrg1837. http://dx.doi.org/10.1038/nrg1837. [DOI] [PubMed] [Google Scholar]
- Kudo H, Senju S, Mitsuya H, Nishimura Y. Mouse and human GTPBP2, newly identified members of the GP-1 family of GTPase. Biochem Biophys Res Commun. 2000;272:456–465. doi: 10.1006/bbrc.2000.2763. http://dx.doi.org/10.1006/bbrc.2000.2763. [DOI] [PubMed] [Google Scholar]
- Kuroda H, Fuentealba L, Ikeda A, Reversade B, De Robertis EM. Default neural induction: neuralization of dissociated Xenopus cells is mediated by Ras/MAPK activation. Genes Dev. 2005;19:1022–1027. doi: 10.1101/gad.1306605. http://dx.doi.org/10.1101/gad.1306605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Latinkic BV, Smith JC. Goosecoid and mix1 repress Brachyury expression and are required for head formation in Xenopus. Development. 1999;126:1769–1779. doi: 10.1242/dev.126.8.1769. [DOI] [PubMed] [Google Scholar]
- Massague J, Seoane J, Wotton D. Smad transcription factors. Genes Dev. 2005;19:2783–2810. doi: 10.1101/gad.1350705. http://dx.doi.org/10.1101/gad.1350705. [DOI] [PubMed] [Google Scholar]
- Moustakas A, Heldin CH. Non-Smad TGF-beta signals. J Cell Sci. 2005;118:3573–3584. doi: 10.1242/jcs.02554. http://dx.doi.org/10.1242/jcs.02554. [DOI] [PubMed] [Google Scholar]
- Moustakas A, Heldin CH. The roles of Smads in TGFβ signaling. Cell Tissue Res. 2012;347:21–36. doi: 10.1007/s00441-011-1190-x. http://dx.doi.org/10.1007/s00441-011-1190-x. [DOI] [PubMed] [Google Scholar]
- Munoz-Sanjuán I, H-Brivanlou A. Early posterior/ventral fate specification in the vertebrate embryo. Dev Biol. 2001;237:1–17. doi: 10.1006/dbio.2001.0350. http://dx.doi.org/10.1006/dbio.2001.0350. [DOI] [PubMed] [Google Scholar]
- Myers CT, Krieg PA. BMP-mediated specification of the erythroid lineage suppresses endothelial development in blood island precursors. Blood. 2013;122:3929–3939. doi: 10.1182/blood-2013-03-490045. http://dx.doi.org/10.1182/blood-2013-03-490045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishimatsu S, Thomsen GH. Ventral mesoderm induction and patterning by bone morphogenetic protein heterodimers in Xenopus embryos. Mech Dev. 1998;74:75–88. doi: 10.1016/s0925-4773(98)00070-7. http://dx.doi.org/10.1016/S0925-4773(98)00070-7. [DOI] [PubMed] [Google Scholar]
- Northrop JL, Kimelman D. Dorsal-ventral differences in Xcad-3 expression in response to FGF-mediated induction in Xenopus. Dev Biol. 1994;161:490–503. doi: 10.1006/dbio.1994.1047. http://dx.doi.org/10.1006/dbio.1994.1047. [DOI] [PubMed] [Google Scholar]
- Osada S, Ohmori SY, Taira M. XMAN1, an inner nuclear membrane protein, antagonizes BMP signaling by interacting with Smad1 in Xenopus embryos. Development. 2003;130:1783–94. doi: 10.1242/dev.00401. http://dx.doi.org/10.1242/dev.00401. [DOI] [PubMed] [Google Scholar]
- Pereira PN, Dobreva MP, Maas E, Cornelis FM, Moya IM, Umans L, Verfaillie CM, Camus A, de Sousa Lopes SM, Huylebroeck D, Zwijsen A. Antagonism of Nodal signaling by BMP/Smad5 prevents ectopic primitive streak formation in the mouse amnion. Development. 2012;139:3343–3354. doi: 10.1242/dev.075465. http://dx.doi.org/10.1242/dev.075465. [DOI] [PubMed] [Google Scholar]
- Riis B, Rattan SI, Clark BF, Merrick WC. Eukaryotic protein elongation factors. Trends Biochem Sci. 1990;15:420–424. doi: 10.1016/0968-0004(90)90279-k. http://dx.doi.org/10.1016/0968-0004(90)90279-K. [DOI] [PubMed] [Google Scholar]
- Rogers CD, Moody SA, Casey ES. Neural induction and factors that stabilize a neural fate. Birth Defects Res C Embryo Today. 2009;87:249–262. doi: 10.1002/bdrc.20157. http://dx.doi.org/10.1002/bdrc.20157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasikumar AN, Perez WB, Kinzy TG. The many roles of the eukaryotic elongation factor 1 complex. Wiley Interdiscip Rev RNA. 2012;3:543–555. doi: 10.1002/wrna.1118. http://dx.doi.org/10.1002/wrna.1118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schier AF, Talbot WS. Molecular genetics of axis formation in zebrafish. Annu Rev Genet. 2005;39:561–613. doi: 10.1146/annurev.genet.37.110801.143752. http://dx.doi.org/10.1146/annurev.genet.37.110801.143752. [DOI] [PubMed] [Google Scholar]
- Schmerer M, Evans T. Primitive erythropoiesis is regulated by Smad-dependent signaling in postgastrulation mesoderm. Blood. 2003;102:3196–3205. doi: 10.1182/blood-2003-04-1094. http://dx.doi.org/10.1182/blood-2003-04-1094. [DOI] [PubMed] [Google Scholar]
- Shi Y, Massagué J. Mechanisms of TGF-β Signaling from Cell Membrane to the Nucleus. Cell. 2003;113:685–700. doi: 10.1016/s0092-8674(03)00432-x. http://dx.doi.org/10.1016/S0092-8674(03)00432-X. [DOI] [PubMed] [Google Scholar]
- Szeto DP, Kimelman D. Combinatorial gene regulation by Bmp and Wnt in zebrafish posterior mesoderm formation. Development. 2004;131:3751–3760. doi: 10.1242/dev.01236. http://dx.doi.org/10.1242/dev.01236. [DOI] [PubMed] [Google Scholar]
- Ten Dijke P, Goumans MJ, Itoh F, Itoh S. Regulation of cell proliferation by Smad proteins. J Cell Physiol. 2002;191:1–16. doi: 10.1002/jcp.10066. http://dx.doi.org/10.1002/jcp.10066. [DOI] [PubMed] [Google Scholar]
- Thomsen GH. Xenopus mothers against decapentaplegic is an embryonic ventralizing agent that acts downstream of the BMP-2/4 receptor. Development. 1996;122:2359–2366. doi: 10.1242/dev.122.8.2359. [DOI] [PubMed] [Google Scholar]
- Trcek T, Larson DR, Moldon A, Query CC, Singer RH. Single-molecule mRNA decay measurements reveal promoter- regulated mRNA stability in yeast. Cell. 2011;147:1484–1497. doi: 10.1016/j.cell.2011.11.051. http://dx.doi.org/10.1016/j.cell.2011.11.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuboi T, Kuroha K, Kudo K, Makino S, Inoue E, Kashima I, Inada T. Dom34:hbs1 plays a general role in quality-control systems by dissociation of a stalled ribosome at the 3’ end of aberrant mRNA. Mol Cell. 2012;46:518–529. doi: 10.1016/j.molcel.2012.03.013. http://dx.doi.org/10.1016/j.molcel.2012.03.013. [DOI] [PubMed] [Google Scholar]
- Uhlen M, Oksvold P, Fagerberg L, Lundberg E, Jonasson K, Forsberg M, Zwahlen M, Kampf C, Wester K, Hober S, Wernerus H, Björling L, Ponten F. Towards a knowledge-based Human Protein Atlas. Nat Biotechnol. 2010;28:1248–50. doi: 10.1038/nbt1210-1248. http://dx.doi.org/10.1038/nbt1210-1248. [DOI] [PubMed] [Google Scholar]
- Uno Y, Nishida C, Takagi C, Ueno N, Matsuda Y. Homoeologous chromosomes of Xenopus laevis are highly conserved after whole-genome duplication. Heredity (Edinb) 2013;111:430–436. doi: 10.1038/hdy.2013.65. http://dx.doi.org/10.1038/hdy.2013.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vonica A, Gumbiner BM. Zygotic Wnt activity is required for Brachyury expression in the early Xenopus laevis embryo. Dev Biol. 2002;250:112–127. doi: 10.1006/dbio.2002.0786. http://dx.doi.org/10.1006/dbio.2002.0786. [DOI] [PubMed] [Google Scholar]
- Vonica A, Brivanlou AH. An obligatory caravanserai stop on the silk road to neural induction: inhibition of BMP/GDF signaling. Semin Cell Dev Biol. 2006;17:117–32. doi: 10.1016/j.semcdb.2005.11.013. http://dx.doi.org/10.1016/j.semcdb.2005.11.013. [DOI] [PubMed] [Google Scholar]
- Watanabe M, Yoshida K, Hida M, Kato H, Uchida K, Yamaguchi R, Tateyama S, Sugano S. Cloning, expression analysis, and chromosomal mapping of GTPBP2, a novel member of the G protein family. Gene. 2000;256:51–58. doi: 10.1016/s0378-1119(00)00346-2. http://dx.doi.org/10.1016/S0378-1119(00)00346-2. [DOI] [PubMed] [Google Scholar]
- Weber JR, Sokol SY. Identification of a phylogenetically conserved activin-responsive enhancer in the Zic3 gene. Mech Dev. 2003;120:955–964. doi: 10.1016/s0925-4773(03)00082-0. http://dx.doi.org/10.1016/S0925-4773(03)00082-0. [DOI] [PubMed] [Google Scholar]
- Weimann M, Grossmann A, Woodsmith J, Özkan Z, Birth P, Meierhofer D, Benlasfer N, Valovka T, Timmermann B, Wanker EE, Sauer S, Stelzl U. A Y2H-seq approach defines the human protein methyltransferase interactome. Nat Methods. 2013;10:339–342. doi: 10.1038/nmeth.2397. http://dx.doi.org/10.1038/nmeth.2397. [DOI] [PubMed] [Google Scholar]
- Wills A, Dickinson K, Khokha M, Baker JC. Bmp signaling is necessary and sufficient for ventrolateral endoderm specification in Xenopus. Dev Dyn. 2008;237:2177–2186. doi: 10.1002/dvdy.21631. http://dx.doi.org/10.1002/dvdy.21631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson PA, Lagna G, Suzuki A, Hemmati-Brivanlou A. Concentration-dependent patterning of the Xenopus ectoderm by BMP4 and its signal transducer Smad1. Development. 1997;124:3177–3184. doi: 10.1242/dev.124.16.3177. [DOI] [PubMed] [Google Scholar]
- Woo KC, Kim TD, Lee KH, Kim DY, Kim S, Lee HR, Kang HJ, Chung SJ, Senju S, Nishimura Y, Kim KT. Modulation of exosome-mediated mRNA turnover by interaction of GTP-binding protein 1 (GTPBP1) with its target mRNAs. Faseb J. 2011;25:2757–2769. doi: 10.1096/fj.10-178715. http://dx.doi.org/10.1096/fj.10-178715. [DOI] [PubMed] [Google Scholar]
- Xanthos JB, Kofron M, Tao Q, Schaible K, Wylie C, Heasman J. The roles of three signaling pathways in the formation and function of the Spemann Organizer. Development. 2002;129:4027–4043. doi: 10.1242/dev.129.17.4027. [DOI] [PubMed] [Google Scholar]
- Yamamoto TS, Takagi C, Hyodo AC, Ueno N. Suppression of head formation by Xmsx-1 through the inhibition of intracellular nodal signaling. Development. 2001;128:2769–2779. doi: 10.1242/dev.128.14.2769. [DOI] [PubMed] [Google Scholar]
- Yoshida Y, Tanaka S, Umemori H, Minowa O, Usui M, Ikematsu N, Hosoda E, Imamura T, Kuno J, Yamashita T, et al. Negative regulation of BMP/Smad signaling by Tob in osteoblasts. Cell. 2000;103:1085–1097. doi: 10.1016/s0092-8674(00)00211-7. http://dx.doi.org/10.1016/S0092-8674(00)00211-7. [DOI] [PubMed] [Google Scholar]
- Zaidi SK, Sullivan AJ, van Wijnen AJ, Stein JL, Stein GS, Lian JB. Integration of Runx and Smad regulatory signals at transcriptionally active subnuclear sites. Proc Natl Acad Sci. 2002;99:8048–8053. doi: 10.1073/pnas.112664499. http://dx.doi.org/10.1073/pnas.112664499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zakin L, Reversade B, Kuroda H, Lyons KM, De Robertis EM. Sirenomelia in Bmp7 and Tsg compound mutant mice: requirement for Bmp signaling in the development of ventral posterior mesoderm. Development. 2005;132:2489–2499. doi: 10.1242/dev.01822. http://dx.doi.org/10.1242/dev.01822. [DOI] [PubMed] [Google Scholar]
- Zhu H, Kavsak P, Abdollah S, Wrana JL, Thomsen GH. A SMAD ubiquitin ligase targets the BMP pathway and affects embryonic pattern formation. Nature. 1999;400:687–693. doi: 10.1038/23293. http://dx.doi.org/10.1038/23293. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.