Abstract
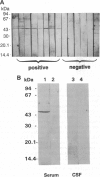
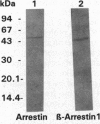
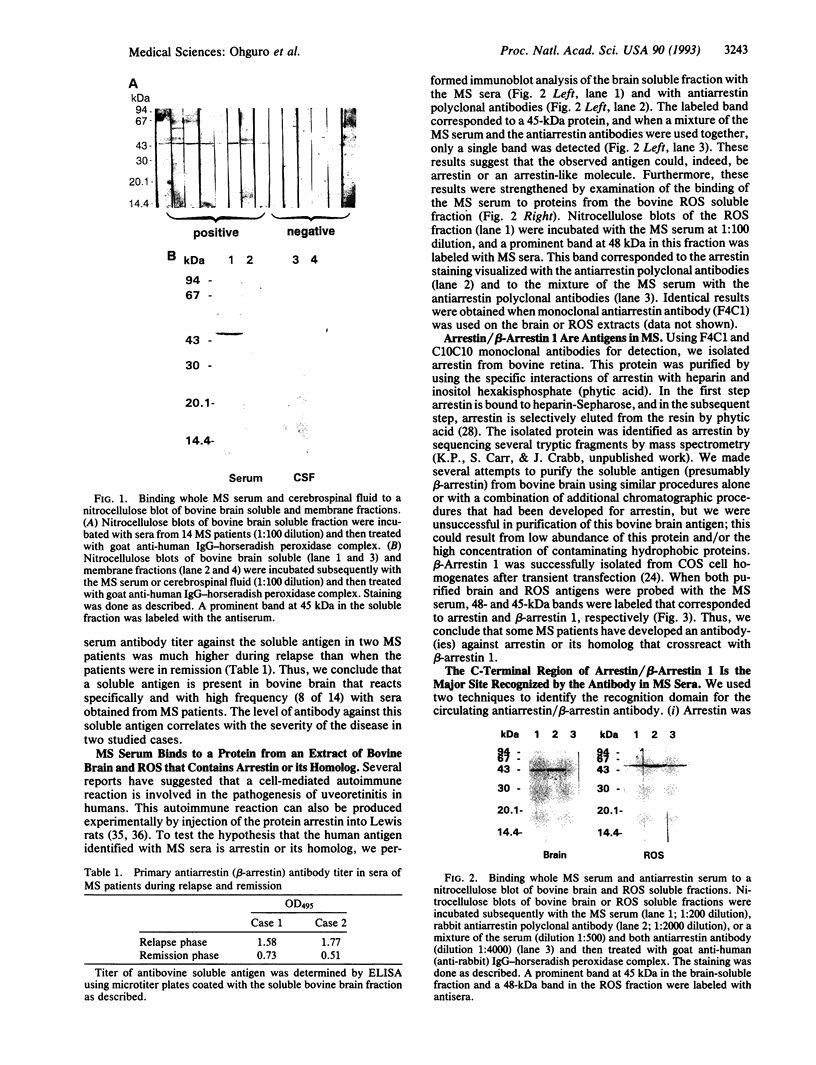
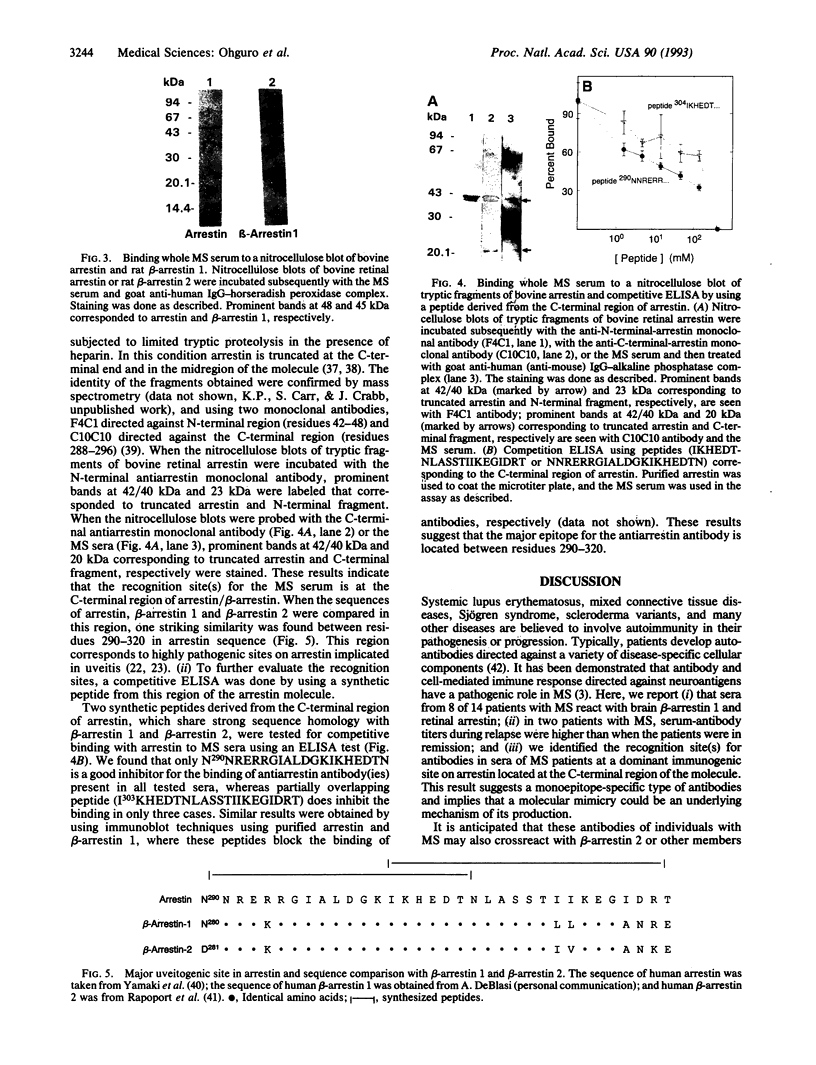
Multiple sclerosis (MS), one of the most common chronic neurologic diseases, is characterized by the presence of multiple plaques of demyelination throughout the central nervous system. Although the etiology of the disease has not been established, it is believed to involve autoimmune mechanisms. We have examined sera from patients with MS for the presence of antibodies to antigens from brain and retina. Immunoblot analysis of soluble fraction of proteins from bovine brain revealed a prominent band at 45 kDa stained with sera of 8-14 patients with MS. In two patients with MS, serum antibody titers during relapse were higher compared with those when the patients were in remission. These antibodies were undetectable in cerebrospinal fluid of our MS patients and additionally were absent in sera of patients with other neurological diseases and normal control subjects. Furthermore, immunoblot analysis of the soluble fraction from bovine retinal rod outer segments revealed a prominent protein band at 48 kDa stained with MS sera. This antigen was purified to homogeneity from bovine retinal outer segments and identified as arrestin. Additionally, sera from MS patients reacted with purified beta-arrestin 1, a 45-kDa protein homologous to arrestin that is found in various tissues. Using limited proteolysis of arrestin and a competitive ELISA test with a synthetic peptide, we identified the recognition site(s) for antibodies in sera of MS patients at a dominant immunogenic site on arrestin located at the C-terminal region of the molecule. We suggest that the presence of circulating antibodies reactive with beta-arrestin or arrestin may be related to the course of MS progression.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abramsky O., Lisak R. P., Silberberg D. H., Pleasure D. E. Antibodies to oligodendroglia in patients with multiple sclerosis. N Engl J Med. 1977 Dec 1;297(22):1207–1211. doi: 10.1056/NEJM197712012972204. [DOI] [PubMed] [Google Scholar]
- Attramadal H., Arriza J. L., Aoki C., Dawson T. M., Codina J., Kwatra M. M., Snyder S. H., Caron M. G., Lefkowitz R. J. Beta-arrestin2, a novel member of the arrestin/beta-arrestin gene family. J Biol Chem. 1992 Sep 5;267(25):17882–17890. [PubMed] [Google Scholar]
- Dua H. S., Abrams M., Barrett J. A., Gregerson D. S., Forrester J. V., Donoso L. A. Epitopes and idiotypes in experimental autoimmune uveitis: a review. Curr Eye Res. 1992;11 (Suppl):59–65. doi: 10.3109/02713689208999512. [DOI] [PubMed] [Google Scholar]
- Gregerson D. S., Fling S. P., Obritsch W. F., Merryman C. F., Donoso L. A. Identification of T cell recognition sites in S-antigen: dissociation of proliferative and pathogenic sites. Cell Immunol. 1989 Oct 15;123(2):427–440. doi: 10.1016/0008-8749(89)90302-x. [DOI] [PubMed] [Google Scholar]
- Gregerson D. S., Obritsch W. F., Fling S. P. Identification of a uveitogenic cyanogen bromide peptide of bovine retinal S-antigen and preparation of a uveitogenic, peptide-specific T cell line. Eur J Immunol. 1987 Mar;17(3):405–411. doi: 10.1002/eji.1830170316. [DOI] [PubMed] [Google Scholar]
- Górny M. K., Wróblewska Z., Pleasure D., Miller S. L., Wajgt A., Koprowski H. CSF antibodies to myelin basic protein and oligodendrocytes in multiple sclerosis and other neurological diseases. Acta Neurol Scand. 1983 Jun;67(6):338–347. doi: 10.1111/j.1600-0404.1983.tb03151.x. [DOI] [PubMed] [Google Scholar]
- Hargrave P. A., Adamus G., Arendt A., McDowell J. H., Wang J., Szaby A., Curtis D., Jackson R. W. Rhodopsin's amino terminus is a principal antigenic site. Exp Eye Res. 1986 Apr;42(4):363–373. doi: 10.1016/0014-4835(86)90030-8. [DOI] [PubMed] [Google Scholar]
- Harris J. O., Frank J. A., Patronas N., McFarlin D. E., McFarland H. F. Serial gadolinium-enhanced magnetic resonance imaging scans in patients with early, relapsing-remitting multiple sclerosis: implications for clinical trials and natural history. Ann Neurol. 1991 May;29(5):548–555. doi: 10.1002/ana.410290515. [DOI] [PubMed] [Google Scholar]
- Knospe V., Donoso L. A., Banga J. P., Yue S., Kasp E., Gregerson D. S. Epitope mapping of bovine retinal S-antigen with monoclonal antibodies. Curr Eye Res. 1988 Nov;7(11):1137–1147. doi: 10.3109/02713688809001885. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lisak R. P. Multiple sclerosis: evidence for immunopathogenesis. Neurology. 1980 Jul;30(7 Pt 2):99–105. doi: 10.1212/wnl.30.7_part_2.99. [DOI] [PubMed] [Google Scholar]
- Lohse M. J., Benovic J. L., Codina J., Caron M. G., Lefkowitz R. J. beta-Arrestin: a protein that regulates beta-adrenergic receptor function. Science. 1990 Jun 22;248(4962):1547–1550. doi: 10.1126/science.2163110. [DOI] [PubMed] [Google Scholar]
- Martin R., McFarland H. F., McFarlin D. E. Immunological aspects of demyelinating diseases. Annu Rev Immunol. 1992;10:153–187. doi: 10.1146/annurev.iy.10.040192.001101. [DOI] [PubMed] [Google Scholar]
- Milligan G., Klee W. A. The inhibitory guanine nucleotide-binding protein (Ni) purified from bovine brain is a high affinity GTPase. J Biol Chem. 1985 Feb 25;260(4):2057–2063. [PubMed] [Google Scholar]
- Nussenblatt R. B., Kuwabara T., de Monasterio F. M., Wacker W. B. S-antigen uveitis in primates. A new model for human disease. Arch Ophthalmol. 1981 Jun;99(6):1090–1092. doi: 10.1001/archopht.1981.03930011090021. [DOI] [PubMed] [Google Scholar]
- Nussenblatt R. B., Rodrigues M. M., Wacker W. B., Cevario S. J., Salinas-Carmona M. C., Gery I. Cyclosporin a. Inhibition of experimental autoimmune uveitis in Lewis rats. J Clin Invest. 1981 Apr;67(4):1228–1231. doi: 10.1172/JCI110138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsson T., Baig S., Höjeberg B., Link H. Antimyelin basic protein and antimyelin antibody-producing cells in multiple sclerosis. Ann Neurol. 1990 Feb;27(2):132–136. doi: 10.1002/ana.410270207. [DOI] [PubMed] [Google Scholar]
- Palczewski K., Buczyłko J., Imami N. R., McDowell J. H., Hargrave P. A. Role of the carboxyl-terminal region of arrestin in binding to phosphorylated rhodopsin. J Biol Chem. 1991 Aug 15;266(23):15334–15339. [PubMed] [Google Scholar]
- Palczewski K., Pulvermüller A., Buczyłko J., Hofmann K. P. Phosphorylated rhodopsin and heparin induce similar conformational changes in arrestin. J Biol Chem. 1991 Oct 5;266(28):18649–18654. [PubMed] [Google Scholar]
- Palczewski K., Riazance-Lawrence J. H., Johnson W. C., Jr Structural properties of arrestin studied by chemical modification and circular dichroism. Biochemistry. 1992 Apr 28;31(16):3902–3906. doi: 10.1021/bi00131a003. [DOI] [PubMed] [Google Scholar]
- Panitch H. S., Hooper C. J., Johnson K. P. CSF antibody to myelin basic protein. Measurement in patients with multiple sclerosis and subacute sclerosing panencephalitis. Arch Neurol. 1980 Apr;37(4):206–209. doi: 10.1001/archneur.1980.00500530044005. [DOI] [PubMed] [Google Scholar]
- Papermaster D. S. Preparation of retinal rod outer segments. Methods Enzymol. 1982;81:48–52. doi: 10.1016/s0076-6879(82)81010-0. [DOI] [PubMed] [Google Scholar]
- Paterson P. Y. Multiple sclerosis: an immunologic reassessment. J Chronic Dis. 1973 Mar;26(3):119–126. doi: 10.1016/0021-9681(73)90085-4. [DOI] [PubMed] [Google Scholar]
- Poser C. M., Paty D. W., Scheinberg L., McDonald W. I., Davis F. A., Ebers G. C., Johnson K. P., Sibley W. A., Silberberg D. H., Tourtellotte W. W. New diagnostic criteria for multiple sclerosis: guidelines for research protocols. Ann Neurol. 1983 Mar;13(3):227–231. doi: 10.1002/ana.410130302. [DOI] [PubMed] [Google Scholar]
- Rapoport B., Kaufman K. D., Chazenbalk G. D. Cloning of a member of the arrestin family from a human thyroid cDNA library. Mol Cell Endocrinol. 1992 Apr;84(3):R39–R43. doi: 10.1016/0303-7207(92)90038-8. [DOI] [PubMed] [Google Scholar]
- Scott C. F., Spitler L. E. Lymphocytotoxic antibody in multiple sclerosis: activity against T cell subsets and correlation with disease activity. Clin Exp Immunol. 1983 Jul;53(1):133–139. [PMC free article] [PubMed] [Google Scholar]
- Sergott R. C., Brown M. J. Current concepts of the pathogenesis of optic neuritis associated with multiple sclerosis. Surv Ophthalmol. 1988 Sep-Oct;33(2):108–116. doi: 10.1016/0039-6257(88)90162-2. [DOI] [PubMed] [Google Scholar]
- Shinohara T., Singh V. K., Yamaki K., Abe T., Tsuda M., Suzuki S. S-antigen: molecular mimicry may play a role in autoimmune uveitis. Prog Clin Biol Res. 1991;362:163–190. [PubMed] [Google Scholar]
- Smith S. C., Allen P. M. The recognition of self-antigens and autoimmune disease. Curr Opin Immunol. 1991 Feb;3(1):22–25. doi: 10.1016/0952-7915(91)90071-8. [DOI] [PubMed] [Google Scholar]
- Tan E. M. Antinuclear antibodies: diagnostic markers for autoimmune diseases and probes for cell biology. Adv Immunol. 1989;44:93–151. doi: 10.1016/s0065-2776(08)60641-0. [DOI] [PubMed] [Google Scholar]
- Thompson E. J. Laboratory diagnosis of multiple sclerosis: immunological and biochemical aspects. Br Med Bull. 1977 Jan;33(1):28–33. doi: 10.1093/oxfordjournals.bmb.a071391. [DOI] [PubMed] [Google Scholar]
- Traugott U., Scheinberg L. C., Raine C. S. Multiple sclerosis: circulating antigen-reactive lymphocytes. Ann Neurol. 1979 Nov;6(5):425–429. doi: 10.1002/ana.410060509. [DOI] [PubMed] [Google Scholar]
- Wacker W. B., Donoso L. A., Kalsow C. M., Yankeelov J. A., Jr, Organisciak D. T. Experimental allergic uveitis. Isolation, characterization, and localization of a soluble uveitopathogenic antigen from bovine retina. J Immunol. 1977 Dec;119(6):1949–1958. [PubMed] [Google Scholar]
- Wacker W. B., Lipton M. M. Experimental allergic uveitis. I. Production in the guinea pig and rabbit by immunization with retina in adjuvant. J Immunol. 1968 Jul;101(1):151–156. [PubMed] [Google Scholar]
- Yamaki K., Tsuda M., Shinohara T. The sequence of human retinal S-antigen reveals similarities with alpha-transducin. FEBS Lett. 1988 Jul 4;234(1):39–43. doi: 10.1016/0014-5793(88)81298-5. [DOI] [PubMed] [Google Scholar]
- de Kozak Y., Sakai J., Thillaye B., Faure J. P. S antigen-induced experimental autoimmune uveo-retinitis in rats. Curr Eye Res. 1981;1(6):327–337. doi: 10.3109/02713688108998359. [DOI] [PubMed] [Google Scholar]